Abstract
Hearing loss often results in tinnitus and auditory cortical map changes, leading to the prevailing view that the phantom perception is associated with cortical reorganization. However, we show here that tinnitus is mediated by a cortical area lacking map reorganization. High-frequency hearing loss results in two distinct cortical regions: a sensory-deprived region characterized by a decrease in inhibitory synaptic transmission and a normal hearing region showing increases in inhibitory and excitatory transmission and map reorganization. Hearing-lesioned animals displayed tinnitus with a pitch in the hearing loss range. Furthermore, drugs that enhance inhibition, but not those that reduce excitation, reversibly eliminated the tinnitus behavior. These results suggest that sensory deprivation-induced homeostatic down-regulation of inhibitory synapses may contribute to tinnitus perception. Enhancing sensory input through map reorganization may plausibly alleviate phantom sensation.
Keywords: auditory cortex, GABA, phantom pain, tonic inhibition, deafness
Tinnitus, the perception of sounds in the absence of acoustic stimuli, often occurs as the result of hearing loss. Despite its simple origins, the mechanisms underlying the phantom perception remain elusive (1–5). Although often arising from peripheral hearing loss, tinnitus persists after auditory nerve transection or lesions of the cochlear nucleus, suggesting the involvement of more central mechanisms (6, 7). Recent studies revealed that abnormal auditory cortex activation and cortical map reorganization are correlated with the occurrence and severity of tinnitus in patients and model animals (8–12). Hearing loss normally associated with tinnitus leads to altered spontaneous activity and map reorganization, both of which are prevented if the trauma is followed by enriched acoustic experience (13–16). These findings suggest that cortical map reorganization may cause abnormal cortical activity and tinnitus, and prevention and reversal of such reorganization could alleviate tinnitus symptoms (5, 16, 17).
Although Hebbian plasticity is believed to be the primary mediator of long-term map reorganization, non-Hebbian homeostatic plasticity may also be activated by altered sensory input (18, 19). Cochlear ablation, for example, weakens inhibitory synapses and strengthens excitatory synapses, resulting in enhanced neuronal excitability in auditory cortex (20). These effects could potentially lead to elevated spontaneous cortical activity and tinnitus (21, 22). Because map reorganization generally increases sensory-driven activity in the previously sensory-deprived neurons, it may attenuate or reverse homeostatic up-regulation of neuronal excitability, thereby reducing or eliminating tinnitus.
In this study, we investigated hearing loss-induced cortical map reorganization, synaptic plasticity and tinnitus behaviors. We found that high-frequency hearing loss differentially alters synaptic transmissions in two zones of primary auditory cortex (AI) that represent the hearing-loss vs. normal-hearing frequency ranges. In the low-characteristic frequency (CF) zone, where neurons represent normal hearing frequencies, both excitatory and inhibitory synaptic transmissions were enhanced. In the high-CF zone, where neurons represent the hearing-loss frequencies, inhibitory synaptic transmission was reduced and excitatory synaptic transmission was not changed. Furthermore, animals with high-frequency hearing loss showed behavioral signs of tinnitus with the pitch in the hearing-loss frequency range. Drugs that enhance GABA-mediated inhibition, not one that reduces excitation, reversibly abolished the tinnitus behavior. These results suggest that tinnitus is likely caused by homeostatic down-regulation of inhibitory synapses in the high-CF zone.
Results
Hearing Lesions Differentially Alter Sound Representations in Low- and High-CF Areas.
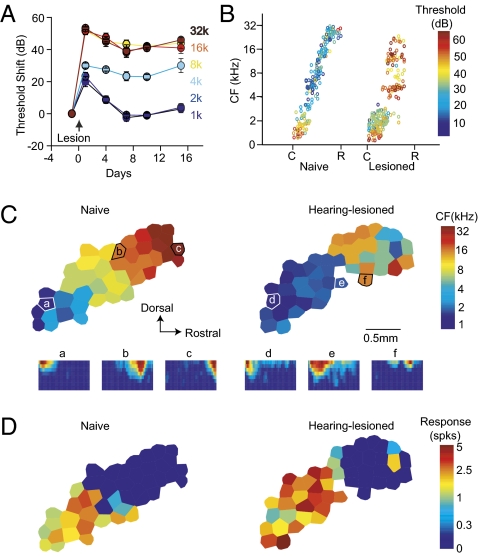
We first examined hearing loss-induced cortical map reorganization. High-frequency hearing loss was made in adult rats by exposing them to a 4-kHz tone at 123 dB for 7 h. The threshold shift, measured from auditory brainstem responses, recovered for low frequencies at 1 and 2 kHz in 7 d, but persisted for high frequencies at 4, 8, 16, and 32 kHz for at least 15 d (Fig. 1A). Cortical mapping 7–15 d after the hearing lesion revealed higher thresholds for high CF neurons in hearing-lesioned animals (P < 0.05 for CFs > 3.5 kHz; P > 0.05 for CFs < 3.5 kHz; Fig. 1B). In addition, the receptive field (RF) of neurons in rostral AI was often discontinuous with multiple peaks (Fig. 1C, f Inset). Consequently, the CF of high frequency-tuned neurons is not always precisely quantifiable. CF maps were thus less tonotopic in rostral AI, and the boundary between rostral AI and the anterior auditory field was determined somewhat arbitrarily (Fig. 1C). Neurons in caudal AI were well tuned with normal thresholds (Fig. 1C, d Inset and e Inset). Cortical areas tuned to frequencies <3.5 kHz expanded in hearing-lesioned animals compared with controls (control, 1.15 ± 0.13 mm2; hearing lesioned, 2.26 ± 0.32 mm2; t8 = 3.753, P < 0.01).
Fig. 1.
Cortical map reorganization after high-frequency hearing loss separates the map into two distinct areas. (A) ABR threshold shift after hearing lesion. (B) CF and threshold (color-coded) as a function of the position along the tonotopic axis. Note the threshold increase for frequencies >4 kHz. C, caudal; R, rostral. (C) Cortical maps and receptive fields in naïve and hearing-lesioned animals. Underneath are receptive fields labeled according to recording sites on the map. (D) Maps of response magnitude to a 2-kHz and 50-dB tone. Both responsive area and response magnitude increased in the hearing-lesioned animal.
Enlarged cortical representations of low-frequency sound in hearing-lesioned animals could result from a cutoff of responses to the hearing loss frequencies (a hearing lesion effect), an increase in low-frequency responses (a plasticity effect), or both. To determine whether the hearing lesion resulted in plasticity effects in low-CF neurons in caudal AI, we examined cortical responses to a 2-kHz tone at 50 dB SPL. The AI area responsive to such a tone was enlarged in hearing-lesioned animals (control, 1.67 ± 0.23 mm2; hearing lesioned, 2.39 ± 0.23 mm2; t8 = 2.474, P < 0.05; Fig. 1D), and the response magnitude also increased (control, 1.81 ± 0.08 spikes per tone; hearing lesioned, 2.44 ± 0.23 spikes per tone; t8 = 3.216, P < 0.05). Thus, the observed expansion of low-frequency representations was, at least partly, a result of enhanced cortical responses to low-frequency tones.
Hearing Lesions Enhance Excitatory Synaptic Transmission in the Low-CF Area.
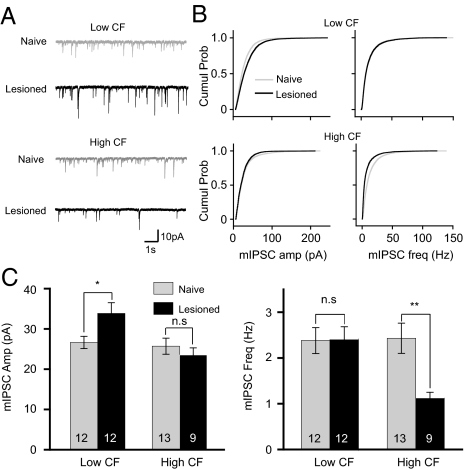
Further probing into the precise type of synaptic plasticity after hearing loss, we next examined miniature excitatory postsynaptic currents (mEPSCs) and miniature inhibitory postsynaptic currents (mIPSCs) in pyramidal neurons in auditory cortical slices 10 d after hearing lesion. Low- and high-CF areas of the auditory cortex were identified by landmarks of adjacent hippocampal structures (Fig. S1A). Pyramidal neurons were identified by their firing pattern and confirmed morphologically (Fig. S1B and see Materials and Methods for details). Amplitude and frequency of mEPSCs both increased in the low-CF area (amplitude, F1,23 = 6.86, P = 0.015; frequency, F1,23 = 9.25, P = 0.005; Fig. 2), but were not altered in the high-CF area (amplitude, F1,21 = 0.78, P = 0.38; frequency, F1,21 = 2.09, P = 0.16; Fig. 2). The potentiation of the excitatory synapses in the low-CF area may have contributed to the observed expansion of low-frequency representations (Fig. 1).
Fig. 2.
High frequency hearing loss induces potentiation of excitatory synaptic transmission in the low-CF area. (A) Example traces of miniature excitatory postsynaptic currents (mEPSCs) recorded from low-CF and high-CF areas of naïve and hearing-lesioned animals. (B) Cumulative histograms of mEPSC amplitude and frequency. (C) Mean mEPSC amplitude and frequency. *P < 0.05; **P < 0.01; n.s., not significant. The number of recorded neurons is indicated on each bar. See also Fig. S1.
Hearing Lesions Differentially Alter Inhibitory Synapses in Low- and High-CF Areas.
The mIPSC amplitude in the low-CF area also increased, but its frequency was not altered (amplitude, F1,22 = 5.47, P = 0.028; frequency, F1,22 = 0.001, P = 0.99; Fig. 3). By contrast, mIPSC frequency was reduced in the high-CF area of hearing-lesioned animals, and mIPSC amplitude was not altered (frequency, F1,20 = 9.81, P = 0.005; amplitude, F1,20 = 0.60, P = 0.44; Fig. 3), indicative of reduced GABA release presumably from fewer functional GABA synapses or lower neurotransmitter release probability.
Fig. 3.
High frequency hearing loss differentially affects inhibitory synaptic transmission in the low-CF and high-CF areas. (A) Example traces of miniature inhibitory postsynaptic currents (mIPSCs) recorded from low-CF and high-CF areas of naïve and hearing-lesioned animals. (B) Cumulative histograms of mIPSC amplitude and frequency. (C) Mean mEPSC amplitude and frequency. *P < 0.05; **P < 0.01; n.s., not significant.
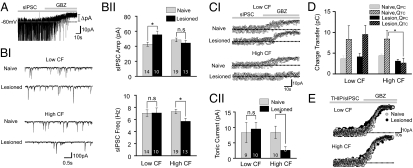
Changes in spontaneous inhibitory postsynaptic currents (sIPSCs) paralleled those in mIPSCs. The sIPSC amplitude in low-CF areas was enhanced (F1,21 = 5.53, P = 0.028) and sIPSC frequency in high-CF areas was reduced (F1,30 = 7. 38, P = 0.01; Fig. 4B). Application of Gabazine, a GABAA receptor blocker, uncovered a tonic form of inhibition, which was reduced only in the high-CF neurons of the hearing-lesioned animals (Fig. 4 A and C). Despite the small amplitude of the tonic inhibitory currents, its impact on neuronal excitability could be significant because, in naïve animals, the charge transfer via tonic GABAA receptor-mediated currents (QTC) was approximately twice that via phasic currents (QPC). In hearing-lesioned animals, QTC was reduced specifically in high-CF neurons but not low-CF neurons (high-CF, F1,16 = 5.36, P = 0.034; low-CF, F1,17 = 0.09, P = 0.76; Fig. 4D). QPC was also slightly reduced in high-CF neurons (F1,17 = 3.54, P = 0.07).
Fig. 4.
High-frequency hearing loss alters both phasic and tonic inhibition. (A) An example trace of spontaneous inhibitory postsynaptic currents (sIPSCs). Bath application of Gabazine abolishes phasic sIPSCs and reveals a tonic inhibitory current. (BI) Example traces showing phasic sIPSCs. (BII) Amplitude and frequency of sIPSCs. (CI) Tonic inhibition measured as the shift in baseline current after Gabazine application. (CII) Hearing loss reduces tonic inhibition in the high-CF area. (D) Charge transfer through phasic and tonic inhibitory currents (QPC and QTC, repectively). (EI) Tonic inhibition activated by an agonist, THIP. The amplitude of the THIP-activated tonic inhibitory current was not altered by hearing loss. *P < 0.05; n.s., not significant.
Tonic inhibition is mediated primarily through activation of noninactivating extrasynaptic GABAA receptors by ambient GABA (23–26). The hearing lesion-induced reduction in tonic inhibition could be due to reduced ambient GABA concentration, reduced extrasynaptic GABAA receptor functions, or both. To separate these pre- and postsynaptic contributions, we recorded the level of tonic inhibition activated by 1 μM THIP, an agonist of tonic/extrasynaptic GABA receptors (27). We found similar levels of THIP-activated tonic inhibition in high-CF and low-CF areas, and in naïve and hearing-lesioned animals (Low-CF, F1,9 = 0.003, P = 0.95; High-CF, F1,14 = 0.13, P = 0.71; Fig. 4E), indicating that extrasynaptic GABAA receptor functions were not altered.
Hearing Lesions Reduce GAD Expression in the High-CF Area.
Our electrophysiological results suggest that presynaptic GABA release is reduced, resulting in a lower concentration of ambient GABA. To identify a biochemical correlate of reduced GABA release, we examined the expression level of the 65-kd isoform of the glutamic acid decarboxylase (GAD65), a GABA-synthesizing enzyme (28). The hearing lesion caused a reduction of GAD65 expression in the high-CF area, but not in the low-CF area (Fig. 5 A and B; high-CF area, t14 = 5.56, P < 0.0001). The overall GAD65 protein level as quantified with Western blot analysis was also significantly reduced in the high-CF area of the hearing lesioned animals (t4 = 7.03, P = 0.002; Fig. 5C). The neuronal density visualized with NeuN, a neuronal marker, was not different between naïve and trauma groups (Low-CF, t4 = 0.78, P = 0.47; High-CF, t7 = 0.29, P = 0.77; Fig. S2), indicating no significant neuronal death in A1 after hearing loss. Thus, the lower GAD65 level was due to reduced protein expression rather than inhibitory neuronal cell loss.
Fig. 5.
High frequency hearing loss reduces GAD65 protein level in the high-CF area. (A) Immunohistochemical staining of auditory cortical neurons (with NeuN green) and GAD65 protein. Note the reduced GAD65 level in high-CF zone of the hearing-lesioned animals. (Scale bar: 100 μm; Inset, 20 μm.) (B) Density of GAD65 puncta was reduced in the high-CF zone of hearing-lesioned animals (n = 8). (C) GAD65 expression level, as measured with Western blot analysis, was reduced in high-CF areas of the hearing-lesioned animals. **P < 0.01; n.s., not significant. See also Fig. S2.
Hearing Lesions Produce a Tinnitus Pitch in the Hearing-Loss Frequency Range.
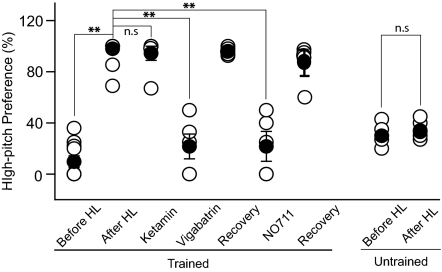
Tinnitus is believed to be caused by increased neuronal excitability, which could be a result of enhanced excitatory synaptic transmissions such as that observed in the low-CF area, or reduced inhibitory transmissions such as that observed in the high-CF area. If tinnitus is caused by enhanced excitatory transmission, its pitch should be in the low-frequency hearing range. However, a tinnitus pitch in the high-frequency hearing-loss range would suggest a role for homeostatic down-regulation of neuronal inhibition. To determine the type of plasticity inolved, we examined the pitch of hearing lesion-induced tinnitus in a novel place preference test. In the first training phase, animals were trained to move into or stay in the light side of the two compartments of a shuttle box when the pitch of a continuously played background sound was ≥4 kHz, and do the same in the dark side when the pitch of the sound was ≤3 kHz. Animal performances were generally similar for the high and the low pitch-cued trials. After the overall performance reached 70% for 3 consecutive days, the animals underwent a second testing phase, in which silent probe trials were randomly interspersed with regular sound-cued trials. In the probe trials, the animals’ preference for the two compartments was recorded, and no foot shock was delivered. In the silent probe trials, animals generally preferred the dark side (Fig. 6). After the hearing lesion, animals consistently increased their preference for the high-pitch compartment (i.e., the light side) in the probe trials (paired Student's t test, t7 = 9.5, P < 0.001). This switch in place preference was guided by the animals’ perception of sounds, because it did not occur in naïve animals without prior training in the task. This behavioral change is not a result of impaired high frequency hearing, because it was reversed by GABAergic enhancers that do not alter the hearing threshold (see below). The animals’ preference for the high-pitch compartment in the absence of external sounds indicates that they perceived tinnitus in the hearing-loss frequency range, which implicates down-regulation of cortical inhibition as a mechansism for tinnitus.
Fig. 6.
Hearing lesion-induced tinnitus is reversibly abolished by an enhancement in GABA-mediated inhibition. Both previously trained (n = 8) and naïve animals (n = 5) naturally prefered the dark, low-pitch side in silent probe trials. After the hearing lesion, trained animals switched their preference to the light, high-pitch side in probe trials, indicating their perception of high-pitch tinnitus. Untrained animals did not switch their preferred side after the hearing lesion. Enhancing GABA-mediated inhibition by Vigabatrin or NO711 reversibly abolished tinnitus behaviors. Ketamine, a NMDA receptor antagonist, did not alter tinnitus behaviors through an excitatory mechanism. **P < 0.01; n.s., not significant.
Tinnitus Behavior Is Abolished by GABAergic Enhancers.
To further determine whether the observed tinnitus in our animal model involves excitatory synaptic potentiation in the low-CF area or inhibitory synaptic depression in the high-CF area, we examined the effects of glutamatergic antagonists and GABAergic enhancers on the tinnitus behavior. Although the tinnitus behaviors were not altered by ketamine, a glutamatergic antagonist, they were reversibly abolished by vigabatrin, an inhibitor of GABA transaminase, and NO711, a GABA uptake blocker (ketamine, t5 = 1.0, P = 0.36; vigabatrin, t4 = 8.1, P = 0.001; NO711, t4 = 7.5, P = 0.001; Fig. 6). These results further suggest that hearing loss-induced tinnitus is likely caused by the reduction of inhibitory synaptic transmission in input-deprived cortical neurons.
Discussion
Earlier studies have implicated cortical map reorganization and homeostatic plasticity as potential mechanisms for hearing loss-induced tinnitus (22, 29–31). After partial hearing lesion, the area of AI representing the normal hearing range increases, and cortical responses to the hearing frequencies are enhanced (8, 32–36). This increased cortical responsiveness has been considered a candidate mechanism for tinnitus. If these map changes cause tinnitus, we would expect the tinnitus pitch to be in the normal-hearing frequency range. However, results from the present study and studies of tinnitus patients indicate that the pitch of tinnitus is in the hearing-loss frequency range (31). The parallel between tinnitus and phantom sensation/pain has often been cited as supporting evidence that sensory map changes may be responsible for both phenomena (1, 37, 38). However, longitudinal studies indicate that amputation-induced phantom pain often becomes weaker with time, during which cortical map reorganization is expected to progress, and may eventually disappear after cortical map reorganization is complete (39, 40). Normal perceptual learning may also lead to large-scale cortical reorganization without causing tinnitus or phantom pain (41, 42). By contrast, tinnitus may develop in animals with mild hearing loss that results in reduced cortical inhibition but not map reorganization (43, 44). These findings, together with the results of the present study, suggest that cortical map reorganization does not directly mediate tinnitus perception.
Homeostatic plasticity has also been implicated in tinnitus (22, 29, 31). Neurons change their synaptic strength and membrane excitability in response to altered sensory input to maintain the overall level of activity (19). Hearing loss increases the excitability of cortical neurons, which could lead to elevated spontaneous activity and tinnitus (20). In the present study, high frequency hearing lesions resulted in homeostatic down-regulation of inhibitory synaptic transmission and tonic inhibition in the high-CF area, where the model tinnitus pitch was represented before hearing loss. We have found that either enhancing GABA release by blocking GABA transaminase with Vigabatrin or reducing GABA uptake with NO711 reversibly eliminated the tinnitus behavior (45). Collectively, these results support the notion that homeostatic plasticity at the inhibitory synapse is a mechanism for chronic tinnitus.
Results of this study suggest that hearing loss-induced down-regulation of GABA release, but not the hearing loss per se, is a cause of tinnitus. Although sound-induced hearing loss in adulthood resulted in reduced GABA release (Figs. 3–5), cochlear removal in early development has been shown to increase GABA release in the auditory cortex (46). Thus, differences in the causes and ages of hearing loss may contribute to the variable outcomes of tinnitus symptoms (43). The higher percentage of hearing-loss animals that developed tinnitus behaviors in this study than in some previous reports (e.g., ref. 47) may also be attributed to our hearing lesion method—i.e., binaural, prolonged (7 h) sound exposure resulting in broad-spectrum (≥4 kHz), long-lasting (up to 180 d), severe (≥40 dB) hearing loss.
Previous attempts to treat tinnitus in animal models and human patients have focused on preventing cortical map reorganization (12, 14, 16). Our findings suggest an alternative approach—preventing or reversing hearing loss-induced homeostatic synaptic plasticity. Because homeostatic down-regulation of inhibitory synapses is caused by a lack of sensory-driven activity, manipulations that increase such activity might attenuate the homeostatic plasticity and reduce tinnitus. Thus, cortical map reorganization, by which previously deafferented neurons become responsive to normal-hearing frequencies, might help reduce or eliminate tinnitus. Pharmacologically targeting GABAergic synapses or the cellular mechanisms underlying homeostatic down-regulation of GABA release might also alleviate tinnitus symptoms (45). Applications of these results in future research offer a paradigmatic shift in treating conditions of phantom perception.
Materials and Methods
Noise Exposure and Auditory Brainstem Response (ABR) Recording.
Adult rats (Sprague–Dawley; Charles River) weighing 250∼300 g were used in all experiments. All experimental procedures were reviewed and approved by the University of California Berkeley Animal Care and Use Committee. Hearing lesions were performed by placing animals, pretreated with buprenorphine, into a small wire mesh cage located in a sound attenuation chamber where a continuous pure tone of 4 kHz was played at 123 dB SPL through a Yorkville loudspeaker for 7 h. The sound level was calibrated with a Bruel and Kjaer 4135 condenser microphone.
Hearing thresholds were assessed by using ABR recorded before and after the hearing lesion. Animals were anesthetized with ketamine and xylazine (50 mg/kg and 10 mg/kg, respectively, i.p.), and were maintained on a heating pad. Tone pips (3-ms full-cycle sine waves at 1, 2, 4, 8, 16, and 32 kHz at 5-dB intensity steps from 70 to 0 dB) were delivered to the left ear at a rate of 19 times per second through a calibrated earphone (Stax), using BioSigRP software on a Tucker Davis Technology Sys3 recording rig. ABR signals were recorded with three electrodes s.c. inserted behind ipsilateral and contralateral ears and at the vertex of the skull. The sound level that activated a minimal discernable response was defined as the auditory threshold. Threshold shift was determined at each tested frequency with the difference between the threshold before and after hearing loss.
Acute Auditory Mapping.
Under pentobarbital (50 mg/kg) (or both ketamine and xylazine) anesthesia, units were evenly sampled from the AI. Their responses to 25-ms tone pips of 51 frequencies (1–32 kHz, 0.1 octaves spacing) and eight sound pressure levels (0–70 dB SPL, 10-dB steps) were recorded to reconstruct the frequency-intensity receptive field. A total of 464 single units were sampled (229 from naive group and 235 from noise-exposed group). Data analysis was done offline with custom Matlab functions.
Slice Preparation and Electrophysiological Recordings.
Animals were anesthetized with CO2 and decapitated. The brain was extracted and immediately placed in an oxygenated (95% O2/5% CO2) external solution (104 mM NaCl, 4 mM KCl, 1.4 mM MgCl2, 10 mM d-glucose, 25 mM NaHCO3, and 2.4 mM CaCl2 at pH 7.4). The brain was sectioned along the transverse plane into 300-μm slices by using a vibrating microtome (Leica; VT1200), and brain slices were immersed in oxygenated external solution in a tissue chamber for at least 1 h at room temperature before electrophysiological recording; the chamber was continuously perfused with external solution at a rate of 2 mL/min. A fixed stage microscope (Olympus; BX50WI) equipped with differential interference contrast optics and a 63× water-immersion objective was used to visualize individual neurons in the auditory cortex.
Patch electrodes were fabricated from 1.5-mm-diameter borosilicate glass micropipettes and had impedance of 3–5 MΩ when back-filled with the internal solutions for mEPSC measurement (117 mM K-gluconate, 13 mM KCl, 1.0 mM MgCl26H2O, 0.07 mM CaCl22H2O, 0.1 mM EGTA, 10 mM Hepes, 3 mM ATP-Mg, and 0.3 mM GTP-Na at pH 7.3 and 290 mOsm) and for mIPSCs, sIPSCs, and tonic inhibition measurement (140 mM CsCl, 1.0 mM MgCl26H2O, 0.07 mM CaCl22H2O, 0.1 mM EGTA, 10 mM Hepes, 3 mM ATP-Mg, and 0.3 mM GTP-Na at pH 7.3 and 290 mOsm). The initial access resistance typically ranged from 15 to 23 MΩ and remained stable during the recording session. Series resistance was also continuously monitored with a brief voltage pulse. Recordings were accepted when a cell had a resting membrane potential of at least –68 mV and a series resistance of 15–25 MΩ (<20% change during the recording session). All recordings were performed at room temperature (≈22.5 °C) by using Multiclamp 700B amplifier (Molecular Devices). Data were collected and analyzed with pCLAMP software (Molecular Devices) and MiniAnalysis (Synaptosoft).
Behavior Test.
Tinnitus behaviors in hearing lesioned rats were tested with a conditioned place preference test. In the first training phase, animals were trained to move into one of two compartments in a shuttle box, depending on the frequency range of a continuously played 1-octave bandpath noise (1–2, 1.2–2.4, 1.5–3, 4–8, 8–16, and 16–32 kHz) or pure tones (1, 1.5, 2, 4, 8, and 16 kHz, and all sounds played at 50, 60, and 70 dB). When the sound frequency was ≥4 kHz, animals had to move into the illuminated light compartment in 10 s to avoid mild foot shocks (0.6 mA, once every 5 s). When the sound frequency range was ≤3 kHz, they had to move into the dark compartment. Sixty trials were given each day in a period of ≈1 h. Training continued until the animal shuttled correctly in 70% of the trials for three consecutive sessions. Then the second testing phase ensued. In each testing session, probe trials were introduced, in which no sound was played and no foot shock was delivered. Animals’ preference for the two compartments in the probe trials was recorded. Hearing loss was then induced as described. Ten days after the hearing lesion, we resumed the testing sessions with sound levels adjusted for their hearing threshold shift (typically an increase of sound level by <20 dB). Their performance in the probe trials was analyzed to determine whether they showed signs of tinnitus and the pitch of the tinnitus percept. In our pilot studies, we also trained animals to move into the dark compartment when high-pitch sounds were played and into the light compartment when low-pitch sounds were played. Those animals did not switch their natural preference for the dark side after hearing loss. Although consistent with high-pitch tinnitus perception, the training paradigm did not allow measurement of the tinnitus behavior and was not pursued further.
The effects of Ketamine on tinnitus behaivors were tested 15 min after drug administration (5 mg/kg, i.p.). Vigabatrin (150 mg/kg, i.p.), and NO-711 (3 mg/kg, i.p.) are slow-acting and, therefore, were given daily for at least 3 d before behavioral tests were conducted.
For additional methods, see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. Lu Chen and David Weisblat for help with the experiments. The work was supported by the American Tinnitus Association and by the National Institute on Deafness and Other Communicative Disorders.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107998108/-/DCSupplemental.
References
- 1.Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci. 2004;27:676–682. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Rauschecker JP, Leaver AM, Mühlau M. Tuning out the noise: Limbic-auditory interactions in tinnitus. Neuron. 2010;66:819–826. doi: 10.1016/j.neuron.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Syka J. Plastic changes in the central auditory system after hearing loss, restoration of function, and during learning. Physiol Rev. 2002;82:601–636. doi: 10.1152/physrev.00002.2002. [DOI] [PubMed] [Google Scholar]
- 4.Lockwood AH, et al. Neuroanatomy of tinnitus. Scand Audiol Suppl. 1999;51:47–52. [PubMed] [Google Scholar]
- 5.Weisz N, et al. The neural code of auditory phantom perception. J Neurosci. 2007;27:1479–1484. doi: 10.1523/JNEUROSCI.3711-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brozoski TJ, Bauer CA. The effect of dorsal cochlear nucleus ablation on tinnitus in rats. Hear Res. 2005;206:227–236. doi: 10.1016/j.heares.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Barrs DM, Brackmann DE. Translabyrinthine nerve section: Effect on tinnitus. J Laryngol Otol. 1984;9:287–293. [Google Scholar]
- 8.Sun W, et al. Noise exposure-induced enhancement of auditory cortex response and changes in gene expression. Neuroscience. 2008;156:374–380. doi: 10.1016/j.neuroscience.2008.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wienbruch C, Paul I, Weisz N, Elbert T, Roberts LE. Frequency organization of the 40-Hz auditory steady-state response in normal hearing and in tinnitus. Neuroimage. 2006;33:180–194. doi: 10.1016/j.neuroimage.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 10.Weisz N, Moratti S, Meinzer M, Dohrmann K, Elbert T. Tinnitus perception and distress is related to abnormal spontaneous brain activity as measured by magnetoencephalography. PLoS Med. 2005;2:e153. doi: 10.1371/journal.pmed.0020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mühlnickel W, Elbert T, Taub E, Flor H. Reorganization of auditory cortex in tinnitus. Proc Natl Acad Sci USA. 1998;95:10340–10343. doi: 10.1073/pnas.95.17.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noreña AJ, Eggermont JJ. Enriched acoustic environment after noise trauma abolishes neural signs of tinnitus. Neuroreport. 2006;17:559–563. doi: 10.1097/00001756-200604240-00001. [DOI] [PubMed] [Google Scholar]
- 13.Komiya H, Eggermont JJ. Spontaneous firing activity of cortical neurons in adult cats with reorganized tonotopic map following pure-tone trauma. Acta Otolaryngol. 2000;120:750–756. doi: 10.1080/000164800750000298. [DOI] [PubMed] [Google Scholar]
- 14.Noreña AJ, Eggermont JJ. Enriched acoustic environment after noise trauma reduces hearing loss and prevents cortical map reorganization. J Neurosci. 2005;25:699–705. doi: 10.1523/JNEUROSCI.2226-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seki S, Eggermont JJ. Changes in spontaneous firing rate and neural synchrony in cat primary auditory cortex after localized tone-induced hearing loss. Hear Res. 2003;180:28–38. doi: 10.1016/s0378-5955(03)00074-1. [DOI] [PubMed] [Google Scholar]
- 16.Engineer ND, et al. Reversing pathological neural activity using targeted plasticity. Nature. 2011;470:101–104. doi: 10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eggermont JJ. Cortical tonotopic map reorganization and its implications for treatment of tinnitus. Acta Otolaryngol Suppl. 2006:9–12. doi: 10.1080/03655230600895259. [DOI] [PubMed] [Google Scholar]
- 18.Buonomano DV, Merzenich MM. Cortical plasticity: From synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 19.Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- 20.Kotak VC, et al. Hearing loss raises excitability in the auditory cortex. J Neurosci. 2005;25:3908–3918. doi: 10.1523/JNEUROSCI.5169-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dominguez M, Becker S, Bruce I, Read H. A spiking neuron model of cortical correlates of sensorineural hearing loss: Spontaneous firing, synchrony, and tinnitus. Neural Comput. 2006;18:2942–2958. doi: 10.1162/neco.2006.18.12.2942. [DOI] [PubMed] [Google Scholar]
- 22.Schaette R, Kempter R. Development of tinnitus-related neuronal hyperactivity through homeostatic plasticity after hearing loss: A computational model. Eur J Neurosci. 2006;23:3124–3138. doi: 10.1111/j.1460-9568.2006.04774.x. [DOI] [PubMed] [Google Scholar]
- 23.Jia F, et al. An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J Neurophysiol. 2005;94:4491–4501. doi: 10.1152/jn.00421.2005. [DOI] [PubMed] [Google Scholar]
- 24.Krook-Magnuson EI, Huntsman MM. Excitability of cortical neurons depends upon a powerful tonic conductance in inhibitory networks. Thalamus Relat Syst. 2005;3:115–120. doi: 10.1017/S1472928807000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belelli D, et al. Extrasynaptic GABAA receptors: Form, pharmacology, and function. J Neurosci. 2009;29:12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farrant M, Nusser Z. Variations on an inhibitory theme: Phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 27.Drasbek KR, Jensen K. THIP, a hypnotic and antinociceptive drug, enhances an extrasynaptic GABAA receptor-mediated conductance in mouse neocortex. Cereb Cortex. 2006;16:1134–1141. doi: 10.1093/cercor/bhj055. [DOI] [PubMed] [Google Scholar]
- 28.Sarro EC, Kotak VC, Sanes DH, Aoki C. Hearing loss alters the subcellular distribution of presynaptic GAD and postsynaptic GABAA receptors in the auditory cortex. Cereb Cortex. 2008;18:2855–2867. doi: 10.1093/cercor/bhn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fröhlich F, Bazhenov M, Sejnowski TJ. Pathological effect of homeostatic synaptic scaling on network dynamics in diseases of the cortex. J Neurosci. 2008;28:1709–1720. doi: 10.1523/JNEUROSCI.4263-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerken GM. Central tinnitus and lateral inhibition: An auditory brainstem model. Hear Res. 1996;97:75–83. [PubMed] [Google Scholar]
- 31.Schaette R, Kempter R. Predicting tinnitus pitch from patients’ audiograms with a computational model for the development of neuronal hyperactivity. J Neurophysiol. 2009;101:3042–3052. doi: 10.1152/jn.91256.2008. [DOI] [PubMed] [Google Scholar]
- 32.Rajan R, Irvine DR, Wise LZ, Heil P. Effect of unilateral partial cochlear lesions in adult cats on the representation of lesioned and unlesioned cochleas in primary auditory cortex. J Comp Neurol. 1993;338:17–49. doi: 10.1002/cne.903380104. [DOI] [PubMed] [Google Scholar]
- 33.Robertson D, Irvine DR. Plasticity of frequency organization in auditory cortex of guinea pigs with partial unilateral deafness. J Comp Neurol. 1989;282:456–471. doi: 10.1002/cne.902820311. [DOI] [PubMed] [Google Scholar]
- 34.Noreña AJ, Tomita M, Eggermont JJ. Neural changes in cat auditory cortex after a transient pure-tone trauma. J Neurophysiol. 2003;90:2387–2401. doi: 10.1152/jn.00139.2003. [DOI] [PubMed] [Google Scholar]
- 35.Eggermont JJ, Komiya H. Moderate noise trauma in juvenile cats results in profound cortical topographic map changes in adulthood. Hear Res. 2000;142:89–101. doi: 10.1016/s0378-5955(00)00024-1. [DOI] [PubMed] [Google Scholar]
- 36.Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hear Res. 2000;147:261–274. doi: 10.1016/s0378-5955(00)00136-2. [DOI] [PubMed] [Google Scholar]
- 37.Rauschecker JP. Auditory cortical plasticity: A comparison with other sensory systems. Trends Neurosci. 1999;22:74–80. doi: 10.1016/s0166-2236(98)01303-4. [DOI] [PubMed] [Google Scholar]
- 38.Flor H, et al. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 1995;375:482–484. doi: 10.1038/375482a0. [DOI] [PubMed] [Google Scholar]
- 39.Houghton AD, Nicholls G, Houghton AL, Saadah E, McColl L. Phantom pain: Natural history and association with rehabilitation. Ann R Coll Surg Engl. 1994;76:22–25. [PMC free article] [PubMed] [Google Scholar]
- 40.Pascual-Leone A, Peris M, Tormos JM, Pascual AP, Catalá MD. Reorganization of human cortical motor output maps following traumatic forearm amputation. Neuroreport. 1996;7:2068–2070. doi: 10.1097/00001756-199609020-00002. [DOI] [PubMed] [Google Scholar]
- 41.Pantev C, et al. Increased auditory cortical representation in musicians. Nature. 1998;392:811–814. doi: 10.1038/33918. [DOI] [PubMed] [Google Scholar]
- 42.Sterr A, et al. Perceptual correlates of changes in cortical representation of fingers in blind multifinger Braille readers. J Neurosci. 1998;18:4417–4423. doi: 10.1523/JNEUROSCI.18-11-04417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hazell JW, McKinney CJ, Aleksy W. Mechanisms of tinnitus in profound deafness. Ann Otol Rhinol Laryngol Suppl. 1995;166:418–420. [PubMed] [Google Scholar]
- 44.Rajan R. Receptor organ damage causes loss of cortical surround inhibition without topographic map plasticity. Nat Neurosci. 1998;1:138–143. doi: 10.1038/388. [DOI] [PubMed] [Google Scholar]
- 45.Brozoski TJ, Spires TJ, Bauer CA. Vigabatrin, a GABA transaminase inhibitor, reversibly eliminates tinnitus in an animal model. J Assoc Res Otolaryngol. 2007;8:105–118. doi: 10.1007/s10162-006-0067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kotak VC, Takesian AE, Sanes DH. Hearing loss prevents the maturation of GABAergic transmission in the auditory cortex. Cereb Cortex. 2008;18:2098–2108. doi: 10.1093/cercor/bhm233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heffner HE, Harrington IA. Tinnitus in hamsters following exposure to intense sound. Hear Res. 2002;170:83–95. doi: 10.1016/s0378-5955(02)00343-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.