Abstract
Aggregation of amyloid-β (Aβ) as toxic oligomers and amyloid plaques within the brain appears to be the pathogenic event that initiates Alzheimer's disease (AD) lesions. One therapeutic strategy has been to reduce Aβ levels to limit its accumulation. Activation of certain neurotransmitter receptors can regulate Aβ metabolism. We assessed the ability of serotonin signaling to alter brain Aβ levels and plaques in a mouse model of AD and in humans. In mice, brain interstitial fluid (ISF) Aβ levels were decreased by 25% following administration of several selective serotonin reuptake inhibitor (SSRI) antidepressant drugs. Similarly, direct infusion of serotonin into the hippocampus reduced ISF Aβ levels. Serotonin-dependent reductions in Aβ were reversed if mice were pretreated with inhibitors of the extracellular regulated kinase (ERK) signaling cascade. Chronic treatment with an SSRI, citalopram, caused a 50% reduction in brain plaque load in mice. To test whether serotonin signaling could impact Aβ plaques in humans, we retrospectively compared brain amyloid load in cognitively normal elderly participants who were exposed to antidepressant drugs within the past 5 y to participants who were not. Antidepressant-treated participants had significantly less amyloid load as quantified by positron emission tomography (PET) imaging with Pittsburgh Compound B (PIB). Cumulative time of antidepressant use within the 5-y period preceding the scan correlated with less plaque load. These data suggest that serotonin signaling was associated with less Aβ accumulation in cognitively normal individuals.
Keywords: microdialysis, selective serotonin reuptake inhibitor antidepressants, late-life depression
Amyloid-β (Aβ) dysregulation appears to initiate the pathogenesis of Alzheimer's disease (AD) with a cascade of downstream factors that exacerbate and propagate neuronal injury (1). Aβ can accumulate as toxic plaques and soluble oligomers in the brains of individuals with AD a decade or more before the initial symptoms are identified (2). The concentration of Aβ is a critical factor determining if and when it will aggregate into these toxic structures; high concentrations of Aβ are more prone to convert from its normal soluble form into these multimeric conformations (3). Aβ is formed within neurons by sequential cleavage of the amyloid precursor protein (APP) by two enzymes, β-secretase and then γ-secretase. Alternatively, α-secretase can cleave APP within the Aβ sequence, which precludes the peptide from being formed at all. The enzymes and mechanisms that produce Aβ have been well characterized; however, the mechanisms that regulate Aβ production and levels are only partly understood. Understanding the cellular processes that regulate Aβ levels may provide greater insight into disease pathogenesis and suggest new avenues to treat or prevent AD.
Synaptic activity is one key regulator of brain Aβ production. Depolarization and subsequent synaptic transmission causes Aβ to be produced presynaptically and then secreted into the brain extracellular fluid or interstitial fluid (ISF) (4–6). Activation of postsynaptic receptors can also modulate Aβ levels. For example, glutamate NMDA receptors activate intracellular signaling cascades that suppress Aβ production (7). Similarly, muscarinic M1 acetylcholine receptors elevate cleavage of APP by α-secretase, which lowers Aβ production and levels (8, 9) (for review, see ref. 10). Chronic administration of M1 receptor agonists reduce brain Aβ levels and plaque load (11–13). Several studies have also assessed the effect of serotonin receptors (5HT-Rs) on APP processing and Aβ levels. Activation of select 5HT-Rs increases nonamyloidogenic processing of APP in vitro (14–16) and chronic administration of selective serotonin reuptake inhibitors (SSRIs) reduces brain Aβ levels in mice (17–19).
Our current studies used animal models of AD to demonstrate that serotonin signaling, including through administration of SSRI antidepressants, rapidly reduced Aβ production in vivo. Serotonin-mediated activation of extracellular regulated kinase (ERK) was necessary for this regulation. Human studies with PET imaging of amyloid plaques and retrospective analysis of antidepressant use suggested that serotonin signaling also was associated with less Aβ accumulation in cognitively normal individuals.
Results
SSRIs Reduce ISF Aβ Levels in a Mouse Model of AD.
We conducted studies in a mouse model of AD to better understand the relationship between serotonin signaling and Aβ generation on an acute timescale in a physiological paradigm. Aβ is primarily made within neurons and then secreted into the brain ISF as part of its normal metabolism. At least a portion of Aβ within the ISF contributes to amyloid plaque growth (20) with higher concentrations of Aβ being more likely to aggregate and deposit as plaques (3). Given that the ISF can be a source of toxic Aβ species, we determined whether antidepressant drugs reduced Aβ levels within this brain fluid. Dynamic changes in ISF Aβ levels can be measured over several days using in vivo microdialysis (21). This technique permits the animals to be awake with freedom of movement during drug administration and sample collection.
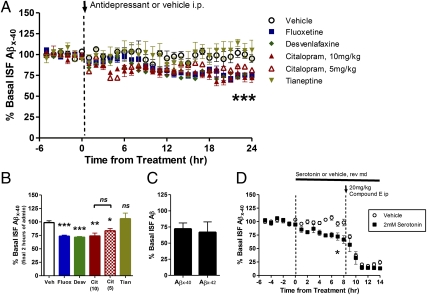
Two- to 3-mo-old presenilin-1 PS1APP transgenic mice (22) were implanted with unilateral microdialysis probes in the hippocampus. At this age, this mouse model of AD does not yet contain insoluble Aβ deposits, thus allowing us to study normal cellular pathways that affect Aβ metabolism. Basal ISF Aβ concentrations were determined in each mouse over a 6-h period followed by i.p. administration of one of several SSRIs; fluoxetine, desvenlafaxine, and citalopram. Compared with vehicle-treated mice, all SSRIs reduced ISF Aβ levels almost immediately following drug administration with significant decreases starting 12–14 h after treatment (Fig. 1 A and B). The effect of SSRIs was long lived with ISF Aβ levels remaining reduced by 25% at 24 h after drug administration. Citalopram was administered at two doses that roughly equate to high and low doses prescribed to human patients for depression (23). Citalopram at 5 mg/kg and 10 mg/kg reduced ISF Aβ by 16 and 26%, respectively (Fig. 1 A and B), both of which were significantly different from vehicle-treated mice, but not statistically different from each other. In contrast, ISF Aβ levels did not change appreciably in vehicle-treated mice compared with the basal Aβ level in each mouse. To determine whether the reduction in ISF Aβ levels was due to either the antidepressant effects of the SSRIs on mood or their action on serotonin receptors, we administered an antidepressant drug that does not act through serotonin receptors. Tianeptine, a non-SSRI antidepressant, had no effect on ISF Aβ levels over a 24-h period (Fig.1 A and B). A subset of PS1APP mice was treated with citalopram during longer ISF sample collections to measure ISF Aβx-40 and Aβx-42 at the same time. Citalopram depressed both species of Aβ to the same extent (Fig. 1C).
Fig. 1.
SSRIs reduce ISF Aβ levels in vivo. Two- to 3-mo-old PS1APP hemizygous mice were administered vehicle (PBS) or one of several antidepressants by i.p. injection: fluoxetine 10 mg/kg, desvenlafaxine 30 mg/kg, citalopram 5 mg/kg and 10 mg/kg, tianeptine 20 mg/kg (n = 5–8 per group). (A) As assessed by in vivo microdialysis, SSRIs reduced ISF Aβx-40 levels significantly beginning between 10 and 14 h after administration. (B) Twenty-four hours after administration, fluoxetine and desvenlafaxine reduced Aβx-40 levels to 73.4 ± 2.0% (P < 0.0001; n = 6) and 71.6 ± 1.2% (P = 0.001; n = 6), respectively, compared with baseline levels in each mouse. Doses of 10 mg/kg and 5 mg/kg citalopram reduced ISF Aβx-40 to 74.0 ± 5.4% (P = 0.004; n = 8) and 83.5 ± 4.2% (P = 0.02; n = 6) of baseline levels, respectively. ISF Aβ levels did not change significantly in tianeptine and vehicle-treated mice (n = 5 per group). (C) PS1APP mice treated with 10 mg/kg citalopram had similar reductions in ISF Aβx-40 and Aβx-42 levels; 71.9 ± 9.4% and 66.7 ± 16.3% of baseline levels, respectively, by 24 h posttreatment (n = 5). (D) PS1APP mice were treated with vehicle (artificial CSF, aCSF) or 2 mM serotonin directly to the hippocampus by reverse microdialysis for 14 h. By 8 h of administration, serotonin reduced ISF Aβx-40 levels to 66.7 ± 7.2% of baseline (P = 0.003, n = 5 per group). After 8 h, mice were administered a γ-secretase inhibitor, Compound E (20 mg/kg i.p.) to assess Aβx-40 half-life. Data presented as mean ± SEM.
Serotonin Reduces ISF Aβ Levels Without Altering Aβ Elimination Half-life.
We hypothesized that serotonin signaling was responsible for reduced ISF Aβ levels in SSRI-treated mice. To this end, we continually infused serotonin directly into the hippocampus by adding the compound to the microdialysis probe perfusion buffer (reverse microdialysis). Serotonin reduced ISF Aβ levels by 35% over an 8-h period compared with vehicle-treated mice (Fig. 1D). After 8 h of treatment, animals were administered a potent γ-secretase inhibitor, Compound E (20 mg/kg i.p.), to rapidly block Aβ production. This approach allows us to assess the elimination rate of existing endogenous Aβ within the brain ISF. Compound E caused ISF Aβ levels to drop rapidly in both vehicle- and serotonin-treated mice (Fig. 1D). In both groups, although the starting concentrations were different, the calculated elimination half-life of ISF Aβ was the same, ∼1 h (Fig. S1 A–C). This suggests that an alteration in Aβ elimination was not responsible for depressed Aβ levels in serotonin or SSRI-treated mice.
Serotonergic Regulation of ISF Aβ Does Not Require Action Potentials.
Serotonin G protein coupled receptors (GPCRs) activate signaling pathways that can mediate both excitatory and inhibitory synaptic transmission primarily by modulating a neuron's release of, or response to, neurotransmitters. The 5HT-R3 subtype, however, is an ionotropic cation channel that directly depolarizes neurons. To determine whether action potentials are required for 5HT-mediated regulation of Aβ, we first infused a sodium channel blocker, tetrodotoxin (TTX), into the hippocampus by reverse microdialysis. TTX dramatically reduces neuronal activity and ISF Aβ levels in vivo (5). After 15 h of TTX infusion, when Aβ levels reached a new steady-state level, mice were coadministered serotonin to the hippocampus (Fig. S1D). Serotonin caused an additional 35% decrease in ISF Aβ compared with TTX-treatment alone, suggesting that action potentials are not required for serotonergic regulation of Aβ production. Instead, serotonin likely is activating a signaling cascade that alters the processing of APP into Aβ.
ERK Is Required for Serotonin-Dependent Depression of ISF Aβ.
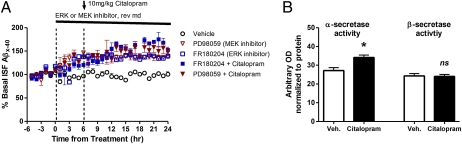
ERK, a mitogen-activated protein kinase (MAPK), has been shown to suppress Aβ production in vitro and in vivo by altering secretase activity (24, 25). Extracellular ligands bind to a GPCR on the cell surface, which leads to sequential activation of Raf, MEK (MAPK kinase), and then ERK. Activated phosho-ERK (pERK) can remain in the cytoplasm to phosphorylate proteins or translocate to the nucleus to alter transcription. We infused inhibitors of two different steps of this signaling cascade, PD98059 and FR180204, to block MEK and ERK, respectively, in vivo. Each inhibitor alone significantly increased ISF Aβ levels by 40%, suggesting basal activity of this signaling pathway suppresses Aβ generation (Fig. 2A). After 6 h of MEK or ERK inhibitor treatment, a subset of mice were then coadministered citalopram. Inhibition of MEK or ERK completely blocked the effect of the SSRI on ISF Aβ levels.
Fig. 2.
ERK-dependent changes in Aβ metabolism. (A) Young PS1APP mice were administered vehicle (aCSF), a MEK inhibitor (PD98059, 100 μM), or an ERK inhibitor (FR180204, 100 μM) by reverse microdialysis (n = 6–8 per group). At 24 h from the beginning of treatment, the MEK and ERK inhibitors alone significantly increased ISF Aβ levels by 37.7 ± 3.9% (P = 0.001) and 39.4 ± 1.6% (P < 0.0001) compared with baseline levels. Coadministration of citalopram (10 mg/kg i.p.) with either of these inhibitors blocked the SSRI-dependent reduction in ISF Aβ. ISF Aβ levels were not significantly different between inhibitor-treated and inhibitor plus citalopram-treated mice. (B) Citalopram increased α-secretase enzymatic activity by 25 ± 4.9% (P = 0.01) within the hippocampus with no change in β-secretase activity (n = 8 per group). Data presented as mean ± SEM.
A separate cohort of 3-mo-old PS1APP mice were administered vehicle or citalopram (10 mg/kg) and then killed 8 h later. Total ERK2 (Fig. S2) and ERK1 levels did not change within the hippocampus; however, activated pERK2 levels significantly increased in the presence of citalopram (Fig. S2). Phosphorylated MEK1/2 (pMEK) had a trend for an increase in SSRI-treated mice (P = 0.103). As assessed by enzymatic cleavage assays, α-secretase activity was significantly higher in citalopram-treated mice; however, β-secretase activity was unchanged (Fig. 2B).
ERK is activated by a wide range of extracellular signals; however, its targets will depend on the cellular context in which it is activated; thus, ERK activation through various receptors can have very precise and distinct effects within a cell (26). Consequently, not all ligands that activate ERK will have the same outcome. We tested several receptors, TrkB receptors, adrenergic neurotransmitter receptors, and NMDA glutamate receptors that are known to modulate ERK activity, for their ability to alter ISF Aβ levels in vivo (27–30). Treatment with BDNF, a TrkB receptor ligand, or norepinephrine, a similar neurotransmitter to serotonin, did not change ISF Aβ levels after direct administration to the hippocampus (Fig. S3). In contrast, infusion of NMDA, which can also activate ERK (29), significantly lowered ISF Aβ levels by over 50% (Fig. S3); this reduction was blocked by ERK and MEK inhibitors (31).
Chronic Administration of Citalopram Reduces Aβ and Plaque Burden in a Mouse Model of AD.
Given that SSRIs acutely reduce ISF Aβ levels, we determined whether chronic administration would impact plaque burden in the PS1APP transgenic mice. Beginning at 3 mo of age, an age before Aβ deposition in this mouse model, littermate females were administered vehicle (water) or citalopram (8 mg/kg/day) in drinking water for 4 mo. As assessed histologically, citalopram significantly reduced plaque burden within the brains of these mice compared with littermate controls that drank only water (Fig. 3 A and B). Cortical and hippocampal plaque load was significantly reduced by 62 and 50%, respectively (Fig. 3C). The contralateral brain regions were processed for biochemical analysis of Aβ protein levels. Insoluble Aβ40 and Aβ42 extracted with 5 M guanidine were significantly reduced (Fig. 3D); however PBS-soluble and Triton X-100–soluble Aβ levels did not change significantly in citalopram-treated mice (Fig. S4). Whereas PBS extraction of whole brain tissue will include Aβ within the ISF, it may also include Aβ loosely associated to membrane or from intracellular locales that are exposed even during the gentle PBS extraction process. That PBS extraction and microdialysis do not measure the same pools of Aβ likely accounts for the differential affects seen on Aβ. Aβ40 and Aβ42 levels within the cerebrospinal fluid (CSF), a truly soluble compartment similar to ISF, were significantly reduced by 28 and 55%, respectively, in these mice (Fig. 3E). Interestingly, the reduction of CSF Aβ42 levels was significantly greater than Aβ40 (P = 0.0004).
Fig. 3.
Chronic SSRI administration reduces plaque load in PS1APP transgenic mice. Beginning at 3 mo of age, PS1APP hemizygous mice were treated with water or citalopram (8 mg/kg/day) in drinking water for 4 mo (n = 10 per group). Representative images of cortex and hippocampus stained for Aβ plaques in (A) water and (B) citalopram-treated mice. (C) Quantification of plaque load in the hippocampus and cortex was performed blinded. Surface area covered by plaques was reduced in citalopram-treated mice to 57.9 ± 6.1% (P = 0.03) and 49.5 ± 6.2% (P = 0.004) of mean levels in water-treated mice. (D) Guanidine-extracted Aβx-40 and Aβx-42 in the hippocampus and cortex was significantly reduced in citalopram-treated mice. (E) Aβx-40 and Aβx-42 levels within the CSF of citalopram-treated mice were reduced to 71.4 ± 7.5% (P = 0.02) and 49.5 ± 6.2% (P < 0.0001) compared with mean levels in water-treated mice. (F) α-Secretase enzymatic activity was significantly increased in chronic citalopram mice (P < 0.001); however, β-secretase activity was unchanged. (G) Quantitative PCR showed that mRNA levels of ADAM10 did not change significantly but that memapsin-2 (P = 0.01) and two components of the γ-secretase complex, presenilin-1 and nicastrin, were significantly reduced in citalopram-treated mice (P = 0.02 and P = 0.01; n = 9–10 per group). APH-1B, neprilysin, and LRP1 mRNA levels did not change following citalopram treatment. Values normalized to mean level in water-treated mice. Data presented as mean ± SEM.
To be sure SSRIs acutely reduce Aβ levels in young and aged mice to a similar extent, we administered vehicle or citalopram (10 mg/kg) to 12-mo-old PS1APP mice (this cohort of mice was naïve to SSRI treatment before the study). Citalopram reduced ISF Aβ40 levels by 25% compared with vehicle (Fig. S5), which was not statistically different from young PS1APP mice treated with the same dose of citalopram (Fig. 1A).
Chronic citalopram treatment significantly increased α-secretase cleavage activity; however, it had no effect on β-secretase activity (Fig. 3F). Similarly, these mice also had a trend for an increase in APP C-terminal fragment-α (CTF-α) levels (P = 0.014) but no change in CTF-β levels (Fig. S6). Citalopram did not, however, alter the mRNA of one putative α-secretase, ADAM10 (Fig. 3G), suggesting that a posttranslational event by ERK is likely responsible for altering α-secretase activity. Memapsin-2 (β-secretase) mRNA levels were significantly reduced in citalopram-treated mice; however, given that β-secretase activity and APP CTF-β levels were not significantly different, the relevance of the transcriptional change in this setting is unknown. Two components of the γ-secretase complex, presenilin-1 (PS1) and nicastrin, had significantly reduced mRNA levels in SSRI-treated mice, which is consistent with the depression in Aβ levels. Levels of mRNA for APH-1B, another component of γ-secretase, however, did not change. Transcripts for other proteins known to alter Aβ metabolism, neprilysin and low-density lipoprotein receptor-1 (LRP1), also did not change following citalopram treatment.
Effect of Antidepressant Drugs on Amyloid Plaque Load in Humans.
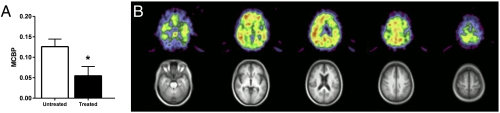
Given that SSRIs reduced Aβ production and plaque load in mouse models, we hypothesized that individuals with a history of antidepressant drug use may have reduced Aβ plaques. A total of 186 cognitively normal participants were recruited into a study for imaging Aβ plaques in late life by PET imaging (32, 33). Participants underwent PET Aβ imaging with Pittsburgh Compound B (PIB) and past use of antidepressant drugs was also ascertained (Table 1). PIB is a radioligand that binds to amyloid plaques and enables quantification of cortical plaque load in the living human brain using PET imaging (34) (SI Materials and Methods for more detail). The majority of antidepressant drugs used by participants were SSRIs, with a rank order of sertraline, fluoxetine, and citalopram (equal), escitalopram, and various other SSRI antidepressants (Table S1). Eight participants had used more than one antidepressant, three were uncertain about the exact antidepressant they had received (“not sure of the name”), and three had been on a combination of antidepressants. Participants who received any antidepressant medication within the past 5 y were considered as “treated” (mean exposure = 34.5 mo). These treated individuals had significantly lower mean cortical binding potential (MCBP) (0.06 ± 0.023) by PET PIB imaging compared with participants who reported no antidepressant medication use in the past 5 y (MCBP = 0.13 ± 0.019; Wilcoxon rank sum test P = 0.01) (Fig. 4A). Participants in the treated and untreated groups (Table 1) did not differ in age, sex, education, or ApoE4 allele status. A t test for the Spearman rank correlation found a significant negative correlation between duration of antidepressant treatment over the 5 y before PIB scan and MCBP (ρ = −0.298, P = 0.02). Fig. 4B shows a normalized Aβ PET subtraction image of PET scans of participants who were not treated with antidepressants within the past 5 y minus those of participants who were treated within the past 5 y, demonstrating that the increased Aβ found in the untreated participants occurred in the same brain regions previously identified in AD (32, 34).
Table 1.
Demographics for participants
| No treatment, n = 134 | Treatment, n = 52 | Statistics | P | |
| Female n (%) | 93 (69.4) | 35 (67.3) | 0.08* | 0.78 |
| ApoE4 positive n (%) | 40 (29.9) | 17 (32.7) | 0.14* | 0.71 |
| Age mean (SD) | 69.46 (8.2) | 69.69 (7.1) | −0.18† | 0.86 |
| Education mean (SD) | 15.59 (2.6) | 15.27 (2.3) | 0.79† | 0.43 |
| MMSE mean (SD) | 29.01 (1.2) | 28.57 (1.8) | 1.53† | 0.13 |
| MCBP mean (SD) | 0.13 (0.2) | 0.05 (0.2) | −2.44‡ | 0.02 |
MCBP, mean cortical binding potential; MMSE, mini mental status examination.
*χ2 test, df = 1.
†t test.
‡Wilcoxon rank-sum test.
Fig. 4.
Antidepressant use is associated with less cortical amyloid in human participants. (A) Mean cortical binding potential of PIB (MCBP) in participants who had taken antidepressants within the past 5 y “treated” versus those who had not been treated “untreated.” Mean exposure time for the treated group = 34.5 mo (SD 23.6). MCBP in untreated participants was 0.13 ± 0.22 (n = 134; mean ± SD) versus 0.06 ± 0.20 (n = 43; P = 0.01). (B) Normalized Aβ PET subtraction image of untreated participants, minus treated participants, showing the pattern of cortical rim Aβ plaque accumulation typical of AD.
Discussion
Serotonin signaling acutely reduced brain Aβ levels and chronically reduced Aβ plaques in a mouse model of AD. The dose of each SSRI administered in mice was within the range of what would be prescribed to human patients to treat depression (taking into account species differences in metabolism) (23). These findings were supported by the observation that cerebral amyloid levels, as depicted by PIB, showed an inverse relationship with chronic use of SSRIs in cognitively normal humans. These findings are preliminary and unreplicated. Should future studies support these data, a randomized controlled clinical trial will be needed to determine whether there is a beneficial effect of SSRIs on cerebral amyloid burden in humans.
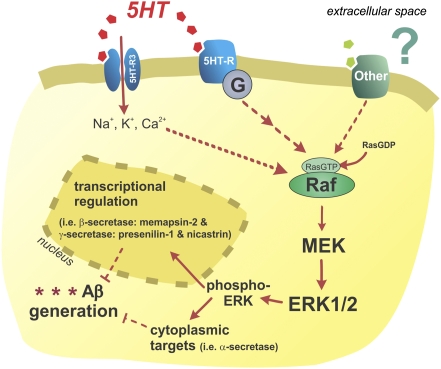
Model of Serotonergic Regulation of Aβ Metabolism.
Synaptic activity is known to regulate Aβ levels through a presynaptic mechanism that is dependent on APP endocytosis and cleavage (4, 5) and through neurotransmitter-activated second messenger signaling pathways that alter APP processing into Aβ (7, 11, 12, 31). Activation of serotonin receptors on the cell surface initiates the ERK signaling cascade, which is required for changes in Aβ generation within this mechanism (Fig. 5). There are seven families of 5HT-Rs with at least 15 unique subtypes. A subset of these receptors have been shown to activate ERK (for review, ref. 35); however, to date it is unknown which particular receptors underlie the change in Aβ metabolism. Once activated, pERK can act within the cytoplasm to phosphorylate proteins and alter their function or translocate to the nucleus to alter transcription of a wide range of genes. We demonstrated that both acute and chronic citalopram treatment increased α-secretase activity, likely through a posttranslational modification, which was responsible for the depression in Aβ levels demonstrated here. Although β-secretase mRNA levels were reduced after chronic citalopram treatment, neither enzymatic activity nor CTF-β levels were different, suggesting this was a transcriptional change that did not manifest past translation. Levels of several mRNAs relating to the γ-secretase complex were slightly, but significantly, depressed by citalopram treatment, suggesting that lower γ-secretase activity could have contributed to a reduction in Aβ levels and plaques. Analysis of APP-CTFs, however, suggests that changes to γ-secretase activity, at most, may play a small role in the reduction of Aβ.
Fig. 5.
Model of serotonergic regulation of Aβ metabolism. Serotonin receptors are activated on the cell surface, which initiates a signaling cascade that leads to ERK phosphorylation and activation. ERK appears to increase α-secretase cleavage and may reduce γ-secretase cleavage of APP. The particular serotonin receptor subtypes responsible for ERK activation in this paradigm are unknown. Not all receptors or ligands that activate ERK will alter APP processing, however similar to serotonin, NMDA receptors can also modulate APP processing through the ERK pathway.
Selectivity of ERK-Dependent Regulation of Aβ Generation.
ERK activation and activity are promiscuous; however, both are highly regulated and can have very precise biological responses, in large part due to cellular context, scaffold proteins, and compartmentalization where it is activated (for review, ref. 26). ERK activation has been shown to modulate APP processing and Aβ generation (24, 25, 36). M1 muscarinic acetylcholine receptors activate the ERK signaling pathway (37), which is at least in part responsible for their regulation of APP processing (38). Similar to our current data with serotonin, M1-mediated down-regulation of Aβ acts by increasing α-secretase cleavage of APP (8, 9). Only certain receptors that activate ERK appear to alter APP processing; serotonin and NMDA receptors regulate Aβ metabolism in an ERK-dependent fashion; however, in the current study norepinephrine and TrkB receptors did not influence Aβ. The particular cellular cues, cofactors, and scaffold proteins that underlie this downstream substrate specificity are still unknown.
Depression as a Risk Factor for AD and the Influence of Antidepressants.
The relationship between major depressive disorder (MDD) and AD is controversial, with several studies reporting contradictory findings. The most consistent observation is that depression itself increases risk of AD (39–41). Interpretation of many MDD/AD risk studies, however, are complicated in that incipient or early AD can present clinically as apparent late-life depression in some cases (42). Recently, several important prospective studies have demonstrated that depression is a predisposing factor for the development of incident dementia (43–45). MDD is also accompanied by an increased risk of comorbid conditions that independently increase risk for AD, such as vascular disease, diabetes, lower cognitive reserve, and stress (39, 46). Therefore, antidepressant use could potentially reduce amyloid load, but not necessarily translate into an observed decrease in risk for AD, particularly in depressed subjects.
Several studies have found that SSRIs reduce risk of AD in depressed individuals. A recent retrospective study of over 1.4 million people in Denmark over a 10-y period suggested that depressed individuals who were treated only once, or for only a short period, had a greater risk of AD than those who had chronic antidepressant use (47); chronic antidepressant use lowered the risk toward that of the general population. Given that AD pathology develops over many years, we postulate that a short-term Aβ-lowering effect from SSRIs may not be sufficient to influence AD risk but that chronic administration of a SSRI may reduce Aβ plaque burden. Consistent with this, in our retrospective study there was an inverse correlation between the amount of time on antidepressants and the degree of amyloid burden as detected by PIB imaging (P = 0.02).
Antidepressants Correlate with Reduced Amyloid Burden in Cognitively Normal Participants.
One strength of the current study is that participants with a history of antidepressant use did not differ in age, sex, and ApoE status from participants with no antidepressant exposure. Increasing age and ApoE genetic background are the strongest known risk factors for AD and plaque load (33, 48); thus it was critical that these parameters were controlled. A limitation of the current study is that, because participants were excluded if their clinical dementia rating (CDR) was greater than zero, antidepressant-treated participants with potential dementia or mild cognitive impairment would have been excluded, producing a selection bias. Another limitation of this retrospective human study is that the participants with a history of antidepressant exposure also had histories of depression or depressive symptoms sufficient to warrant the prescription of antidepressant medication. It was not possible to use retrospective data to separate out the effects of antidepressant exposure from depression. However, given the mouse data demonstrating a direct effect of SSRIs on Aβ levels and on reducing Aβ plaque load, and the negative correlation between length of time on antidepressants and amyloid PIB binding in our human participants, it would appear possible that antidepressants may influence the development of Aβ plaques in humans.
Targeting Serotonin Signaling as a Treatment for AD.
Aβ accumulation and deposition in the AD brain can begin 10 y before the appearance of the first symptoms (2, 32). The concept of “preclinical AD” indicates that AD pathologies are present but AD symptoms are not (49, 50). Several anti-Aβ therapeutic strategies are being pursued to treat AD; however, it is likely that treatment will need to begin during the preclinical phase to prevent or limit plaque accumulation to be beneficial in reducing the risk of developing AD (51, 52). Consequently, if anti-Aβ therapies may be used for years or decades, then very safe compounds will likely be necessary. SSRIs are one of the safest neuroactive classes of compounds approved by the Food and Drug Administration. Whereas SSRIs do have side effects, they are generally well tolerated, even with chronic use. In our current mouse studies, acute treatment with several SSRIs (fluoxetine, desvenlafaxine, and citalopram) reduced ISF Aβ levels by 25% (Fig. 1A) and chronic administration of citalopram reduced brain and CSF Aβ40 and Aβ42 levels by 30–50% (Fig. 3). In in vivo model systems, similar magnitudes of Aβ reductions are sufficient to prevent or halt Aβ plaque accumulation (53, 54).
Our data suggest a potential causal mechanism for antidepressant reduction of Aβ in mice and an association with decreased plaque burden in humans. A direct causal relation between antidepressants and plaque reduction in humans remains to be demonstrated, however.
Materials and Methods
All participants (n = 186) were assessed with the clinical dementia rating (CDR) scale (55), with all human participants scoring CDR = 0 (cognitively normal). All participants were assessed for ApoE genotype, were screened to rule out severe or unstable medical disorders or known primary neurological disorders, received a PIB PET scan (29) (see SI Materials and Methods for more details), and received a structured interview to assess lifetime use of antidepressants (56). This structured interview, adapted from the National Institute for Mental Health (NIMH) Life Chart method (57), incorporated retrospective assessment of cumulative antidepressant use, which we previously demonstrated had an intraclass correlation coefficient of 0.92, when interviews were separated by a mean interval of 3 y (56). All assessment and imaging procedures were approved by Washington University's Human Research Protection Office. Written consent was obtained from each participant.
For statistical analyses, microdialysis, tissue extraction, ELISAs, quantitative PCR, other biochemical assays, and PIB imaging, see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Tony Durbin, Tami Curl, Halley Hindman, and Becky Fierberg for assistance in subject recruitment and Jon Christensen for assistance in PET data processing. This work was supported by National Institutes of Health K01 AG029524 (to J.R.C.), R21 MH77124 and K24 MHO79510 (to Y.I.S.), National Institute on Aging P50 AG05681 (to J.C.M., Y.I.S., and J.R.C.), National Institute of Neurological Disorders and Stroke P30 NS069329 (to J.K.) and P01 AG03991 (to J.C.M. and M.A.M.), and the Charles F. and Joanne Knight Aging and Disability Resource Center at Washington University (J.R.C. and J.C.M.), the McDonnell Center for Cell and Molecular Biology (J.R.C.), and the Shmerler family (J.R.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107411108/-/DCSupplemental.
References
- 1.St George-Hyslop PH, Morris JC. Will anti-amyloid therapies work for Alzheimer's disease? Lancet. 2008;372:180–182. doi: 10.1016/S0140-6736(08)61047-8. [DOI] [PubMed] [Google Scholar]
- 2.Morris JC, Price AL. Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer's disease. J Mol Neurosci. 2001;17:101–118. doi: 10.1385/jmn:17:2:101. [DOI] [PubMed] [Google Scholar]
- 3.Lomakin A, Teplow DB, Kirschner DA, Benedek GB. Kinetic theory of fibrillogenesis of amyloid beta-protein. Proc Natl Acad Sci USA. 1997;94:7942–7947. doi: 10.1073/pnas.94.15.7942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cirrito JR, et al. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cirrito JR, et al. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 6.Kamenetz F, et al. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 7.Hoey SE, Williams RJ, Perkinton MS. Synaptic NMDA receptor activation stimulates alpha-secretase amyloid precursor protein processing and inhibits amyloid-beta production. J Neurosci. 2009;29:4442–4460. doi: 10.1523/JNEUROSCI.6017-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckols K, et al. The muscarinic M1 agonist xanomeline increases soluble amyloid precursor protein release from Chinese hamster ovary-m1 cells. Life Sci. 1995;57:1183–1190. doi: 10.1016/0024-3205(95)02064-p. [DOI] [PubMed] [Google Scholar]
- 9.Nitsch RM, Slack BE, Wurtman RJ, Growdon JH. Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science. 1992;258:304–307. doi: 10.1126/science.1411529. [DOI] [PubMed] [Google Scholar]
- 10.Fisher A. M1 muscarinic agonists target major hallmarks of Alzheimer's disease—an update. Curr Alzheimer Res. 2007;4:577–580. doi: 10.2174/156720507783018163. [DOI] [PubMed] [Google Scholar]
- 11.Caccamo A, et al. M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron. 2006;49:671–682. doi: 10.1016/j.neuron.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 12.Davis AA, Fritz JJ, Wess J, Lah JJ, Levey AI. Deletion of M1 muscarinic acetylcholine receptors increases amyloid pathology in vitro and in vivo. J Neurosci. 2010;30:4190–4196. doi: 10.1523/JNEUROSCI.6393-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nitsch RM, Deng M, Tennis M, Schoenfeld D, Growdon JH. The selective muscarinic M1 agonist AF102B decreases levels of total Abeta in cerebrospinal fluid of patients with Alzheimer's disease. Ann Neurol. 2000;48:913–918. [PubMed] [Google Scholar]
- 14.Nitsch RM, Deng M, Growdon JH, Wurtman RJ. Serotonin 5-HT2a and 5-HT2c receptors stimulate amyloid precursor protein ectodomain secretion. J Biol Chem. 1996;271:4188–4194. doi: 10.1074/jbc.271.8.4188. [DOI] [PubMed] [Google Scholar]
- 15.Arjona AA, Pooler AM, Lee RK, Wurtman RJ. Effect of a 5-HT(2C) serotonin agonist, dexnorfenfluramine, on amyloid precursor protein metabolism in guinea pigs. Brain Res. 2002;951:135–140. doi: 10.1016/s0006-8993(02)03153-0. [DOI] [PubMed] [Google Scholar]
- 16.Shen F, et al. 5-HT(4) receptor agonist mediated enhancement of cognitive function in vivo and amyloid precursor protein processing in vitro: A pharmacodynamic and pharmacokinetic assessment. Neuropharmacology. 2011;61:69–79. doi: 10.1016/j.neuropharm.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 17.Tucker S, et al. RNA therapeutics directed to the non coding regions of APP mRNA, in vivo anti-amyloid efficacy of paroxetine, erythromycin, and N-acetyl cysteine. Curr Alzheimer Res. 2006;3:221–227. doi: 10.2174/156720506777632835. [DOI] [PubMed] [Google Scholar]
- 18.Nelson RL, et al. Prophylactic treatment with paroxetine ameliorates behavioral deficits and retards the development of amyloid and tau pathologies in 3xTgAD mice. Exp Neurol. 2007;205:166–176. doi: 10.1016/j.expneurol.2007.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pákáski M, et al. Imipramine and citalopram facilitate amyloid precursor protein secretion in vitro. Neurochem Int. 2005;47:190–195. doi: 10.1016/j.neuint.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Meyer-Luehmann M, et al. Extracellular amyloid formation and associated pathology in neural grafts. Nat Neurosci. 2003;6:370–377. doi: 10.1038/nn1022. [DOI] [PubMed] [Google Scholar]
- 21.Cirrito JR, et al. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. J Neurosci. 2003;23:8844–8853. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savonenko A, et al. Episodic-like memory deficits in the APPswe/PS1dE9 mouse model of Alzheimer's disease: Relationships to beta-amyloid deposition and neurotransmitter abnormalities. Neurobiol Dis. 2005;18:602–617. doi: 10.1016/j.nbd.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 23.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 24.Kim SK, et al. ERK1/2 is an endogenous negative regulator of the gamma-secretase activity. FASEB J. 2006;20:157–159. doi: 10.1096/fj.05-4055fje. [DOI] [PubMed] [Google Scholar]
- 25.Kojro E, et al. The neuropeptide PACAP promotes the alpha-secretase pathway for processing the Alzheimer amyloid precursor protein. FASEB J. 2006;20:512–514. doi: 10.1096/fj.05-4812fje. [DOI] [PubMed] [Google Scholar]
- 26.Pouysségur J, Volmat V, Lenormand P. Fidelity and spatio-temporal control in MAP kinase (ERKs) signalling. Biochem Pharmacol. 2002;64:755–763. doi: 10.1016/s0006-2952(02)01135-8. [DOI] [PubMed] [Google Scholar]
- 27.Hetman M, Kanning K, Cavanaugh JE, Xia Z. Neuroprotection by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. J Biol Chem. 1999;274:22569–22580. doi: 10.1074/jbc.274.32.22569. [DOI] [PubMed] [Google Scholar]
- 28.Basta-Kaim A, et al. Effects of neurosteroids on glucocorticoid receptor-mediated gene transcription in LMCAT cells—A possible interaction with psychotropic drugs. Eur Neuropsychopharmacol. 2007;17:37–45. doi: 10.1016/j.euroneuro.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Krapivinsky G, et al. The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron. 2003;40:775–784. doi: 10.1016/s0896-6273(03)00645-7. [DOI] [PubMed] [Google Scholar]
- 30.Khan AM, et al. Catecholaminergic control of mitogen-activated protein kinase signaling in paraventricular neuroendocrine neurons in vivo and in vitro: a proposed role during glycemic challenges. J Neurosci. 2007;27(27):7344–7360. doi: 10.1523/JNEUROSCI.0873-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verges DK, Restivo JL, Goebel WD, Holtzman DM, Cirrito JR. Opposing synaptic regulation of amyloid-β metabolism by NMDA receptors in vivo. J Neurosci. 2011;31:11328–11337. doi: 10.1523/JNEUROSCI.0607-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mintun MA, et al. [11C]PIB in a nondemented population: Potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 33.Morris JC, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67:122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klunk WE, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 35.Cowen DS. Serotonin and neuronal growth factors: A convergence of signaling pathways. J Neurochem. 2007;101:1161–1171. doi: 10.1111/j.1471-4159.2006.04420.x. [DOI] [PubMed] [Google Scholar]
- 36.Mills J, et al. Regulation of amyloid precursor protein catabolism involves the mitogen-activated protein kinase signal transduction pathway. J Neurosci. 1997;17(24):9415–9422. doi: 10.1523/JNEUROSCI.17-24-09415.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berkeley JL, et al. M1 muscarinic acetylcholine receptors activate extracellular signal-regulated kinase in CA1 pyramidal neurons in mouse hippocampal slices. Mol Cell Neurosci. 2001;18:512–524. doi: 10.1006/mcne.2001.1042. [DOI] [PubMed] [Google Scholar]
- 38.Haring R, et al. Mitogen-activated protein kinase-dependent and protein kinase C-dependent pathways link the m1 muscarinic receptor to beta-amyloid precursor protein secretion. J Neurochem. 1998;71:2094–2103. doi: 10.1046/j.1471-4159.1998.71052094.x. [DOI] [PubMed] [Google Scholar]
- 39.Geerlings MI, den Heijer T, Koudstaal PJ, Hofman A, Breteler MM. History of depression, depressive symptoms, and medial temporal lobe atrophy and the risk of Alzheimer disease. Neurology. 2008;70:1258–1264. doi: 10.1212/01.wnl.0000308937.30473.d1. [DOI] [PubMed] [Google Scholar]
- 40.Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: Systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63:530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Green RC, et al. Depression as a risk factor for Alzheimer disease: The MIRAGE Study. Arch Neurol. 2003;60:753–759. doi: 10.1001/archneur.60.5.753. [DOI] [PubMed] [Google Scholar]
- 42.Lopez OL, et al. Psychiatric symptoms vary with the severity of dementia in probable Alzheimer's disease. J Neuropsychiatry Clin Neurosci. 2003;15:346–353. doi: 10.1176/jnp.15.3.346. [DOI] [PubMed] [Google Scholar]
- 43.Geda YE. Blowing hot and cold over depression and cognitive impairment. Neurology. 2010;75:12–14. doi: 10.1212/WNL.0b013e3181e8cc2f. [DOI] [PubMed] [Google Scholar]
- 44.Dotson VM, Beydoun MA, Zonderman AB. Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology. 2010;75:27–34. doi: 10.1212/WNL.0b013e3181e62124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saczynski JS, et al. Depressive symptoms and risk of dementia: the Framingham Heart Study. Neurology. 2010;75:35–41. doi: 10.1212/WNL.0b013e3181e62138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. 2005;4:141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 47.Kessing LV, Søndergård L, Forman JL, Andersen PK. Antidepressants and dementia. J Affect Disord. 2009;117:24–29. doi: 10.1016/j.jad.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 48.Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 49.Morris JC, et al. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol. 2009;66:1469–1475. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skoog I, et al. Cerebrospinal fluid beta-amyloid 42 is reduced before the onset of sporadic dementia: A population-based study in 85-year-olds. Dement Geriatr Cogn Disord. 2003;15:169–176. doi: 10.1159/000068478. [DOI] [PubMed] [Google Scholar]
- 51.Holmes C, et al. Long-term effects of Abeta42 immunisation in Alzheimer's disease: Follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 52.Sperling RA, et al. Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging and the Alzheimer's Association workgroup. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McConlogue L, et al. Partial reduction of BACE1 has dramatic effects on Alzheimer plaque and synaptic pathology in APP Transgenic Mice. J Biol Chem. 2007;282:26326–26334. doi: 10.1074/jbc.M611687200. [DOI] [PubMed] [Google Scholar]
- 54.Yan P, et al. Characterizing the appearance and growth of amyloid plaques in APP/PS1 mice. J Neurosci. 2009;29:10706–10714. doi: 10.1523/JNEUROSCI.2637-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 56.Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- 57.Post RM, Roy-Byrne PP, Uhde TW. Graphic representation of the life course of illness in patients with affective disorder. Am J Psychiatry. 1988;145:844–848. doi: 10.1176/ajp.145.7.844. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.