Abstract
The mesenchymal cell is a multipotent stem cell with the capacity to give rise to multiple cell types such as adipocytes, osteoblasts, chondrocytes, and myocytes. However, the molecular events responsible for their lineage specification and differentiation remain obscure. Here we show that inactivation of chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII), a member of the nuclear receptor superfamily, in mesenchymal progenitors favors osteoblast and myoblast development while simultaneously impairing adipogenic and chondrogenic programs. During mouse embryogenesis, COUP-TFII protein is highly detected in the mesenchymal compartment and is involved in mesoderm tissue formation. Ablation of COUP-TFII in mice led to higher bone density, increased muscle mass, and suppression of cartilage and fat formation. We further demonstrate that COUP-TFII directs the plasticity of mesenchymal precursors primarily through the combined modulation of Wnt signaling, Runx2 activity, as well as PPARγ and Sox9 expression. Together, our results provide insight into the mechanisms whereby a single nuclear receptor can fine-tune the lineage-specific differentiation of a progenitor cell.
Mesenchymal stem (stromal) cells (MSCs) are heterogeneous multipotent precursors present in the stromal fraction of many adult tissues. Historically, MSCs were first described within the bone marrow compartment (1, 2) and later were identified from other tissues such as umbilical cord, adipose tissue, connective tissues, and so forth (3, 4). Bone marrow stromal cells (BMSCs) can differentiate into adipocytes, osteoblasts, and chondrocytes when placed under appropriate stimuli (5, 6). C3H10T1/2 cells were originally isolated from C3H mouse embryos and are functionally similar to BMSCs (7, 8). In addition, adult multipotent mesenchymal cells have been identified within human adipose tissue (4). All three cells exhibit multilineage differentiation capacities upon in vitro expansion and in vivo transplantation (9–12). Therefore, they are excellent culture systems to model in vivo mesoderm development.
Differentiation of MSCs into various lineages is mutually exclusive, and inducers of differentiation along one lineage often repress differentiation of alternative paths. An excellent example is the inverse relationship between adipogenesis and osteoblastogenesis (13). PPARγ, a master activator of adipogenesis, inhibits osteoblast differentiation. This competition was consistent with the pathological and epidemiological studies that showed that increased bone marrow adiposity with aging is often associated with osteoporosis (14), leading to the concept that efficient switching of MSC identity from adipocyte to osteoblast may have clinical implication for the treatment of osteoporosis patients (15, 16). Therefore, further studies to uncover the molecular basis controlling mesenchymal cell identity and differentiation are essential for the use of MSCs in clinical applications.
Chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII) is widely detected in the mesenchymal compartment of developing organs and plays a crucial role in mouse embryogenesis (17–22). Although germ-line inactivation of COUP-TFII causes embryonic lethality (17), COUP-TFII heterozygous mice were viable and displayed defects in mesoderm-derived tissues including fat, muscle, and the reproductive tract (20, 21). Here we investigated the role of COUP-TFII in mesenchymal cell development. Using cell-culture models, we show that the depletion of COUP-TFII in precursors led to a preferential switch of lineage allocation from adipocyte and chondrocyte to osteoblast and myoblast. In mouse models, early ablation of the COUP-TFII in limb mesenchyme resulted in cartilage developmental defects, and COUP-TFII heterozygous mice exhibited reduced fat tissue, increased muscle mass, and high bone mineral density. The molecular mechanism by which COUP-TFII effects MSC development is at least partially through the regulation of the Wnt cascade, Runx2 activity, as well as PPARγ and Sox9 expression.
Results
Expression Profiles of the COUP-TFII Gene Indicate a Role in Mesoderm-Derived Tissue Formation.
Immunostaining with COUP-TFII antibody showed that COUP-TFII protein was abundantly detected in the centralized core of the embryonic day (E)11.5 and E12.5 limb mesenchyme (Fig. S1 A and B), which is the common predecessor of several mesodermal tissues. We then assessed COUP-TFII protein levels in mesenchyme-derived organs using COUP-TFII lacZ knock-in mice (22). Oil red O staining clearly indicated the existence of primitive white adipose tissue, and X-gal staining of the consecutive section suggested the fat cells were positive for COUP-TFII expression (Fig. S1 C and D). Interestingly, COUP-TFII protein was predominantly detected in the stromal precursors or immature adipocytes, which were not stained by oil red O (Fig. S1 C and D Lower, solid line). This gradient pattern of COUP-TFII protein was also observed in other tissues. At E14.5, COUP-TFII protein was mainly found on one side of the pelvis (Fig. S1E), and alcian blue staining of the adjacent slide revealed that COUP-TFII–positive cells corresponded to the small early chondroblasts instead of the big prehypertrophic and hypertrophic chondrocytes (Fig. S1F). In muscles, a higher level of COUP-TFII protein was detected in the primitive myoblasts at E15.5 (Fig. S1G) in comparison with the more differentiated multinucleated myotubes at E17.5 (Fig. S1H). At E17.5, β-gal signals were seen in the central ossification area of the developing femur (Fig. S1I) and the periosteum, the committed progenitors of mature osteoblasts (Fig. S1J). Collectively, COUP-TFII protein was high in uncommitted precursor cells but decreased as cells progressed through differentiation. This differential expression profile implicates a potential function of COUP-TFII in mesoderm development, especially in the early-lineage specification stages.
COUP-TFII Promotes Adipogenesis and Attenuates Osteoblast Differentiation from Mesenchymal Cells.
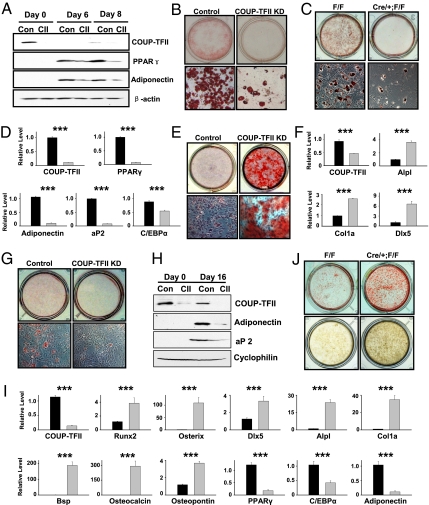
To study the potential role of COUP-TFII in mesenchymal cell differentiation, we used an in vitro differentiation system with three progenitor cells—C3H10T1/2 cells, primary murine BMSCs, and human adipose tissue-derived mesenchymal cells (hAMSCs)—in our studies. C3H10T1/2 cells give rise to lipid-containing adipocytes following hormonal treatment (Fig. S2A). COUP-TFII protein was transiently induced, along with the activation of adipocyte markers PPARγ, aP2, and adiponectin during adipocyte differentiation (Fig. S2B). Next, we knocked down COUP-TFII protein using specific siRNAs to evaluate its function in C3H10T1/2 cells. Loss of COUP-TFII protein at an early stage (Fig. 1A, day 0) impaired fat cell formation (Fig. 1B), leading to the inhibition of PPARγ and adiponectin expression at days 6 and 8 (Fig. 1A). Consistently, COUP-TFII shRNA-transfected C3H10T1/2 cells also displayed drastically reduced lipid accumulation (Fig. S2C). To further establish the importance of COUP-TFII in adipogenesis, we isolated and cultured primary BMSCs from wild-type (WT) and inducible COUP-TFII knockout (COUP-TFII KO) mice as described in Experimental Procedures. COUP-TFII null BMSCs exposed to adipogenic stimuli showed severely compromised formation of oil red O-positive adipocytes (Fig. 1C), and lack induction of adipocyte markers PPARγ, adiponectin, aP2, and C/EBPα (Fig. 1D). The positive role of COUP-TFII in adipogenesis was further substantiated by expression of shRNA targeting COUP-TFII in the hAMSCs (Fig. S2 D and E).
Fig. 1.
COUP-TFII promotes adipogenesis and attenuates osteoblast differentiation from mesenchymal cells. (A and B) Control siRNA and COUP-TFII siRNA-treated C3H10T1/2 cells underwent adipocyte differentiation and cells were stained with oil red O and photographed on day 6 (B). Impaired adipogenesis in COUP-TFII–depleted cells was confirmed by Western blot (A). (C and D) Oil red O stainings of BMSCs cultured under adipogenic stimuli for 12 d (C). Gene expression analysis was performed at day 8 (D). (E and F) C3H10T1/2 cells were stained with alizarin red on day 60 after culturing in an osteogenic medium (E). Enhanced osteoblast differentiation in mutant cells was confirmed by Q-PCR analysis (F). (G and H) COUP-TFII–deficient C3H10T1/2 cells and control cells were cultured in an osteogenic medium for 16 d. Adipocyte formation was assessed by oil red O staining (G) and by Western blot (H). (I and J) Two weeks after osteogenic induction, increased osteoblast markers and decreased adipocyte genes were observed in COUP-TFII null BMSCs (I). Alizarin red and von Kossa staining of BMSCs that underwent osteogenesis for 21 d (J). Gray bars represent COUP-TFII ablation cells (D, F, and I). Data are expressed as mean ± SEM. ***P < 0.001.
Adipocytes and osteoblasts arise from common precursors, and C3H10T1/2 cells form mature osteoblasts under an osteo-inducing medium (Fig. S2F). During this process, COUP-TFII protein gradually declined, accompanied by a transient activation of alkaline phosphatase, an early osteoblast marker (Fig. S2G). aP2 expression was induced and remained elevated, suggesting that both adipogenic and osteogenic programs are activated during osteoblastogenesis (Fig. S2G). We first compared the osteogenic potential of C3H10T1/2 cells treated with COUP-TFII shRNA versus control shRNA. COUP-TFII knockdown cells displayed enhanced alkaline phosphatase activity and widespread formation of mineralized bone nodules (Fig. 1E and Fig. S2H) associated with a higher level of osteoblast markers Alpl, Col1a, and Dlx5 (Fig. 1F). Contrary to the enhanced osteoblastogenesis, adipogenesis was markedly compromised in mutant cells (Fig. 1G), where the levels of adiponectin and aP2 proteins were greatly reduced (Fig. 1H). Because COUP-TFII protein was mostly active at the beginning of osteogenesis (Fig. S2G), we also used specific siRNAs to transiently block COUP-TFII expression in C3H10T1/2 cells. Cells were then placed under osteogenic conditions and assayed 2 mo later, by which time COUP-TFII expression was no longer inhibited. The transient inhibition of COUP-TFII protein at the early phase was sufficient to stimulate osteoblast development (Fig. S2I), implying a role of COUP-TFII in restricting the commitment of precursors toward the osteoblast lineage.
Next, we extended our studies to primary stromal cultures. COUP-TFII–deficient BMSCs grown under osteogenic stimulation showed a dramatic increase of key osteogenic transcriptional factors (Runx2, Osterix, and Dlx5), early osteoblast markers (Alpl and Col1a), and terminal differentiation genes (Bsp, osteocalcin, and osteopontin) associated with impaired adipogenesis, as PPARγ, C/EBPα, and adiponectin were not properly activated (Fig. 1I). Alizarin red and von Kossa staining verified the enhanced bone nodule formation in mutant BMSCs (Fig. 1J). Moreover, shRNA-mediated COUP-TFII inactivation in hAMSCs also resulted in pronounced cell-matrix mineralization, supporting the negative role of COUP-TFII in osteogenesis (Fig. S2J). Because all of the above results were obtained from the bone differentiation condition, we then asked whether in the absence of COUP-TFII a similar switch of multipotency also occurs during adipocyte differentiation. To our surprise, COUP-TFII KO BMSCs exhibited a striking and aberrant increase in osteoblast markers, despite culture under adipogenic conditions (Fig. S2K), and alkaline phosphatase (ALP) staining further supported the increased lineage allocation to an osteoblast program upon COUP-TFII loss (Fig. S2L). Collectively, our data demonstrate the critical role of COUP-TFII in the regulation of adipogenic and osteogenic commitment in progenitor cells.
COUP-TFII Controls Lineage Decision of the Mesenchymal Cell.
It has been suggested that there is a competition or mutual suppression between osteogenesis and chondrogenesis (23, 24). As discussed above, COUP-TFII inhibits bone cell formation; we then asked whether COUP-TFII also affects chondrocyte development. In response to BMP2 treatment, C3H10T1/2 cells were effectively converted into chondrocytes producing cartilage-specific matrix proteins and lipid-filling adipocytes (25) (Fig. S3A). COUP-TFII protein quickly diminished, whereas Sox9, a gene required for chondrogenesis, was transiently up-regulated (Fig. S3B). aP2 protein was activated and persisted throughout differentiation, which correlated with the formation of adipocytes (Fig. S3B).
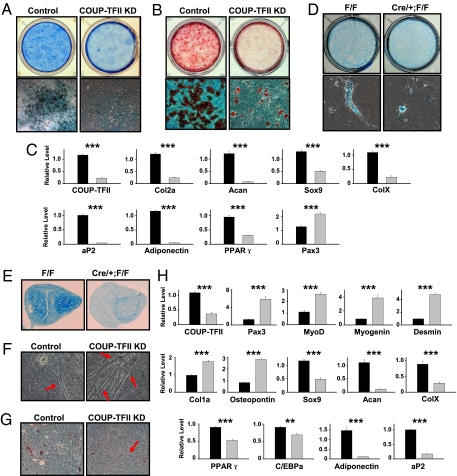
Following chondrogenic stimulation, enormous alcian blue-positive cartilaginous nodules and oil red O-stained fat cells formed in control cultures, whereas C3H10T1/2 cells lacking COUP-TFII protein did not undergo appropriate differentiation (Fig. 2 A and B). The biochemical characterization was corroborated by quantitative (Q-)PCR analysis of genes specific for chondrocytes (Col2a, Acan, Sox9, and ColX) and adipocytes (PPARγ, aP2, and adiponectin) (Fig. 2C). Interestingly, Pax3, a molecule exclusively expressed in muscle precursors, was aberrantly activated, suggesting an increase in myoblast commitment in mutant cells (Fig. 2C). The pronounced increase in myogenic potential and defects in chondrogenesis and adipogenesis were also observed in primary COUP-TFII KO BMSCs, as measured by marker expression (Fig. S3C) and alcian blue staining (Fig. 2D). More importantly, we further demonstrated the positive influence of COUP-TFII in mediating chondrocyte development through micromass culture of primary cells (Fig. 2E and Fig. S3D). Overall, it is clear that COUP-TFII is necessary for appropriate chondrogenesis and adipogenesis in progenitor cells.
Fig. 2.
COUP-TFII controls lineage decision of the mesenchymal cell. (A and B) C3H10T1/2 cells were stained with alcian blue (A) or oil red O (B) 35 d after chondrogenic induction. (C) Q-PCR analysis of differentiation markers specific for chondrocyte, adipocyte, and myocyte development. (D) BMSCs obtained from wild-type and COUP-TFII mutant mice were subjected to chondrogenic stimuli. Alcian blue staining was performed at day 28. (E) Alcian blue staining of a chondrogenic nodule on day 28 of BMSC micromass culture. (F–H) C3H10T1/2 cells were cultured in medium containing 5-azacytidine for 35 d and COUP-TFII–deficient cells displayed increased myotube formation (F) with decreased lipid accumulation (G). Arrows indicate multinucleated myoblast (F and G). Lineage-specific genes were examined by Q-PCR analysis (H). Gray bars represent COUP-TFII–deficient cells (C and H). Data are expressed as mean ± SEM. **P < 0.01, ***P < 0.001.
C3H10T1/2 cells undergo differentiation into multiple cell types with morphological and biochemical characteristics of myoblasts, adipocytes, and chondrocytes when treated with the DNA methyltransferase inhibitor 5-azacytidine (8) (Fig. S3E). With the progression of differentiation, COUP-TFII expression was rapidly extinguished with the concomitant activation of PPARγ and the subsequent appearance of myoblast markers myogenin and Myhc (Fig. S3F). Upon 5-azacytidine treatment, numerous multinucleated myotubes appeared in cultures infected with COUP-TFII shRNA, which is in sharp contrast to control C3H10T1/2 cells, where only a few myoblasts were found (Fig. 2F). Along with the increased myoblast formation, there was a deficiency in lipid accumulation in COUP-TFII–depleted cells (Fig. 2G). Subsequently, we analyzed a panel of lineage-selective genes and identified an elevated myogenic (Pax3, MyoD, myogenin, and Desmin) and osteogenic (Col1a and osteopontin) differentiation accompanied by defective chondrogenic (Sox9, Acan, and ColX) and adipogenic (PPARγ, C/EBPα, adiponectin, and aP2) programming in COUP-TFII–deficient cells (Fig. 2H). Next, we further assayed the differentiation status in COUP-TFII null BMSCs, as exposure of stromal cells to 5-azacytidine also results in the appearance of multinucleated fibers resembling myotubes (26). Significantly, mutant BMSCs cultured with 5-azacytidine exhibited a similar gene expression profile of lineage assignment as shown in C3H10T1/2 cells (Fig. S3G). Taken together, the preceding experiments support a central function of COUP-TFII in mediating lineage specification bias and promoting adipogenesis and chondrogenesis while suppressing myogenesis and osteoblastogenesis of mesenchymal progenitors.
Ablation of COUP-TFII Reveals Its Function in the Control of Mesoderm Development in Vivo.
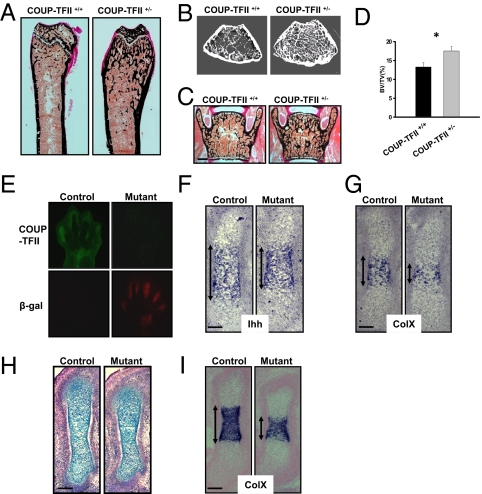
In accordance with our in vitro analysis, COUP-TFII heterozygous mice (COUP-TFII+/−) display less fat tissue with increased muscle mass compared with control littermates (20). Next, we examined the skeletons of 6-wk-old COUP-TFII+/− mice to gain insights into the function of COUP-TFII in bone development. von Kossa staining of nondemineralized femoral sections showed an increase in trabecular bone formation throughout the endocortical compartment in COUP-TFII+/− mice (Fig. 3A). Microcomputed tomography (μCT) analysis of the long bone verified the high bone mass phenotype (Fig. 3B). Effects of COUP-TFII on bone density were not restricted to the femur, as an increase in calcium deposition was also found in the vertebrae of COUP-TFII+/− mice (Fig. 3C). Further quantification demonstrated a 30% higher bone volume in COUP-TFII+/− mice in comparison with control animals (Fig. 3D). Therefore, similar to our in vitro analysis, COUP-TFII indeed suppresses bone formation in vivo.
Fig. 3.
Ablation of COUP-TFII reveals its function in the control of bone and cartilage development. (A and B) von Kossa staining (A) and micro-CT scans (B) of distal femora of wild-type and COUP-TFII+/− mice. (C and D) Histomorphometric analysis of vertebrae from 6-wk-old mice (C) and quantification of changes in bone volume/tissue volume (BV/TV) in COUP-TFII+/− mice (D) (n = 7–9 mice). (Scale bar, 100 μm.) (E) Immunostaining of COUP-TFII and β-galactosidase antibodies in E13.5 limb bud. (F and G) Nonradioactive in situ hybridization on E13.5 mice femora showing expression of Ihh (F) and ColX (G). (Scale bars, 50 μm.) (H and I) Wild-type and COUP-TFII mutant femurs at E14.5. (H) Alcian blue staining. (I) In situ hybridization with ColX probe. (Scale bars, 100 μm.) Double-headed arrows in F, G, and I indicate positive staining by in situ hybridization. *P < 0.05.
Finally, we asked whether COUP-TFII also effects cartilage development in mice. To circumvent the embryonic lethality of the COUP-TFII null mutant, we exploited a tamoxifen-inducible knockout system by crossing ROSA26CRE-ERT2/+; COUP-TFIIflox/flox mice with COUP-TFIIflox/flox female mice. The pregnant female mice were administered tamoxifen at E11.5 to ablate the COUP-TFII gene early in the limb bud. A lacZ reporter was inserted into the 3′ untranslated region of the floxed COUP-TFII locus and can be activated upon COUP-TFII deletion (22). Two days after tamoxifen injection (E13.5), immunohistochemical analysis indicated a drastic reduction of COUP-TFII protein accompanied by the expression of the β-galactosidase gene in the mutant embryo (Fig. 3E), indicating that the COUP-TFII gene was efficiently ablated from the mutant limb mesenchyme.
Using these COUP-TFII mutants, we examined several markers that participated in cartilage development. In situ hybridization showed that Ihh transcripts were expressed at the normal level with a smaller expression domain in the mutant femur, suggesting a defect in chondrocyte maturation in COUP-TFII–deficient mice (Fig. 3F). A similar result was found in the staining for the ColX gene, which was only detected in hypertrophic chondrocytes (Fig. 3G). This retardation of cartilage development was also evident at E14.5, as the COUP-TFII mutant displayed a shorter femoral and smaller ColX expression zone compared with control mice (Fig. 3 H and I). The malformation of cartilage upon COUP-TFII ablation was further demonstrated in the COUP-TFII hypomorphic mutant (COUP-TFIIflox/−), which harbors only one copy of the floxed COUP-TFII allele. At E14.5, the COUP-TFIIflox/− mice exhibited cartilage abnormalities mimicking that observed in COUP-TFII–inducible knockout mice (Fig. S4 A and B), which again supports the notion that COUP-TFII is necessary for appropriate chondrocyte differentiation in vivo.
COUP-TFII Regulates Wnt, Runx2, PPARγ, and Sox9 to Direct Mesenchymal Cell-Fate Decision.
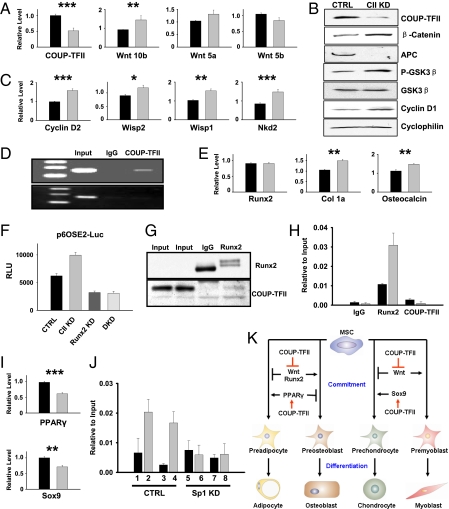
To define the molecular basis of COUP-TFII regulation, we searched for potential COUP-TFII effectors with the ability to modulate MSC lineage allocation. Activation of the canonical Wnt cascade and its downstream targets governs the mesenchymal cell-fate program (27–33). We found that Wnt10b was up-regulated in COUP-TFII–deficient cells (Fig. 4A). Accordingly, ablation of COUP-TFII resulted in acute induction of β-catenin (Fig. 4B) and its endogenous targets, including cyclinD2, Wisp1, Wisp2, and Nkd2 (34) (Fig. 4C). Correspondingly, other components in the Wnt signaling cascade, such as P-GSK-3β, adenomatous polyposis coli (APC), and cyclin D1, were coordinately regulated (Fig. 4B). Finally, we performed an chromatin immunoprecipitation (ChIP) assay and found that COUP-TFII protein was recruited to the Wnt10b regulatory region, which contains a conserved COUP-TFII binding site, suggesting that COUP-TFII directly controls Wnt10b at the transcriptional level (Fig. 4D).
Fig. 4.
COUP-TFII regulates Wnt, Runx2, PPARγ, and Sox9 to direct mesenchymal cell development. (A and B) COUP-TFII controls Wnt10b expression (A) and the expression of components along the Wnt cascade (B). (C) COUP-TFII regulates endogenous Wnt targets. (D) ChIP analysis of COUP-TFII binding to the Wnt10b regulatory region, where a COUP-TFII binding site is located (Upper) and the intron region, lacking COUP-TFII binding sequences, serves as a negative control (Lower). (E–H) COUP-TFII modulates Runx2 activity. (E) COUP-TFII regulates Runx2 target genes. (F) Runx2 is required for the activation of the p6OSE2-luciferase reporter in COUP-TFII knocked-down cells. (G) Western blotting detects COUP-TFII protein in Runx2 immunoprecipitates. (H) ChIP assay showed that Runx2 bound to the known Runx2 response elements of the osteocalcin gene, and increased promoter occupancy of Runx2 was found in COUP-TFII–ablated cells. (I) COUP-TFII promotes PPARγ and Sox9 expression. (J) ChIP analysis of COUP-TFII and Sp1 binding to the Sox9 gene in both control cells and cells treated with Sp1 siRNA. Lanes 1–4, control siRNA treatment; lanes 5–8, Sp1 siRNA-treated samples. Lanes 1 and 5, mouse IgG; lanes 2 and 6, COUP-TFII antibody; lanes 3 and 7, rabbit IgG; lanes 4 and 8, Sp1 antibody. (K) Working model of COUP-TFII as a key regulator of mesenchymal cell commitment and differentiation. Gray bars represent COUP-TFII knockdown cells in A, C, E, H, and I. Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
Runx2 is a master activator of bone development. Although Runx2 transcript was unaffected, two representative Runx2-responsive genes, Col1a and osteocalcin, were up-regulated after COUP-TFII inactivation (Fig. 4E), suggesting COUP-TFII may repress Runx2 transcriptional activity. To further test this hypothesis, we conducted luciferase reporter assays using Runx2-responsive reporter p6OSE2-Luc (35). Depletion of COUP-TFII expression stimulates p6OSE2-Luc activity, and this activation was abolished with the knockdown of Runx2 (Fig. 4F). Moreover, concurrent knockdown of Runx2 and COUP-TFII suppressed the enhanced osteocalcin expression (Fig. S4C), suggesting that COUP-TFII interferes with Runx2 function to regulate Runx2 downstream targets. Next, we asked whether COUP-TFII can physically interact with Runx2 to exert its effect. COUP-TFII protein was associated with Runx2 in a coimmunoprecipitation assay (Fig. 4G), and shRNA-mediated inactivation of COUP-TFII led to a threefold higher recruitment of Runx2 protein to the osteocalcin promoter (Fig. 4H). Therefore, COUP-TFII directly interacts with Runx2 to block its activity by interfering with its binding to the target promoter, thus leading to the decreased commitment of precursors to the osteogenic lineage.
PPARγ, a principal player of adipogenesis, was under the control of Wnt signaling (32–34). In line with the activation of the Wnt pathway, depletion of COUP-TFII resulted in the reduction of PPARγ mRNA and protein levels (Fig. 4I and Fig. S4D). Activation of Sox9 is the first step toward chondrogenesis, and COUP-TFII promotes Sox9 expression (Fig. 4I). It has been shown that COUP-TFII can serve as a repressor through direct binding to COUP-TFII response elements or acts as an activator via tethering to Sp1 (18). Correlated with its positive effect on Sox9 mRNA, COUP-TFII and Sp1 proteins were preferentially bound to a conserved Sp1 binding site within the Sox9 gene, and this recruitment was substantially reduced in Sp1 knockdown cells (Fig. 4J). Thus, COUP-TFII is recruited to the Sox9 regulatory region by Sp1 to activate Sox9 expression, which, in turn, promotes chondrogenesis.
Discussion
One novel function of COUP-TFII is as a molecular rheostat to fine-tune MSC multipotency, both in vitro and in vivo. We show that inactivation of a nuclear receptor can simultaneously influence four distinct differentiation programs, irrespective of the origins of the cells (Figs. 1 and 2). The capacities of MSCs simultaneously converting into multiple cell types provide us with an opportunity to address the question of whether COUP-TFII directly controls lineage assignment of progenitors. Despite different differentiation conditions, loss of COUP-TFII actively reprograms the precursors by shifting the cell identity to osteogenic and myogenic lineages at the expense of adipogenic and chondrogenic programs, supporting the notion that COUP-TFII is essential for fate choice and commitment. This alteration of lineage allocation was also found in vivo. Upon depletion of COUP-TFII in the limb mesenchyme, we observed a smaller Ihh and ColX expression domain without reduction of individual cell signals, suggesting a decrease in the number of progenitors committed to the chondrogenic lineage in the mutant femur (Fig. 3 E–I). Although the phenotypes displayed in mutant mice are consistent with our in vitro observations, cell type-specific ablation of the COUP-TFII gene is needed to further decipher the role of COUP-TFII in vivo.
Wnt signaling molecules not only act as major osteogenic inducers while suppressing adipocyte differentiation but also influence chondrocyte and myocyte development (23, 24, 27–33). In line with its effects on MSC differentiation, COUP-TFII suppresses the canonical Wnt pathway and its downstream molecules in mesenchymal progenitors (Fig. 4 A–D). Aside from our demonstration of Wnt10b as a direct target of COUP-TFII, recent studies have shown that Sox9 can physically interact with β-catenin to promote its degradation (36), and thus the down-regulation of Sox9 expression upon COUP-TFII removal may also contribute to the activation of the Wnt pathway. Studies have suggested that the fine-tuning of the balance between Sox9 and Runx2 as well as PPARγ and Runx2 is what drives mesenchymal cell plasticity. Although Runx2 mRNA remains unchanged, the activation of Runx2 activity as well as the reduction of PPARγ and Sox9 expression in COUP-TFII–deficient cells are sufficient to shift the balance, leading to the changes in lineage decision with respect to adipocytes, osteoblasts, and chondrocytes (Fig. 4 E–J). Moreover, previous reports have indicated that COUP-TFII inhibits myogenesis by transcriptional and posttranscriptional regulation of MyoD (37, 38). Taken together, COUP-TFII modulates multiple master regulators and key pathways, acting together in a combinatorial fashion to achieve lineage specification of MSCs.
In summary, we have identified COUP-TFII as a critical player controlling mesenchymal differentiation. During normal development, COUP-TFII activates PPARγ and Sox9 expression while inhibiting Wnt signaling and Runx2 activity, thus promoting precursor entry to adipocyte and chondrocyte lineage while impeding MSC access to alternative pathways. Removal of COUP-TFII results in a preferential shift of multipotency from adipogenic and chondrogenic programs to osteogenic and myogenic paths (Fig. 4K). Moreover, COUP-TFII–deficient mice display reduced fat tissue, enhanced glucose tolerance and insulin sensitivity, as well as increased bone mineralization and muscle mass (20) (Fig. 3). In this way, pharmacological manipulation of COUP-TFII may open novel therapeutic applications of regenerative medicine and tissue engineering and in the treatment of degenerative diseases of mesenchyme origin such as arthritis, osteoporosis, myocardial infarction, and type 2 diabetes mellitus.
Experimental Procedures
All animal experiments were approved by the Animal Center for Comparative Medicine at Baylor College of Medicine. COUP-TFII lacZ knock-in mice, COUP-TFIIflox/flox mice, COUP-TFII+/− mice, and ROSA26CRE-ERT2/+ mice were used in our studies (22). Detailed materials and methods are provided in SI Experimental Procedures.
Supplementary Material
Acknowledgments
We thank Dr. Yingzi Yang and Dr. Christine Hartmann for the in situ hybridization probes. The p6OSE2-Luc reporter was provided by Dr. Gerard Karsenty. We appreciate technical help from Ms. Wei Qian and Xuefei Tong, and Ms. Jodie R. Hebert for help in preparing this manuscript. X.X. was supported by Rolanette and Berdon Lawrence bone research awards. This work was supported by grants from the National Institutes of Health [DK62434 and DK59820 (to S.Y.T. and M.-J.T.), DK45641 and HD17379 (to M.-J.T.), and HL76448 (to S.Y.T.)].
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1110236108/-/DCSupplemental.
References
- 1.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–247. [PubMed] [Google Scholar]
- 2.Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381–390. [PubMed] [Google Scholar]
- 3.Lee OK, et al. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–1675. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- 4.Zuk PA, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barry FP, Murphy JM. Mesenchymal stem cells: Clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Bianco P, Kuznetsov SA, Riminucci M, Gehron Robey P. Postnatal skeletal stem cells. Methods Enzymol. 2006;419:117–148. doi: 10.1016/S0076-6879(06)19006-0. [DOI] [PubMed] [Google Scholar]
- 7.Reznikoff CA, Brankow DW, Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973;33:3231–3238. [PubMed] [Google Scholar]
- 8.Taylor SM, Jones PA. Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell. 1979;17:771–779. doi: 10.1016/0092-8674(79)90317-9. [DOI] [PubMed] [Google Scholar]
- 9.Dominici M, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 10.Krebsbach PH, et al. Bone formation in vivo: Comparison of osteogenesis by transplanted mouse and human marrow stromal fibroblasts. Transplantation. 1997;6398:1059–1069. doi: 10.1097/00007890-199704270-00003. [DOI] [PubMed] [Google Scholar]
- 11.Cowan CM, et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560–567. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 12.Date T, Doiguchi Y, Nobuta M, Shindo H. Bone morphogenetic protein-2 induces differentiation of multipotent C3H10T1/2 cells into osteoblasts, chondrocytes, and adipocytes in vivo and in vitro. J Orthop Sci. 2004;9:503–508. doi: 10.1007/s00776-004-0815-2. [DOI] [PubMed] [Google Scholar]
- 13.Beresford JN, Bennett JH, Devlin C, Leboy PS, Owen ME. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J Cell Sci. 1992;102:341–351. doi: 10.1242/jcs.102.2.341. [DOI] [PubMed] [Google Scholar]
- 14.Verma S, Rajaratnam JH, Denton J, Hoyland JA, Byers RJ. Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J Clin Pathol. 2002;55:693–698. doi: 10.1136/jcp.55.9.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nuttall ME, Gimble JM. Is there a therapeutic opportunity to either prevent or treat osteopenic disorders by inhibiting marrow adipogenesis? Bone. 2000;27:177–184. doi: 10.1016/s8756-3282(00)00317-3. [DOI] [PubMed] [Google Scholar]
- 16.Phinney DG, Prockop DJ. Concise review: Mesenchymal stem/multipotent stromal cells: The state of transdifferentiation and modes of tissue repair—Current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 17.Pereira FA, Qiu Y, Zhou G, Tsai MJ, Tsai SY. The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev. 1999;13:1037–1049. doi: 10.1101/gad.13.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai SY, Tsai MJ. Chick ovalbumin upstream promoter-transcription factors (COUP-TFs): Coming of age. Endocr Rev. 1997;18:229–240. doi: 10.1210/edrv.18.2.0294. [DOI] [PubMed] [Google Scholar]
- 19.You LR, et al. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005;435:98–104. doi: 10.1038/nature03511. [DOI] [PubMed] [Google Scholar]
- 20.Li L, et al. The nuclear orphan receptor COUP-TFII plays an essential role in adipogenesis, glucose homeostasis, and energy metabolism. Cell Metab. 2009;9:77–87. doi: 10.1016/j.cmet.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takamoto N, et al. Haploinsufficiency of chicken ovalbumin upstream promoter transcription factor II in female reproduction. Mol Endocrinol. 2005;19:2299–2308. doi: 10.1210/me.2005-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takamoto N, et al. COUP-TFII is essential for radial and anteroposterior patterning of the stomach. Development. 2005;132:2179–2189. doi: 10.1242/dev.01808. [DOI] [PubMed] [Google Scholar]
- 23.Hill TP, Später D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/β-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8:727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 25.Fischer L, Boland G, Tuan RS. Wnt-3A enhances bone morphogenetic protein-2-mediated chondrogenesis of murine C3H10T1/2 mesenchymal cells. J Biol Chem. 2002;277:30870–30878. doi: 10.1074/jbc.M109330200. [DOI] [PubMed] [Google Scholar]
- 26.Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18:1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- 27.Ross SE, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 28.Bennett CN, et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci USA. 2005;102:3324–3329. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Longo KA, et al. Wnt10b inhibits development of white and brown adipose tissues. J Biol Chem. 2004;279:35503–35509. doi: 10.1074/jbc.M402937200. [DOI] [PubMed] [Google Scholar]
- 30.Vertino AM, et al. Wnt10b deficiency promotes coexpression of myogenic and adipogenic programs in myoblasts. Mol Biol Cell. 2005;16:2039–2048. doi: 10.1091/mbc.E04-08-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ling L, Nurcombe V, Cool SM. Wnt signaling controls the fate of mesenchymal stem cells. Gene. 2009;433:1–7. doi: 10.1016/j.gene.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Kang S, et al. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein α and peroxisome proliferator-activated receptor γ. J Biol Chem. 2007;282:14515–14524. doi: 10.1074/jbc.M700030200. [DOI] [PubMed] [Google Scholar]
- 33.Takada I, Kouzmenko AP, Kato S. Wnt and PPARγ signaling in osteoblastogenesis and adipogenesis. Nat Rev Rheumatol. 2009;5:442–447. doi: 10.1038/nrrheum.2009.137. [DOI] [PubMed] [Google Scholar]
- 34.Jackson A, et al. Gene array analysis of Wnt-regulated genes in C3H10T1/2 cells. Bone. 2005;36:585–598. doi: 10.1016/j.bone.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 36.Akiyama H, et al. Interactions between Sox9 and β-catenin control chondrocyte differentiation. Genes Dev. 2004;18:1072–1087. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muscat GE, Rea S, Downes M. Identification of a regulatory function for an orphan receptor in muscle: COUP-TF II affects the expression of the myoD gene family during myogenesis. Nucleic Acids Res. 1995;23:1311–1318. doi: 10.1093/nar/23.8.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bailey P, Sartorelli V, Hamamori Y, Muscat GEO. The orphan nuclear receptor, COUP-TF II, inhibits myogenesis by post-transcriptional regulation of MyoD function: COUP-TF II directly interacts with p300 and myoD. Nucleic Acids Res. 1998;26:5501–5510. doi: 10.1093/nar/26.23.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.