Abstract
Background
Admission hyperglycemia impacts ischemic stroke deleteriously but the relative role of acute hyperglycemia (HG) versus diabetes in the pathogenesis of this poor outcome is not clear.
Purpose
To determine the effect of acute HG on neurovascular outcomes of stroke under control and diabetic conditions.
Methods
Moderate acute HG (140-200 mg/dl) was achieved by glucose injection before middle cerebral artery occlusion (MCAO) in control Wistar and diabetic Goto-Kakizaki (GK) rats. Following 3 h MCAO/21 h reperfusion, we measured infarct size, hemorrhagic transformation (HT) frequency, excess hemoglobin, neurobehavioral outcome and plasma and MCA matrix metalloprotease (MMP) activity.
Results
Infarct size was significantly smaller in diabetic rats. Moderate acute HG increased neuronal damage in diabetic but not in control rats. HT frequency and hemoglobin were significantly higher in diabetic rats. HG augmented vascular damage in control rats and had no additional effect on bleeding in diabetic rats. Baseline plasma MMP-9 activity was significantly higher in diabetic rats. HG increased MMP-9 activity in control and diabetic rats. Neurological deficit was greater in diabetic rats and was worsened by HG.
Conclusions
The finding that functional outcome is poorer in both acute HG and diabetes without a significant increase in infarct size suggests that amplified vascular damage contributes to neurological deficit in hyperglycemia. These results highlight the importance of vascular protection to improve neurological outcome in acute ischemic stroke.
Keywords: MMP-9 Metalloproteinase, Minocycline, Cerebral Hemorrhage, Ischemia-Reperfusion Injury, Type 2 Diabetes Mellitus, Hyperglycemia
Introduction
Ischemic stroke is a leading cause of death and disability in the United States and diabetes is the most rapidly increasing risk factor for stroke (1). Stroke risk in patients with diabetes is 2-6 folds higher than age-matched controls (1). The short and long term functional outcomes are worse and mortality is greater in stroke patients with diabetes as compared to the non-diabetic population (2). In addition to increased risk for stroke with diabetes, acute hyperglycemia (HG), which can develop as a stress response exacerbates stroke (3-5). However, it is not clear whether there is a difference between the effect of HG versus diabetes on clinical stroke outcomes (6). This is clinically important as the current stroke guidelines emphasize the need for randomized controlled trials to determine best practice for managing hyperglycemia (7). The GIST-UK randomized clinical trial (8) enrolled predominantly patients without diabetes (83%). Although insulin achieved target blood glucose levels, there was no difference in stroke outcomes. Results from THIS (9) and GRASP (10) pilot trials, both of which enrolled mainly diabetic patients (>50%), have suggested a favorable outcome in patients rapidly treated for hyperglycemia. While needing confirmation by larger trials, these results highlight the importance of targeting the right patient populations and the right blood glucose range to be achieved clinically.
Previous preclinical studies on diabetic and hyperglycemic ischemic brain injury do not provide sufficient information as most studies employed HG induced by either glucose injection or streptozotocin (STZ) injection 2-3 days prior to stroke with very high blood glucose levels (11-13). However, recent clinical evidence suggests that blood glucose levels in stroke patients that present with hyperglycemia at admission range between (140-200 mg/dl) (6, 9, 14).Understanding the mechanisms behind diabetes versus HG-dependent stroke pathology in animal models that closely represent the clinical condition is paramount in improving current preventive and therapeutic interventions.
Matrix metalloproteases (MMP) play an important role in vascular remodeling as well as stroke pathophysiology (15-17). We previously reported that augmented cerebrovascular MMP-2 and MMP-9 activity in the Goto-Kakizaki (GK) model of type-2 diabetes is associated with enhanced remodeling (18) and increased hemorrhagic transformation (HT) following ischemic stroke (19). Clinically, plasma MMP-9 levels are predictive of HT following acute ischemic stroke (16, 20). However, regulation of cerebrovascular and plasma MMP-9 levels in hyperglycemic and diabetic models and the subsequent effects on stroke pathology remain unknown.
Building upon these past findings, this study was designed to address the questions: 1. How do moderate acute hyperglycemia and diabetes affect neurovascular damage and stroke outcome?, 2. Does a further acute elevation in blood glucose at the time of ischemia exacerbate neuronal and vascular injury in diabetes?, and 3. How do HG and diabetes influence vascular and plasma MMP-9 following ischemic stroke?
Materials and methods
Animal models
The institutional animal care and use committees (IACUC) of the Medical College of Georgia and the Charlie Norwood Veterans Affairs approved all protocols. Male Wistar and GK rats were from Harlan (Indianapolis, IN) and Taconic (Hudson, NY), respectively, and weighed (260-310 g). Study groups included 1) Control, 2) Control + acute HG, 3) Diabetes, and 4) Diabetes + acute HG. Acute HG was achieved by a 3 ml IP glucose injection (300 mmol) 20-30 minutes before MCAO and was maintained during ischemia by another injection 1.5 h after MCAO. Two additional groups were: 1) Diabetic rats treated with metformin (300 mg/kg/day) starting at the onset of diabetes till MCAO and 2) Diabetic rats treated with metformin and given acute HG during MCAO. The purpose of the last 2 groups was to determine 1) whether glycemic control prevents/reduces injury if a stroke is superimposed on diabetes, and 2) whether an acute elevation in blood glucose cause the same damage if there was prior glycemic control in diabetes. Metformin was given in drinking water artificially sweetened by non-caloric sweetener for the 4 weeks before the study. Blood glucose was measured from tail vein using a glucometer (Freestyle, Alameda, CA) and reported at baseline, MCAO surgery and sacrifice. MCAO blood glucose levels were given as the average of readings at induction of anesthesia and 1h MCAO.
Experimental temporary focal cerebral ischemia
3 h MCAO was performed under 3% isoflurane anesthesia followed by 21 h reperfusion using a modified Longa method as we reported before (19). Scanning laser Doppler (Pim-3, Perimed, ST) was used to confirm a similar degree of drop in flow between groups. Core body temperature was monitored rectally and maintained using a heating pad under the animal and under the cage till the end of 24 h. Animals were singly housed before and after MCAO with free access to food and water. In a subset of animals, arterial blood gases were measured before and after MCAO as well as post-reperfusion via femoral artery catheterization.
Evaluation of neurovascular injury
Before sacrifice animals underwent intracardiac perfusion of ice-cold saline to flush blood from the vessels. Brains were extracted and MCAs were isolated from ischemic and nonischemic hemispheres. Visual inspection of hemorrhage was done, documented per slice if present and reported in a binary fashion as yes or no to indicate the frequency of macroscopic bleeding. 2, 3, 5-triphenyltetrazolium chloride (TTC) was used to outline the infarct area. Images were analyzed in a blinded fashion using Image-J. Edema is reported as the percent increase in ischemic hemisphere size to the contralateral hemisphere. Infarct volume is reported as the % infarcted area of ipsilateral hemisphere corrected for edema. Indirect infarct size was also calculated as contralateral area minus the area of noninfarcted tissue in the ischemic hemisphere. Following staining, hemispheres were separated and stored at −80°C for later hemoglobin (Hb) ELISA. HT was quantified by measuring excess Hb in the stroked hemisphere compared to the contralateral side as previously published (21).
Evaluation of MMP activity
For vascular MMP-9 activity, MCAs isolated from ischemic and nonischemic hemispheres were homogenized as reported previously (18) and 30 μg homogenate or 20 μl (1:20 diluted) plasma were assayed by gelatin zymography as we reported previously (19). Briefly, samples were loaded directly on SDS-PAGE gel containing 0.1% gelatin and separated under non-reducing conditions. Following electrophoresis, the gel was washed twice in 2.5% “Triton X100” for 20 minutes each, rinsed with ddH20 and incubated for 20 h in a substrate buffer “50 mmol Tris-HCl, 5 mmol CaCl2 + 0.02% NaN3, pH= 7.5” at 37°C. Recombinant MMP-9 active standard was run as positive control (Calbiochem, catalogue# PF024). Following incubation, the gel was stained using “Coomassie blue” for 3 h then destained. The zymogram was digitized and band intensity was quantified by image analysis “GelPro analyzer, Media Cybernetics, MD” and expressed as the % of the recombinant standard.
Evaluation of neurological outcome
Ipsilateral circling, paw grasp and beam walk tests assessed function at baseline, before MCAO and at 24 h. Scores were graded 0-3 (Normal-maximum deficit). Ipsilateral circling (No/ few/ several and continuous circling), paw grasp (Normal grasp/ stroke side can touch/ stroke side hard to touch and unable to touch), beam walk (Animal readily traverses/ walks slowly and shaking/ can stay or eventually falls and unable to stay for 10 seconds). A total score of 9 indicated the worst outcome. Bederson’s score was recorded at baseline and after occlusion to verify proper occlusion (22).
Statistics
The distributions for the measures of stroke severity (Infarct size, percent edema, and bleeding) as well as the measures of behavior were found to be skewed. A rank transformation was used prior to the analysis of these measures. The analysis for the effect of hyperglycemia on Wistar and GK rats was performed using a 2 disease (Wistar vs GK) X 2 treatment (Vehicle vs hyperglycemia) ANOVA. An interaction between disease and treatment would indicate a differential effect of inducing hyperglycemia that is dependent on disease status. The analysis for the effects of hyperglycemia and metformin was performed using a 2 hyperglycemia (No vs yes) X 2 metformin (No vs yes) factorial ANOVA. An interaction between hyperglycemia and metformin would indicate a differential effect of metformin treatment dependent on hyperglycemic status. A Tukey’s test was used to adjust for multiple comparisons when determining mean differences for significant interactions.
Results
Physiological parameters
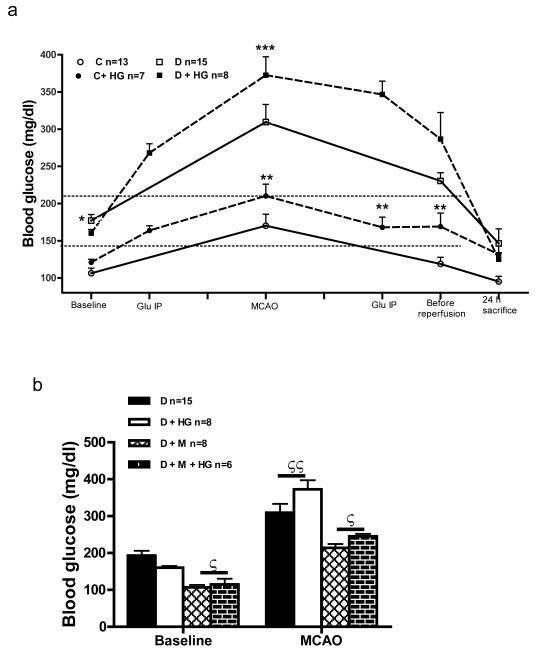
Blood glucose levels over the course of MCAO/reperfusion are depicted in Fig. 1a. During ischemia, we achieved 1) A blood glucose range in control rats similar to the average blood glucose values in diabetic GK rats (140-200 mg/dl), and 2) An acute increase in blood glucose in diabetic rats. Baseline blood glucose was significantly higher in diabetic than in control rats. In all groups, blood glucose increased during MCAO and diabetic rats showed a greater elevation in blood glucose compared to control + HG group (Fig. 1a). The purpose of the second glucose IP injection midway during occlusion was to maintain HG during MCAO. At sacrifice, blood glucose dropped to similar level in all groups. Metformin corrected hyperglycemia in diabetic rats. Glucose injection achieved a similar degree of increase in blood glucose in both vehicle and metformin treated diabetic rats at baseline (Fig. 1b). At sacrifice, blood glucose dropped to control levels in all groups (Fig. 1a).
Fig. 1.
(A) Blood glucose at baseline and during MCAO in Control (C), C+ High Glucose (HG), Diabetes (D), and D+ HG groups. (B) Effect of glycemic control with metformin (M) on blood glucose at baseline and during MCAO. *p<0.05 vs C or C +HG, **p<0.05 vs C, D or D + HG, ***p<0.05 vs D, ζp<0.05 vs D or D + HG, ζζp<0.05 vs baseline. Glu IP= glucose intraperitoneal injection (Dotted horizontal line indicates the range of achieved HG).
Arterial blood gases measurements were similar between control and diabetic rats before and after MCAO as well as post-reperfusion (Table 1).
Table 1.
Arterial blood gases before and after MCAO.
| Controla | Diabetesa | |||||
|---|---|---|---|---|---|---|
| before | after | postReb | before | after | postReb | |
| pH | 7.45±0.01 | 7.45±0.02 | 7.40±0.02 | 7.42±0.02 | 7.39±0.01 | 7.39±0.03 |
| pCO2 | 41.37±0.83 | 40.78±2.10 | 49.12±2.54 | 48.68±2.46 | 51.61±2.60 | 47.91±2.88 |
| pO2 | 163.83±1.5 | 168.50±3.5 | 120.20±11.1 | 150.18±4.1 | 161.00±4.5 | 133.43±9.9 |
| n | 6 | 4 | 5 | 11 | 8 | 7 |
Body temperature 37 ± 0.5 C0.
postRe = post reperfusion.
Neurovascular damage
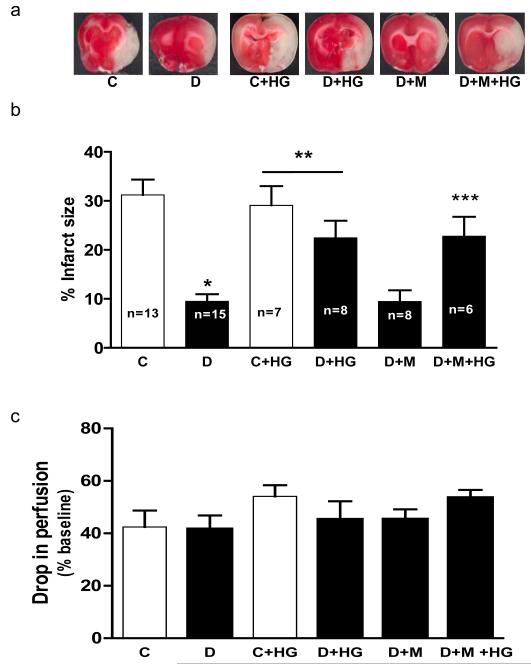
Infarct size was significantly lower in diabetic rats than in controls as we previously reported (Fig. 2a and b) (19). Indirect infarct size measurements showed similar results (Not shown). There was a disease and treatment interaction such that HG increased infarct size in diabetic (30 vs 10%) but not in control (30%) (Fig. 2b). Metformin pretreatment did not affect infarct size in diabetic rats with or without additional acute HG (Fig. 2b). The percent reduction in blood flow from baseline after MCAO was similar in all groups indicating consistent occlusion (Fig. 2c).
Fig. 2.
HG increases infarct size when superimposed on diabetes. Representative images of ischemic damage after TTC stain and quantitative analysis of infarct size are shown in panels A and B, respectively. (C) Drop in flow following MCAO was simialr among groups. *p< 0.05 vs C, **p=0.0062 disease by treatment interaction compared to C and D and ***p=0.0035 vs D or D + M.
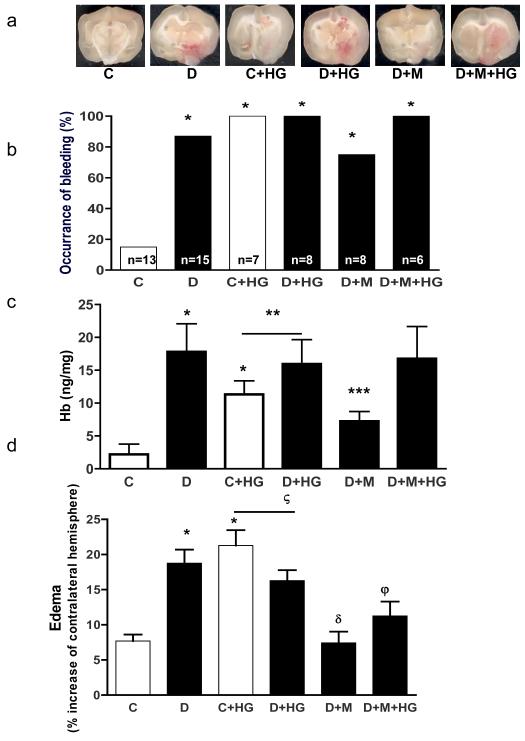
Edema and hemorrhagic transformation (HT) were analyzed as indices of vascular damage. HT was assessed qualitatively by observing macroscopic hemorrhagic transformation and quantitatively by Hb ELISA (ng/mg protein) (Fig. 3b and c). Diabetic rats had a higher rate of HT than the controls (87% vs 15%, p< 0.001). Excess Hb and edema were higher in diabetes indicating vascular damage and there was a disease and treatment interaction such that HG increased both parameters in control but not diabetes (Fig. 3c and d). Metformin reduced brain edema in diabetic rats (Fig. 3d). While it did not affect the rate of HT, bleeding severity was reduced (Fig. 3c).
Fig. 3.
HG and diabetes augment vascular damage. (A) Representative images of hemorrhagic transformation (HT). (B) Frequency of macroscopic HT is significantly higher in diabetes and HG versus control. Severity of bleeding (C) and edema (D) are greater in diabetes and HG. (D). *p< 0.05 vs C, **p=0.016 disease by treatment interaction compared to C and D, p< 0.001 vs D, ***p=0.0089 vs D, ζp<0.0001 disease by treatment interaction compared to C and D, δp<0.0001 vs D and φp<0.01 vs D or D + HG.
MMP activity
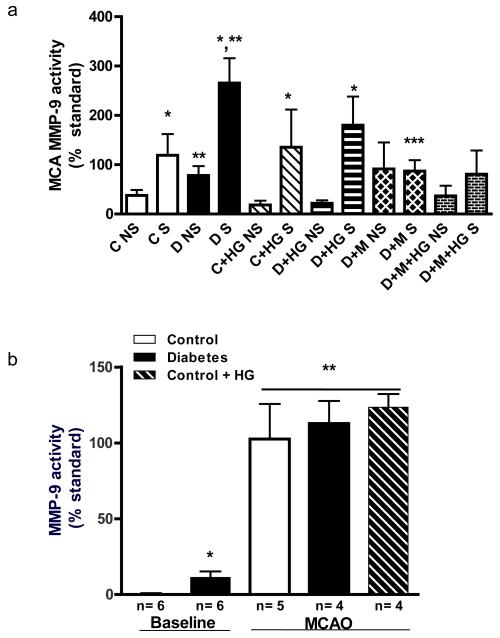
MMP-9 is associated with disruption of vascular integrity in ischemic stroke. Both MCA and plasma MMP-9 activity were measured (Fig. 4). MCA MMP-9 activity was greater in the nonischemic hemisphere of diabetic rats than in control (Fig. 4a). Ischemia enhanced MCA MMP-9 activity in both control and diabetic rats. Metformin pretreatment did not affect MMP-9 activity on the nonischemic side but prevented the increase on the ischemic hemisphere. HG did not change MCA MMP-9 activity. Plasma MMP-9 activity at baseline was higher in diabetes (Fig. 4b). MCAO caused a dramatic increase versus baseline in all groups.
Fig. 4.
Local MCA and circulating MMP-9 activity under basal and ischemic conditions. (A) MCA MMP-9 lytic of non-stroke (NS) and stroke (S) hemispheres by gelatin zymography. Baseline (NS) activity was greater in diabetes than in control and further increased with ischemia. (B) Baseline plasma MMP-9 activity was higher in diabetes and at 24 h after MCAO both diabetes and HG caused a significant increase. *p< 0.05 vs NS, **p< 0.001 vs C S and ***p< 0.001 vs D S.
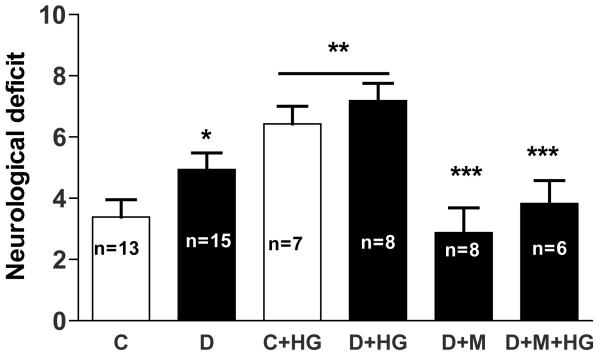
Neurological outcome
Short term (24 h) outcomes were assessed. Neurological deficits were more pronounced in diabetes rats and HG worsened functional outcomes in both control and diabetic rats (Fig. 5). Metformin improved the deficit in diabetic animals.
Fig. 5.
Effect of HG and diabetes on 24 h neurobehavioral outcomes. A greater composite score indicates greater neurological deficit and poor outcome. *p< 0.05 vs C, **p< 0.0001 compared to C and D and ***p< 0.001 vs D or D + HG
Discussion
This study provides several important lines of evidence on the effects of acute hyperglycemia and diabetes (chronic hyperglycemia) on neurovascular and functional outcomes of stroke: 1) Moderate acute hyperglycemia amplifies vascular damage (edema and HT) without increasing infarct size following stroke in otherwise healthy rats. 2) Moderate diabetes is associated with smaller infarcts but greater edema and HT. Overlaying acute hyperglycemia in diabetes exacerbates infarction but does not amplify the HT or edema that is already greater in diabetic rats compared to control rats. 3) Upregulation of MMP-9 activity is a mechanism associated with increased vascular damage in both acute hyperglycemia and diabetes. 4) Glycemic control provides vascular protection in diabetes. The interesting finding that functional outcomes are worse in acute hyperglycemic and diabetic rats despite relatively smaller infarcts strongly suggests that vascular injury contributes to the neurological deficits.
Diabetes and HG are predictors of poor ischemic stroke outcomes. Yet, limited knowledge exists about the different pathophysiology, outcomes and the mechanisms of injury in each case (6, 23). Clinical findings link admission HG, independent of diabetes, to poor outcomes and to increased odds of HT (24, 25). In experimental models, larger infarct size and higher mortality rates were reported after hyperglycemic ischemic stroke. Most of these studies employed acutely-induced severe hyperglycemia (26, 27). Furthermore, diabetes was induced for only a short period of time prior to MCAO rather than being a fully developed disease state (11). Our study expands our current knowledge as GK rats present with mild-moderate hyperglycemia (~ 180 mg/dl) which more closely resembles the clinical situation where admission hyperglycemia is defined as glycemia >140 mg/dl (6, 9, 14). This is in comparison to the STZ model with severe glycemia (~500 mg/dl). In addition, GK rats have been moderately diabetic for a relatively longer period of time, about 5-6 weeks before MCAO.
The infarct size in our diabetic rats was smaller than control as we previously reported (19, 28, 29). This may be due to diabetes-mediated stimulation of survival mechanisms as a compensatory response early in the disease process (30). We had hypothesized that if diabetes activates survival pathways in GK rats, then glucose control would prevent this effect and GKs would develop infarct sizes similar to control. However, there was no significant change in infarct size due to metformin pretreatment. This would suggest that the smaller infarcts in GK are either not due to diabetes-mediated changes in cell survival or that this is a metformin-specific protective effect. Recent studies reported that metformin may also be neuroprotective (31, 32). Future studies are needed to address the preconditioning possibility in GK rats in more depth. At the levels of acute hyperglycemia achieved in our study, ischemic damage in control HG rats was not different from control normoglycemic animals. Yet, when acute HG was superimposed on diabetic rats, ischemic damage increased. This finding may be due to the fact that while baseline blood glucose was similar between control + HG and diabetes groups at the beginning of stroke surgery, diabetic animals showed a greater spike during MCAO indicating a greater stress response. While the reasons are not completely delineated, acute HG in human stroke may be due to stress response by activation of the sympathetic system or the hypothalamic-pituitary-adrenal axis. In our model acute HG was induced by glucose injection but the role(s) of these systems cannot be excluded in our findings.
Neurovascular unit integrity is compromised following prolonged ischemia causing edema and HT due to disruption of the tight junction proteins and vessel breakdown. Admission blood glucose is a predictor of HT in patients given t-PA (3-5). Thus, controlling HG in ischemic stroke is likely to be important in maximizing the therapeutic potential of t-PA. In our study, 87% of diabetic rats exhibited intracerebral bleeding while this was 100% in HG animals versus 15% in control. Upon quantification, severity of HT was higher in diabetic rats compared to control. Edema was higher in diabetic and HG rats compared to control, suggesting that cerebrovasculature is susceptible to damage by hyperglycemia regardless of diabetes. It also points out that the threshold for vascular damage is relatively low and mild-moderate levels of HG are sufficient to induce it.
Upregulation of MMPs may be a common link between diabetes and ischemic stroke pathologies. Increased MMP-9 disrupts basal lamina and neurovascular unit structure promoting edema and HT in ischemic stroke (16, 17, 33-35). MMP-9 knockout mice were shown to be protected against ischemic stroke. It is postulated that the HT complicating tissue plasminogen activator (t-PA) therapy is mediated through upregulation of MMP-9 (36). We investigated the parallel patterns of MMP-9 activity in both cerebral macrovessels and in plasma (tissue versus circulation) in diabetes and HG. Baseline plasma MMP-9 activity was upregulated in diabetes compared to control. At 24 h, MMP-9 plasma activity in all groups increased significantly up to similar level due to the ischemic injury. A similar pattern was observed in macrovessels where type-2 diabetes upregulated MCA MMP-9 activity compared to control in the non-stroke side and ischemic injury caused a further elevation of MMP-9 activity in both control and diabetes macrovessels on the stroke side. The fact that bleeding frequency was significantly higher in both diabetes and HG rats than in controls, yet the 24 h MMP-9 activity was not different between groups, indicates that earlier time points of plasma MMP-9 activity need to be investigated for proper correlation between plasma MMP-9 activity and HT for optimal utilization of MMP-9 as an ischemic stroke biomarker (37).
Our study assessed neurological function at 24 h while the damage is still evolving. The finding that the deficit is greater in the diabetic versus control groups and that HG exacerbated the neurological deficit in both control and diabetic rats suggests that both acute hyperglycemia and diabetes are critical for stroke outcome. An interesting finding is that while glycemic control with metformin did not affect infarct size (Fig. 2b), it improved neurological outcome in diabetes (Fig. 5). Pretreatment with metformin also reduced the deficit mediated by HG in diabetic rats. A novel aspect of the current study is that the functional outcome was worse in both acute and chronic hyperglycemia despite relatively smaller infarct sizes suggesting that vascular injury and potentially subsequent vascular dysfunction in these groups may be as important as the neuronal injury in determining neurological deficit. However, it is recognized that short term 24 h measurement is a limitation of the study and more sensitive methods and longer term outcomes are needed for confirmation.
Conclusion
Therapeutic options for ischemic stroke patients are limited. Given that 40-50% of acute ischemic stroke patients present with admission HG, understanding the mechanisms of neurovascular injury in acute HG and diabetes is very important. While the complexity of human stroke cannot be duplicated in experimental models, these preclinical studies are critical to guide the clinical studies to develop better prevention and intervention strategies in the management of acute ischemic stroke.
Acknowledgments
The authors thank Anna Kozak and Dr Cesar Borlongan for guidance with the MCAO surgery and Dr Hazem Elewa and Dan Wiley for technical assistance with hemoglobin ELISA.
This work was supported in part by grants from NIH [DK074385, NS054688] and American Heart Association Established Investigator Award [0740002N] to A. E. and VA Merit Awards to S.C.F and A.E. AE is a research pharmacologist at the Charlie Norwood Veterans Affairs Medical Center in Augusta, Georgia
Footnotes
Institute of origin: Medical College of Georgia, Address: 1120 15th St, CA2094, Augusta, GA
The authors have no conflict of interests to disclose.
References
- 1.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, et al. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007 Feb 6;115(5):e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Kaarisalo MM, Raiha I, Sivenius J, Immonen-Raiha P, Lehtonen A, Sarti C, et al. Diabetes worsens the outcome of acute ischemic stroke. Diabetes Res Clin Pract. 2005 Sep;69(3):293–8. doi: 10.1016/j.diabres.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Williams LS, Rotich J, Qi R, Fineberg N, Espay A, Bruno A, et al. Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke. Neurology. 2002 Jul 9;59(1):67–71. doi: 10.1212/wnl.59.1.67. [DOI] [PubMed] [Google Scholar]
- 4.Bruno A, Levine SR, Frankel MR, Brott TG, Lin Y, Tilley BC, et al. Admission glucose level and clinical outcomes in the NINDS rt-PA Stroke Trial. Neurology. 2002 Sep 10;59(5):669–74. doi: 10.1212/wnl.59.5.669. [DOI] [PubMed] [Google Scholar]
- 5.McCormick MT, Muir KW, Gray CS, Walters MR. Management of hyperglycemia in acute stroke: how, when, and for whom? Stroke. 2008 Jul;39(7):2177–85. doi: 10.1161/STROKEAHA.107.496646. [DOI] [PubMed] [Google Scholar]
- 6.Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001 Oct;32(10):2426–32. doi: 10.1161/hs1001.096194. [DOI] [PubMed] [Google Scholar]
- 7.Adams HP, Jr., del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007 May;38(5):1655–711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 8.Gray CS, Hildreth AJ, Sandercock PA, O’Connell JE, Johnston DE, Cartlidge NE, et al. Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: the UK Glucose Insulin in Stroke Trial (GIST-UK) Lancet Neurol. 2007 May;6(5):397–406. doi: 10.1016/S1474-4422(07)70080-7. [DOI] [PubMed] [Google Scholar]
- 9.Bruno A, Kent TA, Coull BM, Shankar RR, Saha C, Becker KJ, et al. Treatment of hyperglycemia in ischemic stroke (THIS): a randomized pilot trial. Stroke. 2008 Feb;39(2):384–9. doi: 10.1161/STROKEAHA.107.493544. [DOI] [PubMed] [Google Scholar]
- 10.Johnston KC, Hall CE, Kissela BM, Bleck TP, Conaway MR. Glucose Regulation in Acute Stroke Patients (GRASP) trial: a randomized pilot trial. Stroke. 2009 Dec;40(12):3804–9. doi: 10.1161/STROKEAHA.109.561498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamada H, Yu F, Nito C, Chan PH. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: relation to blood-brain barrier dysfunction. Stroke. 2007 Mar;38(3):1044–9. doi: 10.1161/01.STR.0000258041.75739.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Courten-Myers GM, Kleinholz M, Holm P, DeVoe G, Schmitt G, Wagner KR, et al. Hemorrhagic infarct conversion in experimental stroke. Ann Emerg Med. 1992 Feb;21(2):120–6. doi: 10.1016/s0196-0644(05)80144-1. [DOI] [PubMed] [Google Scholar]
- 13.Martini SR, Kent TA. Hyperglycemia in acute ischemic stroke: a vascular perspective. J Cereb Blood Flow Metab. 2007 Mar;27(3):435–51. doi: 10.1038/sj.jcbfm.9600355. [DOI] [PubMed] [Google Scholar]
- 14.Yong M, Kaste M. Dynamic of hyperglycemia as a predictor of stroke outcome in the ECASS-II trial. Stroke. 2008 Oct;39(10):2749–55. doi: 10.1161/STROKEAHA.108.514307. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg GA, Estrada EY, Dencoff JE. Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke. 1998 Oct;29(10):2189–95. doi: 10.1161/01.str.29.10.2189. [DOI] [PubMed] [Google Scholar]
- 16.Montaner J, Alvarez-Sabin J, Molina CA, Angles A, Abilleira S, Arenillas J, et al. Matrix metalloproteinase expression is related to hemorrhagic transformation after cardioembolic stroke. Stroke. 2001 Dec 1;32(12):2762–7. doi: 10.1161/hs1201.99512. [DOI] [PubMed] [Google Scholar]
- 17.Heo JH, Lucero J, Abumiya T, Koziol JA, Copeland BR, del Zoppo GJ. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J Cereb Blood Flow Metab. 1999 Jun;19(6):624–33. doi: 10.1097/00004647-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Harris AK, Hutchinson JR, Sachidanandam K, Johnson MH, Dorrance AM, Stepp DW, et al. Type 2 diabetes causes remodeling of cerebrovasculature via differential regulation of matrix metalloproteinases and collagen synthesis: role of endothelin-1. Diabetes. 2005 Sep;54(9):2638–44. doi: 10.2337/diabetes.54.9.2638. [DOI] [PubMed] [Google Scholar]
- 19.Ergul A, Elgebaly MM, Middlemore ML, Li W, Elewa H, Switzer JA, et al. Increased hemorrhagic transformation and altered infarct size and localization after experimental stroke in a rat model type 2 diabetes. BMC Neurol. 2007;7:33. doi: 10.1186/1471-2377-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castellanos M, Leira R, Serena J, Pumar JM, Lizasoain I, Castillo J, et al. Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke. 2003 Jan;34(1):40–6. [PubMed] [Google Scholar]
- 21.Hilali HM, Simpkins AN, Hill WD, Waller JL, Knight RA, Fagan SC. Single slice method for quantification of hemorrhagic transformation using direct ELISA. Neurol Res. 2004 Jan;26(1):93–8. doi: 10.1179/016164104773026606. [DOI] [PubMed] [Google Scholar]
- 22.Kozak W, Kozak A, Johnson MH, Elewa HF, Fagan SC. Vascular protection with candesartan after experimental acute stroke in hypertensive rats: a dose-response study. J Pharmacol Exp Ther. 2008 Sep;326(3):773–82. doi: 10.1124/jpet.108.139618. [DOI] [PubMed] [Google Scholar]
- 23.Demchuk AM, Morgenstern LB, Krieger DW, Linda Chi T, Hu W, Wein TH, et al. Serum glucose level and diabetes predict tissue plasminogen activator-related intracerebral hemorrhage in acute ischemic stroke. Stroke. 1999 Jan;30(1):34–9. doi: 10.1161/01.str.30.1.34. [DOI] [PubMed] [Google Scholar]
- 24.Alvarez-Sabin J, Molina CA, Montaner J, Arenillas JF, Huertas R, Ribo M, et al. Effects of admission hyperglycemia on stroke outcome in reperfused tissue plasminogen activator--treated patients. Stroke. 2003 May;34(5):1235–41. doi: 10.1161/01.STR.0000068406.30514.31. [DOI] [PubMed] [Google Scholar]
- 25.Alvarez-Sabin J, Molina CA, Ribo M, Arenillas JF, Montaner J, Huertas R, et al. Impact of admission hyperglycemia on stroke outcome after thrombolysis: risk stratification in relation to time to reperfusion. Stroke. 2004 Nov;35(11):2493–8. doi: 10.1161/01.STR.0000143728.45516.c6. [DOI] [PubMed] [Google Scholar]
- 26.Ennis SR, Keep RF. Effect of sustained-mild and transient-severe hyperglycemia on ischemia-induced blood-brain barrier opening. J Cereb Blood Flow Metab. 2007 Sep;27(9):1573–82. doi: 10.1038/sj.jcbfm.9600454. [DOI] [PubMed] [Google Scholar]
- 27.Vannucci SJ, Willing LB, Goto S, Alkayed NJ, Brucklacher RM, Wood TL, et al. Experimental stroke in the female diabetic, db/db, mouse. J Cereb Blood Flow Metab. 2001 Jan;21(1):52–60. doi: 10.1097/00004647-200101000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Li W, Prakash R, Kelly-Cobbs AI, Ogbi S, Kozak A, El-Remessy AB, et al. Adaptive Cerebral Neovascularization in a Model of Type 2 Diabetes: Relevance to Focal Cerebral Ischemia. Diabetes. 2009 Oct 6; doi: 10.2337/db09-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elewa HF, Kozak A, El-Remessy AB, Frye RF, Johnson MH, Ergul A, et al. Early atorvastatin reduces hemorrhage after acute cerebral ischemia in diabetic rats. J Pharmacol Exp Ther. 2009 Aug;330(2):532–40. doi: 10.1124/jpet.108.146951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stenzel-Poore MP, Stevens SL, King JS, Simon RP. Preconditioning reprograms the response to ischemic injury and primes the emergence of unique endogenous neuroprotective phenotypes: a speculative synthesis. Stroke. 2007 Feb;38(2 Suppl):680–5. doi: 10.1161/01.STR.0000251444.56487.4c. [DOI] [PubMed] [Google Scholar]
- 31.Correia S, Carvalho C, Santos MS, Proenca T, Nunes E, Duarte AI, et al. Metformin protects the brain against the oxidative imbalance promoted by type 2 diabetes. Med Chem. 2008 Jul;4(4):358–64. doi: 10.2174/157340608784872299. [DOI] [PubMed] [Google Scholar]
- 32.Poels J, Spasic MR, Callaerts P, Norga KK. Expanding roles for AMP-activated protein kinase in neuronal survival and autophagy. Bioessays. 2009 Sep;31(9):944–52. doi: 10.1002/bies.200900003. [DOI] [PubMed] [Google Scholar]
- 33.Romanic AM, Graesser D, Baron JL, Visintin I, Janeway CA, Jr., Madri JA. T cell adhesion to endothelial cells and extracellular matrix is modulated upon transendothelial cell migration. Lab Invest. 1997 Jan;76(1):11–23. [PubMed] [Google Scholar]
- 34.Asahi M, Wang X, Mori T, Sumii T, Jung J, Moskowitz MA, et al. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–32. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003 Aug;23(8):879–94. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Lee SR, Arai K, Lee SR, Tsuji K, Rebeck GW, et al. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med. 2003 Oct;9(10):1313–7. doi: 10.1038/nm926. [DOI] [PubMed] [Google Scholar]
- 37.Park KP, Rosell A, Foerch C, Xing C, Kim WJ, Lee S, et al. Plasma and brain matrix metalloproteinase-9 after acute focal cerebral ischemia in rats. Stroke. 2009 Aug;40(8):2836–42. doi: 10.1161/STROKEAHA.109.554824. [DOI] [PMC free article] [PubMed] [Google Scholar]