Abstract
This fMRI study investigates the neural bases of cognitive control of emotion processing in pediatric bipolar disorder (PBD) and attention deficit hyperactivity disorder (ADHD). Seventeen un-medicated PBD patients, 15 un-medicated ADHD patients, and 14 healthy controls (HC) (mean age = 13.78 ± 2.47) performed an emotional valence Stroop Task, requiring them to match the color of an emotionally valenced word to the color of either of two adjacent circles. Both patient groups responded significantly slower than HC, but there were no group differences in accuracy. A voxel-wise analysis of variance on brain activation revealed a significant interaction of group by word valence [F (2,41) = 4.44; p = .02]. Similar group differences were found for negative and positive words. For negative versus neutral words, both patient groups exhibited greater activation in dorsolateral prefrontal cortex (DLPFC) and parietal cortex relative to HC. The PBD group exhibited greater activation in ventrolateral prefrontal cortex (VLPFC) and anterior cingulate cortex (ACC) relative to HC. The ADHD group exhibited decreased VLPFC activation relative to HC and the PBD group. During cognitive control of emotion processing, PBD patients deployed the VLPFC to a greater extent than HC. The ADHD patients showed decreased VLPFC engagement relative to both HC and PBD patients.
Keywords: Functional magnetic resonance imaging (fMRI), Bipolar, ADHD, Attention, Emotion, Stroop, Child, Adolescent, Development
INTRODUCTION
The overlapping symptoms of pediatric bipolar disorder (PBD) and attention deficit hyperactivity disorder (ADHD) can complicate their diagnosis. ADHD is the most frequent comorbid disorder in PBD (Singh, DelBello, Kowatch, & Strakowski, 2006), with comorbidity rates ranging from 60 to 90% (Galanter & Leibenluft, 2008). Even in the absence of comorbidity, PBD and ADHD often share similar behavioral, cognitive, and emotional deficits (Dickstein et al., 2005; Galanter & Leibenluft, 2008) which may complicate diagnosis and pharmacological treatment. Therefore, the present study extends the ongoing effort to disentangle the pathophysiology of these two developmental syndromes to more effectively differentiate between them.
The central clinical features of PBD include emotional dysregulation, rapid mood cycling with mixed episodes, elated mood, grandiosity, irritability, high energy levels, and decreased need for sleep (Geller, Warner, Williams, & Zimerman, 1998; Pavuluri, O’Connor, Harral, & Sweeney, 2007). In addition to deficits in emotional processing (Chang et al., 2004; Dickstein et al., 2007; Pavuluri et al., 2007; Rich et al., 2006), recent studies have also revealed a profile of cognitive deficits in PBD, especially in the domains of executive function, sustained attention, verbal learning, and working memory (Dickstein et al., 2005; Pavuluri et al., 2006, 2009). These deficits are independent of illness status (Pavuluri et al., 2006) and may increase over adolescence relative to healthy peers (Pavuluri et al., 2009). Recent brain imaging studies with PBD patients have implicated dysfunction in a network of cortico-limbic regions. Several functional magnetic resonance imaging (fMRI) studies on cognitive control that used cognitive (Altshuler et al., 2000; Blumberg et al., 2003; Roth et al., 2006; Strakowski, Adler, Holland, Mills, DelBello, & Eliassen, 2005) or emotional (Lagopoulos and Mahli, 2007; Malhi, Lagopoulos, Sachdev, Ivanovski, & Shnier, 2005; Pavuluri, O’Connor, Harral, & Sweeney (2008)). Stroop tasks and response inhibition tasks (Leibenluft et al., 2007; Passarotti, Sweeney, & Pavuluri, in press; Pavuluri et al., 2008) have linked the associated deficits in bipolar disorder (BD) to ventral prefrontal dysfunction, even independently of mood state (Blumberg et al., 2003). Dysfunction of anterior cingulate cortex (ACC), striatum, and limbic regions has also been found in patients with BD compared with healthy controls (HC) in Stroop-like (Gruber, Rogowska, & Yurgelun-Todd, 2004) and face emotion processing tasks (Lawrence et al., 2004; Shah et al., 2009).
ADHD, combined subtype, although diagnosed based on hyperactivity, impulsivity, and inattention, is often accompanied by deficits in behavioral control, executive functions, sustained attention, and response inhibition (Rubia, Taylor, Smith, Oksannen, Overmeyer, & Newman, 2001; Seidman, Valera, & Bush, 2004). These deficits have been attributed to fronto-striatal dysfunction (Rubia et al., 1999; Seidman et al., 2004, 2006). Recent fMRI studies that examined selective attention (Bush et al., 1999; Vaidya et al., 1998) and response inhibition (Casey et al., 1997; Rubia et al., 1999; Tamm, Menon, Ringel, & Reiss, 2004) functions in patients with ADHD found that they exhibited decreased activation in ventrolateral prefrontal cortex (VLPFC), ACC, and striatum, compared with HC. Finally, a study on response inhibition from our laboratory found reduced VLPFC activity in patients with ADHD compared with patients with PBD and HC, possibly suggesting greater VLPFC dysfunction in ADHD than PBD (Passarotti et al., in press).
There are also reports of increased activation in this network of regions in patients with ADHD compared with HC. Schulz et al. (2004) found that adolescents with childhood onset ADHD exhibited increased activation in VLPFC regions, ACC, and fronto-polar regions during a Go/No-Go task compared with healthy adolescents; and a subsequent study (Schulz et al., 2005) found that, during a response conflict task, adolescents with ADHD showed greater VLPFC, ACC, and basal ganglia activation relative to HC.
Preliminary work on the neural interface of cognitive and emotion circuits in PBD patients indicate an interdependence of these two systems (Blumberg et al., 2003; Lagopoulos & Mahli, 2007; Malhi et al., 2005; Pavuluri et al., 2008; Pavuluri & Passarotti, 2008), but the exact nature of the functional interface between cognitive and affective dimensions in PBD is not well-understood. Also with regard to children with ADHD, recent behavioral studies reported that emotional dysfunction may accompany deficits in behavior inhibition (Desman, Schneider, Ziegler-Kirbach, Petermann, Mohr, & Hampel, 2006) and facial affect recognition (Braaten & Rosén, 2000; Casey, 1996; Rapport, Friedman, Tzelepis, & VanVoorhis, 2002). Therefore, it has been suggested that attention and inhibition deficits in ADHD may be associated with poor emotional self-regulation, due to the inability to separate emotion from cognition, especially during emotional challenge (Barkley, 1997; Braaten & Rosén, 2000; Friedman et al., 2003). Indeed, there is a growing need for a better understanding of emotional dysregulation in ADHD, and its relation to severe mood disorders such as BD (Skirrow, McLoughlin, Kuntsi, & Asherson, 2009).
Given the emerging findings of behavioral and neural deficits in cognitive and affective operations in PBD and in ADHD, we sought to characterize the neural function at the interface of these two neural systems, to better elucidate similarities and differences in the two disorders. Emotional valence Stroop tasks have been used to examine how cognition is affected by incidental processing of emotions during a selective attention task. As in an fMRI study by Pavuluri et al. (2008), we adopted a modified version of an emotional valence Stroop task. In this task, subjects matched the color in which a word of positive, negative, or neutral valence was presented to one of two colored circles presented to the left and right of the word. Because automatic attention is usually drawn toward emotional content, typically with this paradigm both healthy adults (Compton et al., 2003; Stormark, Nordy, & Hugdahl, 1995) and clinical populations with emotional dysregulation (Williams, Matthews, & McLead, 1996) show slower response time (RT) for emotional relative to non-emotional words.
Studies with healthy adults suggested that the junction of VLPFC (BA 45, 47, inferior frontal gyrus) and dorsolateral prefrontal cortex (DLPFC) (BA 9, 46, middle frontal gyrus) may play a crucial role at the interface of cognitive and affective processing (Petrides & Pandya, 2002) and may be impaired in PBD patients (Pavuluri et al., 2008; Rich et al., 2006). Therefore, we conducted this fMRI study to compare and contrast the neural bases of cognitive control of automatic emotion processing in un-medicated children and adolescents with PBD (Type I and II), un-medicated children and adolescents with ADHD, Combined Type, and demographically matched HC.
Based on the literature on attention and cognitive control deficits in PBD (Dickstein et al., 2005; Pavuluri et al., 2006, 2009) and ADHD (Rubia et al., 2001; Seidman et al., 2004), we hypothesized that the two patient groups would show slower RT and lower performance accuracy relative to HC. Moreover, based on previous fMRI studies using Stroop-like tasks with PBD (Mahli et al., 2005; Pavuluri et al., 2008;) and ADHD patients (Bush et al., 1999; Rubia et al., 1999; Schulz et al., 2004, 2005; Tamm et al., 2004), we hypothesized greater group differences for negative as compared to positive word valence (Pavuluri et al., 2008; Posner et al., 2009), and prefrontal cortex (PFC) dysfunction in PBD and ADHD patients relative to HC. Specifically, we predicted greater VLPFC effort to modulate emotions in the PBD group relative to ADHD and HC groups, and dysfunction of PFC, ACC, and striatum due to cognitive control problems in the ADHD group relative to the HC.
METHODS
Participants
Patient participants were recruited from the Child Psychiatry Clinics at the University of Illinois at Chicago (UIC), and healthy controls were recruited through advertising and word-of-mouth from the neighboring Chicago community. This study was approved by the Institutional Review Board at UIC. We obtained an assent for children age 15 and younger, and an informed consent for children age 15 or older. Consent of at least one parent or legal guardian was also obtained. Our subjects (mean age = 13.78 years; SD = 2.47) were 17 un-medicated PBD patients (Type I, manic: n = 5, mixed: n = 5; and Type II, hypomanic: n = 7) (11 female, 6 male; mean age: 14.27, SD = 1.98), 15 un-medicated ADHD patients (Type Combined; 3 female, 12 male; mean age: 12.93, SD = 2.60), and 14 HC (7 female, 7 male; mean age: 14.14, SD = 2.42). Efforts were made to match all subjects on age, socioeconomic status (SES, defined in terms of parents’ education and occupation), handedness, race, and IQ as estimated with the Wechsler Abbreviated Scale of Intelligence (WASI, 1999). For each patient with PBD, we tried to find a patient with ADHD and a healthy control with similar demographic characteristics. The subject and a parent or legal guardian were interviewed using the entire Washington University Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) (Geller et al., 1998) to determine the DSM IV (DSM IV, 1994) Axis I clinical diagnoses of PBD or ADHD, and the absence of these and other psychiatric diagnoses in HC. Clinicians rated all subjects on the Young Mania Rating Scale (YMRS; Young, Biggs, Ziegler, & Meyer, 1978) and the Child Depression Rating Scale-Revised (CDRS-R; Poznanski, Grossman, Buchsbaum, Banegas, Freeman, & Gibbons, 1984). A Parent ADHD Rating Scale IV-Revised (DuPaul, Power, Anastopulous, & Reid, 1998) was also administered. A diagnosis of mania was given for YMRS scores > 12, and a diagnosis of depressive symptoms was given for CDRS-R scores > 40. A Handedness questionnaire (Annett, 1970) was also administered to all participants.
Inclusion criteria for PBD and ADHD patients were as follows: age 10–18 years; a diagnosis of either bipolar disorder Type I with mania or hypomania or Type II, or a diagnosis of ADHD, Type Combined; and consent to be scanned in a medication-free state for the study. For PBD, an inclusion criterion was a score > 12 on the YMRS. Patients were studied if they were medication free, or when medication was withdrawn because the current regimen was ineffective and a wash-out before new medication was warranted. When washout was necessary, medication was reduced gradually over a 3-week period so that patients were drug-free before testing. The mean number of medication-free days before scanning for the ADHD group was 4.2 (SD = 1.3), and for the PBD group was 6.83 (SD = 1.6). Clinical supervision and monitoring was provided during drug-free periods. None of the patients were on fluoxetine or aripiprazole that warrant a longer washout period. With regard to medication exposure before the scanning, of the PBD group 8 patients received second generation antipsychotics, 2 received lithium carbonate, 2 received antiepileptic agents, and 2 received stimulants. Five patients with ADHD group received stimulants.
Axis I diagnoses of bipolar disorder Type I and II, and ADHD were based on the DSM-IV (DSM IV, 1994) criteria. Subjects with ADHD who had a co-morbid DSM-IV diagnosis of bipolar disorder or with PBD who had a co-morbid DSM-IV diagnosis of ADHD were excluded from the study. None of the ADHD subjects had a diagnosis of major depression. Subjects were also excluded from the study if they had a history of head trauma with loss of consciousness for more than 10 minutes, neurological symptoms, speech or hearing difficulties, an IQ score of less than 70, a history of substance abuse, and any contraindications to MRI scans (i.e., metal implants, retractors, braces, pregnancy or possible pregnancy, and claustrophobia).
Any human data included in this manuscript were obtained in compliance with the Institutional Review Board (IRB) regulations of our University.
fMRI session and Emotional Valence Stroop Task
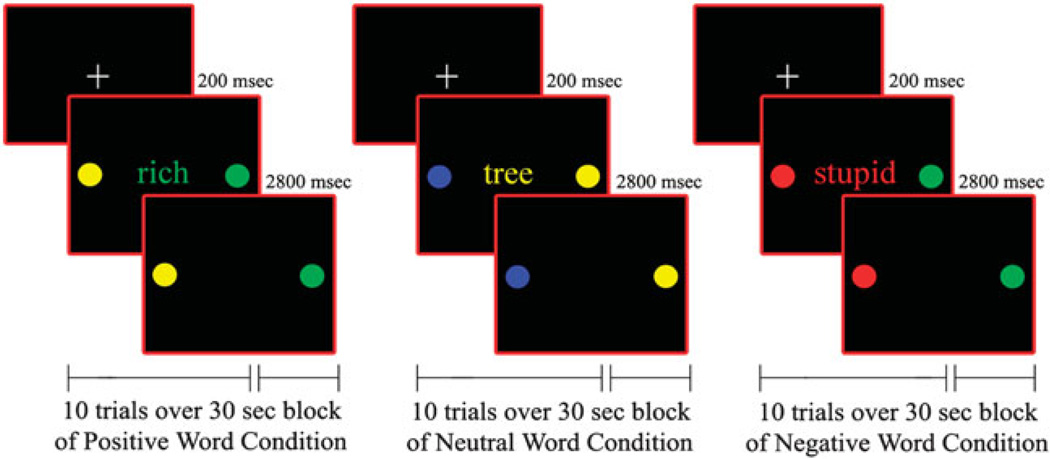
After a brief training session in a mock scanner, participants underwent an fMRI scanning session during which they were administered an emotional valence Stroop Task for approximately 10 min. On each trial participants matched the color (i.e., red, green, yellow, or blue) of a emotional word, presented centrally for 200 ms, to either of two colored circles, presented to the left or right of the word for 3000 ms (Figure 1). There was no inter-stimulus interval. Subjects responded by pressing the left or right response key to match the word color to the correct color dot.
Figure 1.
Illustration of Positive, Neutral and Negative word valence trials for the Color Matching Task.
The words that we adopted in this task were taken from the Lang Affective norms for English Words (ANEW, Bradley & Lang, 1999), were at an 8-year-old reading level, and were equivalent across affect conditions in frequency of usage (Gilhooly & Logie, 1980; Klein, 1964). They had either a negative (e.g., poor), positive (e.g., victory), or neutral (e.g., table) emotional valence (Figure 1). Positive valence words were chosen to reflect feelings of happiness, energy, and accomplishment, whereas negative valence words reflected feelings of sadness, disappointment, and rejection. Neutral valence words were names of objects or common words with no emotional content. No word was repeated during the task. Trials were counterbalanced for matching color and response key.
A block design was used because of the greater statistical power and signal stability it offers relative to an event-related design, especially with clinical populations who have more variable neural activation. Moreover, by summating neural activation over a time period including several consecutive trials, the block design enabled us to examine sustained activation in prefrontal cortex to a greater extent than would be possible using an event-related design. Fifteen 30-s blocks of positive, negative, and neutral words (5 blocks for each word valence) were pseudo-randomly interspersed with fifteen 10-s fixation blocks in a pseudo-random order. There were 10 trials of 3 s each in every word block. A color high-resolution LCD projector projected visual stimuli onto a rear projection screen that was viewed by means of an angled double mirror system mounted on a standard GE head coil.
MRI Protocol
Gradient-echo echo-planar functional imaging and structural acquisitions were performed with a 3.0 Tesla whole body scanner (Signa, General Electric Medical System, Milwaukee, WI). Twenty-five axial slices were acquired (TE = 25 ms; flip angle = 90°; field of view = 20 × 20 cm2; acquisition matrix = 64 × 64; TR = 2.5 s; slice thickness = 5 mm with 1-mm gap). Anatomical images were acquired in the axial plane (three-dimensional spoiled gradient recalled [SPGR], 1.5-mm-thick contiguous axial slices).
Image Processing and Data Analysis
FIASCO software (Functional Imaging Analysis Software-Computational Olio; http://www.stat.cmu.edu/~fiasco/) (Eddy, Figzgerald, Genovese, Mockus, & Noll, 1996) was used to correct the functional neuroimaging data for head motion. This approach implements both 3D motion estimation and correction, removal of slow signal drift, and identification of images with artifacts such as high shot noise or displacement that cannot be readily corrected by motion correction algorithms. We excluded from the analyses individual volumes from the time series if head displacement from the median head position was greater than 1.5 mm, or if head rotation from the median head position was greater than 0.5 degrees. There were no significant group differences in the number of volumes retained after discarding those with motion artifact.
Voxel-wise effect size (r) maps were then calculated for each subject by contrasting activation for Negative and Neutral, as well as Positive and Neutral, word valences. A voxel-wise Fisher’s z transform was also applied to normalize the effect size maps (zr, Rosenthal, 1991). The zr-maps and SPGR anatomical images were imported in Analysis of Functional Neuroimages (AFNI, Cox, 1996) and warped into Talairach space using AFNI’s automated Talairach procedures (Talairach & Tournoux, 1988). Lastly, we resampled each individual talairached functional map to an isotropic 3 × 3 × 3 mm grid.
To examine significant group differences in brain activation across the whole brain a whole-brain voxel-wise analysis of variance (ANOVA) was carried out in AFNI. Group (PBD, ADHD, HC) was the between-subjects factor, and word valence contrast (negative vs. neutral; positive vs. neutral) was the within-subjects factor. A significant group by word valence condition interaction was followed by t tests in AFNI to further examine group differences. To correct Type I error rates for multiple group comparisons (n = 3), we first applied a Bonferroni correction and adopted a voxel-wise probability threshold for significance of p < .016 (i.e., p = .05/3). We then corrected for multiple voxel-wise comparisons in the ANOVA using a contiguity threshold (minimum volume threshold = 297 cubic mm; minimum clustering radius: 3.1 mm, uncorrected p < .01) that ensured an experiment-wise Type 1 error rate of p < .02 (corrected), based on AFNI’s AlphaSim Monte Carlo simulations (Ward, 2000) that were restricted to in-brain voxels. In this way, we identified clusters of voxels for which there were significant group differences for each contrast (negative vs. neutral, positive vs. neutral) with a corrected p < .02.
Finally, based on findings from the whole brain ANOVA, we performed exploratory Spearman correlation analyses to determine the relationship between fMRI activation in relevant anatomical regions of interest (ROIs) and clinical measures (YMRS, CDRS, ADHD Rating Scale IV-R). ROIs were defined in standard Talairach space using AFNI tools. These ROIs in AFNI format, and the rationale for anatomical ROI definition, are available at: http://ccm.psych.uic.edu/Research/NormalBrain/ROI_rules.htm; http://ccm.psych.uic.edu/Research/ResearchProgram/NormalBrain/ROIaffect_rules.aspx.
Demographic, Clinical, and Behavioral Data Analyses
Separate ANOVAs were carried out for each demographic or clinical measure (age, estimated IQ, SES, YMRS, CDRS, ADHD scale) with group (PBD, ADHD, HC) as the between-subjects factor. Significant group effects were followed by planned comparisons between the three groups. Fisher’s exact tests (two-tailed) were carried out for categorical variables (sex, handedness, race). Mean RT and accuracy data were analyzed using repeated measures ANOVAs with group (PBD, ADHD, HC) as a between-subjects factor and word valence (negative, positive, neutral) as a within-subjects factor.
RESULTS
Demographic and Clinical Data
Table 1 summarizes clinical and demographic data for the three groups. Data from one ADHD patient and one of the HC were not available due to technical problems and, therefore, were not included in our analyses. ANOVAs revealed no group differences in age, estimated IQ, or SES. Fisher’s p tests (two-tailed) also failed to reveal group differences in handedness or racial composition, although the ADHD group had a significantly higher proportion of male subjects than the PBD group. The three groups differed in mean YMRS and CDRS-R scores, with significantly higher ratings for the PBD group on the YMRS and the CDRS-R than for the other two groups. While the HC and ADHD groups did not differ on the CDRS-R, the ADHD group had significantly higher YMRS than HC. Group differences were also found on the ADHD Rating Scale IV-R. The PBD and ADHD groups had significantly higher scores on this scale than HC but the former two groups did not differ significantly from one another.
Table 1.
Demographic and clinical characteristics for PBD, ADHD and HC
| PBD (N=17) | ADHD (N=15) | HC (N=14) | ||
|---|---|---|---|---|
| Variable | Mean (SD) | Mean (SD) | Mean (SD) | F, p |
| Age in years (Age range: 10–18) | 14.27 (1.98) | 12.93 (2.60) | 14.14 (2.42) | F(2,43) = 1.37, p=.27 |
| Estimated IQa (IQ range: 80–115) | 98.40 (6.74) | 96.10 (12.69) | 104.5 (10.14) | F(2,43) = 2.84, p=.07 |
| SES | 2.35 (.61) | 2.57 (.94) | 2.00 (.92) | F(2,43) = 2.25, p=.12 |
| YMRS | 14.13 (6.73) | 6.67 (5.25) | .86 (1.51) | F(2,43) = 27.27, p=.000001 |
| PBD>ADHD: p=.001 | ||||
| PBD>HC: p=.001 | ||||
| ADHD>HC: p=.005 | ||||
| CDRS-R | 52.19 (10.12) | 21.25 (7.86) | 19.43 (2.28) | F(2,43) = 95.38, p=.000001 |
| PBD>ADHD: p=.000001 | ||||
| PBD>HC: p=.000001 | ||||
| ADHD vs HC: p=.54. | ||||
| ADHD Rating Scale IV- R | 22.63 (8.63) | 26.93 (8.67) | 2.79 (4.15) | F(2,43) = 41.44, p=.001 |
| PBD vs ADHD: p=.12 | ||||
| PBD>HC: p=.001 | ||||
| ADHD>HC: p=.001 | ||||
| Variable | N (%) | N (%) | N (%) | Fisher’s Exact Test (two-tailed) |
| Sex | ||||
| Male | 6 (35%) | 12 (80%) | 7 (50%) | ADHD vs. PBD: p=.02 |
| Female | 11 (65%) | 3 (20%) | 7 (50%) | ADHD vs. HC: p=.13 |
| PBD vs. HC: p=.50 | ||||
| Handedness | ||||
| Right | 16 (94%) | 15 (100%) | 14 (100%) | ADHD vs. PBD:. p=1.00 |
| Left | 1 (6%) | 0 (0%) | 0 (0%) | ADHD vs. HC: p=1.00 |
| PBD vs. HC: p=1.00 | ||||
| Race Composition | ||||
| Caucasian | 8 (47%) | 3 (20%) | 7 (50%) | ADHD vs. PBD:. p=.15 |
| Other | 9 (53%) | 12 (80%) | 7 (50%) | ADHD vs. HC: p=.13 |
| PBD vs. HC: p=1.00 | ||||
Note. YMRS = Young Mania Rating Scale; CDRS-R = Child Depression Rating Scale-Revised; SES = socioeconomic status; PBD = Pediatric Bipolar Disorder; ADHD = Attention Deficit Hyperactivity Disorder; HC = Healthy Control.
Estimated with Wechsler Abbreviated Scale of Intelligence (WASI; Matrix Reasoning and Vocabulary Subtests).
Behavioral Performance Results
Mean RT and accuracy data for the PBD, ADHD, and HC groups are presented in Table 2. Planned comparisons on the significant group effect [F (2,41) = 5.74; p = .006] indicated that, across all word valence presentations, the PBD [F (1,41) = 5.81; p = .02] and ADHD [F (1,41) = 10.79, p = .002] groups exhibited significantly slower RT than HC, but did not differ from each other. Moreover, there was a significant interaction of group by word valence [F (4,82) = 3.73; p = .008]. To examine the source of this interaction, planned comparisons were conducted to elucidate the effects of word emotional valence on RT for each group. For the PBD group, RT for positive words was significantly slower than RT for neutral [F (1,41) = 4.11; p = .05] and negative [F (1,41) = 7.48; p = .009] words, which did not differ from each other. For the ADHD group, RT for negative words was significantly slower than RT for positive words [F (1,41) = 5.12; p = .03], but no significant differences were found between RT for neutral words and RT for either negative or positive words. For the HC group RT did not differ with word valence.
Table 2.
Mean response time and accuracy for the emotional Stroop task in patients with pediatric bipolar disorder (PBD), attention deficit hyperactivity disorder (ADHD), and healthy controls (HC)
| PBD | ADHD | HC | |
|---|---|---|---|
| RT (in ms) | Mean (SD) | Mean (SD) | Mean (SD) |
| Positive words | 710 (221) | 669 (145) | 525 (96) |
| Negative words | 632 (150) | 734 (189) | 557 (121) |
| Neutral words | 637 (146) | 714 (175) | 517 (106) |
| Accuracy (% correct) | % (SD) | % (SD) | % (SD) |
| Positive words | 88 (22) | 84 (12) | 96 (3) |
| Negative words | 85 (22) | 85 (13) | 95 (8) |
| Neutral words | 84 (22) | 85 (12) | 95 (3) |
Additional planned comparisons were conducted to examine group differences in the effects of word emotional valence on RT. For negative words, the ADHD group had significantly slower RT than HC [F (1,41) = 9.27; p = .004], but differences between the PBD group and the other two groups were not significant. For positive words, the PBD [F (1,41) = 9.21; p = .004] and ADHD [F (1,41) = 5.63; p = .02] groups had significantly slower RT than the HC group, but did not differ from each other. Similarly, RT for neutral words, in the PBD group [F (1,41) = 4.90; p = .03] and the ADHD group [F (1,41) = 13.10; p = .0008] were significantly slower than in HC, but did not differ from each other.
With regard to the accuracy data, there were no significant results.
fMRI Results
The whole-brain ANOVA revealed a significant group by condition interaction [F (2,41) = 4.44; p < .02] that was examined further with planned comparisons. We report results for group differences in brain activation for negative versus neutral and positive versus neutral word condition contrasts.
Group differences for the negative versus neutral word contrast (Table 3; Figure 2)
Table 3.
Talairach coordinates and t values of peak activation for regions (signifi cant clusters at p < .020 with contiguity threshold) for which contrasts (Negative vs. Neutral or Positive vs Neutral) differ between groups
| Talairach Coordinates for peak values | Area | BA | Volume (mm3) | t value for peak values | |
|---|---|---|---|---|---|
| Negative vs. Neutral | |||||
| PBD > ADHD | |||||
| 50, 35, 14 | R inferior FG (VLPFC) | 45/46 | 1080 | 4.10 | |
| 32, −46, 65 | R precuneus | 7 | 405 | 3.10 | |
| 5, −43, 35 | R posterior CG | 31 | 729 | 3.01 | |
| −1, −55, 23 | L posterior CG | 31 | 1269 | 2.85 | |
| 29, −34, −4 | R parahippocampal gyrus | 297 | 2.97 | ||
| ADHD > PBD | |||||
| None | None | None | None | ||
| PBD > HC | |||||
| 2, 62, 12 | R ventral-medial FG | 10 | 351 | 2.57 | |
| −2, 62, 12 | L ventral-medial FG | 10 | 270 | 2.35 | |
| 50, 29, 32 | R middle FG (DLPFC) | 9,46 | 2673 | 3.46 | |
| 41, 35, 14 | R inferior FG (VLPFC) | 47,10 | 297 | 3.38 | |
| 2, 35, 20 | R dorsal ACC | 32 | 297 | 3.40 | |
| 2, −55, 26 | R posterior CG | 31 | 2619 | 2.91 | |
| −61, −13, −4 | L middle TG | 21 | 297 | 2.75 | |
| 2, −64, 56 | R precuneus | 7 | 1755 | 3.02 | |
| −13, −58, 35 | L precuneus | 7 | 999 | 3.43 | |
| 41, −61, 47 | R inferior PL | 40 | 351 | 3.38 | |
| 47, −46, 29 | R supramarginal gyrus | 40 | 351 | 3.81 | |
| HC > PBD | |||||
| None | None | None | None | ||
| ADHD > HC | |||||
| 41, 53, 17 | R superior FG | 10, 46 | 513 | 3.49 | |
| 50, 32, 29 | R middle FG (DLPFC) | 9,46 | 486 | 2.55 | |
| −46, −55, 47 | L inferior PL | 40 | 459 | 4.09 | |
| −25, −64, −31 | L cerebellar tonsil | 324 | 4.32 | ||
| 41, −61, −25 | R cerebellar tuber | 297 | 2.66 | ||
| HC > ADHD | |||||
| 29, 59, −13 | R OFC | 11 | 324 | 3.39 | |
| 50, 32, 11 | R inferior FG (VLPFC) | 45/46 | 999 | 4.20 | |
| −49, 11, −28 | L superior TG | 21 | 432 | 3.09 | |
| Positive vs. Neutral | |||||
| PBD > ADHD | |||||
| −16, 50, 32 | L superior FG | 9 | 351 | 2.44 | |
| −40, 17, −19 | L inferior FG (VLPFC) | 47 | 1107 | 3.12 | |
| −4, −19, 11 | L thalamus | 297 | 3.10 | ||
| 50, −19, −10 | R superior TG | 21 | 837 | 3.06 | |
| 35, −19, −10 | R parahippocampal gyrus | 297 | 2.69 | ||
| ADHD > PBD | |||||
| None | None | None | None | ||
| PBD > HC | |||||
| 5, 62, 14 | R ventro-medial FG | 10 | 378 | 2.99 | |
| −40, 20, −16 | L inferior FG (VLPFC) | 47 | 405 | 4.55 | |
| 44, −55, 50 | R inferior PL | 40 | 1323 | 2.92 | |
| 32, −70, 35 | R precuneus | 19 | 378 | 2.74 | |
| −10, −52, 65 | L precuneus | 7 | 351 | 2.93 | |
| HC > PBD | |||||
| None | None | None | None | ||
| ADHD > HC | |||||
| 29, 56, 32 | R superior/middle FG | 9, 10 | 783 | 3.68 | |
| 47, 29, 35 | R middle FG (DLPFC) | 9 | 351 | 3.31 | |
| −4, 26, −1 | L subgenual ACC | 24 | 324 | 2.97 | |
| −1, 8, 26 | L dorsal ACC | 24 | 486 | 3.61 | |
| 41, −55, 50 | R superior PL | 40 | 1242 | 3.01 | |
| HC > ADHD | |||||
| −34, −19, −13 | L parahippocampal gyrus | 297 | 3.23 | ||
| −49, 5, −28 | L Middle TG | 21 | 378 | 5.08 |
Note. ACC = anterior cingulate cortex; CG = cingulate gyrus; FG = frontal gyrus; TG = temporal gyrus; OG = occipital gyrus; PL = parietal lobule; OFC = orbitofrontal cortex; VLPFC = ventrolateral prefrontal cortex; DLPC = dorsolateral prefrontal cortex; HC = Healthy Control group; PBD = Pediatric Bipolar Disorder group; ADHD = Attention-Deficit Hyperactivity Disorder group; BA = Brodmann’s Area; L = Left; R = Right.
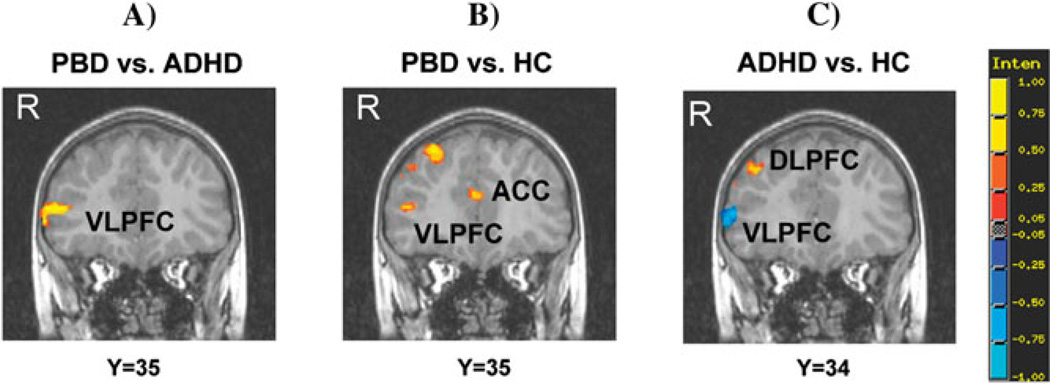
Figure 2.
Group differences in significant clusters of brain activation for the Negative versus Neutral word contrast. Red indicates greater activation in the first group compared with the second group. Blue indicates reduced activation in the first group compared with the second group. PBD = Pediatric Bipolar Disorder group; ADHD = Attention Deficit Hyperactivity Disorder group; HC = Healthy Controls. VLPFC = ventrolateral prefrontal cortex; DLPFC = dorsolateral prefrontal cortex; ACC = anterior cingulate cortex. Y corresponds to the TLRC coordinate in the coronal axis for the coronal brain images presented.
Only the PBD group exhibited greater activation than the ADHD group at the junction of right middle and inferior frontal gyrus (VLPFC) (Figure 2a), in bilateral posterior cingulate gyrus, right precuneus and parahippocampal gyrus. The PBD group also showed greater activation than HC in right and left ventral-medial PFC (VMPFC), VLPFC (inferior frontal gyrus), DLPFC (middle frontal gyrus), and cingulate regions (dorsal anterior cingulate cortex, posterior cingulate gyrus) (Figure 2b), in parietal regions (bilateral precuneus and right inferior parietal lobe), in right supramarginal gyrus, and in left middle temporal gyrus. Relative to HC, the ADHD group exhibited greater activation in right DLPFC (superior and middle frontal gyrus), left inferior parietal lobe, and cerebellar regions (left tonsil and right tuber) and reduced activation in right orbito-frontal cortex, right VLPFC (inferior frontal gyrus) (Figure 2c), and left superior temporal gyrus.
Group differences for the positive versus neutral word contrast (Table 3; Figure 3)
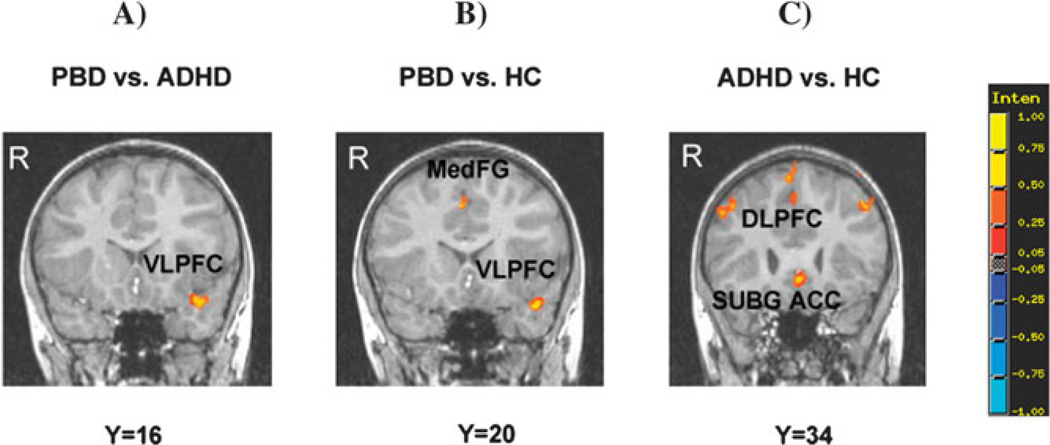
Figure 3.
Group differences in significant clusters of brain activation for the Positive versus Neutral word contrast. Red indicates greater activation in the first group compared with the second group. Blue indicates reduced activation in the first group compared with the second group. Legend as in Figure 2. Subg.ACC = subgenual anterior cingulate cortex; MedFG = medial frontal gyrus.
Compared with the ADHD group, the PBD group exhibited greater activation in left DLPFC (superior frontal gyrus) and left VLPFC (inferior frontal gyrus) (Figure 3a), left thalamus, right parahippocampal gyrus and superior temporal gyrus, and no reduced activation. Compared with HC, the PBD group showed greater activation in right ventro-medial frontal gyrus, left VLPFC (inferior frontal gyrus) (Figure 3b), right supramarginal gyrus and parietal regions (right inferior parietal lobule and bilateral precuneus), and no reduced activation. The ADHD group, relative to the HC group, exhibited greater activation in right DLPFC (superior and middle frontal gyrus), left cingulate regions (left subgenual ACC, left dorsal ACC) (Figure 3c) and in right superior parietal lobule, and reduced activation in left parahippocampal gyrus and left middle temporal gyrus.
Correlations Between ROI Activation and Clinical Measures
Spearman correlation analyses were performed between clinical measures (YMRS, CDRS, and ADHD Rating Scale IV-R) and activation in anatomical DLPFC, VLPFC, and ACC ROIs for the PBD group in the two experimental conditions. After correcting for multiple comparisons no significant correlations were found.
DISCUSSION
To our knowledge, this is the first fMRI study to compare and contrast the impact of emotion on cognitive processing concurrently in un-medicated PBD patients (Type I and II), un-medicated ADHD patients (Combined type), and HC. While it is often difficult to differentiate between PBD and ADHD in terms of behavioral performance, our fMRI results provide preliminary evidence that adolescents with PBD and ADHD may exhibit a phenotypic variation in the way neural circuits engage in emotion regulation, especially with regard to VLPFC functions. The central finding of this study is that for the negative versus neutral word contrast, the PBD group showed greater VLPFC activation than both the ADHD and HC groups, while the ADHD group engaged the VLPFC less than the other two groups. This initial finding suggests increased effort to manage emotional impact on cognitive processing in PBD, and reduced engagement of these circuitries in ADHD. Similar group differences were found during the positive versus neutral word contrast.
With regard to behavioral performance, there were no significant group differences in accuracy. For RT, the two patient groups were significantly slower than HC overall, but did not differ from each other. Also, while RT for the PBD group was significantly faster for negative than positive words, the ADHD group exhibited the opposite pattern, and no difference was observed for the HC. The group differences in behavioral performance, however, were not associated with differences in neural processing. This may be due to the task being too easy to reveal significant group differences in performance, or to power limitations because of the small sample and the small number of task trials due to the need for a short fMRI scanning session. Alternatively, these findings may suggest dissociation between neural activation and behavioral performance. It is possible that the patient groups engaged alternate neural circuits or the same circuits to different extents, relative to HC, to obtain performance levels similar to HC.
Differential VLPFC Dysfunction in PBD and ADHD
In line with previous findings (Dickstein et al., 2005; Galanter & Leibenluft, 2008), the PBD and ADHD groups showed similar RT and accuracy patterns, and similar scores on the ADHD Rating Scale IV-R. These results are also in line with neuropsychological studies on PBD (Dickstein et al., 2005; Pavuluri et al., 2006) and ADHD (Doyle et al., 2005; Rubia et al., 2001), which suggest similar problems with attention and cognitive control in these two disorders. Nevertheless, in the present study, the PBD and ADHD groups differed in terms of VLPFC engagement but were similar in terms of DLPFC engagement during the emotional Stroop task. Specifically, while the PBD group, compared with the ADHD group and HC, showed increased activity in emotion regulation systems including the VLPFC (Lagopoulos & Mahli, 2007; Mahli et al., 2005; Pavuluri et al., 2008), the ADHD group engaged this region less than the PBD group and HC. On the other hand, both patient groups engaged the DLPFC in a similar way, and exhibited greater DLPFC and posterior parietal activation than HC, which can be explained in terms of greater working memory and attention effort in the patient groups relative to HC (Compton et al., 2003; MacDonald, Cohen, Stenger, & Carter, 2000; Manes et al., 2002; Posner et al., 2009).
For negative versus neutral words, the PBD group exhibited greater activation than the ADHD group in right VLPFC, precuneus and parahippocampal gyrus, and bilateral posterior cingulate gyrus. Similarly, for positive versus neutral words, PBD patients had greater activation than ADHD patients in left VLPFC, left thalamus, right parahippocampal gyrus, and right superior temporal gyrus. The VLPFC is involved in response inhibition (Aron et al., 2003; Kemmotsu, Vollalobos, Gaffrey, Courchesne & Muller, 2005; Konishi, Nakajima, Uchida, Kikyo, Kameyama, & Miyashita, 1999; Menon et al., 2001; Pavuluri et al., 2008), as well as evaluation and modulation of emotional processes (Botvinick, Braver, Barch, Carter, & Cohen, 2001). The posterior cingulate gyrus relays valence information to PFC, limbic structures and precuneus (Posner et al., 2009). Moreover, the parahippocampal gyrus is involved in encoding of arousing emotional words (Posner et al., 2009), and the superior and middle temporal gyri monitor the emotional valence of words (Compton et al., 2003).
Previous emotional Stroop studies found decreased VLPFC activation in euthymic, mostly Type I, bipolar patients, relative to HC (Lagopoulos & Mahli, 2007; Malhi et al., 2005; Pavuluri et al., 2008). Conversely, the current study found increased VLPFC and VMPFC, compared with HC, in our sample of manic, mixed, and hypomanic patients, similar to the findings reported by Rich et al. (2006). It is possible that PBD patients may have to expend additional effort for emotion regulation during more severe illness states. A more systematic comparison of neural function across varying mood states in PBD patients compared with HC will be needed to examine this possibility. If the present preliminary evidence is replicated, it would suggest that in PBD the extent of alteration of VLPFC activity relative to HC may depend on severity of illness or mood status.
With regard to the ADHD group, previous studies have observed results similar to ours, with decreased VLPFC activation in ADHD groups compared with HC, on tasks of selective attention (Bush et al., 1999; Vaidya et al., 1998) and response inhibition (Casey et al., 1997; Passarotti et al., in press; Rubia et al., 1999; Tamm et al., 2004). Decreased VLPFC activation in ADHD patients relative to both PBD patients and HC was also found on a motor inhibition task that did not involve emotion processing (Passarotti et al., in press). Taken together, these findings suggest that VLPFC modulation of inhibition processes, whether involving inhibition of cognitive processes (as in previous studies) or inhibition of emotional processes (as in the present study), may be dysfunctional in individuals with ADHD compared with both HC and PBD patients.
The present findings suggest differential engagement of regulatory circuits in patients with PBD and ADHD, relative to one another as well as to HC, on tasks demanding inhibition of automatic emotion processing. In PBD patients relative to HC, a similar pattern of greater VLPFC and VMPFC activation was found for negative and positive words, although neural recruitment was greater for negative words. It is possible that when inhibition of emotion processing is necessary, the neural interface between cognition and emotion processing (i.e., VLPFC and ventral ACC) may become overactive in patients with PBD. This is potentially a compensatory mechanism that over-engages control systems in an attempt to modulate the impact of emotional arousal on cognitive processes in the presence of excessive emotional reactivity (Blumberg et al., 2003, Frangou, Haldane, Roddy, & Kumari, 2005; Pavuluri et al., 2007, 2008). Conversely, the ADHD group may underutilize such higher order control centers. In fact, the ADHD group engaged the VLPFC less than HC for negative words and less than PBD patients for both negative and positive words. This finding may be interpreted as reduced engagement of cortical inhibition and emotion modulating circuits in ADHD patients (Passarotti et al., in press; Rubia et al., 1999; Tamm et al., 2004). Thus, while both patient groups may clinically show increased distractibility or impact of emotions on behavior, they may have this phenotypic deficit for different reasons at the neural level. Future studies should further examine the role of VLPFC dysfunction in PBD and ADHD phenotypes.
Neural Activation and Emotional Valence in PBD and ADHD Patients
Compared with HC, both clinical groups exhibited differential neural engagement for negative and positive words. This finding is preliminary, and its relevance is tempered by the fact that group differences in behavioral performance were not robustly modulated by word valence. The PBD group engaged a more extensive network of regions than HC, including ventral–medial frontal as well as dorsal and posterior cingulate and temporal regions, for negative than for positive words. These findings are in line with evidence that negative words may capture attention and increase arousal to a greater degree than positive words (Compton et al., 2003; Posner et al., 2009; Stormark et al., 1995; Williams et al., 1996). In contrast, the ADHD group showed similar VLPFC activation but increased subgenual and dorsal ACC activation for positive words compared with HC. The dorsal ACC aids the DLPFC in resolving cognitive conflict (Bush, Luu, & Posner, 2000), while the subgenual ACC aids the VLPFC in modulating sub-cortical emotional processes (Botvinick et al., 2001; Bush et al., 2000). It is possible that the ADHD patients were more able to engage VLPFC and rostral ACC when arousal was reduced, as can occur with positive versus negative words (Posner et al., 2009). Moreover, the ADHD group exhibited greater cerebellar activity than HC only for negative words, which may be associated with greater effort for cognitive control (Rubia et al., 1999) in response to these stimuli (Stormark et al., 1995).
Study Limitations and Future Directions
Some limitations of the present study offer opportunities for further investigations. The present study did not find significant correlations between clinical measures and brain activation in ROIs that yielded significant group differences, possibly because of power limitations. Therefore, future studies need larger patient samples to further explore patterns of brain dysfunction in PBD and ADHD and how they relate to symptom measures. Also, despite our efforts to control for sex composition across groups, we had a higher proportion of males in the ADHD group compared with the PBD group. A better control for sex composition will help disentangle possible gender effects in PFC dysfunction in these disorders. Future studies should also use an event-related fMRI design, instead of a block design, to differentiate between neural activation for correct and incorrect responses. An experimental paradigm that directly contrasts cognitive control processes with and without emotional interference will also help clarify whether in PBD and ADHD emotional dysregulation disrupts cognitive functions, or there is an inherent deficit in the cognitive control of emotion processing. Finally, the present study did not examine comorbidity effects. Adler, DelBello, Mills, Schmithorst, Holland, and Strakowski (2005) found reduced prefrontal activation and increased recruitment of posterior brain regions during an attention task in bipolar adolescents with comorbid ADHD as compared to bipolar adolescents without ADHD. Furthering our understanding of how a combined presence of these disorders (or high degree of shared symptomatology) relates to brain dysfunction is crucial and has important implications for treatment.
CONCLUSIONS
Our results provide preliminary evidence for phenotypic differences in neural response during cognitive control of emotion processing in patients with PBD compared with patients with ADHD. Increased VLPFC and ACC activation in PBD patients compared with HC suggest compensatory over-activity of the cognitive and emotional interface because of underlying circuitry dysfunction. Conversely, reduced VLPFC activation in ADHD compared with both HC and PBD patients may indicate a deficient function of the underlying regulation systems in ADHD.
ACKNOWLEDGMENTS
We are grateful to the children and families who participated in this study. Thanks also to Erin M. Harral for her help with subject testing and data analyses. This work is supported by NIH K23 RR18638-01, the Dana Foundation and NARSAD. Information in this manuscript and the manuscript itself has never been published either electronically or in print.
Dr. Pavuluri’s work unrelated to this manuscript is supported by NARSAD Independent Investigator Award, NICHD, GlaxoSmithKline-NeuroHealth, Abbott Pharmaceuticals, and Janssen Research Foundation. Dr. Sweeney, also unrelated to this work, has received support from NIH, GlaxoSmithKline, Astra-Zeneca, Janssen and Eli Lilly.
Footnotes
Dr. Passarotti has no financial relationships to disclose.
REFERENCES
- Adler CM, DelBello MP, Mills NP, Schmithorst V, Holland S, Strakowski SM. Comorbid ADHD is associated with altered patterns of neuronal activation in adolescents with bipolar disorder performing a simple attention task. Bipolar Disorders. 2005;7:577–588. doi: 10.1111/j.1399-5618.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Bartzokis G, Grieder T, Curran J, Jimenez T, Leight K, et al. An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biological Psychiatry. 2000;48:147–162. doi: 10.1016/s0006-3223(00)00836-2. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders IV. 4th ed. Washington, DC: American Psychiatric Association Press; 1994. [Google Scholar]
- Annett M. A classification of hand preference by association analysis. British Journal of Psychology. 1970;61:303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Leung HC, Skudlarski P, Lacadie C, Fredericks CA, Harris BC, et al. A functional magnetic resonance imaging study of bipolar disorder: State and trait-related dysfunction in ventral prefrontal cortices. Archives of General Psychiatry. 2003;60:599–607. doi: 10.1001/archpsyc.60.6.601. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Braaten EB, Rosén LA. Self-regulation of affect in attention-deficit hyperactivity disorder (ADHD) and non-ADHD boys: Differences in empathic responding. Journal of Consulting and Clinical Psychology. 2000;68:313–321. doi: 10.1037/0022-006X.68.2.313. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Affective Norms for English Words (ANEW): Stimuli, Instruction Manual and Affective Ratings. Gainsville, FL: University of Florida Center for Research in Psychophysiology; 1999. [Google Scholar]
- Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, et al. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the counting Stroop. Biological Psychiatry. 1999;45:1542–1552. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Science. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Casey RJ. Emotional competence in children with externalizing and internalizing disorders. In: Lewis M, Sullivan M, editors. Emotional Development in Atypical Children. Hillsdale, NJ: Erlbaum; 1996. pp. 161–183. [Google Scholar]
- Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, et al. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: a functional magnetic resonance imaging investigation. Archives of General Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- Compton RJ, Banich MT, Mohanty A, Milham MP, Herrington J, Miller GA, et al. Paying attention to emotion: An fMRI investigation of cognitive and emotional Stroop task. Cognitive Affective and Behavioral Neuroscience. 2003;3:81–96. doi: 10.3758/cabn.3.2.81. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI. Software for analysis and visualization of functional magnetic resonance neuroimages. Computational Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Desman C, Schneider A, Ziegler-Kirbach E, Petermann F, Mohr B, Hampel P. Behavioural inhibition and emotion regulation among boys with ADHD during a go-/nogo-task. Praxis der Kinderpsychologie und Kinderpsychiatrie. 2006;55:328–349. [PubMed] [Google Scholar]
- Dickstein DP, Garvey M, Pradella AG, Greenstein DK, Sharp WS, Castellanos FX, et al. Neurologic examination abnormalities in children with bipolar disorder or attention deficit/hyperactivity disorder. Biological Psychiatry. 2005;58:517–524. doi: 10.1016/j.biopsych.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Dickstein DP, Rich BA, Roberson-Nay R, Berghorst L, Vinton D, Pine DS, et al. Neural activation during encoding of emotional faces in pediatric bipolar disorder. Bipolar Disorders. 2007;9:679–692. doi: 10.1111/j.1399-5618.2007.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle AE, Willcutt EG, Seidman LJ, Biederman J, Chouinard VA, Silva J, et al. Attention-deficit/hyperactivity disorder endophenotypes. Biological Psychiatry. 2005;57:1324–1335. doi: 10.1016/j.biopsych.2005.03.015. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopulous AD, Reid R. ADHD Rating Scale, Vol. IV. New York: Guilford Press; 1998. [Google Scholar]
- Eddy WF, Fitzgerald M, Genovese CR, Mockus A, Noll DC. Functional image analysis software - computational olio. In: Prat A, editor. Proceedings in Computational Statistics. Heidelberg: Physica-Verlag; 1996. pp. 39–49. [Google Scholar]
- Frangou S, Haldane M, Roddy D, Kumari V. Evidence for deficit in tasks of ventral, but not dorsal, prefrontal executive function as an endophenotypic marker for bipolar disorder. Biological Psychiatry. 2005;58:838–839. doi: 10.1016/j.biopsych.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Friedman SR, Rapport LJ, Lumley M, Tzelepis A, VanVoorhis A, Stettner L, et al. Aspects of social and emotional competence in adult attention-deficit/hyperactivity disorder. Neuro-psychology. 2003;17:50–58. [PubMed] [Google Scholar]
- Galanter CA, Leibenluft E. Frontiers between attention deficit hyperactivity disorder and bipolar disorder. Child and Adolescent Psychiatric Clinics of North America. 2008;17:325–346. doi: 10.1016/j.chc.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Geller B, Warner K, Williams M, Zimerman B. Prepubertal and young adolescent bipolarity versus ADHD: Assessment and validity using the WASH-U-KSADS, CBCL, and TRF. Journal of Affective Disorders. 1998;51:93–100. doi: 10.1016/s0165-0327(98)00176-1. [DOI] [PubMed] [Google Scholar]
- Gilhooly KJ, Logie RH. Age of acquisition, imagery, concreteness, familiarity and ambiguity measures for 1,944 words. Behaviour Research Methods and Instrumentation. 1980;12:395–427. [Google Scholar]
- Gruber SA, Rogowska J, Yurgelun-Todd DA. Decreased activation of the anterior cingulate in bipolar patients: An fMRI study. Journal of Affective Disorders. 2004;82:191–201. doi: 10.1016/j.jad.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Kemmotsu N, Villalobos ME, Gaffrey MS, Courchesne E, Muller RA. Activity and functional connectivity of inferior frontal cortex associated with response conflict. Cognitive Brain Research. 2005;24:335–342. doi: 10.1016/j.cogbrainres.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Klein GS. Semantic power measured through the interference of words with color-naming. American Journal of Psychology. 1964;77:576–588. [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain. 1999;122:981–991. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- Lagopoulos J, Malhi GS. A functional magnetic resonance imaging study of emotional Stroop in euthymic bipolar disorder. NeuroReport. 2007;18:1583–1587. doi: 10.1097/WNR.0b013e3282efa07a. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biological Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Lagopoulos J, Sachdev PS, Ivanovski B, Shnier R. An emotional Stroop functional MRI study of euthymic bipolar disorder. Bipolar Disorders. 2005;7 Suppl. 5:58–69. doi: 10.1111/j.1399-5618.2005.00255.x. [DOI] [PubMed] [Google Scholar]
- Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, et al. Decision-making processes following damage to the prefrontal cortex. Brain. 2002;125:624–639. doi: 10.1093/brain/awf049. [DOI] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a go/no-go response inhibition task. Human Brain Mapping. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN. Neural correlates of response inhibition deficits in pediatric bipolar disorder and attention deficit hyperactivity disorder. Psychiatry Research: Neuroimaging. doi: 10.1016/j.pscychresns.2009.07.002. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, O’Connor MM, Harral EM, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biological Psychiatry. 2007;62:158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Shenkel LS, Aryal S, Harral E, Hill K, Herbener ES, et al. Neurocognitive function in unmedicated manic and medicated euthymic pediatric bipolar patients. American Journal of Psychiatry. 2006;163:286–293. doi: 10.1176/appi.ajp.163.2.286. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, O’Connor MM, Harral EM, Sweeney JA. An fMRI study of the interface between affective and cognitive neural circuitry in pediatric bipolar disorder. Psychiatry Research. 2008;162:244–245. doi: 10.1016/j.pscychresns.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti AM. Neural bases of emotional processing in pediatric bipolar disorder. Expert Review of Neurotherapeutics. 2008;8:1381–1387. doi: 10.1586/14737175.8.9.1381. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. An fMRI study of the neural correlates of incidental versus directed emotion processing in Pediatric Bipolar Disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(3):308–318. doi: 10.1097/CHI.0b013e3181948fc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and cortico-cortical connection patterns in the monkey. European Journal of Neuroscience. 2002;16:291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- Posner J, Russel JA, Gerber A, Gorman D, Colibazzi T, Yu S, et al. The neurophysiological bases of emotion: An fMRI study of affective circumplex using emotion-denoting words. Human Brain Mapping. 2009;30:883–895. doi: 10.1002/hbm.20553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanski E, Grossman J, Buchsbaum Y, Banegas M, Freeman L, Gibbons R. Preliminary studies of the reliability and validity of the children’s depression rating scale. Journal of the American Academy of Child and Adolescent Psychiatry. 1984;23:191–197. doi: 10.1097/00004583-198403000-00011. [DOI] [PubMed] [Google Scholar]
- Psychological Corporation. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Harcourt Brace & Company; 1999. [Google Scholar]
- Rapport LJ, Friedman S, Tzelepis A, VanVoorhis A. Experienced emotion and affect recognition in adult attention-deficit hyperactivity disorder. Neuropsychology. 2002;16:102–110. doi: 10.1037//0894-4105.16.1.102. [DOI] [PubMed] [Google Scholar]
- Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berg-horst LH, McClure EB, et al. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proceedings of the National Academy of Sciences of the United States America. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. Meta-analytic procedures for social research. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Roth RM, Koven NS, Randolph JJ, Flashman LA, Heather S, Ricketts SM, et al. Functional magnetic resonance imaging of executive control in bipolar disorder. NeuroReport. 2006;17:1085–1089. doi: 10.1097/01.wnr.0000227979.06013.57. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, et al. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: A study with functional MRI. American Journal of Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- Rubia K, Taylor E, Smith H, Oksannen H, Overmeyer S, Newman S. Neuropsychological analyses of impulsiveness in childhood hyperactivity. British Journal of Psychiatry. 2001;179:138–143. doi: 10.1192/bjp.179.2.138. [DOI] [PubMed] [Google Scholar]
- Shah MP, Wang F, Kalmar JH, Chepenik LG, Tie K, Pittman B, et al. Role of variation in the serotonin transporter protein gene (SLC6A4) in trait disturbances in the ventral anterior cingulate in bipolar disorder. Neuropsychopharmacology. 2009;34:1301–1310. doi: 10.1038/npp.2008.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KP, Fan J, Tang CY, Newcorn JH, Buchsbaum MS, Cheung AM, et al. Response inhibition in adolescents diagnosed with attention deficit hyperactivity disorder during childhood: An event-related FMRI study. American Journal of Psychiatry. 2004;161:1650–1657. doi: 10.1176/appi.ajp.161.9.1650. [DOI] [PubMed] [Google Scholar]
- Schulz KP, Tang CY, Fan J, Marks DJ, Newcorn JH, Cheung AM, et al. Differential prefrontal cortex activation during inhibitory control in adolescents with and without childhood attention-deficit/hyperactivity disorder. Neuropsychology. 2005;19:390–402. doi: 10.1037/0894-4105.19.3.390. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Bush G. Brain function and structure in adults with attention-deficit/hyperactivity disorder. Psychiatry Clinics of North America. 2004;27:323–347. doi: 10.1016/j.psc.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Macris N, Monuteux MC, Boriel DL, Kelkar K, Kennedy DN, Caviness VS, Bush G, Aleardi M, Faraone SV, Biederman J. Dorsolateral prefrontal and anterior cingulated cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biological Psychiatry. 2006;60:1071–1080. doi: 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Singh MK, DelBello MP, Kowatch RA, Strakowski SM. Co-occurrence of bipolar and attention-deficit hyperactivity disorder in children. Bipolar Disorders. 2006;8:710–720. doi: 10.1111/j.1399-5618.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- Skirrow C, McLoughlin G, Kuntsi J, Asherson P. Behavioral, Neurocognitive and treatment overlap between attention-deficit/hyperactivity disorder and mood instability. Expert Review of Neurotherapeutics. 2009;9:489–503. doi: 10.1586/ern.09.2. [DOI] [PubMed] [Google Scholar]
- Stormark KM, Nordy H, Hugdahl K. Attentional shifts to emotionally charged cues: Behavioral and ERP data. Cognition and Emotion. 1995;9:507–523. [Google Scholar]
- Strakowski SM, Adler CM, Holland SK, Mills NP, DelBello MP, Eliassen JC. Abnormal FMRI brain activation in euthymic bipolar disorder patients during a counting Stroop interference task. American Journal of Psychiatry. 2005;162:1697–1705. doi: 10.1176/appi.ajp.162.9.1697. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotactic Atlas of the Human Brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Tamm L, Menon V, Ringel J, Reiss AL. Event-related fMRI evidence of frontotemporal involvement in aberrant response inhibition and task switching in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:1430–1440. doi: 10.1097/01.chi.0000140452.51205.8d. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, et al. Selective effects of methylphenidate in attention deficit hyperactivity disorder: A functional magnetic resonance study. Proceedings of The National Academy of Sciences of the United States America. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Ward B. Bethesda: National Institute Of Health; 2000. ALPHASIM. http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf. [Google Scholar]
- Williams JM, Matthews A, McLead C. The emotional Stroop task and psychopathology. Psychological Bulletin. 1996;120:3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]