Abstract
Several pathogenic bacteria have adopted effector proteins that, upon delivery into mammalian cells, undergo tyrosine phosphorylation at the Glu-Pro-Ile-Tyr-Ala (EPIYA) or EPIYA-like sequence motif by host kinases such as Src family kinases (SFKs). This EPIYA phosphorylation triggers complex formation of bacterial effectors with SH2 domain-containing proteins that results in perturbation of host cell signaling and subsequent pathogenesis. Although the presence of such an anomalous protein interaction suggests the existence of a mammalian EPIYA-containing protein whose function is mimicked or subverted by bacterial EPIYA effectors, no molecule that uses the EPIYA motif for biological function has so far been reported in mammals. Here we show that mammalian Pragmin/SgK223 undergoes tyrosine phosphorylation at the EPIYA motif by SFKs and thereby acquires the ability to interact with the SH2 domain of the C-terminal Src kinase (Csk), a negative regulator of SFKs. The Pragmin–Csk interaction prevents translocalization of Csk from the cytoplasm to the membrane and subsequent inactivation of membrane-associated SFKs. As a result, SFK activity is sustained in cells where Pragmin is phosphorylated at the EPIYA motif. Because EPIYA phosphorylation of Pragmin is mediated by SFKs, cytoplasmic sequestration of Csk by Pragmin establishes a positive feedback regulation of SFK activation. Remarkably, the Helicobacter pylori EPIYA effector CagA binds to the Csk SH2 domain in place of Pragmin and enforces membrane recruitment of Csk and subsequent inhibition of SFKs. This work identifies Pragmin as a mammalian EPIYA effector and suggests that bacterial EPIYA effectors target Pragmin to subvert SFKs for successful infection.
Keywords: mammalian proteomes, bacterial virulence factors
A number of pathogenic bacteria possess effector proteins that are delivered into mammalian host cells via a bacterial type III or type IV secretion system. Among the various effectors, attention has been paid to those that undergo tyrosine phosphorylation in mammalian host cells (1). Helicobacter pylori CagA, a paradigm of such bacterial effectors, plays an important role in the development of human diseases including atrophic gastritis, peptic ulceration, and gastric adenocarcinoma (2, 3). Upon delivery into gastric epithelial cells via type IV secretion, CagA is localized to the inner side of the plasma membrane, where it is tyrosine-phosphorylated by Src family kinases (SFKs) or c-Abl kinase at the Glu-Pro-Ile-Tyr-Ala (EPIYA) sequence that is present in variable numbers in its C-terminal region (4). Once tyrosine-phosphorylated, CagA EPIYA sequences serve as nonphysiological docking sites for the SHP2 protein tyrosine phosphatase, a bona fide oncoprotein, and the C-terminal Src kinase (Csk), which inhibits SFK activity and thus deregulates their functions (5, 6). Hence, CagA functionally mimics mammalian scaffold/adopter proteins, such as Gab, and thereby perturbs host cell signaling (7).
Recent studies have revealed a number of CagA-like bacterial effectors that may also act as nonphysiological scaffold/adopter proteins inside the delivered host cells. These effectors include Anaplasma phagocytophilum AnkA (8, 9), enteropathogenic Escherichia coli Tir (10), Citrobacter rodentium Tir (11), Chlamydia trachomatis Tarp (12), Haemophilus ducreyi LspA (13), and Bartonella henselae BepD, BepE, and BepF (14). Here we designate these proteins as “bacterial EPIYA effectors” because they are characterized by the presence of an EPIYA or EPIYA-like sequence (hereafter, the term “EPIYA motif” includes both the EPIYA sequence and EPIYA-like sequences), tyrosine phosphorylation of which enables them to hijack host-signaling pathways (Fig. S1). Notably, these bacterial effectors do not share sequence homology at both DNA and protein levels outside the EPIYA motif, arguing against the idea that they have a common ancestral gene that was transmitted through horizontal transfer among distinct bacterial species. An alternative idea is therefore that these EPIYA effectors are incidentally selected among distinct bacterial species because of a special role of the EPIYA motif in successful bacterial infection. This in turn raises the possibility that mammalian cells also possess EPIYA-containing effector proteins (mammalian EPIYA effectors), the functions of which are exploited or subverted by bacterial EPIYA effectors.
Each SH2 domain acquires selectivity for specific phosphorylation sites by recognizing the phosphorylated tyrosine residue together with several additional flanking residues, usually three to five residues C-terminal to the phosphotyrosine (15). Recently Selbach et al. (16) proposed that the bacterial EPIYA motif acts as a promiscuous “master key” that can interact with and simultaneously perturb a number of host cell-derived SH2 domain-containing proteins. From the bacterial side, the versatile binding properties may be beneficial in exerting virulence. In mammals, however, proteins with such a master key function may be harmful when cells need to execute finely tuned regulation of intracellular signaling. Consistent with this hypothesis, the incidence of proteins having a consensus EPIYA-like sequence (EPxYAxV) is significantly underrepresented in mammalian proteomes, suggesting negative selection of the EPIYA motif in mammals (16).
In this work we explored a mammalian protein possessing a functional EPIYA motif that may be exploited or evaded by bacterial EPIYA effectors, and we found that Pragmin, a cytoplasmic pseudokinase also known as SgK223, undergoes tyrosine phosphorylation by SFKs at the EPIYA motif. Unlike bacterial EPIYA motifs, however, the Pragmin EPIYA motif exhibited strict binding specificity to the SH2 domain of Csk. We also found that the Pragmin–Csk interaction establishes a positive feedback regulation of SFK activation, which is disrupted by the H. pylori EPIYA effector, CagA.
Results
Exploration of Mammalian EPIYA-Containing Proteins.
To seek mammalian EPIYA effectors, we focused on H. pylori CagA, an archetypical bacterial EPIYA effector (Fig. S1) (3, 7). CagA contains variable numbers of EPIYA motifs in its C-terminal region. On the basis of their flanking sequences, four distinct EPIYA segments (EPIYA-A, -B, -C, and -D segments), each of which contains a single EPIYA motif (EPIYA-A, -B, -C, and -D motifs), have been described (7). Upon tyrosine phosphorylation, EPIYA-A or -B motif serves as a docking site for Csk, whereas EPIYA-C or -D motif serves as a binding site for SHP2. Because H. pylori has been associated with humans for more than 58,000 y (17), incorporation of multiple EPIYA motifs into CagA may have given a selective advantage to H. pylori in adapting in the human stomach during a long period of coexistence. This in turn raises the idea that human cells possess a protein(s) with a functional EPIYA motif, which is used or evaded by H. pylori. A search of the human proteome with the National Center for Biotechnology Information BLAST program led to the identification of only six proteins that have a perfect EPIYA sequence (Table S1). These candidates contained Pragmin (also known as SgK223), a cytoplasmic pseudokinase originally isolated as a downstream effector of Rnd2, a Rho family GTPase predominantly expressed in neurons (18). Rnd2-associated Pragmin stimulates RhoA activity and thereby induces cell contraction. However, expression of Pragmin is not limited to neuronal cells, indicating that the protein has a more general role. Also notably, the EPIYA sequence is perfectly conserved among mammalian Pragmin orthologs, indicating that it has an important role in the function of Pragmin. In addition to the EPIYA motif, Pragmin possesses a pseudokinase domain, which does not seem to have intrinsic kinase activity, in the C-terminal region, although the function remains unknown (Fig. S2). We hypothesized that mammalian EPIYA effectors, if they exist, should have a scaffold/adopter function involved in intracellular signal transduction. Because many pseudokinases have been known to act as scaffolds or adaptors in the cells (19), we chose Pragmin for further analysis in this study.
Tyrosine Phosphorylation of the Pragmin EPIYA Motif.
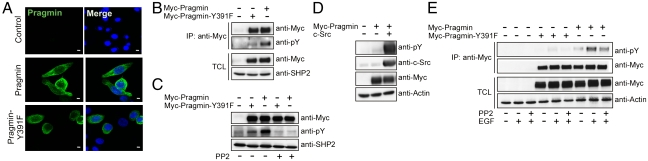
To elucidate the function of the Pragmin EPIYA motif, we sought to construct a mammalian expression vector for Pragmin. Because of the technical difficulty in cloning a human Pragmin cDNA into plasmid vectors, we used a pCMV-based mammalian expression vector for Myc-epitope-tagged wild-type rat Pragmin (Myc-Pragmin) (18). The rat and human Pragmins show 76% and 79% identities at the amino acid and nucleotide levels, respectively. A cDNA encoding a rat Pragmin mutant (Pragmin-Y391F) having a tyrosine-to-phenylalanine substitution at residue 391 in the EPIYA motif, which corresponds to Y411 in human Pragmin, was also generated and, after Myc-epitope tagging, was cloned into the pCMV vector (Fig. S2). The Pragmin expression vectors were transiently transfected into AGS human gastric epithelial cells. In cells, both ectopically expressed Pragmin and Pragmin-Y391F were localized to the cytoplasm, indicating that the Y391F substitution did not influence the intracellular distribution of Pragmin (Fig. 1A).
Fig. 1.
Tyrosine phosphorylation of Pragmin at the EPIYA motif. (A) AGS cells were transfected with a Myc-tagged wild-type Pragmin (Myc-Pragmin) or Myc-tagged Pragmin-Y391F (Myc-Pragmin-Y391F) vector and were stained with an anti-Myc antibody (green). Nuclei are visualized by DAPI staining (blue). (Scale bar, 10 μm.) (B) AGS cells were transfected with a Myc-Pragmin or Myc-Pragmin-Y391F vector. Total cell lysates (TCLs) were immunoprecipitated with an anti-Myc antibody, and the immunoprecipitates (IPs) were subjected to immunoblotting with the indicated antibodies. Anti-pY, anti-phosphotyrosine antibody. (C) AGS cells transfected with a Myc-Pragmin or Myc-Pragmin-Y391F vector were treated with or without 10 μM PP2, a SFK inhibitor. Total cell lysates were subjected to immunoblotting with the indicated antibodies. (D) Lysates of AGS cells transfected with a Myc-Pragmin and/or c-Src vector were subjected to immunoblotting with the indicated antibodies. (E) AGS cells transiently transfected with a Myc-Pragmin or Myc-Pragmin-Y391F vector were treated with 100 ng/mL EGF for 6 h in the presence or absence of 100 nM PP2. Cell lysates were subjected to immunoblotting with the indicated antibodies.
We first wished to know whether Pragmin is tyrosine-phosphorylated at the EPIYA motif. In AGS cells, Myc-Pragmin was efficiently tyrosine-phosphorylated, whereas Myc-Pragmin-Y391F was much less phosphorylated, as determined by antiphosphotyrosine immunoblotting (Fig. 1B). Hence, Pragmin undergoes tyrosine phosphorylation by endogenous kinases, and the EPIYA motif is the major if not the only site of Pragmin phosphorylation. In this regard, mammalian Pragmins contain more than 20 tyrosine residues in addition to one in the EPIYA motif. Some of those tyrosines may also undergo tyrosine phosphorylation, although to a much lesser extent than that at the EPIYA site. Next, we sought to identify tyrosine kinases that mediate Pragmin phosphorylation. Because H. pylori CagA is tyrosine-phosphorylated at EPIYA motifs by host SFKs (4, 7), we treated AGS cells expressing Myc-Pragmin with PP2, a specific inhibitor of SFKs, for 4 h before harvest and found that the PP2 treatment abolished tyrosine phosphorylation of Myc-Pragmin (Fig. 1C). Conversely, coexpression of c-Src markedly increased the level of Myc-Pragmin tyrosine phosphorylation (Fig. 1D). These results indicated that SFKs, which are constitutively activated in AGS cells, mediate EPIYA phosphorylation of Pragmin in the cells.
To investigate the physiological relevance for the tyrosine phosphorylation of Pragmin, we next tested whether the phosphorylation status of Pragmin is modified in response to external stimuli. To this end, AGS cells transiently transfected with the Myc-Pragmin or Myc-Pragmin-Y391F vector were cultured in the absence of serum for 24 h. The serum starvation substantially reduced the level of Pragmin tyrosine phosphorylation (Fig. 1E), indicating that decreased tyrosine kinase activity in serum-starved cells resulted in reduced tyrosine phosphorylation of Pragmin. Serum-starved AGS cells were then stimulated with EGF for 6 h. EGF treatment potently increased the level of tyrosine phosphorylation of Myc-Pragmin (Fig. 1E). In contrast, Myc-Pragmin-Y391F was hardly tyrosine-phosphorylated in serum-starved AGS cells before and after EGF treatment. Of note, EGF-dependent tyrosine phosphorylation of Myc-Pragmin was not inhibited by treatment of cells with 100 nM PP2, the concentration of which almost completely suppressed SFK activity in cells (Fig. 1E and Fig. S3) (20). This observation indicated that the ligand-activated EGF receptor itself or a non-SFK kinase(s) lying downstream of the EGF receptor also phosphorylates the EPIYA motif of Pragmin.
Pragmin Specifically Binds to Csk in an EPIYA Phosphorylation-Dependent Manner.
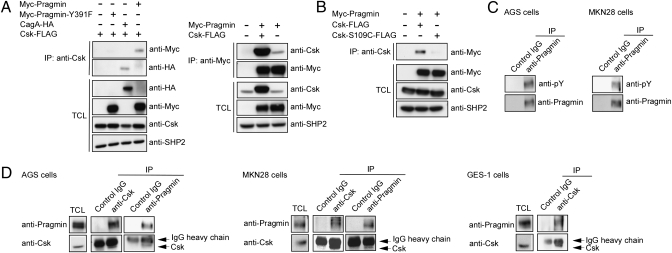
The above-described observations prompted us to search for a protein that can specifically interact with the Pragmin EPIYA motif in a tyrosine phosphorylation-dependent manner. During the course of this study, we noticed that the sequence distal to the Pragmin EPIYA motif (EPIYAESAK) is similar to that located downstream of the CagA EPIYA-B motif (EPIYAQVAK), to which the Csk SH2 domain binds in an EPIYA phosphorylation-dependent manner (6). We thus transiently transfected a FLAG-tagged Csk (Csk-FLAG) vector together with a Myc-Pragmin or HA-tagged CagA (CagA-HA) vector into AGS cells and found that Csk immunoprecipitation coprecipitated Myc-Pragmin or CagA-HA (Fig. 2A, Left). Reciprocally, Myc-Pragmin immunoprecipitation coprecipitated Csk-FLAG as well as endogenous Csk (Fig. 2A, Right). On the other hand, Myc-Pragmin-Y391F immunoprecipitation failed to coprecipitate Csk (Fig. 2A). Inactivation of the SH2 domain of Csk by a point mutation (S109C) also abolished the ability of Csk to coprecipitate Pragmin (Fig. 2B). Given these, we next focused on endogenous Pragmin and found that it was tyrosine-phosphorylated in two human gastric epithelial cell lines, AGS and MKN28 (Fig. 2C). Interaction of endogenous Csk with endogenous Pragmin was confirmed by reciprocal coimmunoprecipitation using an anti-Csk or anti-Pragmin antibody in both AGS and MKN28 cells (Fig. 2D). The Pragmin-Csk complex was also detected in a normal human gastric epithelial cell line, GES-1 (Fig. 2D). These results indicated that Pragmin associates with Csk via the tyrosine-phosphorylated EPIYA motif of Pragmin and the SH2 domain of Csk.
Fig. 2.
Interaction of Pragmin with Csk in a tyrosine phosphorylation-dependent manner. (A) AGS cells were transfected with the indicated vectors. Total cell lysates (TCLs) were immunoprecipitated with an anti-Csk antibody (Left) or an anti-Myc antibody (Right). The immunoprecipitates (IPs) were subjected to immunoblotting with the respective antibodies. (B) AGS cells were transfected with the indicated vector. Total cell lysates were immunoprecipitated with an anti-Csk antibody, and the immunoprecipitates were subjected to immunoblotting with the indicated antibodies. (C) Total cell lysates prepared from AGS or MKN28 gastric epithelial cells were immunoprecipitated with an anti-Pragmin antibody or control IgG. The immunoprecipitates were subjected to immunoblotting with an anti-phosphotyrosine (pY) antibody or anti-Pragmin antibody. (D) Total cell lysates prepared from AGS, MKN28, or GES-1 cells were immunoprecipitated with an anti-Csk antibody, anti-Pragmin antibody, or control IgG, and the immunoprecipitates were subjected to immunoblotting with an anti-Pragmin antibody or anti-Csk antibody.
To investigate whether the Pragmin EPIYA motif can display promiscuous specificity for multiple SH2 domains like bacterial EPIYA motif, we transiently coexpressed Pragmin and one of the SH2-containing proteins (SHP1, Grb7, c-Abl, CrkII, Grb2, and PI3-kinase p85 subunit), which were reported to interact with the EPIYA motifs of CagA (16), in AGS cells and examined their interaction with Pragmin through a sequential immunoprecipitation and immunoblotting technique. The results of the experiment revealed that Pragmin bound none of these SH2 domain-containing proteins (Fig. S4), indicating that the tyrosine-phosphorylated EPIYA motif of Pragmin is highly specific to the SH2 domain of Csk. It has been reported that SH2-binding specificity is primarily determined by three to five residues C-terminal to the phosphotyrosine (15). Considering the similarity of residues C-terminal to the EPIYA motif of Pragmin (EPIYAESAK) with that of the CagA EPIYA-B motif (EPIYAQVAK), degenerated SH2-binding specificity of the CagA EPIYA-B motif may be due to the difference in residues at +2 and +3 positions from the phosphotyrosine.
Elevated SFK Activity in Cells Expressing Pragmin.
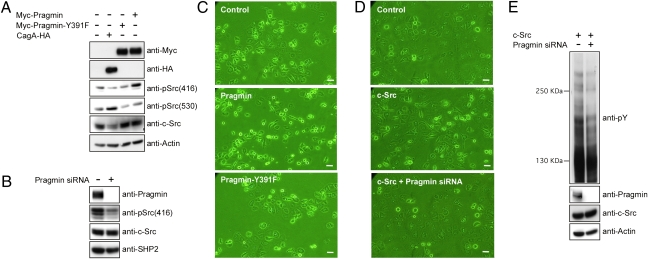
Csk inactivates SFKs by phosphorylating a consensus tyrosine residue near the C terminus (Y530 and Y527 in human and chicken c-Src, respectively) (21). Upon tyrosine phosphorylation, Y530 engages in intramolecular interaction that locks SFKs in an inactive conformation. To elucidate the biological consequences of Pragmin–Csk interaction, we investigated the activation status of SFKs in cells with or without Pragmin expression. To this end, we used an anti-Phospho-Src (Y530) antibody, which recognizes an inactive form of SFKs, and anti-Phospho-Src (Y416) antibody, which reacts with an active form of SFKs. In AGS cells expressing Myc-Pragmin, the level of Y530-phosphorylated c-Src was decreased, whereas the level of active c-Src was increased (Fig. 3A). Such changes in the level of c-Src phosphorylation were not observed in AGS cells expressing Myc-Pragmin-Y391F. Conversely, inhibition of endogenous Pragmin expression in AGS cells by specific siRNA reduced the level of active c-Src. Taken together, these results indicated that Pragmin potentiates kinase activity of SFKs upon complex formation with Csk (Fig. 3B).
Fig. 3.
Activation of c-Src in cells expressing Pragmin. (A) AGS cells were transfected a Myc-Pragmin, Myc-Pragmin-Y391F, or CagA-HA vector. Cell lysates prepared were subjected to immunoblotting with the indicated antibodies: anti-pSrc(416), anti-Phospho-Src (Y416) antibody; anti-pSrc(530), anti-Phospho-Src (Y530) antibody. (B) AGS cells were treated with Pragmin-specific siRNA. Cell lysates prepared were immunoblotted with the indicated antibodies. (C) AGS cells were transfected with a Myc-Pragmin or Myc-Pragmin-Y391F vector. Morphology of transfected cells was analyzed by microscope. (Scale bars, 10 μm.) (D) AGS cells treated with or without the Pragmin-specific siRNA were transfected with a c-Src vector. Cells were subjected to morphological investigation using microscopy. (Scale bars, 10 μm.) (E) AGS cells treated with or without the Pragmin-specific siRNA were transfected with a c-Src vector. Cell lysates were subjected to immunoblotting with the indicated antibodies. Anti-pY, anti-phosphotyrosine antibody.
In addition to SFK activation, cells expressing Myc-Pragmin, but not Myc-Pragmin-Y391F, displayed a morphological change, which was characterized by a polygonal cell shape with multiple protrusions (Fig. 3C and Fig. S5A). The morphogenetic activity of Pragmin was inhibited by treatment of cells with PP2. Similar morphological change was also induced by ectopic expression of c-Src in AGS cells, and inhibition of Pragmin expression by specific siRNA attenuated the c-Src-mediated morphological change (Fig. 3D and Fig. S5B). The overall levels of protein tyrosine phosphorylation in cells expressing c-Src were also reduced after inhibition of Pragmin expression (Fig. 3E). These results collectively indicated that Pragmin provokes cell morphological transformation by potentiating SFK kinase activity.
Colocalization of Pragmin and Csk in the Cytoplasm.
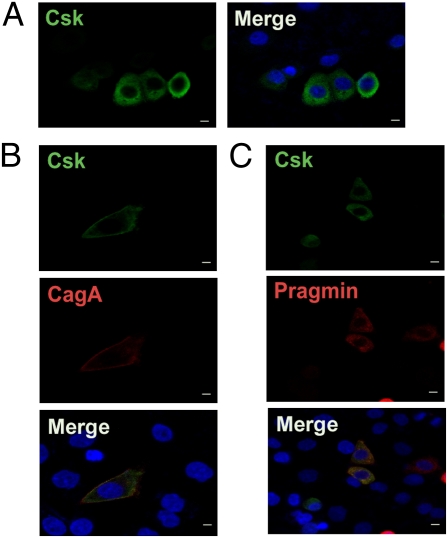
Csk is localized predominantly in the cytoplasm because it lacks fatty acid acylation domain for anchoring to the plasma membrane (21, 22). Given that all SFKs are anchored to the inner plasma membrane via myristoylation/palmitoylation, Csk needs to translocate from the cytoplasm to the membrane to inhibit SFKs. Membrane-recruitment of Csk is in most cases mediated via interaction with membrane-associated proteins such as Cbp (Csk-binding protein)/PAG (phosphoprotein associated with glycosphingolipid-enriched microdomains) and caveolin-1 via the SH2, SH3, and/or kinase domain of Csk (22). We therefore wished to know whether subcellular localization of Csk is altered upon complex formation with Pragmin. First, as a control experiment, we expressed Csk in AGS cells alone or together with CagA, which has been shown to interact with Csk and thereby recruit it to the plasma membrane (6). In the absence of CagA, Csk was broadly distributed to the cytoplasm (Fig. 4A). In the presence of CagA, however, Csk was translocalized to the membrane, where it was colocalized with CagA (Fig. 4B). We then examined the effect of Pragmin on Csk localization. In AGS cells, both Csk and Pragmin were localized in the cytoplasm when singly expressed, and coexpression of Pragmin and Csk in the same cell did not alter cytoplasmic localization of Csk (Fig. 4C). These observations indicate that, in contrast to the CagA–Csk complex, the Pragmin–Csk complex keeps Csk in the cytoplasm, preventing SH2 domain-mediated interaction of Csk with other molecules.
Fig. 4.
Subcellular localization of Pragmin-Csk complex. (A) AGS cells transfected with a Csk-FLAG vector were stained with an anti-FLAG antibody. (B) AGS cells cotransfected with Csk-FLAG and CagA-HA vectors were double-stained with anti-FLAG (Csk; green) and anti-HA (CagA; red) antibodies. (C) AGS cells cotransfected with Csk-FLAG and Myc-Pragmin vectors were double-stained with anti-FLAG (Csk; green) and anti-Myc (Pragmin; red) antibodies. Nuclei (blue) were visualized by DAPI staining. (Scale bars, 10 μm.)
H. pylori CagA Inhibits the Pragmin-Csk Interaction.
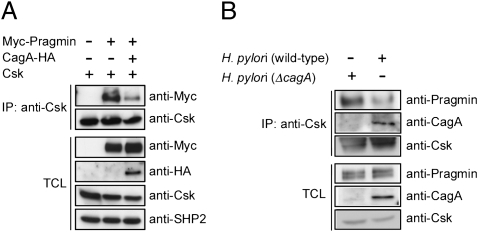
The finding that Pragmin specifically binds to the SH2 domain of Csk in a tyrosine phosphorylation-dependent manner prompted us to investigate the effect of H. pylori CagA, which also binds to the Csk SH2 domain upon EPIYA phosphorylation, on the Pragmin–Csk complex. To this end, AGS cells were transiently cotransfected with Pragmin and Csk vectors in the presence or absence of a CagA vector, and the amount of the Pragmin–Csk complex formed in cells was examined. As a result, expression of CagA not only led to generation of the CagA–Csk complex but also substantially reduced the level of the Pragmin–Csk complex (Fig. 5A). In this regard, Pragmin has been reported to activate RhoA GTPases (18). Additionally, H. pylori CagA is capable of stimulating RhoA (23). However, inhibition of RhoA activity by Clostridial C3 transferase did not influence the level of the Pragmin–Csk complex in cells, arguing against an active role of RhoA in the Pragmin–Csk interaction (Fig. S6). To further elucidate the pathophysiological relevance of the above-described observations, we next performed an H. pylori infection experiment in AGS cells and found that infection with the H. pylori cagA-positive strain, but not that with the isogenic cagA-negative strain, reduced the level of the endogenous Pragmin–Csk complex, which was concomitantly associated with the appearance of the CagA–Csk complex (Fig. 5B). From these observations, we concluded that H. pylori CagA inhibits Pragmin–Csk interaction while simultaneously forming a complex with Csk. Because both H. pylori CagA and Pragmin commonly bind to the same SH2 domain of Csk upon EPIYA-tyrosine phosphorylation, it was thought that direct competition between CagA and Pragmin for Csk binding underlies the CagA-mediated inhibition of Pragmin–Csk complex formation.
Fig. 5.
Disruption of the Pragmin–Csk complex by H. pylori CagA. (A) AGS cells were transfected with the indicated vectors. Total cell lysates (TCLs) were immunoprecipitated with an anti-Csk antibody, and the immunoprecipitates (IPs) were subjected to immunoblotting with the indicated antibodies. (B) AGS cells were infected with the H. pylori cagA-positive or the cagA-negative isogenic strain (ΔcagA) for 5 h. Total cell lysates were immunoprecipitated with an anti-Csk antibody, and the immunoprecipitates were subjected to immunoblotting with the indicated antibodies.
Discussion
We demonstrated in this work that mammalian Pragmin undergoes tyrosine phosphorylation at the EPIYA motif by SFKs or in response to EGF stimulation. Upon EPIYA phosphorylation, Pragmin acquires the ability to interact with the SH2 domain of Csk. The Pragmin–Csk interaction is inhibited by H. pylori CagA, which also binds to the Csk SH2 domain in an EPIYA-dependent manner. Consistent with our present work, a comprehensive proteomic study by Zhang et al. (24) also revealed human Pragmin to be one of 76 proteins that are tyrosine-phosphorylated in response to EGF stimulation. They further confirmed by mass spectrometry analysis that Pragmin was tyrosine-phosphorylated on Y411 at the EPIYA motif, which corresponds to Y391 in rat Pragmin.
The EPIYA motif is evolutionally conserved throughout mammalian Pragmin orthologs and is also present in Xenopus Pragmin, indicating that the sequence plays an important role in the function of Pragmin. Now, the present work revealed a heretofore unidentified role of Pragmin in the regulation of SFKs, composed of nine members of Src-related nonreceptor tyrosine kinases that are membrane-tethered via lipid modification. Csk phosphorylates the C-terminal inhibitory tyrosine residue of SFKs and thereby inactivates their kinase activity. To accomplish this task, Csk must be translocated from the cytoplasm to the membrane, a process mediated by various membrane-associated proteins such as Cbp/PAG and caveolin-1 that, upon tyrosine phosphorylation, bind to the Csk SH2 domain (21, 22). We show that Pragmin is localized to the cytoplasm and, through complex formation, Pragmin anchors Csk in the cytoplasm, preventing its relocation to the membrane and subsequent inactivation of SFKs. Because SFKs phosphorylate the EPIYA motif of Pragmin, SFKs positively regulate their own kinase activity by sequestrating Csk via EPIYA-phosphorylated Pragmin. Also notably, Pragmin is phosphorylated at the EPIYA motif by the activated EGF receptor or its downstream kinases distinct from SFKs. This finding indicates the presence of a positive feedback regulation that ensures sustained SFK activation in cells stimulated with a growth factor such as EGF (Fig. S7). Recently, Leroy et al. (25) reported that ectopic expression of c-Src in human colon carcinoma cells induces phosphorylation of a cluster of tyrosine kinases and pseudokinases that include Pragmin. They also showed that, upon tyrosine phosphorylation on Y391 (the EPIYA motif), Pragmin stimulates c-Src activity in colon carcinoma cells, although the underlying mechanism was not known (25). The results of the study by Leroy et al. are consistent with those of the present work and can be fully explained by the positive feedback regulation of SFKs via the Pragmin–Csk interaction.
It has been proposed that bacterial EPIYA motifs act as promiscuous master keys that can interact with and therefore simultaneously disturb a number of mammalian SH2 domain-containing proteins (16). For bacteria, the versatile binding properties may be beneficial in exerting virulence. On the other hand, such a master key function may not be allowed in mammals in which intracellular signaling circuits are subjected to strict regulation. Consistent with this idea, mammalian proteomes are significantly depleted of EPIYA-like sequences (16). In the present study we found that Pragmin EPIYA binds to Csk in an EPIYA phosphorylation-dependent manner. However, in contrast to bacterial EPIYA motifs, which are characterized by promiscuous SH2 binding, the Pragmin EPIYA motif did not exhibit diverse SH2-binding activity. Because SH2-binding specificity is governed by three to five residues C-terminal to the phosphotyrosine (15), degenerated SH2 binding in bacterial EPIYA effectors could be attributable to subtle differences within these particular residues. The molecular basis that confers promiscuous SH2 binding on bacterial EPIYA effectors obviously warrants further investigation.
Pragmin potentiates SFK activity by sequestrating Csk, an inhibitor of SFKs, to the cytoplasm. On the other hand, H. pylori-delivered CagA, which competes with Pragmin for Csk binding, inhibits Pragmin–Csk complex formation. Furthermore, CagA recruits Csk to the membrane upon complex formation, where Csk inactivates SFKs. The effect of CagA on SFKs (inhibition) is therefore opposite to that of Pragmin (activation). Because EPIYA tyrosine phosphorylation is required for full activation of CagA virulence (2, 3, 7), feedback inhibition of SFK activity by CagA may be beneficial for H. pylori in preventing excess CagA virulence, as previously proposed (6). Also notably, in epithelial cells, SFKs are required for the induction of antibacterial peptides such as defensins (26) and are involved in the activation of NF-κB in response to bacterial infection (27). It is therefore intriguing to speculate that CagA-mediated SFK inhibition enables H. pylori to evade innate defense mechanisms of host epithelial cells.
In the immune system, SFKs regulate antigen receptor signaling in T and B lymphocytes (28), mediate cytokine receptor signaling in various hematopoietic lineage cells (29), and promote bacterial phagocytosis by macrophages (30). Thus, sustained SFK activation, which is ensured by the Pragmin–Csk interaction, may play an important role in priming antibacterial immune responses. In turn, aberration in the SFK activity in immune cells might result in a wide array of immune dysfunctions that help bacteria to achieve immune evasion. In this regard, we and others have previously suggested that H. pylori CagA could also be delivered into immune cells that have migrated to the inflammatory region of gastric mucosa (31, 32). This raises the idea that, upon delivery into immune cells, CagA suppresses SFKs to dampen host immune systems.
Given that SFKs comprise nine members, Csk must be an ideal target for bacteria to collectively inhibit these SFK members. The EPIYA motifs of B. henselae BepD and BepF, which are delivered into human endothelial cells, also bind to Csk upon tyrosine phosphorylation (16). H. ducreyi inhibits macrophage-mediated phagocytosis, and this inhibition requires an H. ducreyi EPIYA effector, LspA (13, 33). By analogy with CagA, it is possible that EPIYA-phosphorylated LspA binds Csk to inhibit SFKs in macrophages. Simultaneous development of such EPIYA effectors in different bacteria may be an excellent example of “genetic convergence,” in which EPIYA motif-containing proteins were incidentally selected as bacterial EPIYA effectors, most probably because of the special advantage in perturbing host cell functions, such as those mediated by SFKs for successful bacterial infection.
Here we identified a mammalian EPIYA effector, Pragmin. Elucidation of a prototypic EPIYA effector protein that acts as a scaffold/adaptor in mammalian signal transduction may give further insights into the mechanisms underlying generation and evolution of bacterial EPIYA effectors during the long and highly balanced degree of coevolution of bacteria with mammalian hosts.
Materials and Methods
Expression Vectors.
The pCMV, pSP65SRα, and pcDNA3-derived mammalian expression vectors for Pragmin, Csk, and H. pylori CagA are described in SI Materials and Methods.
Antibodies and Reagents.
Antibodies and reagents used in this work are described in SI Materials and Methods.
Bacteria.
AGS cells were infected with H. pylori NCTC11637 or its isogenic strain lacking the cagA gene (ΔcagA) for 5 h at a multiplicity of infection of 200 before harvest.
Cell Culture and Transfection.
AGS and MKN28 human gastric carcinoma cells were cultured in RPMI medium 1640 supplemented with 10% FCS. GES-1 human normal gastric epithelial cells were cultured in DMEM containing 10% FCS. Transient transfection was performed according to standard protocol using Lipofectamine 2000 reagent (Invitrogen).
Immunoprecipitation and Immunoblotting.
Immunoprecipitation and immunoblotting were performed as previously described (5, 6). Refer to SI Materials and Methods for a detailed description. Intensity of protein band was quantitated by a luminescent image analyzer.
Immunofluoresence Microscopy.
Cells fixed with 3% paraformaldehyde were treated with primary antibodies and were then visualized with Alexa Fluor-conjugated secondary antibodies, as previously described (5, 6). Images were acquired using a confocal microscope.
Data Analysis.
All of the DNA and protein sequences were retrieved from the National Center for Biotechnology database (www.ncbi.nlm.nih.gov) and were analyzed using the BLAST search program. BioEdit and WebLogo were used to align and display protein sequences (http://weblogo.berkeley.edu). ClustalW and TreeView (http://align.genome.jp/) were applied to build and view phylogenic trees.
Supplementary Material
Acknowledgments
We thank Drs. M. Negishi, A. Villalobo, and M. Fukayama for cDNAs and cells. This work was supported by Grants-in-Aid for the Scientific Research on Innovative Area from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to M.H.). F.S. is the recipient of a Japanese Government (Monbukagakusho) Scholarship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107740108/-/DCSupplemental.
References
- 1.Backert S, Selbach M. Tyrosine-phosphorylated bacterial effector proteins: The enemies within. Trends Microbiol. 2005;13:476–484. doi: 10.1016/j.tim.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Peek RM., Jr Orchestration of aberrant epithelial signaling by Helicobacter pylori CagA. Sci STKE. 2005;2005:pe14. doi: 10.1126/stke.2772005pe14. [DOI] [PubMed] [Google Scholar]
- 3.Hatakeyama M. SagA of CagA in Helicobacter pylori pathogenesis. Curr Opin Microbiol. 2008;11:30–37. doi: 10.1016/j.mib.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Tegtmeyer N, Backert S. Role of Abl and Src family kinases in actin-cytoskeletal rearrangements induced by the Helicobacter pylori CagA protein. Eur J Cell Biol. 2011 doi: 10.1016/j.ejcb.2010.11.006. 10.1016/j.ejcb.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Higashi H, et al. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori Cag. Protein Sci. 2002;295:683–686. doi: 10.1126/science.1067147. [DOI] [PubMed] [Google Scholar]
- 6.Tsutsumi R, Higashi H, Higuchi M, Okada M, Hatakeyama M. Attenuation of Helicobacter pylori CagA x SHP-2 signaling by interaction between CagA and C-terminal Src kinase. J Biol Chem. 2003;278:3664–3670. doi: 10.1074/jbc.M208155200. [DOI] [PubMed] [Google Scholar]
- 7.Hatakeyama M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat Rev Cancer. 2004;4:688–694. doi: 10.1038/nrc1433. [DOI] [PubMed] [Google Scholar]
- 8.IJdo JW, Carlson AC, Kennedy EL. Anaplasma phagocytophilum AnkA is tyrosine-phosphorylated at EPIYA motifs and recruits SHP-1 during early infection. Cell Microbiol. 2007;9:1284–1296. doi: 10.1111/j.1462-5822.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- 9.Lin M, den Dulk-Ras A, Hooykaas PJ, Rikihisa Y. Anaplasma phagocytophilum AnkA secreted by type IV secretion system is tyrosine phosphorylated by Abl-1 to facilitate infection. Cell Microbiol. 2007;9:2644–2657. doi: 10.1111/j.1462-5822.2007.00985.x. [DOI] [PubMed] [Google Scholar]
- 10.Campellone KG, Giese A, Tipper DJ, Leong JM. A tyrosine-phosphorylated 12-amino-acid sequence of enteropathogenic Escherichia coli Tir binds the host adaptor protein Nck and is required for Nck localization to actin pedestals. Mol Microbiol. 2002;43:1227–1241. doi: 10.1046/j.1365-2958.2002.02817.x. [DOI] [PubMed] [Google Scholar]
- 11.Deng W, Vallance BA, Li Y, Puente JL, Finlay BB. Citrobacter rodentium translocated intimin receptor (Tir) is an essential virulence factor needed for actin condensation, intestinal colonization and colonic hyperplasia in mice. Mol Microbiol. 2003;48:95–115. doi: 10.1046/j.1365-2958.2003.03429.x. [DOI] [PubMed] [Google Scholar]
- 12.Clifton DR, et al. A chlamydial type III translocated protein is tyrosine-phosphorylated at the site of entry and associated with recruitment of actin. Proc Natl Acad Sci USA. 2004;101:10166–10171. doi: 10.1073/pnas.0402829101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng K, Mock JR, Greenberg S, van Oers NS, Hansen EJ. Haemophilus ducreyi LspA proteins are tyrosine phosphorylated by macrophage-encoded protein tyrosine kinases. Infect Immun. 2008;76:4692–4702. doi: 10.1128/IAI.00513-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulein R, et al. A bipartite signal mediates the transfer of type IV secretion substrates of Bartonella henselae into human cells. Proc Natl Acad Sci USA. 2005;102:856–861. doi: 10.1073/pnas.0406796102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machida K, Mayer BJ. The SH2 domain: Versatile signaling module and pharmaceutical target. Biochim Biophys Acta. 2005;1747:1–25. doi: 10.1016/j.bbapap.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Selbach M, et al. Host cell interactome of tyrosine-phosphorylated bacterial proteins. Cell Host Microbe. 2009;5:397–403. doi: 10.1016/j.chom.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Linz B, et al. An African origin for the intimate association between humans and Helicobacter pylori. Nature. 2007;445:915–918. doi: 10.1038/nature05562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka H, Katoh H, Negishi M. Pragmin, a novel effector of Rnd2 GTPase, stimulates RhoA activity. J Biol Chem. 2006;281:10355–10364. doi: 10.1074/jbc.M511314200. [DOI] [PubMed] [Google Scholar]
- 19.Boudeau J, Miranda-Saavedra D, Barton GJ, Alessi DR. Emerging roles of pseudokinases. Trends Cell Biol. 2006;16:443–452. doi: 10.1016/j.tcb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Kong L, Deng Z, Shen H, Zhang Y. Src family kinase inhibitor PP2 efficiently inhibits cervical cancer cell proliferation through down-regulating phospho-Src-Y416 and phospho-EGFR-Y1173. Mol Cell Biochem. 2011;348:11–19. doi: 10.1007/s11010-010-0632-1. [DOI] [PubMed] [Google Scholar]
- 21.Roskoski R., Jr Src protein-tyrosine kinase structure and regulation. Biochem Biophys Res Commun. 2004;324:1155–1164. doi: 10.1016/j.bbrc.2004.09.171. [DOI] [PubMed] [Google Scholar]
- 22.Ingley E. Src family kinases: Regulation of their activities, levels and identification of new pathways. Biochim Biophys Acta. 2008;1784:56–65. doi: 10.1016/j.bbapap.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Saito Y, Murata-Kamiya N, Hirayama T, Ohba Y, Hatakeyama M. Conversion of Helicobacter pylori CagA from senescence inducer to oncogenic driver through polarity-dependent regulation of p21. J Exp Med. 2010;207:2157–2174. doi: 10.1084/jem.20100602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, et al. Time-resolved mass spectrometry of tyrosine phosphorylation sites in the epidermal growth factor receptor signaling network reveals dynamic modules. Mol Cell Proteomics. 2005;4:1240–1250. doi: 10.1074/mcp.M500089-MCP200. [DOI] [PubMed] [Google Scholar]
- 25.Leroy C, et al. Quantitative phosphoproteomics reveals a cluster of tyrosine kinases that mediates SRC invasive activity in advanced colon carcinoma cells. Cancer Res. 2009;69:2279–2286. doi: 10.1158/0008-5472.CAN-08-2354. [DOI] [PubMed] [Google Scholar]
- 26.Moon SK, et al. Activation of a Src-dependent Raf-MEK1/2-ERK signaling pathway is required for IL-1α-induced upregulation of β-defensin 2 in human middle ear epithelial cells. Biochim Biophys Acta. 2002;1590:41–51. doi: 10.1016/s0167-4889(02)00196-9. [DOI] [PubMed] [Google Scholar]
- 27.Li JD, et al. Activation of NF-kappaB via a Src-dependent Ras-MAPK-pp90rsk pathway is required for Pseudomonas aeruginosa-induced mucin overproduction in epithelial cells. Proc Natl Acad Sci USA. 1998;95:5718–5723. doi: 10.1073/pnas.95.10.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chow LM, Veillette A. The Src and Csk families of tyrosine protein kinases in hemopoietic cells. Semin Immunol. 1995;7:207–226. doi: 10.1006/smim.1995.0026. [DOI] [PubMed] [Google Scholar]
- 29.Ingley E, Klinken SP. Cross-regulation of JAK and Src kinases. Growth Factors. 2006;24:89–95. doi: 10.1080/08977190500368031. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki T, et al. Differential involvement of Src family kinases in Fc γ receptor-mediated phagocytosis. J Immunol. 2000;165:473–482. doi: 10.4049/jimmunol.165.1.473. [DOI] [PubMed] [Google Scholar]
- 31.Umehara S, Higashi H, Ohnishi N, Asaka M, Hatakeyama M. Effects of Helicobacter pylori CagA protein on the growth and survival of B lymphocytes, the origin of MALT lymphoma. Oncogene. 2003;22:8337–8342. doi: 10.1038/sj.onc.1207028. [DOI] [PubMed] [Google Scholar]
- 32.Lin WC, et al. Translocation of Helicobacter pylori CagA into Human B lymphocytes, the origin of mucosa-associated lymphoid tissue lymphoma. Cancer Res. 2010;70:5740–5748. doi: 10.1158/0008-5472.CAN-09-4690. [DOI] [PubMed] [Google Scholar]
- 33.Mock JR, et al. Haemophilus ducreyi targets Src family protein tyrosine kinases to inhibit phagocytic signaling. Infect Immun. 2005;73:7808–7816. doi: 10.1128/IAI.73.12.7808-7816.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.