Abstract
We examined the neural basis of self-regulation in individuals from a cohort of preschoolers who performed the delay-of-gratification task 4 decades ago. Nearly 60 individuals, now in their mid-forties, were tested on “hot” and “cool” versions of a go/nogo task to assess whether delay of gratification in childhood predicts impulse control abilities and sensitivity to alluring cues (happy faces). Individuals who were less able to delay gratification in preschool and consistently showed low self-control abilities in their twenties and thirties performed more poorly than did high delayers when having to suppress a response to a happy face but not to a neutral or fearful face. This finding suggests that sensitivity to environmental hot cues plays a significant role in individuals’ ability to suppress actions toward such stimuli. A subset of these participants (n = 26) underwent functional imaging for the first time to test for biased recruitment of frontostriatal circuitry when required to suppress responses to alluring cues. Whereas the prefrontal cortex differentiated between nogo and go trials to a greater extent in high delayers, the ventral striatum showed exaggerated recruitment in low delayers. Thus, resistance to temptation as measured originally by the delay-of-gratification task is a relatively stable individual difference that predicts reliable biases in frontostriatal circuitries that integrate motivational and control processes.
Keywords: reward, behavioral suppression, functional MRI, inferior frontal gyrus, longitudinal
The ability to resist temptation in favor of long-term goals is an essential component of individual, societal, and economical success. Developmentally, this ability has been assessed by measuring how long a young child can resist an immediate reward (e.g., a cookie) in favor of a larger, later reward (e.g., two cookies) (1). Even as adults we vary in our ability to resist temptations. Alluring situations can diminish our control (2–4); what serves as an alluring situation that requires a capacity to control our impulses, however, changes as a function of age (e.g., from cookies to social acceptance). In the present study we examined the extent to which individual differences in delay of gratification assessed when participants were in preschool and in their 20s and 30s predict control over impulses and sensitivity to social cues at the behavioral and neural level when the participants were in their 40s.
Delay of gratification depends importantly on cognitive control (5). Cognitive control refers to the ability to suppress competing inappropriate thoughts or actions in favor of appropriate ones (6–11). Previously, we have shown that performance on the delay-of-gratification task in childhood predicts the efficiency with which the same individuals perform a cognitive control task (the go/nogo task) as adolescents and young adults (5). Individuals who as preschoolers directed their attention toward rewarding aspects of the classic delay-of-gratification situation, such as focusing on the cookies (high-temptation-focus group), had more difficulty suppressing inappropriate actions than did their low-temptation-focus counterparts, especially for the most difficult trials. Difficulty was manipulated by increasing the number of “go” trials preceding a “nogo” trial, thus making the “go” response more salient and automated. Differences between the high- and low-temptation-focus groups increased as the number of preceding “go” trials increased, with the high-temptation-focus group having more difficulty, reflected in slower response times, suppressing responses. These findings suggest that performance in preschool delay of gratification may predict the capacity, in adulthood, to control thoughts and actions, as reflected in performance on cognitive control tasks, and that the ability to control one's thoughts and actions can vary by the potency of interfering information (12). Likewise, alluring or social contexts can diminish self-control (4, 13, 14).
Early experiments on delay of gratification demonstrated that part of the contextual effect was due to the different cognitive strategies that individuals used. For example, “cooling” the hot, appealing, or appetitive features of tempting stimuli by reappraisal or reframing strategies to focus on their cool, cognitive features (e.g., to envision the marshmallow as a cloud or a little cotton ball, rather than as a sweet, delectable treat) has been shown to be highly effective in enhancing delay of gratification (e.g., 1, 15–17). The same preschool child who yielded immediately to the temptation by representing the hot, appetitive features of the reward (e.g., its yummy, sweet, chewy taste) could wait for long periods for the same tempting stimulus by focusing on its cool qualities (e.g., its shape). At the same time, there seem to be important, naturally existing individual differences in the spontaneous use of such strategies (e.g., 5, 18).
Indeed, Metcalfe and Mischel (2) proposed “cool” and “hot” systems to explain the dynamics of resisting temptation during the delay-of-gratification task. These two interacting neurocognitive systems are implicated in self-control. Whereas the first, a “cool” system, involves cognitive control-related neural circuitry, the second, a “hot” system (19), involves desires and emotions that are under stimulus control and are associated with emotional brain regions. Recent brain imaging studies have provided evidence for dissociable brain systems related to immediate over long-term choice behavior consistent with the notion of interacting “hot” and “cool” systems. Specifically, whereas top-down prefrontal regions have been shown to be involved in cognitive control during delay of rewards, limbic or emotional brain regions have been shown to be associated with more immediate choices (20–24). Complementary imaging studies have shown that a region of the prefrontal cortex, the inferior frontal gyrus, is critically involved in resolving interference among competing actions [e.g., to go or not to go (10, 25)] and among competing representations in memory (e.g., 11). In each case, prepotent information interferes with other goal-specific information, thus requiring cognitive control processes to resolve the interference.
In the present longitudinal study, we manipulated the alluring qualities of targets in an impulse control task to examine behavioral and neural correlates of delay of gratification using functional magnetic resonance imaging (fMRI). Participants were individuals whose ability to delay gratification was tested as 4-y-olds on the original delay-of-gratification task and whose self-control abilities remained consistent in follow-up assessments. Two experiments were conducted to investigate the ability of these individuals, now in their 40s, to refrain from responding to alluring cues. We developed two tasks to examine impulse control—one in the presence of neutral (“cool”) stimuli and one containing compelling (“hot”) stimuli. Because marshmallows and cookies are unlikely to be as rewarding to individuals now as adults as they were when they were young children, we used the social cues of faces with emotional expressions (happy faces relative to neutral and fearful faces), shown to bias behavior similarly to secondary reinforcers (4, 13, 26). Experiment 1 tested whether individuals who were less able to delay gratification as children and young adults (low delayers) would, as adults in their 40s, show less impulse control in suppression of a response to “hot” relative to “cool” cues.
In experiment 2, we used fMRI to examine neural correlates of delay of gratification. We hypothesized that participants with consistently low levels of self-control from young childhood to early adulthood (low delayers), compared with their consistently high-control counterparts, would be characterized by diminished activity in the right prefrontal cortex, implicated in response inhibition (13, 27–29), and by amplified activity in the ventral striatum, implicated in processing of positive or rewarding cues (13, 26, 30).
Result and Discussion
Experiment 1 Results.
In experiment 1, 59 participants classified as low or high delayers (Table 1) completed a behavioral version of the “hot” and “cool” impulse control tasks. Reaction times for trials that required a response (“go” trials) and accuracy for “go” and “nogo” trials were compared by delay group and task.
Table 1.
Subject demographics
| Group | n | Female (n) | Age (y) | Age 4 y delay score | Adult self-report | Raven's score* |
| Experiment 1 sample | ||||||
| High delaying | 32 | 20 | 44.6 ± 2.1 | 332.3 ± 147.0 | 7.61 ± 0.54 | |
| Low delaying | 27 | 16 | 44.3 ± 1.6 | −284.2 ± 145.2 | 5.91 ± 1.04 | |
| Experiment 2 sample | ||||||
| High delaying | 15 | 10 | 44.8 ± 1.8 | 304.2 ± 145.2 | 7.39 ± 0.45 | 25.5 ± 5.0 |
| Low delaying | 11 | 4 | 44.2 ± 1.8 | −222.8 ± 145.3 | 6.18 ± 0.58 | 22.9 ± 4.6 |
*Raven's data were collected on the imaging subjects only.
Reaction times.
There were no effects of delay group on reaction time measures to correct “go” trials [main effect of group, F(1,57) = 2.23, P > 0.1; group × task interaction, F(1,57) = 0.002, P > 0.9].
Accuracy.
Participants performed with a high level of accuracy for correctly responding to “go” trials during both the “cool” (99.8% correct) and “hot” tasks (99.5% correct). Low and high delayers performed with comparable accuracy on “go” trials [neither the main effect of group, F(1,57) = 1.08, P > 0.3, nor the interaction of group and task, F(1,57) = 0.05, P > 0.8, was significant].
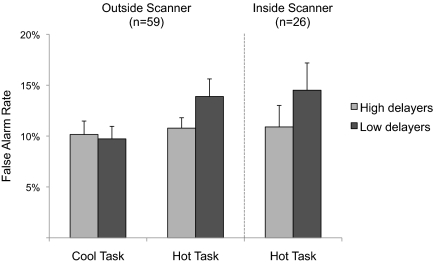
Accuracy for “nogo” trials was more variable [mean false alarm rate for “cool” task, 9.96%; for “hot” task, 12.2%; main effect of task, F(1,57) = 7.89, P = 0.007, η2partial = 0.122] and yielded a significant interaction of group and task [F(1,57) = 4.312, P = 0.042, η2partial = 0.070; Fig. 1]. Whereas low and high delayers performed comparably on the “cool” task [t(57) = −0.24, P > 0.8], the low delayers trended toward performing more poorly on the “hot” task than did the high delayers [t(57) = 1.64, P = 0.11, d = 0.43]. Further, only the low delay group showed a significant decrement in performance for the “hot” trials relative to the “cool” trials [t(26) = 3.09, P = 0.005, d = 0.89; high delay group P > 0.5]. Additional planned analyses separated “hot” task “nogo” trials into fear and happy subcategories. The decrement in performance for low delayers was driven largely by commission errors on the happy “nogo” trials [low delayers, 15.7% errors; high delayers, 11.2% errors; t(57) = 2.06, P = 0.044, d = 0.55], whereas low and high delaying groups performed at equivalent levels of accuracy for the fear “nogo” trials [12.0% and 10.4%, respectively, t(57) = 0.804, P > 0.4].
Fig. 1.
Left: Experiment 1 (outside the scanner). High and low delayers do not differ in performance on a go/nogo task when cues are “cool” stimuli (neutral facial expressions), but low delayers make more errors when the cues are “hot” (emotional faces). Right: Experiment 2 (inside the scanner). A similar pattern is observed. Error bars denote SEM.
Experiment 1 Discussion.
In this experiment the go/nogo task produced differences between the two delay groups only in the presence of emotional (“hot”) cues. Specifically, individuals who, as a group, had more difficulty delaying gratification at 4 y of age showed more difficulty as adults in suppressing responses to happy faces. The findings are consistent with previous work suggesting that the capacity to resist temptation varies by context; the more tempting the choice for the individual, the more predictive are the individual differences in people's ability to regulate their behavior (e.g., 12). Thus, behavioral correlates of delay ability are a function not only of cognitive control but also of the compelling nature of the stimuli that must be suppressed. Because behavioral differences between the low and high delayers only emerged on the “hot” task, we scanned participants during this task in experiment 2.
Experiment 2 Results.
Reaction times.
As with the behavioral findings outside of the scanner, the two delay groups did not differ significantly in reaction times to correct “go” trials [t(24) = 0.81, P > 0.4].
Accuracy.
Overall, accuracy rates for the “hot” go/nogo task in the scanner were uniformly high for “go” trials (mean 98.2% correct hits), with more variable performance to “nogo” trials (12.4% false alarm rate). Differences between the two delay groups in “nogo” accuracy were consistent with the observed differences in the ”hot” task performance in experiment 1, with low-delay participants committing more false alarms than high-delay participants (low delayers, 14.5%; high delayers, 10.9%; Fig. 1, Right). This performance difference, however, did not reach statistical significance [t(24) = 1.08, P = 0.29, d = 0.44], likely owing to fewer data points given the smaller sample and fewer trials in the imaging version of the task.
Imaging results.
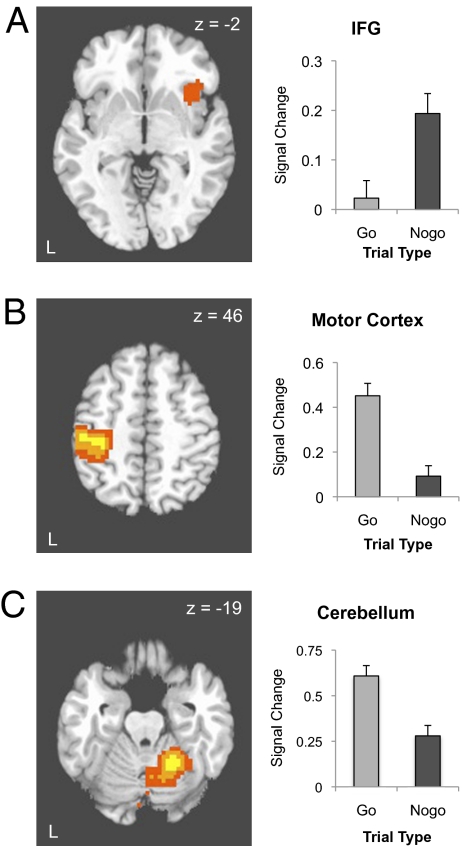
The 2 (delay group: high, low) × 2 (trial type: nogo, go) × 2 (emotion: happy, fear) voxelwise ANOVA was conducted to identify brain regions differentially active by task conditions and delay group. The comparison of “nogo” vs. “go” trials identified candidate regions differentially engaged as a function of cognitive control demands, including the right inferior frontal gyrus (x = 35, y = 11, z = −4), showing significantly greater responses to “nogo” relative to “go” trials (P < 0.05, whole-brain corrected; Fig. 2A and Table 2). As expected, both left primary motor cortex (x = −44, y = −23, z = 54) and ipsilateral cerebellum (x = 23, y = −47, z = −19) were more active for “go” trials, which required a response, vs. “nogo” trials, which did not (Ps < 0.05, whole-brain corrected; Fig. 2 B and C). No regions reached significance for the main effect of or two-way interactions with delay group. Regions identified for the main effect of emotion included bilateral portions of the temporal lobes (Table 2).
Fig. 2.
Main effects of the go/nogo task. (A) The right inferior frontal cortex was associated with correct inhibition of a response (nogo) relative to making a correct response (go). Left: Activation map depicting right inferior frontal gyrus activation, thresholded at P < 0.05, whole-brain corrected, displayed on a representative high-resolution T1-weighted axial image. Right: Percentage change in MR signal for “go” and “nogo” trials in the inferior frontal gyrus. (B) Left primary motor cortex was associated with making a correct response (go) relative to correctly withholding a response (nogo). Left: Activation map depicting activation in left precental gyrus, thresholded at P < 0.05, whole-brain corrected, displayed on a representative high-resolution T1-weighted axial image. Right: Percentage change in MR signal for “go” and “nogo” trials in the left precentral gyrus. (C) Left cerebellum was associated with making a correct response (go) relative to correctly withholding a response. Left: Activation map depicting left cerebellum activation, thresholded at P < 0.05, whole-brain corrected, displayed on a representative high-resolution T1-weighted axial image. Right: Percentage change in MR signal for “go” and “nogo” trials in the right cerebellum.
Table 2.
Brain activations observed for main effects of trial type and emotion
| Region | x | y | Z | F |
| Trial type (nogo > go) | ||||
| Inferior parietal cortex | 53 | −56 | 42 | 25.36 |
| Inferior frontal gyrus | 35 | 11 | −4 | 24.92 |
| Trial type (go > nogo) | ||||
| Cerebellum | 23 | −47 | −19 | 111.48 |
| Postcentral gyrus | −44 | −23 | 54 | 97.93 |
| Thalamus | −2 | −14 | 15 | 33.23 |
| Putamen | −26 | −8 | 3 | 26.23 |
| Cerebellum | −50 | −59 | −22 | 20.55 |
| Emotion (fear > happy) | ||||
| Middle temporal gyrus | 56 | −56 | 3 | 19.0 |
| Middle temporal gyrus | −44 | −44 | 6 | 19.0 |
Threshold: P < 0.05, whole-brain corrected.
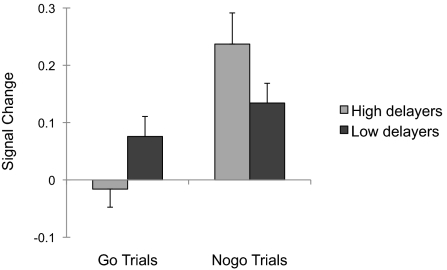
Although the interaction of delay group and response was not significant in whole-brain analyses, we hypothesized that the inferior frontal gyrus would show subtle differences in recruitment to nogo trials as a function of delay group (5). To test this prediction, parameter estimates from the inferior frontal gyrus region functionally defined by the main effect of “nogo” relative to “go” trials were extracted for each individual and evaluated for potential interactions by delay group in post hoc statistical analyses. This analysis yielded a significant interaction of trial type and delay group in inferior frontal gyrus recruitment [F(1,24) = 7.05, P = 0.014, η2partial = 0.23; Fig. 3]. Follow-up t tests indicated that high-delay participants showed greater polarization of inferior frontal gyrus response to “nogo” relative to “go” trials [t(14) = 4.33, P = 0.001, d = 2.6)], whereas differential inferior frontal gyrus recruitment was less pronounced in low delayers [t(10) = 1.93, P = 0.082, d = 0.82]. No other region for the main effect of “nogo” vs. “go” trials differentiated the delay groups (i.e., parietal cortex; Table 2).
Fig. 3.
Differential inferior frontal gyrus recruitment between nogo and go trials is more pronounced in high delayers than in low delayers. The right inferior gyrus (region shown in Fig. 2A) showed a significant interaction between group and trial type, with greater polarization of inferior frontal gyrus response to “nogo” relative to “go” trials for high delayers. Error bars denote SEM.
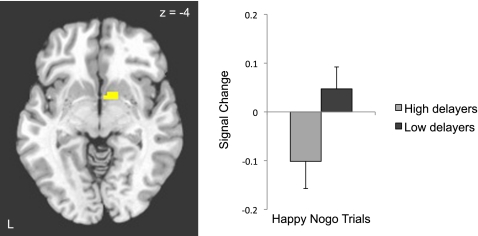
In the primary analysis of interest, we examined group differences in the ability to suppress a response to a positive cue, as tested by the three-way interaction of delay group (high, low), trial type (nogo, go), and emotion (happy, fear). This analysis revealed a cluster in the ventral striatum (x = 11, y = 2, z = −4; P < 0.05 small volume corrected; Fig. 4). Planned follow-up analyses of this interaction showed greater ventral striatal activity in low delayers relative to high delayers overall during task runs in which happy faces served as the “nogo” stimulus [t(24) = 2.20, P = 0.038, d = 0.90], with low delayers engaging the ventral striatum more than high delayers specifically for happy “nogo” trials [t(24) = 1.96, P = 0.06, d = 0.80; Fig. 4].
Fig. 4.
Low delay ability in early childhood predicts greater recruitment of ventral striatum when inhibiting responses to positive social cues 40 y later. Left: Activation map for the three-way interaction of task, emotion, and delay group depicting ventral striatum activity thresholded at P < 0.05, small volume corrected, displayed on a representative high-resolution T1-weighted axial image. Right: Ventral striatal response to happy “nogo” trials in high and low delayers.
Experiment 2 Discussion.
Resisting temptation, as measured by the “hot” go/nogo task, was supported by frontostriatal circuitry. Specifically, the right inferior frontal gyrus was involved in accurately withholding a response. Interrogation of this region showed that compared with high delayers, low delayers had diminished recruitment of this region for correct “nogo” relative to “go” trials. In addition, the ventral striatum demonstrated a significant difference in recruitment between high and low delay groups. This reward-related region showed a three-way interaction of group × trial type × emotion, with elevated activity to happy “nogo” trials for low delayers relative to high delayers. This finding highlights the importance of the qualities of the stimulus people have to resist, such as its salience or allure, in modulating cognitive control ability.
General Discussion.
The results from both experiments together suggest three key findings. First, resistance to temptation as measured originally by the delay-of-gratification task, and in the present study by a “hot” version of an impulse control task, is a relatively stable individual difference characteristic. Second, and consistent with delay experiments on the value of “cooling” the hot features of temptations (1), behavioral correlates of delay ability involve not cognitive control in general, but in particular in response to positive compelling cues. Third, resisting temptation is supported by ventral frontostriatal circuitry, with the inferior frontal gyrus showing lesser recruitment in low delayers and the ventral striatum showing exaggerated recruitment in low delayers when resisting alluring cues. These findings suggest that sensitivity to positive social cues influences an individual's ability to suppress thoughts and actions and thus can undermine self-regulation.
The relative stability in the capacity to resist temptation was shown over a 40-y span. Specifically, individuals who, as a group, had more difficulty delaying gratification at 4 y of age and continued to show reduced self-control abilities subsequently exhibited more difficulty as adults in suppressing responses to positive social cues. Previous research has documented that higher delay ability promotes the development of better social–cognitive and emotional coping in adolescence and buffers against the development of a variety of dispositional physical and mental health vulnerabilities in middle age, such as high BMI, cocaine/crack use, features of borderline personality disorder, anxious overreactions to rejection, and marital divorce/separation (17, 18, 31–33), even when controlling for childhood social environment and child health (34). Thus, our findings confirm the significance and predictive validity of delay ability in preschoolers for behaviors in later life. These findings complement prior work characterizing developmental features of self-regulation (35–37) by providing further evidence for their predictive validity in subsequent stages of life (38).
An important construct related to resisting temptation is that of impulse control (5). The go/nogo task is considered a reliable measure of impulsivity (25, 39). In the present study, the “cool” version of the go/nogo task did not differentiate the low- and high-delay groups. Only in the presence of positive social (“hot”) cues did the go/nogo task reveal differences between the two delay groups. These findings are consistent with our previous study showing associations between performance on the delay of gratification task and the go/nogo task, which were particularly apparent in certain contexts (5). In that study, the differences between groups were most pronounced on difficult trials, “nogo” trials preceded by several “go” trials, such that the “go” response became more compelling (28). Thus, the behavioral correlates of delay ability are a function not only of impulse control but also of the salience of the stimulus one has to resist.
Two neurocognitive systems that rely on distinct neural systems have been proposed to enable self-control (2). Whereas the “cool” system involves cortical control regions, including the prefrontal cortex, the “hot” system involves deep brain structures such as the ventral striatum that are implicated in the processing of desires and rewards. Resisting temptation, as measured by the “hot” go/nogo task in the present study, supports this view, with the prefrontal cortex and the ventral striatum differentiating low- and high-delay participants. The difference in inferior frontal gyrus recruitment for “nogo” relative to “go” trials was reduced in low delaying participants, potentially reflecting reduced ability in these individuals to invoke cognitive control in the context of emotional or “hot” cues. The ventral striatum has been implicated in reward and in immediate, as opposed to delayed, choice behavior (20, 21, 26, 40). Thus, sensitivity to environmental cues influences an individual's ability to suppress thoughts and actions, such that control systems may be “hijacked” by a primitive limbic system, rendering control systems unable to appropriately modulate behavior. Similar analogies of imbalances between these neural systems in the literature suggest that addiction (41) and adolescence (13, 42, 43) may be contexts when cognitive control may be particularly vulnerable to alluring environmental cues.
Resisting temptation in favor of long-term goals is important for individual, societal, and economic functioning. Our findings provide a neurobiological basis for differences in this ability. Together the behavioral and imaging findings provide evidence that the ability to delay gratification assessed early in life predicts how well individuals can regulate behavior years later, particularly when they are required to suppress thoughts and actions toward alluring social cues.
Materials and Methods
We contacted 117 individuals from more than 500 original participants who completed the delay-of-gratification task at age 4 y at Stanford's Bing Nursery School during the late 1960s and early 1970s and were above or below average in their original delay-of-gratification performance as well as in self-report measures of self-control administered in their 20s and 30s (44). More than half of these individuals agreed to participate in a longitudinal behavioral study (experiment 1), and of these 59 subjects, nearly half agreed to be part of a functional neuroimaging study (experiment 2). Both the behavioral and imaging experiments received institutional review board approval, and all participants provided consent before testing and scanning.
Experiment 1.
Participants.
Participants were selected on the basis of their scores on two types of self-control measures: (i) the length of time they waited for the more desirable but delayed reward at age 4 y, and (ii) their score(s) on a self-control scale in adulthood. The time spent delaying a reward at age 4 y (see ref. 1 for review) was recorded in seconds and normalized by calculating a difference score of the participant's delay time relative to the average delay time of children in the same experimental condition (see ref. 33 for details). The self-control scale used in the adulthood assessments consisted of eight items [e.g., “Unable to delay gratifications; cannot wait for satisfactions” (reverse coded); “Is attentive and able to concentrate”] from the modified shortened version of the California Child Q-set (45). This self-report measure was administered in 1993 (when participants were in their 20s) and in 2003 (when participants were in their 30s). Internal consistency of this scale was relatively high (α = 0.74 in 1993 and 0.82 in 2003).
Of the 562 participants for whom we had preschool delay scores at age 4 y, 155 completed the 1993 follow-up and 135 completed the 2003 follow-up. For the present study, we targeted individuals who obtained consistently high or consistently low scores on measures of self-control. The high-delay group was composed of individuals who scored above the mean in their preschool delay time as well as in their subsequent self-control assessments in adulthood (whichever was available, or the average of the two when both were available), whereas the low-delay group was composed of individuals who scored consistently below the mean on these measures. Sixty participants met the criterion for the high-delay group, and 57 participants met the criterion for the low-delay group; all of these individual were sent invitation letters. The final sample (n = 59) consists of those who accepted our invitation to participate in the present follow-up. As expected, high- and low-delay groups differed significantly in delay score [t(57) = 16.1, P < 0.001] and reported self-control as adults [t(57) = 8.06, P < 0.001]; they did not differ in age [t(50) = 0.69, P > 0.49] (age information was not obtained from four high delayers and three low delayers, but all participants are known to have been born between 1964 and 1970) or sex distribution [χ2(1) = 0.07, P > 0.8; Table 1].
Behavioral tasks.
Participants completed two versions of a go/nogo task (4, 46, 47). The “cool” version of the go/nogo task consisted of male and female stimuli with neutral expressions. Within a single run, both male and female stimuli were presented, one sex as a “go” (i.e., target) stimulus to which participants were instructed to press a button, and the other sex as a “nogo” (i.e., nontarget) stimulus to which participants were instructed to withhold a button press. Before the onset of each run, a screen appeared indicating which stimulus category served as the target. Participants were instructed to respond as quickly and accurately as possible. During the task, a face appeared for 500 ms, followed by a 1-s interstimulus interval. A total of 160 trials were presented per run in pseudorandomized order (120 go, 40 nogo). Thus, the task was a 2 (trial type: go, nogo) × 2 (stimulus sex: male, female) factorial design. Accuracy and response latency data were acquired in four runs representing each combination of stimulus sex (male, female) and trial type (go, nogo). The “hot” version of the go/nogo task was identical to the “cool” version, except that for this task fearful and happy facial expressions served as stimuli. Both expressions were used as targets (go) and nontargets (nogo) in a 2 (trial type: go, nogo) × 2 (emotion: fear, happy) factorial design.
Stimuli and apparatus.
Stimuli consisted of happy, fearful, and neutral faces of unique identities from the NimStim set of facial expressions (48). The tasks were presented using Inquisit software on laptop computers that were sent to participants’ homes.
Data analysis.
Behavioral data were analyzed for two accuracy measures on the go/nogo tasks: hit rate for “go” trials (correctly pressing) and correct suppression rate for “nogo” trials (correctly withholding press). Because delay score was nonnormally (bimodally) distributed (Table 1), delay score was treated as a categorical rather than a continuous variable. Analyses of response accuracy (correct “go,” correct “nogo”) were performed with 2 (task: “hot,” “cool”) × group (low delayer, high delayer) ANOVAs. Reaction times for correct “go” trials were trimmed for outlying responses within each participant (±3 SDs from the mean, resulting in a mean 1.23% data excluded). Mean reaction time data were analyzed at the group level with a 2 (task: “hot,” “cool”) × group (low delayer, high delayer) ANOVA. Behavioral effects relevant to “nogo” trials are presented graphically as false alarm rates, the mathematical inverse of correct suppression. For significant effects, effect sizes were calculated using the η2partial metric for ANOVA and Cohen's d for t test results (correcting for shared variance in the case of paired t tests).
Experiment 2.
Participants.
Of the 59 individuals who completed experiment 1, 27 agreed to complete the imaging study. One 41-y-old man, a low delayer on the original delay-of-gratification task, was excluded from all analyses because of poor performance (59% hit rate). The high- and low-delay groups differed significantly in delay score [t(24) = 9.1, P < 0.001] but did not differ in age [t(21) = 0.87, P > 0.390] (age information was not obtained from one high delayer and two low delayers, but all participants are known to have been born between 1964 and 1970), sex distribution [χ2(1) = 2.3, P > 0.1], or their scores on Raven's progressive matrices [t(22) = 1.26, P > 0.2].
Behavioral paradigm.
The “hot” version of the go/nogo task was similar to the task described in experiment 1. Differences were in the timing, number of trials, and apparatus. The timing of the task included the presentation of the face stimulus for 500 ms, followed by a jittered intertrial interval ranging from 2 to 14.5 s in duration (mean, 5.2 s), during which participants rested while viewing a fixation crosshair. A total of 48 trials were presented per run in pseudorandomized order (35 “go,” 13 “nogo”). In total, imaging data were acquired for 26 nogo trials and 70 go trials for each expression. The stimuli and instructions were identical to those used in experiment 1. E-Prime software was used to display the task (viewable by a rear projection screen) and to record button responses and reaction times on a Neuroscan five-button response pad.
Image acquisition.
Participants were scanned with a General Electric Signa 3.0-T fMRI scanner (General Electric Medical Systems) with an eight-channel head coil. A high-resolution, T1-weighted anatomical spoiled gradient sequence (256 × 256 in-plane resolution, 240-mm field of view, 136 × 1.2-mm axial slices) was used to acquire an anatomical scan for each subject for transformation and localization of data to Talairach grid space. A spiral in-and-out T2*-weighted sequence (49) was used to acquire four runs of functional data (repetition time 2,500 ms, echo time 30 ms, flip angle 90°, skip 0, 64 × 64 matrix) with 34 4-mm slices per volume.
Behavioral data analysis.
Behavioral differences between the low- and high-delay groups were analyzed on two accuracy measures on the go/nogo tasks: hit rate for “go” trials (correctly pressing) and correct suppression rate for “nogo” trials (correctly withholding press). Group behavioral analysis was conducted as described in experiment 1. Mean reaction times for correct “go” trials were calculated and analyzed as described in experiment 1 (mean 1.24% responses trimmed).
Imaging data processing.
The fMRI data analysis was performed using Analysis of Functional Neuroimages software (50). Functional datasets were corrected for slice acquisition time and realigned within and across runs to correct for head motion. All subjects moved less than 2.5 mm in all planes, with the exception of one participant who had 3.8% of their functional data excluded for motion during scan runs. Functional datasets were coregistered to each participant's high-resolution anatomical scan, converted to percentage signal change, transformed into the standard coordinate space of Talairach and Tournoux (51), and smoothed with a 6-mm FWHM Gaussian kernel.
Imaging data analysis.
A voxelwise general linear model analysis was conducted for each participant by convolving task regressors of interest (happy-go, happy-nogo, fear-go, fear-nogo, and errors) with a γ-variate hemodynamic response function. Motion parameters, linear and quadratic trends were modeled as regressors of noninterest. To identify regions of interest of further analysis, parameter estimate (β) maps for the conditions happy-go, happy-nogo, fear-go, and fear-nogo were carried to a random-effects group analysis.
As described earlier, one participant was excluded for excessively poor behavioral performance on the fMRI version of the task, leaving 26 participants for group analyses. A 2 × 2 × 2 group linear mixed-effects model was conducted with factors of trial type (within subjects: go, no-go), emotion (within subjects: happy, fearful), and group (between subjects: high-delayer, low-delayer). Candidate regions of interest were identified by the main effect of response map and by the trial type × emotion × group interaction map. Resulting clusters considered statistically significant exceeded whole-brain correction for multiple comparisons using a P value and cluster combination identified by Monte Carlo simulations to preserve a corrected α < 0.05 (53 voxels at P < 0.005 thresholding). On the basis of our prior work (13), the striatum was targeted as an a priori structure of interest for voxelwise analysis. Task effects in the striatum from the present experiment were queried within an anatomically masked area (1,060 voxels) including the dorsal and ventral striatum and achieved P < 0.05 corrected thresholding on the basis of this search volume (13 voxels at P < 0.005, uncorrected). Regions of interest were created as spheres around peak voxels with a radius of 4 mm, each containing 10 3 × 3 × 3 voxels. Parameter estimates were extracted for the four conditions for each participant and plotted for descriptive purposes.
Acknowledgments
This work was supported in part by National Institutes of Health Grants R01 DA018879 (to B.J.C.), R01 HD069178 (to B.J.C. and W.M.), and by National Science Foundation Grant 06-509 (to Y.S.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Mischel W, Shoda Y, Rodriguez MI. Delay of gratification in children. Science. 1989;244:933–938. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- 2.Metcalfe J, Mischel W. A hot/cool-system analysis of delay of gratification: dynamics of willpower. Psychol Rev. 1999;106:3–19. doi: 10.1037/0033-295x.106.1.3. [DOI] [PubMed] [Google Scholar]
- 3.Somerville LH, Casey BJ. Developmental neurobiology of cognitive control and motivational systems. Curr Opin Neurobiol. 2010;20:236–241. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hare TA, Tottenham N, Davidson MC, Glover GH, Casey BJ. Contributions of amygdala and striatal activity in emotion regulation. Biol Psychiatry. 2005;57:624–632. doi: 10.1016/j.biopsych.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 5.Eigsti IM, et al. Predicting cognitive control from preschool to late adolescence and young adulthood. Psychol Sci. 2006;17:478–484. doi: 10.1111/j.1467-9280.2006.01732.x. [DOI] [PubMed] [Google Scholar]
- 6.Kahneman D, Treisman A, Burkell J. The cost of visual filtering. J Exp Psychol Hum Percept Perform. 1983;9:510–522. doi: 10.1037//0096-1523.9.4.510. [DOI] [PubMed] [Google Scholar]
- 7.Allport A. Selection for action: Some behavioral and neurophysiological considerations of attention and action. In: Sanders HHAF, editor. Perspectives on Perception and Action. Hillsdale, NJ: Laurence Erlbum Associates; 1987. pp. 395–419. [Google Scholar]
- 8.Cohen JD, Servan-Schreiber D. Context, cortex, and dopamine: A connectionist approach to behavior and biology in schizophrenia. Psychol Rev. 1992;99:45–77. doi: 10.1037/0033-295x.99.1.45. [DOI] [PubMed] [Google Scholar]
- 9.Casey BJ, et al. Dissociation of response conflict, attentional control, and expectancy with functional magnetic resonance imaging. Proc Natl Acad Sci USA. 2000;97:8727–8733. doi: 10.1073/pnas.97.15.8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casey BJ, Tottenham N, Fossella J. Clinical, imaging, lesion, and genetic approaches toward a model of cognitive control. Dev Psychobiol. 2002;40:237–254. doi: 10.1002/dev.10030. [DOI] [PubMed] [Google Scholar]
- 11.Jonides J, Nee DE. Brain mechanisms of proactive interference in working memory. Neuroscience. 2006;139:181–193. doi: 10.1016/j.neuroscience.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 12.Shoda Y, Mischel W, Peake PK. Predicting adolescent cognitive and self-regulatory competencies from preschool delay of gratification: Identifying diagnostic conditions. Dev Psychol. 1990;26:978–986. [Google Scholar]
- 13.Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J Cogn Neurosci. 2011;23:2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardner M, Steinberg L. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: An experimental study. Dev Psychol. 2005;41:625–635. doi: 10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- 15.Mischel W, Ebbesen EB, Zeiss AR. Cognitive and attentional mechanisms in delay of gratification. J Pers Soc Psychol. 1972;21:204–218. doi: 10.1037/h0032198. [DOI] [PubMed] [Google Scholar]
- 16.Mischel W, Underwood B. Instrumental ideation in delay of gratification. Child Dev. 1974;45:1083–1088. [PubMed] [Google Scholar]
- 17.Mischel W, Ayduk O. Willpower in a cognitive-affective processing system: The dynamics of delay of gratification. In: Baumeister RF, Vohs KD, editors. Handbook of Self-Regulation: Research, Theory, and Applications. New York: Guilford; 2004. pp. 99–129. [Google Scholar]
- 18.Rodriguez ML, Mischel W, Shoda Y. Cognitive person variables in the delay of gratification of older children at risk. J Pers Soc Psychol. 1989;57:358–367. doi: 10.1037//0022-3514.57.2.358. [DOI] [PubMed] [Google Scholar]
- 19.Abelson RP. Computer simulation of “hot cognition”. In: Tomkins SS, Mesick S, editors. Computer Simulation of Personality. New York: Wiley; 1963. pp. 277–302. [Google Scholar]
- 20.McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time discounting for primary rewards. J Neurosci. 2007;27:5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci. 2008;9:545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanfey AG. Social decision-making: Insights from game theory and neuroscience. Science. 2007;318:598–602. doi: 10.1126/science.1142996. [DOI] [PubMed] [Google Scholar]
- 23.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Heatherton TF, Wagner DD. Cognitive neuroscience of self-regulation failure. Trends Cogn Sci. 2011;15:132–139. doi: 10.1016/j.tics.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Galvan A, et al. The role of ventral frontostriatal circuitry in reward-based learning in humans. J Neurosci. 2005;25:8650–8656. doi: 10.1523/JNEUROSCI.2431-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casey BJ, et al. A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. J Cogn Neurosci. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- 28.Durston S, Thomas KM, Worden MS, Yang Y, Casey BJ. The effect of preceding context on inhibition: An event-related fMRI study. Neuroimage. 2002;16:449–453. doi: 10.1006/nimg.2002.1074. [DOI] [PubMed] [Google Scholar]
- 29.Ochsner KN, et al. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 30.Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- 31.Ayduk O, et al. Regulating the interpersonal self: Strategic self-regulation for coping with rejection sensitivity. J Pers Soc Psychol. 2000;79:776–792. doi: 10.1037//0022-3514.79.5.776. [DOI] [PubMed] [Google Scholar]
- 32.Ayduk O, et al. Rejection sensitivity and executive control: Joint predictors of borderline personality features. J Res Pers. 2008;42:151–168. doi: 10.1016/j.jrp.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mischel W, Shoda Y, Peake PK. The nature of adolescent competencies predicted by preschool delay of gratification. J Pers Soc Psychol. 1988;54:687–696. doi: 10.1037//0022-3514.54.4.687. [DOI] [PubMed] [Google Scholar]
- 34.Kubzansky LD, Martin LT, Buka SL. Early manifestations of personality and adult health: A life course perspective. Health Psychol. 2009;28:125–130. doi: 10.1037/a0014428. [DOI] [PubMed] [Google Scholar]
- 35.Posne MI, Rothbart MK. Developing mechanisms of self-regulation. Dev Psychopathol. 2000;12:427–441. doi: 10.1017/s0954579400003096. [DOI] [PubMed] [Google Scholar]
- 36.Carlson SM, Moses LJ. Individual differences in inhibitory control and children's theory of mind. Child Dev. 2001;72:1032–1053. doi: 10.1111/1467-8624.00333. [DOI] [PubMed] [Google Scholar]
- 37.Rothbart MK, Ahadi SA. Temperament and the development of personality. J Abnorm Psychol. 1994;103:55–66. doi: 10.1037//0021-843x.103.1.55. [DOI] [PubMed] [Google Scholar]
- 38.Moffitt TE, et al. A gradient of childhood self-control predicts health, wealth, and public safety. Proc Natl Acad Sci USA. 2011;108:2693–2698. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 40.O'Doherty JP, Buchanan TW, Seymour B, Dolan RJ. Predictive neural coding of reward preference involves dissociable responses in human ventral midbrain and ventral striatum. Neuron. 2006;49:157–166. doi: 10.1016/j.neuron.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 41.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: A neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 42.Galvan A, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chein J, Albert D, O'Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain's reward circuitry. Dev Sci. 2011;14:F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mischel W, et al. ‘Willpower’ over the life span: Decomposing self-regulation. Soc Cogn Affect Neurosci. 2011;6:252–256. doi: 10.1093/scan/nsq081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Block J, Block JH. The California Child Q-set. Palo Alto, CA: Consulting Psychologists Press; 1980. [Google Scholar]
- 46.Hare TA, et al. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tottenham N, Hare TA, Casey BJ. Behavioral assessment of emotion discrimination, emotion regulation and cognitive control in childhood, adolescence, and adulthood. Front Psychol. 2011;2:39. doi: 10.3389/fpsyg.2011.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tottenham N, et al. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Res. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glover GH, Thomason ME. Improved combination of spiral-in/out images for BOLD fMRI. Magn Reson Med. 2004;51:863–868. doi: 10.1002/mrm.20016. [DOI] [PubMed] [Google Scholar]
- 50.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 51.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]