Abstract
The proinflammatory and catabolic cytokine IL-1β has been implicated in the pathogenesis of osteoarthritis (OA) by mediating synovial inflammation and cartilage degeneration. Although synovial macrophages are suggested to be the source of IL-1β, the mechanism remains unclear. Ectopic deposition of hydroxyapatite (HA) crystals in joints is closely associated with OA and other arthropathies, but the precise role of HA in arthritis pathogenesis has not been clearly demonstrated. Here we show that HA crystals of a particular size and shape can stimulate robust secretion of proinflammatory cytokines IL-1β and IL-18 from murine macrophages in a NLRP3 inflammasome-dependent manner. HA-induced inflammasome activation is dependent on potassium efflux, generation of reactive oxygen species (ROS), and lysosomal damage, but independent of cell death. Mice lacking the inflammasome components are protected against HA-induced neutrophilic inflammation in the air-pouch model of synovitis, and they show decreased joint pathology accompanying spontaneous HA deposition in the ank-deficient mouse model of arthritis. Moreover, calcium crystal positive synovial fluids from some OA patients exhibited inflammasome-stimulatory activity in vitro. These results demonstrate that the NLRP3 inflammasome mediates the pathological effect of HA crystals in vitro and in vivo and suggest a critical role for the inflammasome in the pathogenesis of OA.
Keywords: caspase-1, ASC, basic calcium phosphate crystals
Osteoarthritis (OA) is a progressive joint disease characterized by cartilage degeneration, subchondral bone remodeling, and inflammation of the synovial membrane. Although it is the most common form of arthritis in humans and a leading cause of disability worldwide, its complex pathogenesis remains poorly understood and no disease-modifying drug is currently available (1, 2). Synovial inflammation has been implicated in the development of OA, and the proinflammatory cytokine IL-1β has been identified as one of the key mediators produced by activated synovial macrophages to promote the inflammatory and destructive responses in OA (3, 4). The level of IL-1β is elevated in synovial fluids and the cartilage of OA patients (5). Numerous studies have demonstrated the ability of IL-1β to up-regulate the expression of cartilage-degrading proteases such as matrix metalloproteinases (MMPs) and aggrecanases (A Disintegrin And Metalloproteinase with Thrombospondin Motifs, ADAMTSs), to suppress the biosynthesis of extracellular matrix, and to induce inflammatory mediators and cell infiltration in joints (4, 5). IL-1β antagonists have shown some efficacy in the treatment of OA in animal models (2), and genomic studies have identified the association of genes encoding IL-1α, IL-1β, and IL-1 receptor antagonist (IL1RN) with the development of human OA (1, 6). In addition to IL-1β, IL-18, another member of the IL-1 family cytokines, is also up-regulated in synovial fluids of OA patients and strongly associated with OA severity (7, 8). IL-18 has been shown to inhibit aggrecan synthesis and chondrocyte proliferation as well as induce prostaglandin production and chondrocyte apoptosis (8–10). However, the origin of inflammation in OA and the molecular mechanism by which IL-1β and IL-18 production is stimulated in OA synovium are largely unknown.
Both IL-1β and IL-18 are primarily produced as precursors, pro–IL-1β and pro–IL-18, and require active caspase-1 to cleave them to their mature form and mediate their secretion. Caspase-1 activity is tightly controlled by innate immune complexes defined as inflammasomes. The NLR family member NLRP3, along with the adaptor protein ASC, can activate caspase-1 via inflammasome assembly and result in the secretion of mature IL-1β and IL-18 (11). Thus far, a number of crystalline and particulate substances have been identified as agonists for the NLRP3 inflammasome, such as monosodium urate crystals (MSU), calcium pyrophosphate dihydrate crystals (CPPD), silica, asbestos, aluminum hydroxide (alum), and malaria hemozoin (12). Notably, activation of the NLRP3 inflammasome by intraarticular MSU and CPPD has been indicated to be responsible for the development of arthritis symptoms in gout and pseudogout, respectively (13).
The predominant crystal found in OA joints is hydroxyapatite (HA), a type of basic calcium phosphate crystal and the primary mineral of bones and teeth. Intraarticular deposition of HA crystals occurs in up to 70% of OA cases and strongly correlates with the severity of cartilage deterioration (14–16). Abnormal accumulation of HA can provoke an acute arthritis in some occasions (17) and is also associated with other destructive arthropathies such as calcific periarthritis and Milwaukee shoulder syndrome (18). In addition, as a widely used biomaterial in orthopedics, synthetic HA crystals released from implanted prosthesis were known to cause tissue inflammation and destruction (19). However, currently there is no effective therapy to prevent the deposition or modify the biological effect of HA, nor is the cellular and molecular mechanism underlying HA-associated pathogenesis well understood.
In this study we investigate the mechanism of HA-induced inflammation and demonstrate the essential role of the NLRP3 inflammasome in mediating the pathological response to HA crystals in vitro and in vivo. We showed that synthetic HA crystals of size and shape similar to those found in diseased joints stimulated robust proinflammatory response in a NLRP3 inflammasome-dependent manner both in cultured primary macrophages and in experimental models of arthritis. We found further that activation of the inflammasome by HA shared a common cellular mechanism with other crystalline inflammasome agonists. Collectively, our data suggest that the NLRP3 inflammasome plays a pivotal role in the development of HA deposition diseases of joints. This study might reveal novel targets for therapeutic intervention in the treatment of osteoarthritis.
Results
Specific Forms of HA Crystals Induce the Secretion of IL-1β and IL-18, but Not TNF-α in Vitro.
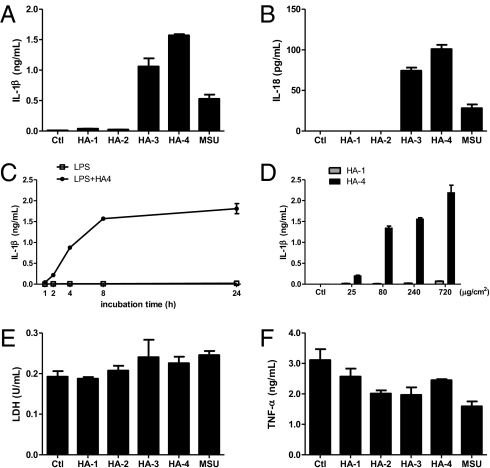
First as a proof of principle that OA involves the induction of IL-1β, we found that synovial fluid from some OA patients (four out of eight) could stimulate murine macrophages to secrete this proinflammatory and catabolic cytokine (Fig. S1). Alizarin Red S staining revealed the presence of calcium crystals in these synovial fluid samples. NLRP3 and ASC, components of the NLRP3 inflammasome, which can activate IL-1β secretion in response to a number of crystalline agonists, were required for this inflammatory response. As hydroxyapatite crystals have been described to be predominant in OA joints (15), we speculated that HA crystals might be the factor within OA synovial fluid that stimulates IL-1β production via activation of the NLRP3 inflammasome. To characterize the inflammatory property of HA crystals, peritoneal macrophages derived from wild-type (WT) mice were primed with LPS and stimulated with different forms of synthetic HA crystals for 8 h (Fig. S2A). Although HA-1, HA-2, HA-3, and HA-4 all shared the same chemical composition, their proinflammatory activities varied greatly with their physical parameters. Whereas crystals of spherical shape (HA-1) and large size (HA-2) exhibited minimal effect on cytokine induction, HA-3 and HA-4 crystals, which resembled the needle-shaped or irregular clump or rod forms of HA aggregates found in the OA synovium (17), stimulated robust secretion of the proinflammatory cytokines IL-1β and IL-18 from LPS-primed macrophages (Fig. 1 A and B). The kinetics of this HA-induced response was examined (Fig. 1C and Fig. S3D), and both HA-3 and HA-4 were observed to trigger IL-1β and IL-18 production in a dose-dependent manner when applied in a pathological range (20) (Fig. 1D and Fig. S3 B, C, and E). In contrast, all four forms of HA crystals displayed equivalent, low levels of cytotoxicity as determined by lactate dehydrogenase (LDH) release assay, and none of them stimulated TNF-α secretion from LPS-primed macrophages (Fig. 1 E and F). Treatment of macrophages with HA crystals alone in the absence of LPS priming did not result in detectable IL-1β production (Fig. S2), excluding the possibility of microbial contamination in these crystal preparations and also in agreement with the “two signal” model in which LPS first stimulated the expression of pro–IL-1β and pro–IL-18, and HA crystals acted as the second hit to induce the processing and secretion of mature forms of cytokines (21).
Fig. 1.
Specific forms of HA crystals induce IL-1β and IL-18 secretion from LPS-primed macrophages. (A, B, E, and F) Macrophages from wild-type mice were primed with 50 ng/mL LPS for 14 h and then either left untreated (Ctl group) or stimulated with different forms of HA crystals (240 μg/cm2) or MSU (200 μg/mL) for 8 h. IL-1β (A), IL-18 (B), and TNF-α (F) released into culture supernatants was measured by ELISA. The cytotoxicity of different treatments was determined by lactate dehydrogenase (LDH) release (E). (C) LPS-primed macrophages were stimulated with HA-4 crystals (240 μg/cm2) and culture supernatants were collected at the indicated time of incubation with HA-4. (D) LPS-primed macrophages were stimulated with the indicated amount of HA-1 or HA-4 crystals for 8 h and IL-1β secretion was measured by ELISA. Determinations were performed in triplicate and expressed as the mean ± SEM; several error bars in C are too small to be shown in the graph. Results are representative of at least four independent experiments.
HA-Induced IL-1β and IL-18 Production Depends on NLRP3, ASC, and Caspase-1, but Not NLRC4.
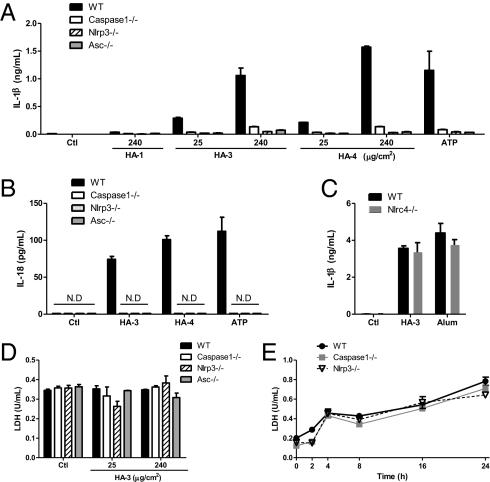
The NLRP3 inflammasome can mediate caspase-1 activation in response to various stimuli including pathogen-associated moleculars as well as host-derived stress signals such as extracellular ATP and MSU. Because HA crystals specifically induced the secretion of caspase-1–dependent cytokines IL-1β and IL-18, and their crystalline nature was reminiscent of a number of identified agonists of the NLRP3 inflammasome, we investigated whether the NLRP3 inflammasome was required for HA-induced inflammatory response in vitro. HA-induced IL-1β and IL-18 secretion was markedly reduced in LPS-primed macrophages derived from NLRP3-, ASC-, and caspase-1–deficient mice (Fig. 2 A and B and Fig. S4), but not affected in macrophages lacking NLRC4, another NLR family member that mediates caspase-1 activation in response to some Gram-negative bacteria via the assembly of a different inflammasome (11, 22) (Fig. 2C). Moreover, the degree of cell death in WT and inflammasome-deficient macrophages after HA treatment did not differ (Fig. 2 D and E), suggesting that HA-induced IL-1β/IL-18 release was not a consequence of cell lysis, and that the NLRP3 inflammasome was not required for HA-induced cytotoxicity.
Fig. 2.
HA-induced cytokine production depends on NLRP3, ASC, and caspase-1, but not NLRC4. (A) LPS-primed peritoneal macrophages from WT, ASC-deficient, NLRP3-deficient, and caspase-1–deficient mice were stimulated with HA-1, HA-3, or HA-4 crystals of indicated dose. As a positive control, cells were pulsed with ATP (5 mM) for 20 min. Culture supernatants were collected 8 h later to analyze IL-1β secretion. (B and C) LPS-primed WT, Asc−/−, Nlrp3−/−, caspase-1−/−, or Nlrc4−/− macrophages were treated with HA crystals (240 μg/cm2), ATP (5 mM), or Imject alum (500 μg/mL) for 8 h. IL-1β (C) or IL-18 (B) released into culture supernatants was measured by ELISA. (D and E) LPS-primed WT, caspase-1−/−, and Nlrp3−/− macrophages were stimulated with HA-3 crystals of indicated dose for 8 h (D) or 240 μg/cm2 HA-3 crystals for the indicated period (E). The degree of cell death was measured by the amount of LDH released into culture supernatant. N.D, nondetectable. Determinations were performed in triplicate and expressed as the mean ± SEM. Results are representative of at least four independent experiments.
HA-Induced IL-1β Requires Potassium Efflux, Reactive Oxygen Species (ROS) Generation, Phagocytosis, and Lysosomal Protease Release.
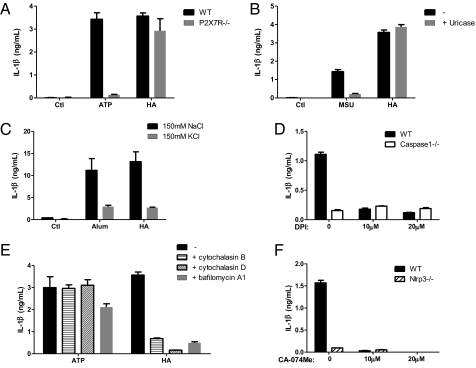
ATP and MSU released from dying or injured cells into the extracellular milieu may activate the NLRP3 inflammasome (13, 23, 24), and the inflammatory action of some particulates such as alum and hemozoin has been related to cell death and release of such factors (25, 26). To exclude the possibility that NLRP3 inflammasome activation was in response to ATP release caused by HA-induced cellular damage, we used macrophages deficient in P2X7R, a key molecule for ATP sensing, and found that they responded normally to HA crystals (Fig. 3A). Moreover, treatment with uricase, which degraded MSU, did not impair HA-induced IL-1β release (Fig. 3B). Thus, by eliminating the effect of ATP and MSU, we conclude that HA crystals stimulate the activation of inflammasome directly, independently of its cytotoxicity. Next, to elucidate the cellular mechanism by which HA triggers the NLRP3 inflammasome, we assessed the requirement of potassium efflux and generation of ROS, both of which have been implicated in inflammasome activation in response to the majority of known NLRP3 agonists (12). HA-induced IL-1β secretion was markedly suppressed when potassium efflux was prevented by culturing cells in a medium that contained a high concentration of K+ (Fig. 3C). Pretreatment of macrophages with diphenyleneiodonium chloride (DPI), a chemical inhibitor of ROS production, also diminished inflammasome-dependent IL-1β secretion in response to HA (Fig. 3D). Together, these suggest that both K+ efflux and ROS production are critical for HA-induced inflammasome activation. We then examined whether the phagocytic function of macrophages was required for HA-induced inflammasome activation, as for other crystalline NLRP3 agonists like alum and MSU. Either inhibiting actin polymerization with cytochalasin B or cytochalasin D, or inhibiting lysosome acidification with bafilomycin A1 effectively blocked HA-induced IL-1β secretion; meanwhile, IL-1β production triggered by extracellular ATP, which engages P2X7R but not the phagocytic machinery to activate the NLRP3 inflammasome, remained largely unaffected under these treatments, confirming that cells were viable with competent inflammasome in the presence of these drugs (Fig. 3E). Moreover, in accordance with previous studies showing the importance of cathepsin B or other protease release upon crystal-induced lysosomal damage in inflammasome activation, we found that HA-induced IL-1β secretion was abrogated in the presence of the cathepsin B inhibitor CA-074Me (Fig. 3F). Collectively, these data demonstrate that HA-induced inflammasome activation depends on K+ efflux, ROS production, phagocytosis, and cathepsin B-like activity, sharing a common cellular mechanism with other crystalline agonists of the NLRP3 inflammasome.
Fig. 3.
HA-induced IL-1β secretion requires potassium efflux, ROS generation, actin polymerization, lysosome acidification, and lysosomal protease activity. (A) LPS-primed macrophages from WT or P2X7R−/− mice were treated with HA-3 (240 μg/cm2) for 8 h or pulsed with ATP (5 mM) for 20 min. (B) LPS-primed WT macrophages were stimulated 8 h with HA-3 (240 μg/cm2) or MSU (200 μg/mL) in the presence or absence of 2 U/mL uricase. (C) LPS-primed WT macrophages were stimulated with HA-3 crystals (240 μg/cm2) or Imject alum (500 μg/mL) in serum-free buffer containing either 150 mM NaCl or 150mM KCl. (D and F) LPS-primed macrophages from WT mice and caspase1−/− or Nlrp3−/− mice were incubated with the indicated dose of diphenyleneiodonium chloride (DPI) or CA-074Me for 30 min before the addition of HA-3 crystals (240 μg/cm2). (E) LPS-primed WT macrophages were pretreated with cytochalasin B (20 μM), or cytochalasin D(10 μM), or bafilomycin A1 (50 nM) 30 min before the addition of HA-3 crystals (240 μg/cm2) or ATP (5 mM). (A–F) IL-1β release was measured by ELISA from culture supernatant 8 h poststimulation. Determinations were performed in triplicate and expressed as the mean ± SEM. Results are representative of at least two independent experiments.
HA-Induced Inflammation Depends on NLRP3, ASC, and Caspase-1 in the Air Pouch Model of Synovitis.
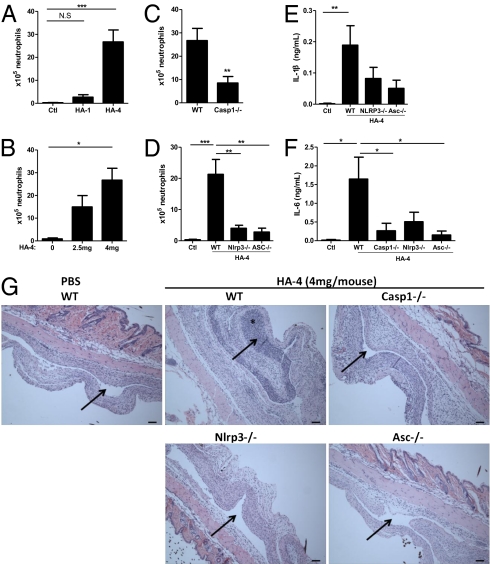
Our in vitro results presented above have revealed the potent capability of certain forms of HA crystals to induce a proinflammatory response via activation of the NLRP3 inflammasome. To validate the physiological relevance of this, especially in the context of HA-associated joint disease, we next studied HA-induced in vivo responses using a murine model of joint inflammation. Because it proved to be difficult to inject a reproducible dose of crystals into mouse joints and assess the resulting phenotype in a quantitative manner, we applied the established air pouch model in which an accessible space lined with a synovium-like membrane containing fibroblastic and phagocytic cells was generated to mimic the local environment of a joint (27). HA crystals were suspended in PBS and injected into the air pouches, and the pouch exudates were analyzed 8 h postinjection for leukocyte infiltration and cytokine production in comparison with PBS-injected controls. Injection of the small, fibrous HA-4 crystals into wild-type mice evoked a robust influx of neutrophils into the pouch cavity dose dependently, whereas HA-1 crystals of similar size but spherical shape displayed little ability to induce this neutrophilic response, in strong accordance with their different proinflammatory effect in vitro (Fig. 4 A and B). Wild-type mice also secreted a significant amount of IL-1β and its downstream effector cytokine IL-6 into the pouch cavity in response to HA-4 (Fig. 4 E and F). In contrast, both HA-induced neutrophil recruitment and cytokine production were largely abrogated in mice lacking the inflammasome components NLRP3, ASC or caspase-1 (Fig. 4 C–F). These reactions were also reflected in the histological features of air pouches. As shown in Fig. 4G, the PBS-injected pouches were lined with a thin synovium-like membrane and a layer of vascularized fibrous and adipose tissue underneath, whereas wild-type pouches injected with HA-4 crystals showed a disrupted architecture with massive cell infiltration throughout the pouch cavity and the membrane lining, accompanied by swelling of the surrounding tissues (Fig. 4G). By comparison, there was marked attenuation in both leukocyte ingress and swelling of the synovium-like membrane in the HA-4 crystal-injected air pouches of Nlrp3−/−, Asc−/−, and Caspase-1−/− mice. These results correlated well with our in vitro data, demonstrating that the NLRP3 inflammasome is indispensable for HA-induced tissue inflammation in vivo and thus might play a pivotal role in the pathogenesis of HA deposition disease of the joint, including OA.
Fig. 4.
HA-induced inflammation requires NLRP3, ASC and caspase-1 in the air pouch model of synovitis. The s.c. air pouches were raised on the dorsum of 10- to 12-wk-old mice of indicated genotypes (Materials and Methods). 1 mL suspension of HA crystals (4 mg, unless indicated) in PBS or PBS alone was injected into the air pouches. 8 hours postinjection, mice were killed and the pouch exudates were harvested by a lavage with 3 mL PBS containing 5 mM EDTA. (A–D) The infiltrated neutrophils were identified with hematoxylin and eosin (H&E) staining and counted using a hemocytometer. (C–G) 4 mg HA-4 crystals in suspension were injected into air pouches of age- and sex-matched WT, Casp1−/−, Nlrp3−/−, or Asc−/− mice. (E and F) Supernatants of air pouch exudates were collected by centrifugation. IL-1β and IL-6 production were measured by ELISA. Values are shown as the mean ± SEM of at least seven mice per group. *P < 0.05, **P < 0.01, ***P < 0.001; unpaired Student's t test or one-way ANOVA and post hoc test. (G) Sagittal sections of the air pouches with surrounding tissues were stained with H&E. (Scale bars, 100 μm.) Arrows, pouch cavities; asterisk, neutrophil infiltration. Images are representative of at least seven mice per group. All results were repeated by at least two separate experiments.
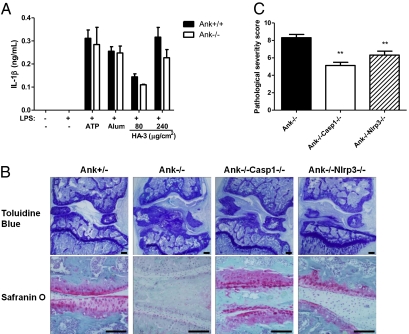
Mice Lacking NLRP3 or Caspase-1 Showed Decreased Joint Pathology in an ANK-Deficient Model of Arthritis.
Formation of HA crystals in vivo is potently inhibited by extracellular inorganic pyrophosphate (ePPi), the secretion of which relies on a membrane transporter encoded by the ANK gene in mice and the ANKH gene in humans (28). Regulatory mutations and altered expression levels of ANKH have been observed in patients with sporadic forms of osteoarthritis or chondrocalcinosis (29, 30). In the ANK knockout mice described previously, HA crystals are extensively deposited in articular cartilage and synovial fluid, resulting in joint space narrowing, cartilage erosion, formation of bony outgrowths, and eventually joint immobility (31). Although the disease distribution and severity in this murine model do not precisely mimic any single form of human arthritis, many of the pathological features in ANK-deficient mice are reminiscent of the hallmark features in OA and other HA deposition diseases of joint, including ectopic calcification, fibrosis, cartilage destruction, and osteophyte formation (32). We found that macrophages derived from Ank−/− mice did not secrete IL-1β spontaneously in the absence of in vitro stimuli, but they showed a normal response to NLRP3 inflammasome agonists including extracellular ATP, alum, and HA crystals (Fig. 5A). Therefore, we crossed ANK-deficient mice with caspase-1–deficient or NLRP3-deficient mice to assess whether the lack of inflammasome activation in double knockout mice was able to suppress the progression of OA-like arthropathy induced by ectopic HA deposition. Three-month-old Ank−/− mice developed severe joint pathology, manifested by mineral deposition on the articular surface, massive cell infiltration, and formation of extra fibrous and bony tissues in the synovial space. The integrity of articular cartilage was also disrupted, accompanied by the loss of proteoglycan as revealed by Safranin O staining (Fig. 5B). By comparison, Ank−/−Casp1−/− and Ank−/−Nlrp3−/− mice displayed significantly decreased joint pathology (Fig. 5 B and C). Although HA accumulation was not prevented in these double knockout mice, there was less fibrosis and cell infiltration in the synovial cavity, suggesting a blunted inflammatory response to the HA crystals. In addition, proteoglycan deposition along the articular surface was preserved and the overall joint architecture was largely maintained in double knockout mice. Therefore, lack of inflammasome activation attenuated the arthritis-like phenotype in the ANK-deficient model. Altogether, our results suggest that the NLRP3 inflammasome might be critical for mediating synovitis and joint destruction in response to ectopic HA deposition during OA pathogenesis.
Fig. 5.
Mice lacking NLRP3 or caspase-1 showed decreased joint pathology in an ank-deficient model of arthritis. (A) Peritoneal macrophages from Ank+/+ or Ank−/− mice were either primed with 50 ng/mL LPS for 14 h or left untreated. LPS-primed macrophages were stimulated with ATP (5 mM), Imject alum (500 μg/mL), or indicated dose of HA-3 crystals. Culture supernatants were harvested 8 h later and IL-1β secretion was measured by ELISA. (B) Ank−/−Casp1−/− and Ank−/−Nlrp3−/− mice were generated and analyzed in comparison with Ank−/− or Ank+/− mice of the same generation (Materials and Methods). Frontal sections of the knee joints from 3-mo-old mice of indicated genotype were stained with toluidine blue to reveal gross morphology and cellularity (B, Upper) or stained with Safranin O to examine proteoglycan deposition (B, Lower). Images were obtained at 4× (Upper) and 20× (Lower) magnification and are representative of five mice per group for Ank+/− and at least seven mice per group for Ank−/−, Ank−/−Casp1−/−, and Ank−/−Nlrp3−/−. (Scale bars, 150 μm.) (C) The pathological severity of each joint was scored blindly according to the degree of ectopic mineral deposition, cell infiltration, and proteoglycan loss. **P < 0.01, one-way ANOVA and post hoc test.
Discussion
Deposition of HA crystals in joints is uniquely associated with OA but not other forms of arthritis like rheumatoid arthritis (16). Although the presence of intraarticular HA crystals has been correlated with severe joint pathology characterized by marked synovial hyperplasia, aggravated joint degeneration, and large joint effusions, it is still controversial whether such crystals are a primary cause of arthritis or a secondary consequence of joint damage (14, 33), and the mechanism of their pathogenicity has not been clarified in vivo. While this manuscript was in preparation, a recent study was published which showed the involvement of the NLRP3 inflammasome in basic calcium phosphate crystal-induced IL-1β secretion in vitro (34). However, an in vivo study by the same group did not reveal an effect of the inflammasome on octacalcium phosphate crystal-induced murine peritonitis (35). Using primary macrophages and experimental models of arthritis, both our in vitro and in vivo data presented here clearly identify the NLRP3 inflammasome as an essential player that mediates the pathological response to HA crystals by activating caspase-1 and resulting in the secretion of IL-1β and IL-18. The IL-1β/IL-18–dependent proinflammatory and catabolic activity of HA crystals then provides a mechanism for ectopic deposition of HA crystals as a primary event that drives synovitis and cartilage degradation during the initiation and progression of OA.
As current treatments for OA are predominantly directed toward relief of pain and maintenance of quality of life, and also no known drug prevents the formation of HA crystals (2, 16), our findings imply that therapeutic approaches to block the action of IL-1β or to inhibit the activation of inflammasome might be capable of protecting articular tissues from the detrimental effects of HA crystals and therefore ameliorating OA or other HA-associated arthropathies. On the other side, it is likely that certain mutations in NLRP3, like those resulting in autoinflammatory cryopyrin-associated periodic syndromes (CAPS) (11), might also predispose patients to developing severe OA.
Expression of the inflammasome components in OA synovium has been described and active caspase-1 has been detected with markedly increased cellularity in articular cartilage of human OA (36–38). Here we demonstrated that in comparison with the human monocytic cell line THP1, the expressions of ASC, caspase-1, and especially NLRP3 mRNA were very low in CD33− nonhematopoietic cells, including primary human chondrocytes and synoviocytes derived from OA patients (Fig. S5). This suggests that synovial macrophages are the principal source of IL-1β and IL-18 in joints in response to HA crystals and indicates that the previously described ability of HA to stimulate the synthesis and secretion of prostaglandin, cytokines, and MMPs in vitro (14, 39) is likely a consequence of HA-induced IL-1β/IL-18 from macrophages and not a direct result of HA's action upon chondrocytes or synoviocytes. On the basis of these findings, we postulate a model for the pathogenesis of HA deposition disease of joint, in which accumulation of intraarticular HA crystals resulting from unregulated calcification or mechanical friction activates the NLRP3 inflammasome in synovial macrophages, leading to IL-1β and IL-18 release; IL-1β and IL-18 then target chondrocytes and synovial lining cells to up-regulate cartilage-degrading enzymes and suppress extracellular matrix synthesis, resulting in joint destruction. As active caspase-1 has been implicated in the processing of multiple protein substrates and mediating unconventional protein secretion (40), further study is necessary to examine whether additional factors regulated by HA-induced inflammasome activity contribute to OA pathogenesis.
Synthetic HAs have been widely applied as bioceramics in orthopedics surgery for several decades. In light of our data presented above, the use of HA particles as bone substitutes or coating material of prosthetic implants requires caution, for the wear- induced debris of HA might stimulate potent inflammatory and destructive response in surrounding tissues. Indeed, HA particles released from arthroplasty material could be a cause of implant failure depending on their characteristics (19). In our study, HA crystals with identical chemical composition but different physical parameters exhibited varied capacity to induce inflammasome activation both in vitro and in vivo. Therefore, the heterogeneity in size or shape of intraarticular HA crystals might account for the variable clinical manifestations of cartilage calcification from asymptomatic to highly pathogenic. It is tempting to speculate that activation of the NLRP3 inflammasome might involve a sensing mechanism that allows the host to recognize and distinguish certain physical features of an incipient insult. Elucidating such a mechanism might shed light on the development of safer bioceramics.
Materials and Methods
Mice.
The generation of mice deficient in NLRP3, ASC, caspase-1, NLRC4, P2X7R, and Ank has been described previously (31, 41–44). NLRP3-, caspase-1– and ASC-deficient mice were fully backcrossed to C57BL/6 background, and NLRC4-deficient mice were backcrossed six generations. Age- and sex-matched C57BL/6 mice from the National Cancer Institute were used as WT controls. Ank+/− mice were kindly provided by Dr. D. Kingsley (Stanford University, Stanford, CA); generation of Ank−/−, Ank−/−Casp1−/−, and Ank−/−Nlrp3−/− mice are detailed in SI Materials and Methods. All procedures used in this study complied with federal guidelines and were approved by the Yale Animal Care and Use Committee.
HA Crystals.
The synthesis and verification of HA-1 (0–25 μm, spheric), HA-2 (80–160 μm, irregular), and HA-4 (0–25 μm, needle-shaped) crystals were described previously (19). HA-3 (5–30 μm, heterogeneous in shape) crystals were purchased from Cementek. All crystals are endotoxin-free.
Air Pouch.
The generation of s.c. air pouch with an accessible space and a synovium-like lining membrane has been described previously (27). Briefly, air pouches were raised on the dorsum of 10- to 12-wk-old WT, Caspase-1−/−, Asc−/−, and Nlrp3−/− mice by s.c. injection of 4 mL of sterile air on day 0, followed by a second injection of 3 mL of sterile air into the pouch on day 3. On day 7, indicated doses of HA crystals suspended in 1-mL endotoxin-free PBS were injected into the pouch cavity. Eight hours postinjection, the mice were killed and the air pouches were lavaged with 3 mL PBS containing 5 mM EDTA. IL-1β and IL-6 were measured in the supernatants of pouch exudates by ELISA. Leukocyte population in the exudates was manually counted with a hemocytometer following hematoxylin and eosin (H&E) staining with Diff-Quick (Dade Behring) of cytospin slides. For histological analysis, air pouches with surrounding tissues were fixed and embedded in paraffin. Sagittal sections were stained with H&E.
Joint Histology.
Knee joints from Ank+/−, Ank−/−, Ank−/−Casp1−/− and Ank−/−Nlrp3−/− mice were dehydrated in a graded ethanol series and embedded without decalcification in methylmethacrylate as described previously (45). Frontal sections were stained with toluidine blue (pH 3.7) to reveal general morphology and cellularity, or stained with Safranin O to show the deposition of proteoglycan. The pathological changes of joints were evaluated blindly, taking into account the degree of inflammation and cartilage destruction. Ectopic mineral deposition, cell infiltration, and proteoglycan loss were scored separately on a scale of 1–3 units and the scores were pooled to represent the overall pathological severity.
Statistical Analysis.
Data are expressed as mean ± SEM. Unpaired Student's t tests were performed for two-group analysis; one-way ANOVA and post hoc analysis were used for multigroup comparison. P values <0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank Peter Cresswell for discussion, Joseph Craft for discussion and critical review of the manuscript, and Fran Manzo for manuscript preparation. We thank Tuere Wilder of Bruce Cronstein's laboratory at New York University Medical Center for technical assistance with the murine air pouch model. We thank the Physiology Core in Yale Core Center for Musculoskeletal Disorders for histological analysis of joints. We are grateful to David Kingsley at Stanford University School of Medicine for providing Ank-deficient mice. R.A.F. is an investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1111101108/-/DCSupplemental.
References
- 1.Abramson SB, Attur M. Developments in the scientific understanding of osteoarthritis. Arthritis Res Ther. 2009;11:227. doi: 10.1186/ar2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iqbal I, Fleischmann R. Treatment of osteoarthritis with anakinra. Curr Rheumatol Rep. 2007;9:31–35. doi: 10.1007/s11926-007-0019-9. [DOI] [PubMed] [Google Scholar]
- 3.Bondeson J, et al. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum. 2010;62:647–657. doi: 10.1002/art.27290. [DOI] [PubMed] [Google Scholar]
- 4.Pujol JP, et al. Interleukin-1 and transforming growth factor-beta 1 as crucial factors in osteoarthritic cartilage metabolism. Connect Tissue Res. 2008;49:293–297. doi: 10.1080/03008200802148355. [DOI] [PubMed] [Google Scholar]
- 5.Daheshia M, Yao JQ. The interleukin 1beta pathway in the pathogenesis of osteoarthritis. J Rheumatol. 2008;35:2306–2312. doi: 10.3899/jrheum.080346. [DOI] [PubMed] [Google Scholar]
- 6.Loughlin J, Dowling B, Mustafa Z, Chapman K. Association of the interleukin-1 gene cluster on chromosome 2q13 with knee osteoarthritis. Arthritis Rheum. 2002;46:1519–1527. doi: 10.1002/art.10260. [DOI] [PubMed] [Google Scholar]
- 7.Denoble AE, et al. Uric acid is a danger signal of increasing risk for osteoarthritis through inflammasome activation. Proc Natl Acad Sci USA. 2011;108:2088–2093. doi: 10.1073/pnas.1012743108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inoue H, et al. High levels of serum IL-18 promote cartilage loss through suppression of aggrecan synthesis. Bone. 2008;42:1102–1110. doi: 10.1016/j.bone.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 9.John T, Kohl B, Mobasheri A, Ertel W, Shakibaei M. Interleukin-18 induces apoptosis in human articular chondrocytes. Histol Histopathol. 2007;22:469–482. doi: 10.14670/HH-22.469. [DOI] [PubMed] [Google Scholar]
- 10.Futani H, et al. Relation between interleukin-18 and PGE2 in synovial fluid of osteoarthritis: A potential therapeutic target of cartilage degradation. J Immunother. 2002;25(Suppl 1):S61–S64. doi: 10.1097/00002371-200203001-00009. [DOI] [PubMed] [Google Scholar]
- 11.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 12.Jin C, Flavell RA. Molecular mechanism of NLRP3 inflammasome activation. J Clin Immunol. 2010;30:628–631. doi: 10.1007/s10875-010-9440-3. [DOI] [PubMed] [Google Scholar]
- 13.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 14.Cheung HS. Role of calcium-containing crystals in osteoarthritis. Front Biosci. 2005;10:1336–1340. doi: 10.2741/1623. [DOI] [PubMed] [Google Scholar]
- 15.McCarthy GM, Cheung HS. Point: Hydroxyapatite crystal deposition is intimately involved in the pathogenesis and progression of human osteoarthritis. Curr Rheumatol Rep. 2009;11:141–147. doi: 10.1007/s11926-009-0020-6. [DOI] [PubMed] [Google Scholar]
- 16.Whelan LC, Morgan MP, McCarthy GM. Basic calcium phosphate crystals as a unique therapeutic target in osteoarthritis. Front Biosci. 2005;10:530–541. doi: 10.2741/1549. [DOI] [PubMed] [Google Scholar]
- 17.Simon RR, Koenigsknecht SJ. Emergency Orthopedics: The Extremities. 4th Ed. New York: McGraw-Hill; 2001. p. 39. [Google Scholar]
- 18.Molloy ES, McCarthy GM. Hydroxyapatite deposition disease of the joint. Curr Rheumatol Rep. 2003;5:215–221. doi: 10.1007/s11926-003-0070-0. [DOI] [PubMed] [Google Scholar]
- 19.Laquerriere P, et al. Importance of hydroxyapatite particles characteristics on cytokines production by human monocytes in vitro. Biomaterials. 2003;24:2739–2747. doi: 10.1016/s0142-9612(03)00089-9. [DOI] [PubMed] [Google Scholar]
- 20.McCarthy GM, Cheung HS, Abel SM, Ryan LM. Basic calcium phosphate crystal-induced collagenase production: Role of intracellular crystal dissolution. Osteoarthritis Cartilage. 1998;6:205–213. doi: 10.1053/joca.1998.0113. [DOI] [PubMed] [Google Scholar]
- 21.Latz E. The inflammasomes: Mechanisms of activation and function. Curr Opin Immunol. 2010;22:28–33. doi: 10.1016/j.coi.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mariathasan S, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 23.Mariathasan S, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 24.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 25.Kool M, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205:869–882. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Griffith JW, Sun T, McIntosh MT, Bucala R. Pure hemozoin is inflammatory in vivo and activates the NALP3 inflammasome via release of uric acid. J Immunol. 2009;183:5208–5220. doi: 10.4049/jimmunol.0713552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu-Bryan R, Scott P, Sydlaske A, Rose DM, Terkeltaub R. Innate immunity conferred by Toll-like receptors 2 and 4 and myeloid differentiation factor 88 expression is pivotal to monosodium urate monohydrate crystal-induced inflammation. Arthritis Rheum. 2005;52:2936–2946. doi: 10.1002/art.21238. [DOI] [PubMed] [Google Scholar]
- 28.Ryan LM. The ank gene story. Arthritis Res. 2001;3:77–79. doi: 10.1186/ar143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes AE, McGibbon D, Woodward E, Dixey J, Doherty M. Localisation of a gene for chondrocalcinosis to chromosome 5p. Hum Mol Genet. 1995;4:1225–1228. doi: 10.1093/hmg/4.7.1225. [DOI] [PubMed] [Google Scholar]
- 30.Zaka R, Williams CJ. Role of the progressive ankylosis gene in cartilage mineralization. Curr Opin Rheumatol. 2006;18:181–186. doi: 10.1097/01.bor.0000209432.36355.6e. [DOI] [PubMed] [Google Scholar]
- 31.Gurley KA, et al. Mineral formation in joints caused by complete or joint-specific loss of ANK function. J Bone Miner Res. 2006;21:1238–1247. doi: 10.1359/jbmr.060515. [DOI] [PubMed] [Google Scholar]
- 32.Ho AM, Johnson MD, Kingsley DM. Role of the mouse ank gene in control of tissue calcification and arthritis. Science. 2000;289:265–270. doi: 10.1126/science.289.5477.265. [DOI] [PubMed] [Google Scholar]
- 33.Pritzker KP. Counterpoint: Hydroxyapatite crystal deposition is not intimately involved in the pathogenesis and progression of human osteoarthritis. Curr Rheumatol Rep. 2009;11:148–153. doi: 10.1007/s11926-009-0021-5. [DOI] [PubMed] [Google Scholar]
- 34.Pazár B, et al. Basic calcium phosphate crystals induce monocyte/macrophage IL-1β secretion through the NLRP3 inflammasome in vitro. J Immunol. 2011;186:2495–2502. doi: 10.4049/jimmunol.1001284. [DOI] [PubMed] [Google Scholar]
- 35.Narayan S, et al. Octacalcium phosphate (OCP) crystals induce inflammation in vivo through IL-1 but independent of the NLRP3 inflammasome. Arthritis Rheum. 2010;63(2):422–433. doi: 10.1002/art.30147. [DOI] [PubMed] [Google Scholar]
- 36.Kolly L, et al. Expression and function of the NALP3 inflammasome in rheumatoid synovium. Immunology. 2010;129:178–185. doi: 10.1111/j.1365-2567.2009.03174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosengren S, Hoffman HM, Bugbee W, Boyle DL. Expression and regulation of cryopyrin and related proteins in rheumatoid arthritis synovium. Ann Rheum Dis. 2005;64:708–714. doi: 10.1136/ard.2004.025577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saha N, et al. Interleukin-1beta-converting enzyme/caspase-1 in human osteoarthritic tissues: Localization and role in the maturation of interleukin-1beta and interleukin-18. Arthritis Rheum. 1999;42:1577–1587. doi: 10.1002/1529-0131(199908)42:8<1577::AID-ANR3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 39.Schumacher HR. Osteoarthritis: The role of articular crystals. Ariz Med. 1978;35:23–25. [PubMed] [Google Scholar]
- 40.Keller M, Rüegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 41.Sutterwala FS, et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Lara-Tejero M, et al. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J Exp Med. 2006;203:1407–1412. doi: 10.1084/jem.20060206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuida K, et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 44.Solle M, et al. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- 45.Kacena MA, Troiano NW, Coady CE, Horowitz MC. HistoChoice as an alternative to formalin fixation of undecalcified bone specimens. Biotech Histochem. 2004;79:185–190. doi: 10.1080/10520290400015506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.