Abstract
The CCAAT/enhancer binding proteins (C/EBPs) are differentially expressed throughout mammary gland development and interact with binding sites within the promoter of a milk protein gene, β-casein. The specific roles of C/EBPβ and C/EBPα in mouse mammary gland development and differentiation have been investigated in mice that carry targeted deletions of these genes. C/EBPβ−/− virgin mice exhibited cystic, enlarged mammary ducts with decreased secondary branching. Transplantation of C/EBPβ−/− mammary epithelium into the cleared mammary fat pads of nude mice confirmed that this defect in ductal morphogenesis was intrinsic to the epithelium. When treated with estrogen/progesterone (E+P) to simulate pregnancy, C/EBPβ−/− mammary glands displayed only limited lobuloalveolar development and ductal side branching. Primary mammary epithelial cells obtained from E+P-treated C/EBPβ−/− mice that were cultured on extracellular matrix gels did not functionally differentiate in response to lactogenic hormones despite their organization into three-dimensional structures. Expression of β-casein protein was inhibited 85%–100% and whey acidic protein (WAP) was undetectable. In contrast, no detectable alterations in mammary development or β-casein expression were observed in mammary outgrowths derived from newborn C/EBPα−/− mammary epithelium transplanted into the cleared mammary fat pads of syngeneic hosts. These results demonstrate that C/EBPβ, but not C/EBPα, is required for ductal morphogenesis, lobuloalveolar development, and functional differentiation of mammary epithelial cells.
Keywords: Mammary development, C/EBP, ductal growth, lactogenesis, β-casein, whey acidic protein
The CCAAT/enhancer binding protein (C/EBP) family of leucine-zipper DNA binding (bZIP) proteins regulates the transcription of a variety of target genes in multiple tissues by binding as homo- or heterodimers to a common nucleotide consensus site (Landschulz et al. 1988; Williams et al. 1991). The specificity of gene regulation by the C/EBPs is thought to result from both the tissue-restricted and temporal expression of individual family members, differences in their amino-terminal transactivation domains, and their ability to heterodimerize with other C/EBP and bZIP proteins (Yeh et al. 1995). In addition, alternative translation of the intronless C/EBPα and C/EBPβ genes produces different protein isoforms via a leaky ribosome scanning mechanism (Descombes and Schibler 1991; Lin et al. 1993; Ossipow et al. 1993). Translation of C/EBPα mRNA produces two protein isoforms, 42 and 30 kD, which differ in their transcriptional activities. C/EBPβ may be translated into three different protein isoforms; the liver-enriched transcriptional activating proteins (LAPs), 39 kD (LAP1) and 36 kD (LAP2), which function as activators of transcription and the liver-enriched transcriptional inhibitory protein (LIP, 20 kD), which lacks most of the transactivation domain and, therefore, acts as a dominant-negative transcriptional repressor (Descombes and Schibler 1991). LIP not only binds to the C/EBP consensus with a higher affinity than the LAPs, but also forms heterodimers with LAP to inhibit transactivation at substoicheometric ratios (Descombes and Schibler 1991). Therefore, the ratio of LIP/LAP is an important determinant of overall C/EBPβ function.
Individual C/EBPs have been implicated as critical regulators of proliferation versus differentiation in multiple tissues. C/EBPα, the first family member to be discovered (Landschulz et al. 1988), functions as both an inhibitor of proliferation and facilitator of terminal differentiation (Umek et al. 1991; Hendricks-Taylor and Darlington 1995). Experiments in several systems support a model of cascade regulation in which C/EBPδ and C/EBPβ expression precede expression of C/EBPα and correlate with induction of proliferative and/or early differentiative events (Cao et al. 1991; Alam et al. 1992; Yeh et al. 1995). C/EBPα expression is restricted to late in the differentiation process and induces terminal differentiation (Cao et al. 1991; Lin and Lane 1994).
Three C/EBPs, C/EBPα, C/EBPβ, and C/EBPδ, are differentially expressed in the rat mammary gland during the course of postnatal development (Raught et al. 1995). This complex process consists of four tightly regulated stages: ductal outgrowth into the stromal fat pad during puberty, lobuloalveolar proliferation and differentiation during pregnancy, synthesis and secretion of milk by epithelial cells at lactation, and involution of the secretory epithelium following weaning. Each stage depends on a critical balance between proliferation, differentiation, and apoptosis orchestrated by multiple signaling pathways (Hennighausen and Robinson 1998). The C/EBPs may play a pivotal role in coordinating these processes in the mammary gland as has been demonstrated in liver, adipocytes, intestine, ovary, and the immune system (Cao et al. 1991; Piontkewitz et al. 1993; Diehl et al. 1994; Blais et al. 1995; Yeh et al. 1995; Zhang et al. 1997).
On the basis of Western blot analysis with two different anti-C/EBPα antibodies, C/EBPα was reported previously to be expressed maximally at lactation and decreased during involution (Raught et al. 1995). The expression of LAP2, which is thought to be the most transcriptionally active form of C/EBPβ (Williams et al. 1995), occurs throughout mammary gland development from the virgin to lactation and involution with only a modest increase in expression during pregnancy and a modest decrease at lactation (Raught et al. 1995). LIP, however, is primarily expressed during pregnancy, a period of rapid proliferation of epithelial cells. The high ratio of the dominant-negative LIP isoform to LAP2 observed during pregnancy suggests that LAP2 may have a reduced ability to transactivate target genes. Because LIP can also inhibit C/EBPα through heterodimerization, the increase of LIP during pregnancy may also reduce transactivation of C/EBPα’s target genes. At lactation, LIP is rapidly downregulated, resulting in a dramatic decrease in the LIP/LAP ratio (Raught et al. 1995). In support of the hypothesis that LIP is expressed during periods of proliferation in the mammary gland, high levels of LIP expression have been observed in both murine mammary tumors and in high-grade infiltrating ductal carcinomas from human patients, whereas low to undetectable levels of LIP were expressed in hyperplastic or normal tissue surrounding the tumors (Raught et al. 1996; Zahnow et al. 1997).
Deletion of either C/EBPα or C/EBPβ in mice results in pleiotropic disorders. C/EBPα−/− mice die shortly after birth as a result of the failure of the liver to store glycogen (Wang et al. 1995). In addition, they do not accumulate white adipose tissue or mature granulocytes in the blood (Zhang et al. 1997). Animals lacking C/EBPβ are viable, but are more susceptible to bacterial infections because of immune system defects (Screpanti et al. 1995; Tanaka et al. 1995), and females are sterile as a result of defects in ovarian granulosa cell differentiation (Sterneck et al. 1997). Most mice lacking both C/EBPβ and C/EBPδ genes die shortly after birth (Tanaka et al. 1997). Newborns that survive fail to accumulate normal levels of lipids and have defects in brown fat development. Primary embryonic fibroblasts isolated from embryos fail to differentiate from preadipocytes to adipocytes (Tanaka et al. 1997), confirming that C/EBPβ and C/EBPδ cooperate during adipocyte differentiation in agreement with the model of cascade regulation.
Mice carrying targeted deletions of either C/EBPβ or C/EBPα have been used to facilitate the assignment of specific regulatory roles to the individual C/EBPs in mammary development and function. To study mammary gland development in these null mice with postnatal viability and reproductive problems, several different techniques have been used. To rescue the mammary epithelium from newborn C/EBPα−/− mice, mammary rudiments were isolated and transplanted into the cleared fat pads of syngeneic 3-week-old host mice similar to the method developed by DeOme et al. (1959). After a period of outgrowth, the morphology of the C/EBPα+/+ and −/− epithelium-transplanted glands were compared. To simulate pregnancy in the sterile C/EBPβ mutant females, animals were injected subcutaneously with estrogen followed by insertion of slow-release estrogen/progesterone (E+P) pellets for 21 days. Finally, primary mammary epithelial cells (MEC) from E+P-treated females were isolated and cultured on extracellular matrix (Matrigel) gels to analyze β-casein and whey acidic protein (WAP) expression in response to lactogenic hormones (prolactin, Prl, insulin, I, and hydrocortisone, H). These studies indicate that ductal morphogenesis and branching, and lobuloalveolar proliferation and functional differentiation are critically dependent on C/EBPβ expression. C/EBPα, however, does not appear to be essential for these processes.
Results
Expression pattern of C/EBPβ during mouse mammary gland development
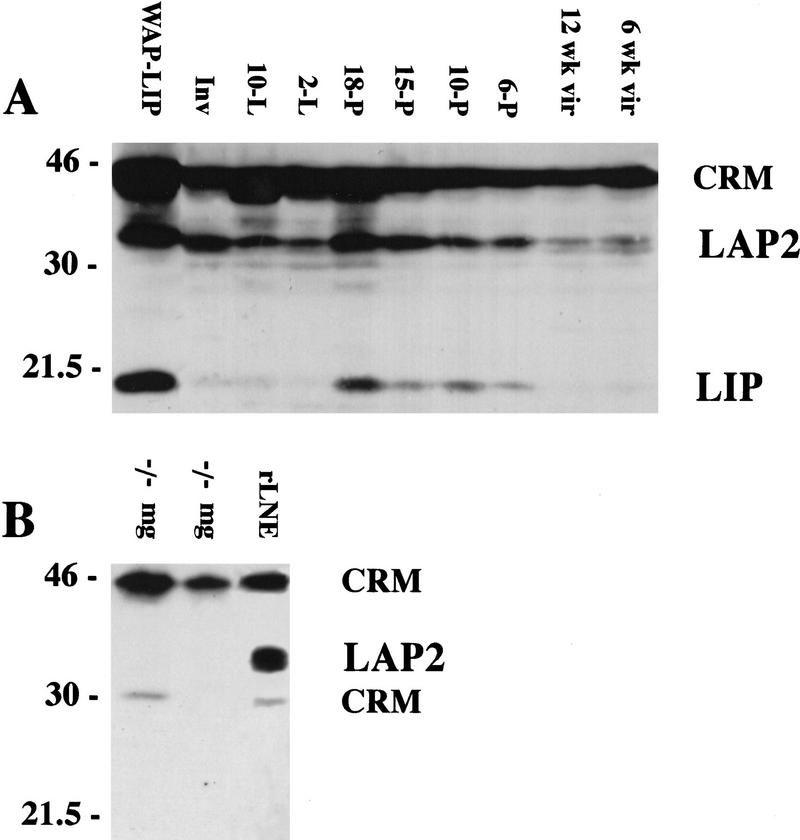
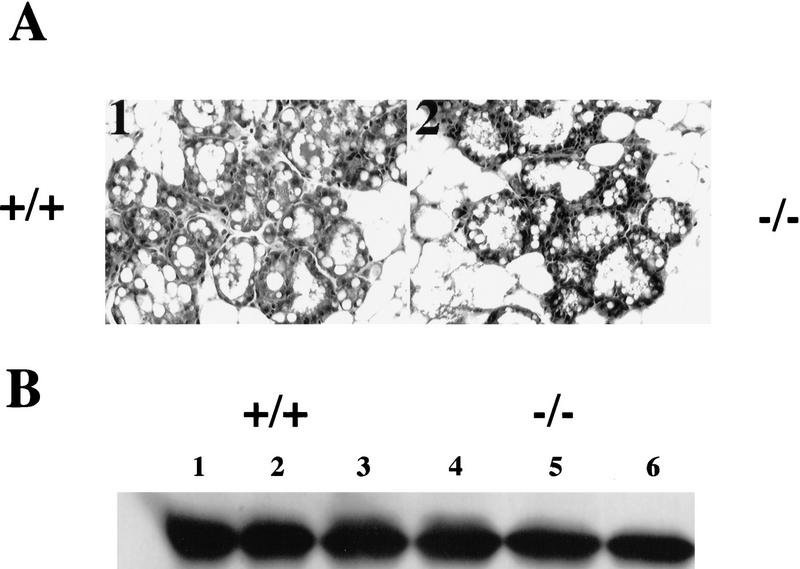
Western blot analyses of whole cell extracts of mouse mammary tissue collected at intervals during development confirmed that the expression pattern of C/EBPβ protein in the mouse mimics that observed during rat mammary gland development (Raught et al. 1995). Note the increased expression of the C/EBPβ LIP isoform (20 kD) during pregnancy (Fig. 1A). Consistent with previous reports of expression in the rat mammary gland, the LAP2 (36 kD) isoform of C/EBPβ is expressed at low levels in both immature and mature virgin mice, its expression increases during pregnancy and decreases again at lactation. The 45-kD band referred to previously as LAP1 in the rat (Raught et al. 1995) represents instead cross-reactive material (CRM) and not a specific C/EBPβ isoform despite specific competition by an excess of the C/EBPβ peptide to which the antisera was raised. This has been established definitively by Western blot analysis of mammary gland extracts prepared from the C/EBPβ−/− mice in which the entire gene has been deleted (Fig 1B).
Figure 1.
Expression of the C/EBPβ isoforms in C57/Bl6 mice during mammary development. (A) Mammary tissue was isolated from mice at the following developmental stages: immature virgin (6 week vir), mature virgin (12 week vir), 6 days of pregnancy (6-P), 10 days of pregnancy (10-P), 15 days of pregnancy (15-P), 18 days of pregnancy (18-P), 2 days of lactation (2-L), 10 days of lactation (10-L), and 10 days of lactation followed by 4 days of involution induced by removal of pups (Inv). Aliquots of WCE (100 μg) were separated by SDS-PAGE, transferred to membrane, and probed with antibodies against rat C/EBPβ (Santa Cruz, sc-150-x) as described in Materials and Methods. The LAP2 (36 kD) and LIP (20 kD) isoforms of C/EBPβ are shown (A,B). As a positive control for expression of LIP (A), an aliquot (100 μg) from a late pregnant WAP-driven LIP transgenic mouse (WAP–LIP) was included. (B) The 45-kD band referred to previously as LAP1 (Raught et al. 1995) is CRM as indicated by probing C/EBPβ−/− virgin mg (−/−mg) with C/EBPβ antibody. In addition, CRM at 30 kD was noted in some C/EBPβ−/− mammary gland extracts and in the rat liver nuclear extract (rLNE) included as a positive control (B).
Deletion of C/EBPβ alters ductal morphogenesis as a result of a defect in the mammary epithelium
To determine the contribution of C/EBPβ to mammary gland function, ductal development was analyzed in virgin C/EBPβ−/− mice (Fig. 2). Whole inguinal mammary glands were isolated, whole mounted, and compared in the developing (5 week) and mature (8–12 week) mammary glands from wild-type (Fig. 2A,C,E), heterozygous and C/EBPβ−/− (Fig. 2B,D,F) female virgin mice. At 5 weeks of age, terminal end buds were present and appeared normal in the C/EBPβ−/− mice (data not shown). At maturity, however, all virgin C/EBPβ−/− glands and ∼20% of C/EBPβ+/− glands (data not shown) were filled with abnormally large, bloated ducts, which terminated with bloated ends. In addition, fewer secondary and tertiary ductal branches were observed in the glands of mature C/EBPβ-deficient virgins (Fig. 2B,D) as compared with heterozygous or wild-type littermates (Fig. 2A,C). The increase in the width of all of the ducts in the C/EBPβ−/− virgins did not appear to be a result of secreted proteinaceous products collected in the duct or a hyperplasia of ductal luminal cells (Fig. 2F).
Figure 2.
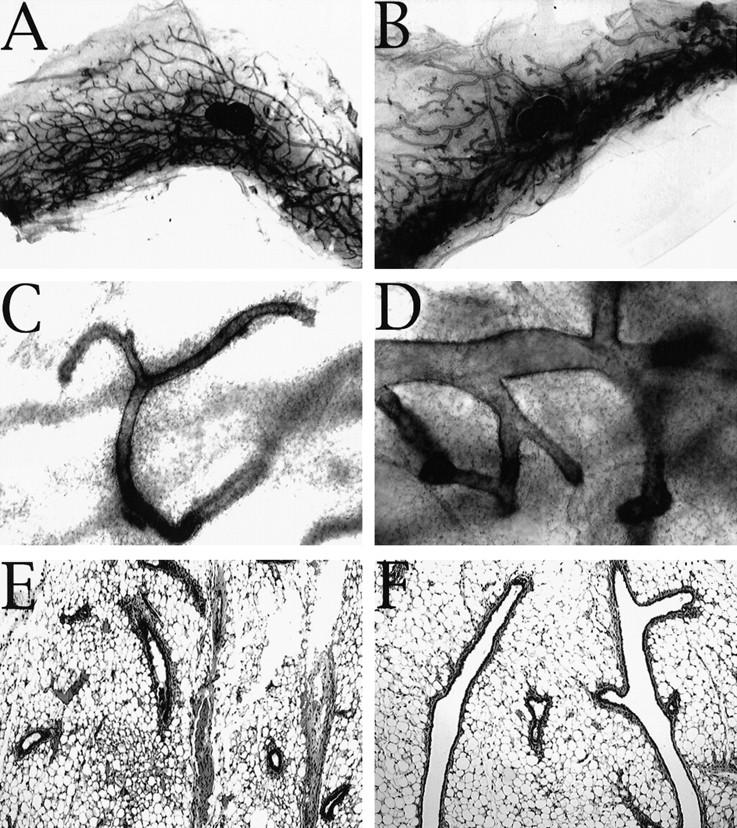
C/EBPβ−/− virgins exhibit abnormal, cystic ducts compared with wild-type littermates. Very large bloated ducts are obvious in whole mount preparations taken from mature C/EPBβ−/− mice (B,D) compared with glands isolated from wild-type littermates that contained normal ducts (A,C,E). Analysis of hematoxylin and eosin-stained paraffin-embedded sections (E,F) indicates that the enlarged ducts present in C/EBPβ nulls (F) are not the result of proteinaceous material trapped within the ducts or hyperplasia of the ductal epithelium. Images were captured at either 1.5× (A,B) or 10× (C–F) magnification.
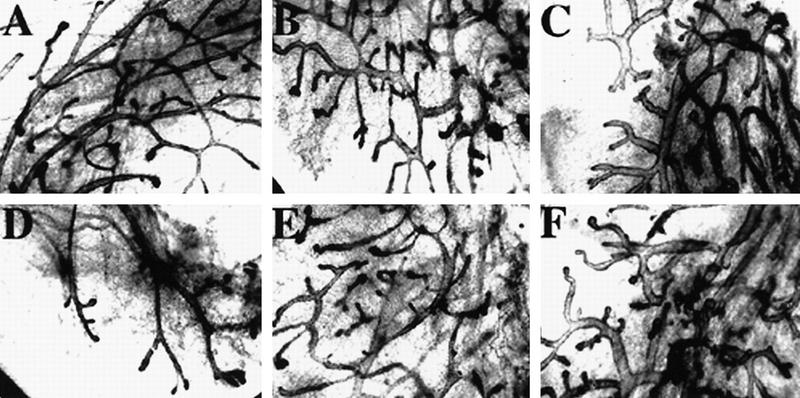
To investigate whether the defect in mammary ductal morphogenesis was a result of a systemic effect in the C/EBPβ−/− mice, or a direct result of a defect residing in the mammary epithelium, donor tissue from mature C/EBPβ+/+, C/EBPβ+/−, and C/EBPβ−/− females was transplanted into the cleared fat pads of 3-week-old nude (nu/nu) host mice. Because the C/EBPβ females are maintained in a mix of inbred and outbred mouse strains, transplantation into nude mice was required to prevent immunorejection of transplanted tissue. After 6 weeks of ductal outgrowth, both inguinal transplanted glands were harvested, fixed, and stained for whole mount analysis. The C/EBPβ+/+ transplants displayed normal ducts, whereas the C/EBPβ−/− transplants exhibited the bloated ducts resembling those observed in the intact virgin C/EBPβ−/− mice (Fig. 3A,D vs. C,F). Transplants containing heterozygous epithelium displayed an intermediate phenotype in that some ducts appeared to be normal whereas others were enlarged (Fig. 3B,E). These results confirm that the defects observed in ductal development reside in the mammary epithelium of the C/EBPβ−/− and are not a result of systemic deficiencies.
Figure 3.
Transplantation of epithelium from C/EBPβ donors of each genotype into the cleared fat pads of nu/nu recipients localizes the defect in ductal morphogenesis to the mammary epithelium. Six weeks post-transplantation, whole transplanted inguinal mammary glands were isolated from recipients, fixed and stained with hematoxylin for whole mount analysis. The C/EBPβ+/+ transplants (A,D) contain normal narrow branched ducts, whereas the C/EBPβ+/− (B,E) exhibit an intermediate phenotype with some bloated ducts, and the C/EPBβ−/− transplants (C,F) contain primarily the bloated ducts observed in intact C/EBPβ−/− virgins.
Lobuloalveolar development in response to E+P treatment is limited in C/EBPβ−/− mice
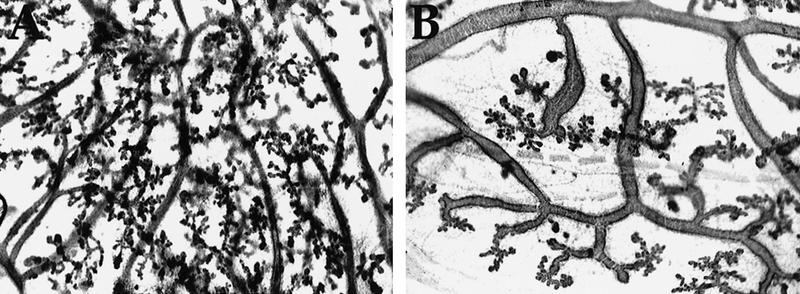
Because C/EBPβ−/− females are sterile, pregnancy was simulated by E+P treatment of sexually mature virgins (8–10 weeks of age). Animals were injected once subcutaneously with estradiol benzoate, and three days following the injection, slow-release E+P beeswax pellets were inserted under the skin behind the neck and left for 21 days. Following treatment, the thoracic mammary glands were isolated, fixed, and whole mounted. In response to E+P during pregnancy, alveoli composed of a single-layer of epithelial cells bud from the ductal tree. An obvious impairment of lobuloalveolar development was evident in the C/EBPβ−/− mice (Fig. 4B). Large areas of the ducts did not contain either side branches or alveoli. Although the alveoli that developed in the nulls appeared normal, the density of alveoli in the nulls was sparse compared with the C/EBPβ+/− mice (Fig. 4A). In heterozygous mice this protocol of estrogen priming followed by E+P treatment stimulated development similar to that observed at mid-pregnancy in wild-type C57 mice (day 10–11, mid-pregnancy). In contrast, the extent of development in the majority of the C/EBPβ−/− mouse mammary glands resembled that of a mouse at 6–8 days of pregnancy (early pregnancy). Although relatively few alveoli are present at 6 days of pregnancy, they are usually distributed evenly along the ducts. In comparison with development that occurs in C57 mice, development in the C/EBPβ−/− mice was unusual because of the continued presence of enlarged ducts and the sporadic development of alveoli along the ducts (Fig. 4B). In a small percentage of the C/EBPβ-deficient mice studied to date, a complete lack of alveolar development was noted. Although the degree of impairment of lobuloalveolar budding varied among individual C/EBPβ−/− mice, all nulls consistently exhibited a diminished response to E+P treatment as compared with heterozygous controls.
Figure 4.
Lobuloalveolar development is impaired in the C/EBPβ−/− mice following E+P treatment. The thoracic glands from E+P-treated C/EBPβ+/− or C/EBPβ−/− mice were fixed and stained with hematoxylin by whole mount preparation. In contrast to extensive lobuloalveolar development observed in the C/EBPβ+/− glands (A), large areas of ductal epithelium in the C/EBPβ−/− glands (B) did not contain either secondary/tertiary side branches or alveoli.
β-casein expression is dramatically inhibited and WAP is absent in primary cultures of MEC isolated from E+P-primed C/EBPβ−/− mice
Next, the epithelial cells from the C/EBPβ−/− mice were assayed for their ability to express milk protein genes, classical markers of differentiation in the mammary gland. The process of functional differentiation of the murine mammary secretory epithelium is typically measured by the induction of the abundant milk proteins, β-casein and WAP. The regulation of both WAP and β-casein expression has been extensively characterized in both cell culture and transgenic mice (Rosen et al. 1997). Primary MEC from E+P-treated C/EBP-β+/− and C/EBPβ−/− mice were cultured on a thick layer of extracellular matrix (Matrigel) in the presence of the lactogenic hormones (Prl, I, H) required for β-casein expression. Cells cultured without prolactin served as negative controls. MEC isolated from both heterozygous and null mice were morphologically indistinguishable in culture, forming the proper three-dimensional structures on Matrigel regardless of the extent of alveolar development detected in parallel whole mounts (Fig. 5A). The ability of the alveolar structures to make β-casein protein was detected by Western blotting of whole cell extracts by use of a monoclonal antibody to β-casein (Fig. 5B,C). Strikingly, β-casein expression in response to lactogenic hormones was inhibited 85%–100% in primary cells from the C/EBPβ−/− mice. The extent of inhibition varied somewhat between different animals from which the MEC were prepared (−/− #2 vs. −/− #3), but was not related to the degree of alveolar development observed in whole mounts of portions of glands isolated from the same animals. For example, the whole mount of the gland taken from C/EBPβ−/− #2 exhibited almost no alveolar buds (Fig. 5A). The primary cultures derived from this C/EBPβ−/− mouse, however, expressed β-casein at 15% of the heterozygous control (Fig. 5B,C). In contrast, the whole mount of the gland taken from C/EBPβ−/− #3 contains alveoli, but MEC isolated from this animal only expressed 2% the level of β-casein as the heterozygous control (Fig. 5B).
Figure 5.
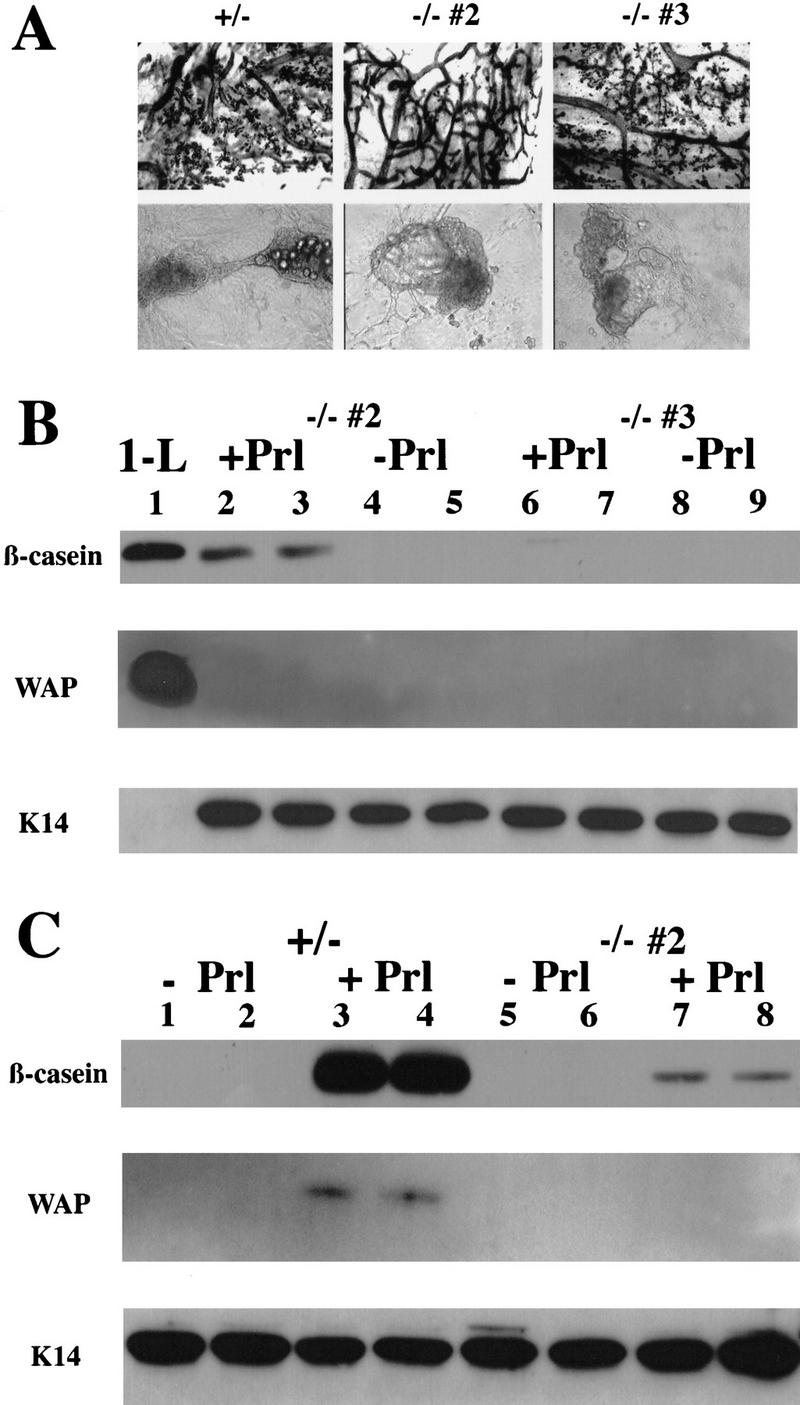
C/EBPβ−/− MECs isolated from E+P-treated females fail to functionally differentiate in response to lactogenic hormones (Prl, I, H). Primary mammary epithelial cells isolated from E+P-treated C/EBPβ+/− and two individual C/EPBβ−/− females (−/− #2 and −/− #3) were cultured on Matrigel (A) in duplicate sets of wells and treated with Prl+I+H as indicated in Materials and Methods. Portions of E+P-treated glands were whole mounted to confirm extent of lobuloalveolar development (A). Duplicate wells of cells isolated from each animal treated with I+H but without prolactin (−Prl) were included as noninduced controls (B, lanes 4,5,8,9; C, lanes 1,2,5,6). Both the heterozygous (+/−) and null (−/−) cells are capable of organizing into three-dimensional structures on Matrigel (A), however, the MECs from C/EBPβ−/− mice fail to produce WAP and expression of β-casein is inhibited 85%–100% (B, lanes 2,3,6,7; C, lanes 7,8). Expression of milk protein genes in the C/EBPβ−/− mice was compared with a heterozygous control (+/−, C, lanes 1–4) as described in Materials and Methods. Equivalent loading was determined by Western blotting with K14 antisera (B,C). No K14 was detected in the control from day 1 of lactation (B, 1-L) because of the high ratio of epithelial cells to myoepithelial cells at this stage of development and the low amounts of protein analyzed.
To determine whether C/EBPβ was also required for WAP expression, Western blots probed previously with β-casein were subsequently probed with either WAP or cytokeratin 14 (K14) antibodies (Fig. 5B,C). No WAP was detectable in the MEC derived from the C/EBPβ−/− mice, but WAP was readily detectable in the control mammary gland extracts prepared from tissue at day 1 of lactation and in the MEC from the C/EBPβ+/− control. The equivalent loading of the Western blots was established by probing the blots with an antibody to K14, an intermediate filament marker of the myoepithelial cells that line the ductal and alveolar epithelial cells.
Northern analysis of C/EBPα expression in the mouse mammary gland
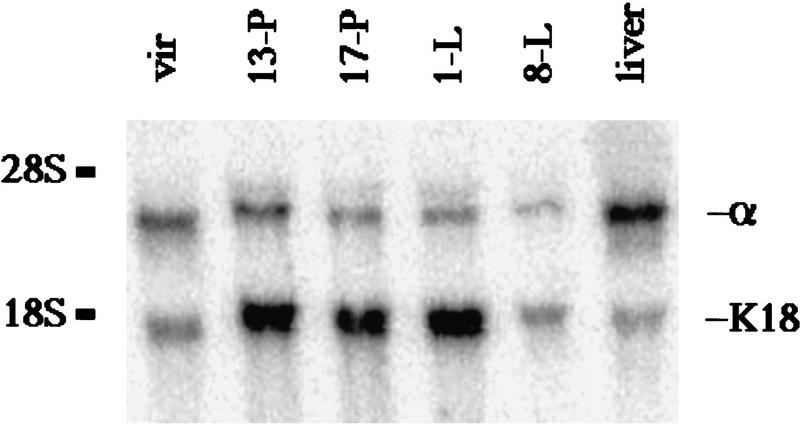
To confirm the expression pattern of C/EBPα during development in the mouse mammary gland (Raught et al. 1995), whole-cell extract (WCE) from C57/Bl6 mammary glands were analyzed by Western blotting. Both polyclonal antisera that were utilized previously to determine expression of C/EBPα in the mammary gland were no longer available (Raught et al. 1995). Therefore, Western blotting was attempted with three different polyclonal antibodies prepared against different epitopes of rat C/EBPα (data not shown). Because of problems with CRM observed with each of these C/EBPα antibodies, similar to those described for C/EBPβ, C/EBPα expression was re-evaluated during mammary gland development by Northern blot analysis of mouse RNA (Fig. 6). C/EBPα mRNA was detectable throughout development in the mammary gland and was expressed at 20%–25% of the levels of mature rat liver. In agreement with recently published studies (Gigliotti and DeWille 1998), C/EBPα mRNA levels appear to decrease during lactation. To normalize for the increase in the epithelial cell population that occurs during pregnancy and lactation, the ratio of C/EBPα to cytokeratin 18 (K18), a marker of luminal/alveolar epithelium, was evaluated. When the expression of C/EBPα was normalized to K18, no significant change in the ratio of C/EBPα to K18 was observed during the transition from pregnancy to lactation. A twofold decrease in C/EBPα mRNA was noted from the mature virgin gland to mid-pregnancy. The decrease in C/EBPα mRNA signal observed during lactation is most likely caused by dilution effects of the abundant milk protein mRNAs. Because C/EBPα expression does not appear to be restricted to pregnancy and lactation, these results do not support a cascade model of C/EBP regulation during mammary gland development. Instead, both C/EBPβ and C/EBPα mRNAs are expressed coordinately during mid-to-late pregnancy and lactation (see Robinson et al. 1998).
Figure 6.
C/EBPα mRNA is expressed throughout development of the murine mammary gland. C/EBPα mRNA is detected during all stages of mammary development including in the mature virgin (vir), and during mid-pregnancy (13-P), late pregnancy (17-P), day 1 lactation (1-L), and mid-lactation (8-L). The expression of C/EBPα mRNA in the mammary gland is ∼20%–25% the level of C/EBPα detected in the mature rat liver (liver). When corrected for the increase of epithelial cells that occurs during development of the mammary gland, measured by the levels of K18 mRNA, the ratio of C/EBPα/K18 remains fairly constant during development. The ratio of C/EBPα/K18 at day 1 lactation (0.20) is not significantly different from that observed during mid-pregnancy (0.30) or late pregnancy (0.24). The apparent decrease in both C/EBPα and K18 that occurs at mid-lactation (8-L) is most likely a dilutional effect of the abundant milk protein mRNAs.
Deletion of C/EBPα does not alter mammary gland development or β-casein expression
To determine the effects of deletion of C/EBPα in mammary epithelium on mammary gland development and differentiation, mammary anlage from a total of five C/EBPα−/− newborns (F0) were transplanted into the cleared fat pads of 3-week-old syngeneic 129-Sv hosts (F1). The mammary epithelial stem cells present in small portions of tissue isolated from adult donors, such as the mature wild-type 129-Sv females used in our study, will regenerate an entire ductal tree within 6–8 weeks after transplantation. Because mammary development occurs primarily after birth, however, only a small fraction of the newborn mammary fat pad contains ductal epithelium, and, therefore, the stem cells. Transplantation of donor epithelium from newborns to hosts usually results in <50% of the transplants producing ductal epithelial outgrowths. To achieve more reproducible transplants, tissue was isolated from the F1 hosts that contained successful outgrowths 6–8 weeks following transplantation and, subsequently, serially transplanted into cleared fat pads of additional 3-week-old 129-Sv recipients (F2). No discernible differences in mammary development were noted by whole gland staining between C/EBPα+/+ (F1) and C/EBPα−/− transplants (F2) in virgin, day 13 pregnant, day 17 pregnant (data not shown), day 1 lactating, or day 4 involuting animals (Fig. 7). Both the C/EBPα+/+ and C/EBPα−/− transplants exhibited the normal ductal (D) branching originating from the site of tissue transplant (Fig. 7A,B). Glands taken prior to 8 weeks of outgrowth contained normal terminal end buds (EB), the club-shaped structures located at the tips of growing ducts (Fig. 7B). On reaching the edges of the fat pad, the terminal end buds disappeared. Alveolar budding was comparable between the C/EBPα+/+ and C/EBPα−/− transplants at days 13 (Fig. 7C,D) and 17(data not shown) of pregnancy.
Figure 7.
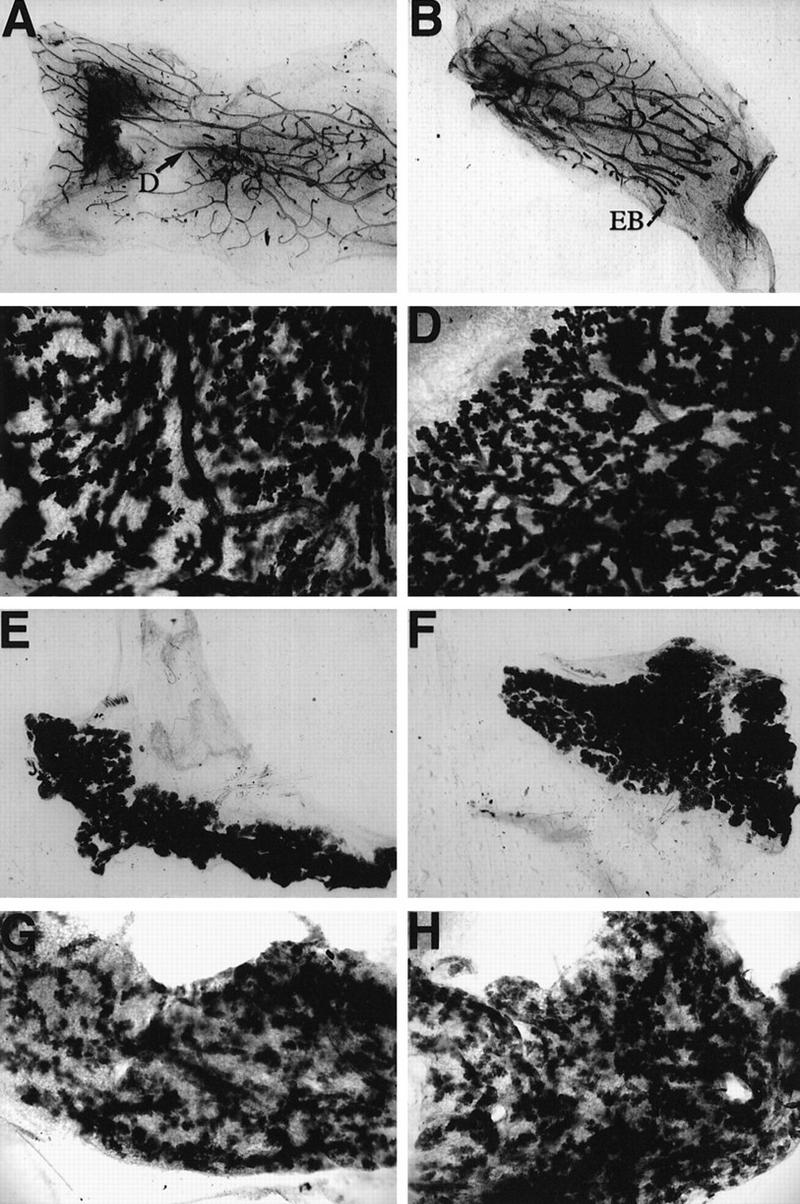
Mammary development is normal in the C/EBPα−/− transplants. Portions of mammary tissue isolated from wild-type (A,C,E,G) or C/EBPα null (B,D,F,H) transplanted glands were fixed and stained with Harris hematoxylin according to standard whole mount procedure. Glands from virgin (A,B), day 13 pregnant (C,D), day 1 lactation (E,F), and day 4 involuted mice (G,H) are included. The whole mounts from 17 days of pregnancy (data not shown) were omitted because they closely resembled the glands isolated at day 1 of lactation. Images of the glands were directly captured from a Sony video camera at 4× (A,B,E,F) or at 10× (C,D,G,H) magnification. Apparent differences in magnification are a result of the original size of each transplant gland. Note the presence of normal ducts (D) in the virgin outgrowths and the presence of terminal end buds in the portion of the ductal tree that has not yet reached the edge of the fat pad in the C/EBPα−/− gland taken at 6 weeks post-transplantation (B).
The C/EBPα−/− transplants exhibited normal amounts of alveoli at day 1 of lactation (Fig. 7E,F) and appeared to be capable of producing milk. A milk-like secretion was observed at day 1 of lactation in the C/EBPα−/− transplants during surgical removal of the glands. Analysis of hematoxylin and eosin-stained sections from these lactating animals confirmed that the C/EBPα−/− transplants contained normal, polarized, secretory alveoli as indicated by proteinaceous/lipid secretions in the lumen (Fig. 8A). To determine effects of C/EBPα deletion on β-casein expression at day 1 of lactation, Western blot analysis was performed on whole cell extracts isolated from both C/EBPα+/+ and C/EBPα−/− transplants by use of a monoclonal antibody to β-casein (Fig. 8B). No significant difference in β-casein expression was observed in the C/EBPα−/− transplants, indicating that the deletion of C/EBPα in the epithelium does not inhibit β-casein gene expression at lactation.
Figure 8.
Histology of C/EBPα−/− transplants at day 1 of lactation is normal (A) and the C/EBPα−/− transplants make normal levels of β-casein (B). Paraffin-embedded sections from 1-day lactating animals stained with hematoxylin and eosin reveal that normal, secretory alveoli are present in both the C/EBPα−/− (−/−; 2) and wild-type transplants (+/+; 1). The C/EBPα+/+ (1–3) and C/EBPα−/− (4–6) transplants also express equivalent levels of β-casein as determined by Western blotting of 1 μg of WCE from these transplants (B).
Proliferation and apoptosis are unaffected in the C/EBPα−/− transplants
One additional hypothesis for the function of C/EBPα is that it might be important to promote or maintain terminal differentiation in mammary cells as suggested previously by studies in liver and adipocytes (Umek et al. 1991; Rana et al. 1994). Normally, MEC at lactation that have exited the cell cycle exhibit barely detectable levels of bromodeoxyuridine (BrdU) incorporation (0.1%, data not shown). To determine if deletion of C/EBPα resulted in an increase in cellular proliferation at day 1 of lactation, BrdU incorporation was quantitated by immunohistochemical staining performed on paraffin-embedded sections. No increase in proliferation was observed in the C/EBPα−/− transplants as compared with the C/EBPα+/+ transplants or with the control thoracic mammary glands of the hosts (data not shown).
If C/EBPα plays an important role in terminal differentiation, and only cells at lactation that have terminally differentiated undergo apoptosis, then involution might be delayed in the C/EBPα−/− transplants. In the transplant model, the gland will naturally involute as lactation proceeds, because the ducts are no longer connected to a nipple and milk stasis occurs. To circumvent this problem, pups were removed from day 1 lactating mothers to induce involution before milk stasis occurred. By whole gland staining, involution of the gland at 4 days of forced involution did not appear to be delayed in the gland containing C/EBPα null epithelium (Fig. 7G,H). To quantitate the number of apoptotic cells in the C/EBPα+/+ versus C/EBPα−/− transplants, the TUNEL assay was performed on paraffin-embedded sections. By this assay, the same percentage of cells were observed undergoing apoptosis (2.5%) at 4 days of involution in the wild-type and null epithelium transplants and in intact control glands (data not shown).
Discussion
These studies reveal that C/EBPβ is essential for ductal morphogenesis and proliferation of lobuloalveolar secretory units. In addition, C/EBPβ is required for functional differentiation of secretory epithelium because both β-casein and WAP expression were inhibited or absent in C/EBPβ−/− mice. The multiple defects observed during mammary development in C/EBPβ-deficient mice infer that several signaling pathways are altered by deletion of all of the C/EBPβ isoforms in the mammary gland. The absence of a phenotype in the transplants carrying C/EBPα−/− epithelium suggests that C/EBPα is not critical for mammary development, but these observations may be complicated by potential compensation for lack of C/EBPα by the LAP2 isoform of C/EBPβ.
C/EBPβ function in the murine mammary gland
Individual C/EBPβ isoforms may repress and/or activate different sets of target genes in the mammary gland in a temporal fashion because the ratios of positive (LAP2) to negative (LIP) isoforms change during mammary development. Because LIP was not detectable in the virgin mammary gland, the defect in ductal morphogenesis is likely to be a result of inappropriate regulation of genes normally positively regulated by LAP2. Because both the LAP2 and LIP isoforms are expressed coordinately during pregnancy, interpretation of how target genes are affected in the C/EBPβ−/− mice is more complex. LIP may serve two functions during pregnancy, to push cells into S phase as has been demonstrated in HepG2 cells (Buck et al. 1994) and to negatively regulate expression of milk protein genes in cells that are still dividing (Rosen et al. 1997). LIP may accomplish these processes by either antagonizing the transcriptional activity of the LAP2 or other C/EBPs in the mammary gland or by recruiting proteins that are able to bind specifically to LIP but not to LAP2. In contrast, LAP2 may facilitate both the process of differentiation and the expression of milk proteins in the differentiated cells (Rosen et al. 1997). At onset of lactation, relatively low levels of LIP protein are expressed, resulting in the dramatic decrease of the LIP/LAP ratio. The absence of both LIP and LAP in the C/EBPβ−/− mice may explain why both proliferation (positively influenced by LIP) and differentiation (positively influenced by LAP2) of the secretory epithelium are impaired in the mammary gland.
In addition to regulation of target gene transcription, it is likely that C/EBPβ plays a direct role in cell cycle regulation in the mammary gland. All three isoforms of the human homolog of C/EBPβ, NF-IL6, have been demonstrated to bind directly to the T-antigen binding pocket of the hypophosphorylated form of the retinoblastoma (Rb) protein in differentiating adipocytes (Chen et al. 1996b). Interestingly, Rb has also been demonstrated to act as a transient coactivator of C/EBPβ by increasing the transactivation potential of a NF-IL6-responsive reporter construct in a dose-dependent manner (Chen et al. 1996b). Rb−/− fibroblast cells do not differentiate into adipocytes following hormonal treatment, suggesting Rb’s interactions with the C/EBPβ are required to push cells towards adipocyte terminal differentiation (Chen et al. 1996a). Similar interactions between C/EBPβ and Rb in the mammary gland may contribute to lobuloalveolar differentiation.
Potential C/EBPβ target genes in the mammary gland
Because the C/EBP (bZIP) consensus is the most commonly found transcription factor binding site that occurs in composite response elements (CoRE) of promoters (Kel et al. 1995), it is likely that multiple genes are regulated in part by C/EBPβ. Several potential target genes expressed in the mammary gland have been proposed or demonstrated to contain at least one C/EBP binding site in their promoter. These include the prolactin receptor (PrlR) (Hu et al. 1997) and the Drosophila homolog of the fibroblast growth factor receptor (FGFR), breathless (Murphy et al. 1995). Aberrant regulation of these potential candidate genes may contribute to the mechanisms behind the abnormal ductal and lobuloalveolar development of the C/EBPβ−/− mice.
Improper expression and regulation of the FGF family of ligands and/or FGFRs, in particular keratinocyte growth factor (KGF, FGF7) and its receptor (KGFR, FGFR2), may be responsible for the ductal and lobuloalveolar developmental defects observed in the C/EBPβ null mice. Evidence for FGFR2 as a target of C/EBPβ has been provided by genetic epistasis and biochemical experiments in Drosophila. The Drosophila homolog of C/EBP (slbo) has been demonstrated to bind directly to the promoter of breathless, the FGFR homolog responsible for branching morphogenesis in the trachea/lung (Reichman-Fried et al. 1994; Murphy et al. 1995; Reichman-Fried and Shilo 1995; Samakovlis et al. 1996). It is likely that C/EBPs regulate FGFR2 expression in mammals as well, although little is known about the regulation of the mouse FGFRs. Transgenic mice carrying a mouse mammary tumor virus (MMTV) driven dominant-negative form of FGFR2 (Jackson et al. 1997), which lacks the tyrosine kinase domain, display delayed lobuloalveolar development during early pregnancy similar to the E+P-stimulated C/EBPβ nulls. No effect of the dominant-negative MMTV–FGFR construct was observed in the mammary gland in virgin mice most likely because of the low level of expression of the construct driven by the MMTV long-terminal repeat (LTR) at this stage of mammary gland development. Intriguingly, the cystic dilation of mammary ducts composed of a single layer of epithelium similar to the phenotype observed in the C/EBPβ−/− mice has been reported previously in mice treated systemically with KGF (Yi et al. 1994), which activates FGFR2.
As phenotypes are not often observed in heterozygous animals, the defects in ductal development that occur in some C/EBPβ+/− mice indicate that a threshold for C/EBPβ-mediated expression of critical target genes may exist. In the case of the prolactin receptor, heterozygous mice displayed severely impaired lobuloalveolar development resulting in a failure to lactate after the first pregnancy, indicating a threshold level of prolactin receptor is necessary for proper development (Ormandy et al. 1997). The ability of the females to successfully nurse after successive pregnancies was directly related to the degree of lobuloalveolar development observed, presumably because of varying expression levels of the prolactin receptor.
Delayed or inhibited lobuloalveolar development has also been reported in other mice carrying targeted gene deletions, including the progesterone receptor (Lydon et al. 1995), cyclin D1 (Fantl et al. 1995; Sicinski et al. 1995) and A-myb (Toscani et al. 1997) knockouts. The role, if any, of C/EBPβ in the regulation of these target genes has yet to be definitively established.
Model for regulation of the β-casein promoter
The analysis of functional differentiation in primary MEC derived from the C/EBPβ−/− mice indicates that C/EBPβ expression is absolutely required in vivo for β-casein expression. These results are in contrast to results obtained with either the Stat5a−/− or Stat5b−/− mice that exhibited varying degrees of impairment of lobuloalveolar development, but still were capable of expressing β-casein mRNA at wild-type levels (Liu et al. 1997; Udy et al. 1997). Because the inhibition of β-casein synthesis in the C/EBPβ nulls does not correlate with the degree of inhibition of alveolar proliferation, the decrease observed in β-casein synthesis cannot be simply explained by fewer alveoli secreting β-casein. Loss of C/EBPβ may affect factors required for alveolar proliferation as well as differentiation and/or directly affect signaling at the β-casein promoter CoRE. Loss of C/EBPβ binding to the CoRE may inhibit the recruitment of other proteins to the promoter. Protein–protein interactions between C/EBP, GR, and Stat5, as well as the common coactivator, p300, may be required to maintain an open chromatin structure at the β-casein promoter (Rosen et al. 1997).
Regulation of WAP expression
A hierarchy of milk protein gene expression based on hormonal, cell–cell, and cell–substratum interactions has been proposed to account for the differential regulation between β-casein and WAP gene expression (Bissell and Aggeler 1987; Roskelley et al. 1995). Because C/EBPβ appears to be required for differentiation, and WAP is expressed later in the mammary gland differentiation program than β-casein, then either the C/EBPβ−/− cells are sufficiently undifferentiated so as not to respond to these multiple signals required for WAP expression, or C/EBPβ may indirectly influence the unique transacting factors required for WAP expression.
Lack of a mammary phenotype in C/EBPα−/− mice
Despite the similarities in their DNA binding sites, the C/EBPβ−/− and C/EBPα−/− mice exhibit significant phenotypic differences that most likely reflect the unique temporal and spatial expression patterns of these transcription factors, the post-transcriptional regulation of different isoforms, as well as the presence of unique target genes (Screpanti et al. 1995; Wang et al. 1995; Sterneck et al. 1997; Zhang et al. 1997). Recent studies have revealed differences in the synergism of C/EBPβ and C/EBPα with the Sp1 transcription factor that is involved in the regulation of a liver-specific P-450 gene (Lee et al. 1997). In liver, C/EBPα expression is thought to contribute to terminal differentiation through its direct interactions with the cell cycle inhibitor p21 (Timchenko et al. 1997). Thus, it was surprising that no detectable differences in mammary gland development, proliferation, or functional differentiation were observed in the C/EBPα−/− transplants, suggesting that C/EBPα does not play a critical role in mammary gland development. Furthermore, in the mammary gland, expression of C/EBPα mRNA does not appear to be restricted to periods of differentiation (late pregnancy and lactation). These results are in contrast to the reported late expression of C/EBPα during liver and adipocyte differentiation, inferring that C/EBPα does not conform to the cascade model of regulation in the mammary gland. However, conclusions based on observations of single gene deletion transplants may be complicated by potential compensation of LAP2 for C/EBPα in the transplanted C/EBPα−/− epithelium. In addition, these studies have not addressed the role of any C/EBPα expressed in the wild-type stroma of the syngeneic recipients.
Conclusions
The results from these studies have demonstrated that C/EBPβ, but not C/EBPα, is required for normal mammary gland ductal and lobuloalveolar development and the expression of two classical markers of functional differentiation in the mouse mammary gland, β-casein, and WAP. The mechanisms underlying how the C/EBPs specifically modulate the processes of proliferation and differentiation in the mammary gland remain to be investigated. Further characterization of the targets of the C/EBPs in transgenic mice will provide insight into how each C/EBP functions in a tissue-specific and developmentally regulated manner. Ultimately, an understanding of the mechanisms that modulate development of the normal mammary gland will provide clues to understanding how aberrant regulation of signaling events such as those regulated by the C/EBPs may lead to breast cancer.
Materials and methods
C/EBPα−/− and C/EBPβ−/− mice and screening
The generation of both the C/EBPβ and C/EBPα-deficient mice have been described previously (Screpanti et al. 1995; Wang et al. 1995). Prior to use in transplantation experiments, the original strain of C/EBPα mice (129-Sv × C57/Bl6) were bred into pure 129-Sv background in the laboratory of Dr. Gretchen Darlington. All newborn mice used as donors for the initial C/EBPα transplants were subsequently screened by Southern blotting as described previously (Wang et al. 1995). Breeding pairs were provided (V. Poli, pers. comm.) to establish a colony of the outbred C/EBPβ-deficient line (genotype of progeny ∼70% C57, 20% 129-Sv, 10% MF-1). Genomic tail DNA was screened to determine genotype of C/EBPβ animals by Southern blotting as described previously (Screpanti et al. 1995).
Care and treatment of animals
Animals were housed in a AAALAC approved facility of Baylor College of Medicine in pathogen-free rooms in filter-topped cages and provided with food and water ad libitum. Nude (nu/nu) mice (Taconic or B&K Universal) received autoclaved bedding, food, and water. All animal experiments were conducted by use of the highest standards for humane care in accordance with the the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Transplantation technique
C/EBPα null transplants
Approximately 1.5 × 1.5-mm portions of mammary glands isolated from newborn null C/EBPα pups (F0) were transplanted into the cleared inguinal (#4) fat pads of 129/Sv 3-week-old females (F1) (Taconic) according to a protocol described previously (DeOme et al. 1959). Epithelium deleted for C/EBPα that grew out in the inguinal glands of the F1 host females was then used as donor epithelium by serial transplantation to a second set of cleared fat pads from 3-week-old 129-Sv females (F2). The thoracic (#3) glands of the wild-type host served as normal controls.
Wild-type transplants
Approximately 1.5 × 1.5 mm portions of the inguinal gland from a 8- to 12-week-old virgin (F0) 129-Sv female (Taconic) were transplanted into the cleared fat pads of 3-week-old 129-Sv hosts (F1) (Taconic). The thoracic (#3) glands again served as normal controls.
Collection of and analysis of tissue from C/EBPα transplants
Wild-type (F1) or mutant (F2) host females were sacrificed between 6 and 8 weeks post-transplantation or bred with ICR males (Taconic). Tissue was harvested from pregnant females at 13 and 17 days of pregnancy based on the date at which plugs were first observed designated as day 0. Tissue was collected from female mice following 12–24 hr of lactation (1 day lactation) or following 12–24 hr of lactation and 4 days of forced involution (removal of pups after 12–24 hr of lactation).
Two hours prior to tissue collection, all animals were injected with 0.1 ml/10 g weight of BrdU (3 mg/ml) cell proliferation labeling reagent (Amersham). To confirm outgrowth and the extent of development in all transplants, each transplanted gland was cut into three pieces; two pieces each, about one-quarter of the size of the transplanted gland, and one piece about one-half the size of the gland. The largest portion of the gland was flash frozen in foil in liquid nitrogen and stored at −80°C for future analysis. The two individual quarters of the original transplanted gland were then spread onto a tissue cassette and fixed in 10% neutral buffered formalin (Richard Allen). One-quarter was then stained following the whole-mount protocol with Harris hematoxylin (Fisher), dehydrated, and stored in methyl salicylate. Whole-mounted glands were photographed by Elite 100 Ektachrome Slide film (Kodak) or were directly imaged with a Sony video camera mounted directly to an Olympus microscope by use of Adobe Photoshop 3.0 software. The other quarter of fixed tissue was embedded in paraffin followed by standard preparation of 5 μm sections that were stained with hematoxylin and eosin for histological analysis or used for BrdU and TUNEL immunohistochemical staining protocols as described previously (Humphreys et al. 1996).
E+P treatment
At 8–10 weeks of age, virgin C/EBPβ+/− or C/EBPβ−/− females were injected with 1 μg of estradiol benzoate (Sigma) in sesame oil (Sigma) subcutaneously behind the neck. Three days later, E+P (2 μg of E/20 mg of P) slow-release beeswax pellets were inserted into a small incision made under the skin behind the neck followed by closure with 9-mm wound clips. After 10–11 days, the first pellet was removed and replaced with an identical E+P pellet for a total of 21 days treatment.
Primary MEC isolation and culture
The #2, #3, #4 and #5 pairs of mammary glands were isolated from E+P-treated animals and epithelial cell fractions prepared for primary culture as described previously (Pullan and Streuli 1996). Briefly, equal volumes of epithelial cells were plated in individual wells of 24-well dishes that had been precoated with 60 μl of Matrigel (Fisher). Cells were allowed to plate in the plating medium for 2 days, gently washed twice with DMEM/F12 (Life Technologies), and switched to growth medium for 24–48 hr. To induce differentiation, cells were then gently washed three times with DMEM/F12 and cultured for 4 subsequent days in medium containing 1 μg/ml hydrocortisone, 5 μg/ml insulin, 50 μg/ml gentamicin in DMEM/F12 ± 3 μg/ml of ovine prolactin (lot AFP-10677C, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Child Health and Human Development, and the U.S. Department of Agriculture).
RNA extraction and Northern blotting
Snap-frozen tissue was pulverized with a mortar and pestle directly in liquid nitrogen and homogenized briefly with a Kinematica Polytron homogenizer in chilled RNAzol B (Tel-Test) followed by isolation of RNA according to protocol provided by the manufacturer. Total RNA (20 μg) was separated by electrophoresis of a 1.2% agarose/3% formaldehyde gel and transferred to Hybond N+ (Amersham) membrane. After blotting, the membrane was probed simultaneously with 1.0-kb fragments from both rat C/EBPα gene and rat K18 cDNA that were labeled (Prime-a-Gene kit, Promega) to high specific activity (1 × 109 cpm/μg) and hybridized at 65°C according to Church and Gilbert (1984). The membrane was exposed to a PhosphorImager cassette (Molecular Dynamics) at room temperature, read by a Storm 860 scanner, followed by quantitation of bands corresponding to C/EBPα and K18 by use of ImageQuant 1.1 software.
Protein extraction and immunoblotting
Tissue
To analyze C/EBP protein expression, WCEs were prepared from frozen tissue isolated from C57/Bl6 females (Taconic) as described previously (Zahnow et al. 1997). WCE (100 μg) were separated by 12% SDS-polyacrylamide gels and transferred in Towbin buffer overnight at 4°C at 100 mA to Immobilon-P membrane (Millipore). Western blotting for C/EBPα and C/EBPβ was performed as described previously (Raught et al. 1995) except that the Super Signal enhanced chemiluminescent (ECL) substrate (Pierce) was used to develop the blots for 1 min prior to exposing Hyperfilm-ECL (Amersham). To determine C/EBPα expression, three rabbit polyclonal antibodies raised against different regions of rat C/EBPα were used. (1) Residues 253–265 (Santa Cruz sc-61-x, 1:2000 dilution). (2) Antibodies to either residues 247–358 or 342–357 at 1:1000 dilutions (both a gift of Dr. Steven McKnight, UT Southwestern Medical Center). To analyze β-casein expression from mammary tissue, ∼1 μg of WCE was separated by 12% SDS-polyacrylamide gel electrophoresis and transferred to Immobolin-P. The membrane was blocked for 1 hr at room temperature in 5% bovine serum albumin (BSA)/Tris-buffered saline (TBS) + 0.1% Tween 20 (TBST), and then incubated for 1 hr at room temperature with a 1:2000 dilution of anti-rat β-casein monoclonal antibody (Kaetzel and Ray 1984) in 5% BSA (provided by Dr. Mina Bissell, Life Sciences Division, Lawrence Berkeley Laboratories, CA). After washing in TBST, the blot was incubated for 30 min at room temperature in a 1:20,000 dilution of a sheep anti-mouse IgG–horseradish peroxidase (HRP) secondary antibody (Amersham) in 5%BSA/TBST followed by washing and chemiluminescent detection as described above except that incubation in substrate was limited to 30–40 sec.
Primary culture cell extracts
Primary cells cultured on Matrigel were directly lysed by three cycles of freeze/thaw (−80°C for 5 min, 37°C for 5 min) in RIPA buffer (without SDS or Antifoam) followed by centrifugation and collection of the supernatant that was immediately flash frozen in liquid nitrogen. Approximately 1.5–2 μg of RIPA extract from primary cells was analyzed for β-casein expression as described above. β-Casein levels were quantitated by densitometric analysis of multiple exposures of scanned films with Adobe Photoshop 4.0. All bands were normalized for film background and compared with the same heterozygote control run on each blot. Membranes blotted previously for β-casein were washed three times for 20 min with TBST, cut into two portions just above the 30-kD molecular weight Rainbow marker standard (Amersham) and then reprobed with antibodies to either WAP (14 kD) or cytokeratin 14 (K14, 55 kD) without prior stripping.
To detect WAP, the blot was blocked for 90 min at room temperature in 5% BSA in TBST (0.05% Tween) followed by incubation in a 1:2500 dilution of rabbit polyclonal antibody to mouse WAP (Shamay et al. 1992) (kindly provided by Dr. Lothar Hennighausen, NIDDK, Bethesda, MD) in 5% BSA/TBST overnight at 4°C, washed, and incubated with 40 ng/ml (in 2.5% BSA/TBST) of anti-rabbit IgG–HRP secondary antibody (Calbiochem) followed by washing and incubation in the Super Signal substrate for 2 min prior to exposure to film. The top portion of the blot to be probed with rabbit polyclonal anti-rat K14 (Roop et al. 1984) was blocked for 90 min in 3% nonfat dry milk (NFDM, Carnation) in TBS plus 0.1% Tween followed by overnight incubation of a 1:500 dilution of K14 antibody in 3% NFDM (kindly provided by Dr. Dennis Roop, Baylor College of Medicine, Houston, TX). After washing, the blot was then incubated with 100 ng/ml of biotinylated anti-rabbit IgG secondary antibody (Calbiochem) in 3% NFDM for 1 hr at room temperature, washed, then incubated with 80 ng/ml of strepavidin–HRP (Calbiochem) in 3% NFDM for 30 min at room temperature. After washing, the blot was incubated for 5 min in Super Signal (Pierce) substrate prior to exposure to film.
Acknowledgments
We thank Dr. Valeria Poli for generously providing breeding pairs to generate our colony of C/EBPβ−/− mice and Michelle Hibbard and Saleen Chenevert of the laboratory of Dr. Gretchen Darlington who screened the founders and provided the probe for Southern blot screening. Dr. Cindy Zahnow kindly provided extracts from her WAP–LIP overexpressing transgenic mice. We also thank Liz Hopkins for embedding, sectioning, and hematoxylin and eosin staining. Mr. Lester Patton and Dr. Jean-Louise Eynard of the Taub Center for Comparative Medicine provided excellent animal care and veterinarian support. Finally, we thank Dr. Charles Streuli, Manchester University, for providing protocols and advice for the preparation of primary epithelial cells. These studies were supported by grant CA 16303 from the National Cancer Institute.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL jrosen@bcm.tmc.edu; FAX (713) 798-8012.
References
- Alam T, An MR, Papaconstantinou J. Differential expression of three C/EBP isoforms in multiple tissues during the acute phase response. J Biol Chem. 1992;267:5021–5024. [PubMed] [Google Scholar]
- Bissell MJ, Aggeler J. Dynamic reciprocity: How do extracellular matrix and hormones direct gene expression? Prog Clin Biol Res. 1987;249:251–262. [PubMed] [Google Scholar]
- Blais S, Boudreau F, Beaulieu JF, Asselin C. CCAAT/enhancer binding protein isoforms expression in the colon of neonatal mice. Dev Dyn. 1995;204:66–76. doi: 10.1002/aja.1002040109. [DOI] [PubMed] [Google Scholar]
- Buck M, Turler H, Chojkier M. LAP (NF-IL-6), a tissue-specific transcriptional activator, is an inhibitor of hepatoma cell proliferation. EMBO J. 1994;13:851–860. doi: 10.1002/j.1460-2075.1994.tb06328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Umek RM, McKnight SL. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes & Dev. 1991;5:1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- Chen PL, Riley DJ, Chen Y, Lee WH. Retinoblastoma protein positively regulates terminal adipocyte differentiation through direct interaction with C/EBPs. Genes & Dev. 1996a;10:2794–2804. doi: 10.1101/gad.10.21.2794. [DOI] [PubMed] [Google Scholar]
- Chen PL, Riley DJ, Chen-Kiang S, Lee WH. Retinoblastoma protein directly interacts with and activates the transcription factor NF-IL6. Proc Natl Acad Sci. 1996b;93:465–469. doi: 10.1073/pnas.93.1.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church GM, Gilbert W. Genomic sequencing. Proc Natl Acad Sci. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeOme KB, Fauklin LJ, Bern HA, Blair PB. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959;78:515–520. [PubMed] [Google Scholar]
- Descombes P, Schibler U. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell. 1991;67:569–579. doi: 10.1016/0092-8674(91)90531-3. [DOI] [PubMed] [Google Scholar]
- Diehl AM, Michaelson P, Yang SQ. Selective induction of CCAAT/enhancer binding protein isoforms occurs during rat liver development. Gastroenterology. 1994;106:1625–1637. doi: 10.1016/0016-5085(94)90420-0. [DOI] [PubMed] [Google Scholar]
- Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes & Dev. 1995;9:2364–2372. doi: 10.1101/gad.9.19.2364. [DOI] [PubMed] [Google Scholar]
- Gigliotti AP, DeWille JW. Lactation status influences expression of CCAAT/enhancer binding protein isoform mRNA in the mouse mammary gland. J Cell Physiol. 1998;174:232–239. doi: 10.1002/(SICI)1097-4652(199802)174:2<232::AID-JCP10>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Hendricks-Taylor LR, Darlington GJ. Inhibition of cell proliferation by C/EBPα occurs in many cell types, does not require the presence of p53 or Rb, and is not affected by large T-antigen. Nucleic Acids Res. 1995;23:4726–4733. doi: 10.1093/nar/23.22.4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW. Think globally, act locally: The making of a mouse mammary gland. Genes & Dev. 1998;12:449–455. doi: 10.1101/gad.12.4.449. [DOI] [PubMed] [Google Scholar]
- Hu Z, Zhuang L, Guan X, Yneng J, Dufau ML. Steroidogenic factor-1 is an essential transcriptional activator for gonad-specific expression of promoter I of the rat prolactin receptor gene. J Biol Chem. 1997;272:14263–14271. doi: 10.1074/jbc.272.22.14263. [DOI] [PubMed] [Google Scholar]
- Humphreys RC, Krajewska M, Krnacik S, Jaeger R, Weiher H, Krajewski S, Reed JC, Rosen JM. Apoptosis in the terminal endbud of the murine mammary gland: A mechanism of ductal morphogenesis. Development. 1996;122:4013–4022. doi: 10.1242/dev.122.12.4013. [DOI] [PubMed] [Google Scholar]
- Jackson D, Bresnick J, Rosewell I, Crafton T, Poulsom R, Stamp G, Dickson C. Fibroblast growth factor receptor signalling has a role in lobuloalveolar development of the mammary gland. J Cell Sci. 1997;110:1261–1268. doi: 10.1242/jcs.110.11.1261. [DOI] [PubMed] [Google Scholar]
- Kaetzel CS, Ray DB. Immunochemical characterization with monoclonal antibodies of three major caseins and alpha-lactalbumin from rat milk. J Dairy Sci. 1984;67:64–75. doi: 10.3168/jds.S0022-0302(84)81267-9. [DOI] [PubMed] [Google Scholar]
- Kel OV, Romaschenko AG, Kel AE, Wingender E, Kolchanov NA. A compilation of composite response regulatory elements affecting gene transcription in vertebrates. Nucleic Acids Res. 1995;23:4097–4103. doi: 10.1093/nar/23.20.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landschulz WH, Johnson PF, Adashi EY, Graves BJ, McKnight SL. Isolation of a recombinant copy of the gene encoding C/EBP. Genes & Dev. 1988;2:786–800. doi: 10.1101/gad.2.7.786. [DOI] [PubMed] [Google Scholar]
- Lee YH, Williams SC, Baer M, Sterneck E, Gonzalez FJ, Johnson PF. The ability of C/EBP beta but not C/EBP alpha to synergize with an Sp1 protein is specified by the leucine zipper and activation domain. Mol Cell Biol. 1997;17:2038–2047. doi: 10.1128/mcb.17.4.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FT, Lane MD. CCAAT/enhancer binding protein alpha is sufficient to initiate the 3T3- L1 adipocyte differentiation program. Proc Natl Acad Sci. 1994;91:8757–8761. doi: 10.1073/pnas.91.19.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FT, MacDougald OA, Diehl AM, Lane MD. A 30-kDa alternative translation product of the CCAAT/enhancer binding protein alpha message: Transcriptional activator lacking antimitotic activity. Proc Natl Acad Sci. 1993;90:9606–9610. doi: 10.1073/pnas.90.20.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Robinson GW, Wagner KU, Garrett L, Wynshaw-Boris A, Hennighausen L. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes & Dev. 1997;11:179–186. doi: 10.1101/gad.11.2.179. [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O’Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes & Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Murphy AM, Lee T, Andrews CM, Shilo BZ, Montell DJ. The breathless FGF receptor homolog, a downstream target of Drosophila C/EBP in the developmental control of cell migration. Development. 1995;121:2255–2263. doi: 10.1242/dev.121.8.2255. [DOI] [PubMed] [Google Scholar]
- Ormandy CJ, Camus A, Barra J, Damotte D, Lucas B, Buteau H, Edery M, Brousse N, Babinet C, Binart N, Kelly PA. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes & Dev. 1997;11:167–178. doi: 10.1101/gad.11.2.167. [DOI] [PubMed] [Google Scholar]
- Ossipow V, Descombes P, Schibler U. CCAAT/enhancer-binding protein mRNA is translated into multiple proteins with different transcription activation potentials. Proc Natl Acad Sci. 1993;90:8219–8223. doi: 10.1073/pnas.90.17.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piontkewitz Y, Enerback S, Hedin L. Expression and hormonal regulation of the CCAAT enhancer binding protein-alpha during differentiation of rat ovarian follicles. Endocrinology. 1993;133:2327–2333. doi: 10.1210/endo.133.5.8404685. [DOI] [PubMed] [Google Scholar]
- Pullan SE, Streuli CH. The mammary gland epithelial cell. In: Harris A, editor. Epithelial cell culture. Cambridge, UK: Cambridge University Press; 1996. pp. 97–121. [Google Scholar]
- Rana B, Mischoulon D, Xie Y, Bucher NL, Farmer SR. Cell-extracellular matrix interactions can regulate the switch between growth and differentiation in rat hepatocytes: Reciprocal expression of C/EBPα and immediate-early growth response transcription factors. Mol Cell Biol. 1994;14:5858–5869. doi: 10.1128/mcb.14.9.5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raught B, Liao WS, Rosen JM. Developmentally and hormonally regulated CCAAT/enhancer-binding protein isoforms influence beta-casein gene expression. Mol Endocrinol. 1995;9:1223–1232. doi: 10.1210/mend.9.9.7491114. [DOI] [PubMed] [Google Scholar]
- Raught B, Gingras AC, James A, Medina D, Sonenberg N, Rosen JM. Expression of a translationally regulated, dominant-negative CCAAT/enhancer-binding protein β isoform and up-regulation of the eukaryotic translation initiation factor 2α are correlated with neoplastic transformation of mammary epithelial cells. Cancer Res. 1996;56:4382–4386. [PubMed] [Google Scholar]
- Reichman-Fried M, Shilo BZ. Breathless, a Drosophila FGF receptor homolog, is required for the onset of tracheal cell migration and tracheole formation. Mech Dev. 1995;52:265–273. doi: 10.1016/0925-4773(95)00407-r. [DOI] [PubMed] [Google Scholar]
- Reichman-Fried M, Dickson B, Hafen E, Shilo BZ. Elucidation of the role of breathless, a Drosophila FGF receptor homolog, in tracheal cell migration. Genes & Dev. 1994;8:428–439. doi: 10.1101/gad.8.4.428. [DOI] [PubMed] [Google Scholar]
- Robinson, G.W., P.F. Johnson, L. Hennighausen, and E. Sterneck. 1998. The C/EBPβ transcription factor regulates epithelial cell proliferation and differentiation in the mammary gland. Genes & Dev. (this issue). [DOI] [PMC free article] [PubMed]
- Roop DR, Cheng CK, Titterington L, Meyers CA, Stanley JR, Steinert PM, Yuspa SH. Synthetic peptides corresponding to keratin subunits elicit highly specific antibodies. J Biol Chem. 1984;259:8037–8040. [PubMed] [Google Scholar]
- Rosen JM, Zahnow C, Kazansky A, Raught B. Composite response elements mediate hormonal and developmental regulation of milk protein gene expression. Biochem Soc Symp. 1997;63:101–113. [PubMed] [Google Scholar]
- Roskelley CD, Srebrow A, Bissell MJ. A hierarchy of ECM-mediated signalling regulates tissue-specific gene expression. Curr Opin Cell Biol. 1995;7:736–747. doi: 10.1016/0955-0674(95)80117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samakovlis C, Hacohen N, Manning G, Sutherland DC, Guillemin K, Krasnow MA. Development of the Drosophila tracheal system occurs by a series of morphologically distinct but genetically coupled branching events. Development. 1996;122:1395–1407. doi: 10.1242/dev.122.5.1395. [DOI] [PubMed] [Google Scholar]
- Screpanti I, Romani L, Musiani P, Modesti A, Fattori E, Lazzaro D, Sellitto C, Scarpa S, Bellavia D, Lattanzio G, et al. Lymphoproliferative disorder and imbalanced T- helper response in C/EBPβ-deficient mice. EMBO J. 1995;14:1932–1941. doi: 10.1002/j.1460-2075.1995.tb07185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay A, Pursel VG, Wilkinson E, Wall RJ, Hennighausen L. Expression of the whey acidic protein in transgenic pigs impairs mammary development. Transgenic Res. 1992;1:124–132. doi: 10.1007/BF02528777. [DOI] [PubMed] [Google Scholar]
- Sicinski P, Donaher JL, Parker SB, Li T, Fazelli A, Gardner H, Haslam SZ, Bronson RT, Elledge SJ, Weinberg RA. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell. 1995;82:621–630. doi: 10.1016/0092-8674(95)90034-9. [DOI] [PubMed] [Google Scholar]
- Sterneck E, Tessarollo L, Johnson PF. An essential role for C/EBPβ in female reproduction. Genes & Dev. 1997;11:2153–2162. doi: 10.1101/gad.11.17.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Akira S, Yoshida K, Umemoto M, Yoneda Y, Shirafuji N, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T. Targeted disruption of the NF-IL6 gene discloses its essential role in bacteria killing and tumor cytotoxicity by macrophages. Cell. 1995;80:353–361. doi: 10.1016/0092-8674(95)90418-2. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Yoshida N, Kishimoto T, Akira S. Defective adipocyte differentiation in mice lacking the C/EBPβ and/or C/EBPδ gene. EMBO J. 1997;16:7432–7443. doi: 10.1093/emboj/16.24.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timchenko NA, Harris TE, Wilde M, Bilyeu TA, Burgess-Beusse BL, Finegold MJ, Darlington GJ. CCAAT/enhancer binding protein alpha regulates p21 protein and hepatocyte proliferation in newborn mice. Mol Cell Biol. 1997;17:7353–7361. doi: 10.1128/mcb.17.12.7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscani A, Mettus RV, Coupland R, Simpkins H, Litvin J, Orth J, Hatton KS, Reddy EP. Arrest of spermatogenesis and defective breast development in mice lacking A-myb. Nature. 1997;386:713–717. doi: 10.1038/386713a0. [DOI] [PubMed] [Google Scholar]
- Udy GA, Towers RP, Snell RG, Wilkins RJ, Park S-H, Ram P, Waxman DJ, Davey HW. Requirement for STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci. 1997;94:7239–7244. doi: 10.1073/pnas.94.14.7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umek RM, Friedman AD, McKnight SL. CCAAT-enhancer binding protein: A component of a differentiation switch. Science. 1991;251:288–292. doi: 10.1126/science.1987644. [DOI] [PubMed] [Google Scholar]
- Wang ND, Finegold MJ, Bradley A, Ou CN, Abdelsayed SV, Wilde MD, Taylor LR, Wilson DR, Darlington GJ. Impaired energy homeostasis in C/EBP alpha knockout mice. Science. 1995;269:1108–1112. doi: 10.1126/science.7652557. [DOI] [PubMed] [Google Scholar]
- Williams SC, Cantwell CA, Johnson PF. A family of C/EBP-related proteins capable of forming covalently linked leucine zipper dimers in vitro. Genes & Dev. 1991;5:1553–1567. doi: 10.1101/gad.5.9.1553. [DOI] [PubMed] [Google Scholar]
- Williams SC, Baer M, Dillner AJ, Johnson PF. CRP2 (C/EBP beta) contains a bipartite regulatory domain that controls transcriptional activation, DNA binding and cell specificity. EMBO J. 1995;14:3170–3183. doi: 10.1002/j.1460-2075.1995.tb07319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh WC, Cao Z, Classon M, McKnight SL. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes & Dev. 1995;9:168–181. doi: 10.1101/gad.9.2.168. [DOI] [PubMed] [Google Scholar]
- Yi ES, Bedoya AA, Lee H, Kim S, Housley RM, Aukerman SL, Tarpley JE, Starnes C, Yin S, Pierce GF, et al. Keratinocyte growth factor causes cystic dilation of the mammary glands of mice. Interactions of keratinocyte growth factor, estrogen, and progesterone in vivo. Am J Pathol. 1994;145:1015–1022. [PMC free article] [PubMed] [Google Scholar]
- Zahnow CA, Younes P, Laucirica R, Rosen JM. Overexpression of C/EBPβ-LIP, a naturally occurring, dominant-negative transcription factor, in human breast cancer. J Natl Cancer Inst. 1997;89:1887–1891. doi: 10.1093/jnci/89.24.1887. [DOI] [PubMed] [Google Scholar]
- Zhang DE, Zhang P, Wang ND, Hetherington CJ, Darlington GJ, Tenen DG. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein alpha-deficient mice. Proc Natl Acad Sci. 1997;94:569–574. doi: 10.1073/pnas.94.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]