Summary
Background
Atrial fibrillation (AF) is the most common sustained arrhythmia affecting over 700,000 individuals in Japan and 2.2 million in the USA. The proper management of patients with AF is critical due to the well-documented association with heart failure and stroke. A strategy to better define the emergency department (ED) management, admission decisions, and spectrum of risk from low to high is needed.
Methods and subjects
The Atrial Fibrillation and Flutter Outcomes & Risk Determination investigation is a prospective, observational cohort study to develop a multivariable clinical prediction rule that accurately estimates risk for adverse outcomes in patients presenting to the ED with symptomatic AF. We will enroll 430 patients at 2 sites who present to the ED with symptomatic AF defined as a new or established diagnosis of AF or atrial flutter that require ED evaluation for a complaint thought related to their rhythm disturbance. The study’s endpoint is to develop an accurate, objective, internally validated, reliable clinical prediction rule to risk-stratify ED patients presenting with AF exacerbations. The rule will incorporate patient history and examination findings and laboratory studies obtained upon ED presentation, as well as trends over the first 2 hours of care. This investigation’s primary outcome is the incidence of any AF-related adverse event at 5 days and 30 days. We expect to complete the study by the end of 2014. The study was registered at Clinicaltrials.gov NCT01138644.
Keywords: Atrial fibrillation, Atrial flutter, Prognosis, Emergency care
Introduction
Atrial fibrillation (AF), the most common sustained cardiac arrhythmia in clinical practice affects more than 700,000 individuals in Japan and 2.2 million in the USA [1,2]. AF is associated with a 5-fold increase in the risk of stroke and 1.5- to 1.9-fold increased risk of death [1–6]. Nearly 1% of all US emergency department (ED) visits are for complaints related to AF [7]. The ability to accurately risk-stratify AF patients presenting to the ED is poor and more than 65% of these ED visits result in hospital admission and contribute to healthcare expenditures ranging from $6 to $26 billion [8,9]. Studies have shown substantial variations in the ED treatment of AF [10] and that nearly half of these admissions could be avoided and patients safely discharged home [11–14]. These have incorporated ED practice guidelines, observation units, and expedited cardioversion, but have suffered from retrospective methodology, small sample sizes, and a focus on identification of high-risk features only. A strategy to better define the ED management, admission decisions, and spectrum of risk from low to high is sorely needed [15]. A review of 12-years of ED visits for AF from the National Hospital Ambulatory Medical Care Survey (NHAMCS) database found that admitted patients with symptomatic AF were similar to those discharged home from the ED with respect to age, sex, comorbidities, and whether ED rate control, cardioversion or anticoagulation were attempted [15].
We recently reported the first clinical prediction model for predicting 30-day adverse events in ED patients with symptomatic AF [16]. This rule, however, was derived from a retrospective cohort sample and we were not able to control for all potential confounders and effect modifiers. The Atrial Fibrillation and Flutter Outcomes & Risk Determination (AFFORD) study’s objective is to develop and internally validate a multivariable clinical prediction rule based on established clinical and biostatistical standards [17–22] that accurately estimates risk for adverse outcomes in ED patients with symptomatic AF. This paper presents the rationale and the design of the AFFORD study.
Methods
Study design
The AFFORD study (Clinicaltrials.gov identifier: NCT01138644) is a prospective, observational cohort study designed to develop and validate a multivariable clinical prediction rule for ED patients with symptomatic AF or atrial flutter. Figure 1 details a schematic flow chart for AFFORD patient enrollment and data collection. The study’s primary outcome is the incidence of any AF-related adverse event at 5 days and 30 days.
Figure 1.
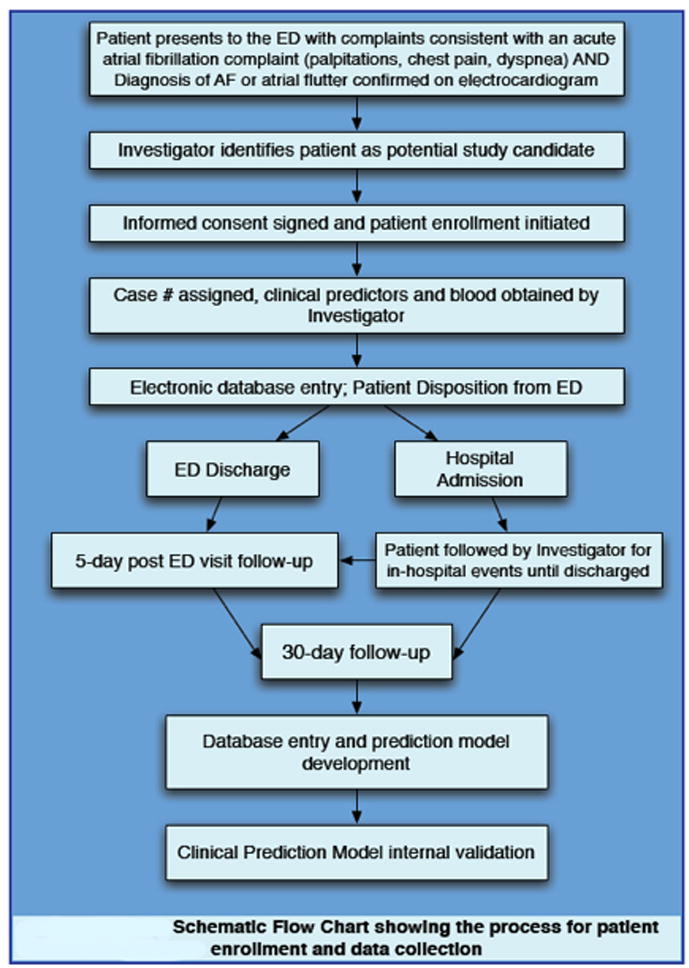
Schematic flow chart showing the process for Atrial Fibrillation and Flutter Outcomes & Risk Determination patient enrollment and data collection. AF, atrial fibrillation; ED, emergency department.
The AFFORD study will enroll subjects at 2 centers: a single, tertiary care, university-affiliated hospital’s adult ED (55,000 adult ED visits/year) and a large, academic, veterans administration hospital ED (20,000 ED visits/year). We will enroll a convenience sample of 430 adult ED patients over a period of 36 months. The inclusion and exclusion criteria are detailed in Table 1. The study investigators will screen all ED patients presenting with signs (e.g. tachycardia, dyspnea) and symptoms (e.g. palpitations, chest pain, shortness of breath, weakness, lightheadedness, pre-syncope, or syncope) consistent with potential diagnoses of symptomatic AF or atrial flutter. The diagnosis of AF requires electrocardiographic evidence of AF or atrial flutter patterns performed on the date of the patient encounter in the ED. The electrocardiographic diagnosis of AF or atrial flutter will be verified with an ED attending prior to enrollment. The final electrocardiogram interpretation is subsequently confirmed by an independent cardiologist review of the electrocardiogram. We will withdraw patients who are erroneously diagnosed with AF or atrial flutter by the ED attending interpretation but later determined to have an alternative heart rhythm by the cardiologist interpretation. We will use the operational definition for the subtypes of AF (new onset, paroxysmal, persistent, and permanent AF) as defined by the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences [23–25].
Table 1.
Atrial Fibrillation and Flutter Outcomes & Risk Determination inclusion and exclusion criteria
| Inclusion criteria |
|
| Exclusion criteria |
|
AF, atrial fibrillation; ED, emergency department.
Study procedures
Patients who consent to participate will be asked a series of standardized questions and candidate predictors will be obtained through direct questioning of the subject and review of the subject’s electronic medical record. We will collect and process blood and urine specimens for laboratory testing. Medical record reviews will be conducted using standardized criteria and their accuracy will be verified by a repeat review [26]. The investigators will be blinded to a patient’s outcome status for the medical record reviews and will use a data dictionary with precise definitions for the candidate predictors and outcomes created prior to study initiation.
Participants will be contacted at 5 and 30 days following their initial ED visit to assess for adverse outcomes. The 5-day follow up period was chosen to enhance the proposed prediction rule’s face validity and represent a more realistic association of adverse events and the treatment received at the ED visit. For comparison to other studies, we will also document follow-up at 30 days. Investigators will use a standard telephone communication data collection form for all interviews. In addition to the telephone interviews, the investigators will review the patient’s electronic medical record and the Social Security Death Index to assess for outcomes. Admitted patients will receive follow-up telephone communication at both 5 days and 30 days from the date of their initial ED visit. If the patient is hospitalized for more than 5 days, the investigators will review the admission medical records and document any adverse events.
Candidate predictor variables
Predictor variables for an AF prediction rule must be readily available to physicians in the routine management of ED patients with symptomatic AF and enter the model in the same temporal manner that the predictor would be available in the clinical arena [17–22, 27]. We will limit the AF prediction rule to include only candidate predictors whose information is available within the first 2 hours of ED management. This time limitation accurately mirrors ED clinical practice and will significantly increase the rule’s utility in real practice. We pre-selected the primary candidate predictor variables for the AFFORD study, listed in Table 2, in accordance with established standards [17–22, 27]. Detailed definitions for each of these variables are available in the electronic supplemental table. However, because of the dynamic nature of this research area and the length of time over which patients are being recruited, we are also collecting data on additional variables as well [28–32].
Table 2.
Atrial Fibrillation and Flutter Outcomes & Risk Determination candidate predictors
| Predictor | Ascertainment & definition | Operational definition | Type* | Degrees of freedom |
|---|---|---|---|---|
| Age | Interview and EMR review | Value in years | c | 3 |
| Sex | Interview | Female/Male | d | 1 |
| 2 hour resting heart rate | Bedside monitor | Value in beats per minute | c | 3 |
| Maximum heart rate in first 2 hours of ED treatment | Bedside monitor | Value in beats per minute | C | 3 |
| Requires iv drug infusion | Review of EMR and electronic order tracker | Yes/No | D | 1 |
| Heart failure | EMR & Echo results | Yes/No | D | 1 |
| Diabetes | Interview and EMR review | Yes/No | D | 1 |
| Valvular heart disease | EMR & Echo results | Yes/No | D | 1 |
| History of CVA or TIA | Interview and EMR review | Yes/No | D | 1 |
| Takes >2 AV node blocker medications | Interview and EMR review | Yes/No | D | 1 |
| Home warfarin use | Interview and EMR review, INR | Yes/No | D | 1 |
| Home digoxin use | Interview and EMR review, digoxin level | Yes/No | D | 1 |
| Dyspnea in ED | Interview and EMR review | Yes/No | D | 1 |
| Palpitations in ED | Interview and EMR review | Yes/No | D | 1 |
| Atrial flutter on ECG | Review of ECG and EMR | Yes/No | D | 1 |
| BNP | Core laboratory test | Laboratory value | C | 2 |
| BUN | Core laboratory test | Laboratory value | C | 2 |
| Troponin I | Core laboratory test | Laboratory value | C | 2 |
| Overall Degrees of Freedom | 27 | |||
Type of variable: c = continuous; d = dichotomous; AV, atrioventricular; BNP, B-type natriuretic peptide; BUN, blood urea nitrogen; CVA, cerebrovascular accident; ECG, electocardiography; Echo, echocardiography; ED, emergency department; EMR, electronic medical record; INR, international normalized ratio; TIA, transient ischemic attack.
Study outcomes
The primary outcome for the AFFORD study is measuring the incidence of adverse events at 5 and 30 days from the ED evaluation. A hierarchical listing of these specific adverse events with a priori proposed ordinal scale severity value assignments is presented in Figure 2. The accurate determination of whether the adverse events are related to AF is of utmost importance for this study. Two faculty investigators, specialists in arrhythmia and emergency medicine, will review each reported 5-day and 30-day adverse event and make a consensus determination on whether it was AF-related. A third senior arrhythmia faculty investigator will adjudicate any disagreements between the two primary reviewers. This consensus outcome determination will be made prior to any analysis of the candidate predictor variables.
Ethical conduct
The study is conducted with Good Clinical Practice and in accordance with our institutional and federal Responsible Conduct of Research guidelines. Our medical center institutional review board approved the study protocol prior to the commencement of study enrollment. At the time of manuscript submission, we are awaiting formal review of our study protocol by the Veterans Administration Hospital Institutional Review Board.
Statistical analysis
The development of an accurate, validated clinical prediction rule requires that there must be 15 subjects or events per degree of freedom for the rule to be reliable and not at risk of overfitting. Thus for the 27 degrees of freedom noted in Table 2, we should enroll 405 subjects [17,27]. We increased the sample size estimate to 430 subjects to account for a potential 5% loss to follow-up. The entire sample will be used in model development.
Analysis strategy
The investigators assessing the presence of each outcome event will be masked to the predictor variables and vice versa. This is done to adhere to the standards for development of clinical prediction rules. The statistical analysis is focused on the development of multivariable models relating predictor variables to the outcome events with the goal of developing the AF prediction rule. Candidate models may then be used to estimate adjusted effects of predictor variables of interest, after controlling for the effects of other predictors [17–23,27].
Potential confounding variables of the rule include demographic features, access to medical care, home medication regimen, other comorbidities, and other predictor variables. We will evaluate for confounding by comparing the bivariate (unadjusted) regression coefficients with the adjusted coefficients of the multiple regression models. Our rule will incorporate effect modifiers in addition to our primary outcomes. These effect modifiers are listed in the electronic supplemental figure.
The AFFORD prediction rule development will follow the established strategies and include the following critical steps [17–23,27,33]. We clearly identified a priori the relationships of interest and defined the candidate predictor variables and outcome variables for each multivariable regression analysis. Regression splines will be used to relax the linearity assumption of continuous predictors [34]. Ideally, we would have no missing data, especially for key predictors. When we have missing data on predictor variables, we will use multiple imputation techniques to make optimal use of partial information recorded for each subject [35]. We will incorporate pre-specified interactions when biologically plausible. The composite outcome variables (incidence of 5-day and 30-day adverse events) have been structured as a hierarchy of ordinal outcomes and proportional odds logistic regression will be used for analysis [36]. The predictive accuracy of the rule will be measured by calculating the rule’s discrimination using area under receiver operating characteristic (ROC) curve or concordance (c-index). We will calculate the rule’s calibration and demonstrate it with a smooth nonparametric calibration curve or scatter plot of predicted versus observed outcome. We will create these plots for a variety of cutoffs in the ordinal outcome level. We will internally validate the calibration and discrimination of the rule using bootstrap resampling. We will review the AFFORD prediction rule with cardiologists and emergency physicians who are masked to the rule’s predictive discrimination (c index). We will derive multiple scoring systems based on the regression coefficients, and trial these systems with clinicians to choose the most “sensible” rule. This will maximize the AFFORD rule’s potential impact factor.
Future prospective, international, multicenter investigations will be planned to externally validate the AFFORD prediction rule.
Timelines
The first AFFORD patient was enrolled on June 8, 2010 and at time of manuscript submission, 221 patients have been enrolled. We anticipate completing patient recruitment by April 2014 and finalizing prediction rule development and internal validation by December 2014.
Discussion
Emergency medicine research has focused on developing alternatives to admitting patients through the use of practice policies and observation units. Physicians have accepted prior clinical decision rules into their practice, so an accurate AF prediction rule would be valuable to clinical practice. The exceedingly high admission rate suggests that physicians are not confident stratifying patients to “low risk” and thus choose to admit the vast majority of patients with symptomatic AF. In addition, the ED return rate within 7 days among discharged patients is reported to be between 3–5% [12–14]. These recidivism rates suggest that the current criteria used by emergency physicians to identify patients safe for ED discharge are deficient.
The proposed project is unique in that most AF prediction rules have focused on long-term thromboembolic events, maintenance of sinus rhythm, or overall mortality. The ED-based rules have chosen to predict hospital admission; no prospective study has yet identified the “low-risk” patient who can be safely discharged from the ED. Furthermore, there is a lack of AF prediction rules that adhere to the strict study design and biostatistical methodology advocated by prognostic modeling experts.
Several key aspects of the study are strengths. We will collect most data in a real-time, prospective manner thus limiting the number of missing values of key candidate predictors that are vital to development of a reliable prediction rule. We will incorporate information available to the physician within the first 2 hours of ED treatment. This time limitation accurately reflects actual management and disposition decision-making. The AFFORD rule will include the most up-to-date diagnostic studies that have demonstrated prognostic association with AF and associated cardiovascular diseases. We will develop a risk stratification rule for the entire AF population. Specifically, we are interested in the low-risk group as this identification might significantly decrease hospital admissions. This study includes an additional 5-day adverse outcome measurement period that is more likely associated with ED management of the patient’s acute AF episode than the common 30-day measurements.
In conclusion, the AFFORD study’s objective is to develop an accurate, objective risk-stratification rule for ED patients with symptomatic AF. An AF prediction rule is a necessary addition to the acute management of AF in order to improve patient care and safety, more efficiently utilize healthcare resources, provide emergency physicians with a standardized decision aid for risk stratifying this common disease, and further clinical research into AF treatment and prevention.
Supplementary Material
Acknowledgments
No industry financial support or compensation has been or will be received for conducting this study. Dr Barrett and this study are funded by NIH grant K23 HL102069 from the National Heart, Lung and Blood Institute. Dr Storrow is supported in part by NIH grant R01 HL088459. Dr Darbar and Dr Roden are supported in part by NIH grants U01 HL65962 and R01 HL092217. The study was also supported in part by Vanderbilt CTSA grant 1 UL1 RR024975 from NCRR/NIH and the Department of Emergency Medicine Research Division.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Inoue H, Fujiki A, Origasa H, Ogawa S, Okumura K, Kubota I, Aizawa Y, Yamashita T, Atarashi H, Horie M, Ohe T, Doi Y, Shimizu A, Chishaki A, Saikawa T, et al. Prevalence of atrial fibrillation in the general population of Japan: an analysis based on periodic health examination. Int J Cardiol. 2009;137:102–7. doi: 10.1016/j.ijcard.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 4.Atarashi H, Inoue H, Okumura K, Yamashita T, Origasa H J-RHYTHM Registry Investigators. Investigation of optimal anticoagulation strategy for stroke prevention in Japanese patients with atrial fibrillation--the J-RHYTHM Registry study design. J Cardiol. 2011;57:95–9. doi: 10.1016/j.jjcc.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113:359–64. doi: 10.1016/s0002-9343(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 6.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–8. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 7.Scott PA, Pancioli AM, Davis LA, Frederiksen SM, Eckman J. Prevalence of atrial fibrillation and antithrombotic prophylaxis in emergency department patients. Stroke. 2002;33:2664–9. doi: 10.1161/01.str.0000035260.70403.88. [DOI] [PubMed] [Google Scholar]
- 8.Coyne KS, Paramore C, Grandy S, Mercader M, Reynolds M, Zimetbaum P. Assessing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value Health. 2006;9:348–56. doi: 10.1111/j.1524-4733.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- 9.Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–20. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- 10.Stiell I, Clement CM, Brison RJ, Rowe BH, Borgundvaag B, Langhan T, Lang E, Magee K, Stenstrom R, Perry JJ, Birnie D, Wells GA. Variation in management of recent-onset atrial fibrillation and flutter among academic hospital emergency departments. Ann Emerg Med. 2011;57:13–21. doi: 10.1016/j.annemergmed.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Zimetbaum P, Reynolds MR, Ho KK, Gaziano T, McDonald MJ, McClennen S, Berezin R, Josephson ME, Cohen DJ. Impact of a practice guideline for patients with atrial fibrillation on medical resource utilization and costs. Am J Cardiol. 2003;92:677–81. doi: 10.1016/s0002-9149(03)00821-x. [DOI] [PubMed] [Google Scholar]
- 12.Decker WW, Smars PA, Vaidyanathan L, Goyal DG, Boie ET, Stead LG, Packer DL, Meloy TD, Boggust AJ, Haro LH, Laudon DA, Lobi JK, Sadosty AT, Schears RM, Schiebel NE, et al. A prospective, randomized trial of an emergency department observation unit for acute onset atrial fibrillation. Ann Emerg Med. 2008;52:322–8. doi: 10.1016/j.annemergmed.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 13.Koenig BO, Ross MA, Jackson RE. An emergency department observation unit protocol for acute-onset atrial fibrillation is feasible. Ann Emerg Med. 2002;39:374–81. doi: 10.1067/mem.2002.122785. [DOI] [PubMed] [Google Scholar]
- 14.Stiell IG, Clement CM, Symington C, Perry JJ, Vaillancourt C, Wells GA. Emergency department use of intravenous procainamide for patients with acute atrial fibrillation or flutter. Acad Emerg Med. 2007;14:1158–64. doi: 10.1197/j.aem.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 15.McDonald AJ, Pelletier AJ, Ellinor PT, Camargo CA., Jr Increasing US emergency department visit rates and subsequent hospital admissions for atrial fibrillation from 1993 to 2004. Ann Emerg Med. 2008;51:58–65. doi: 10.1016/j.annemergmed.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Barrett TW, Martin AR, Storrow AB, Jenkins CA, Harrell FE, Russ S, Roden DM, Darbar D. A clinical prediction model to estimate risk for thirty day adverse events in emergency department patients with symptomatic atrial fibrillation. Ann Emerg Med. 2011;57:1–12. doi: 10.1016/j.annemergmed.2010.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrell FE, Jr, Lee KL, Califf RM, Prtor DB, Rosati RA. Regression modelling strategies for improved prognostic prediction. Stat Med. 1984;3:143–52. doi: 10.1002/sim.4780030207. [DOI] [PubMed] [Google Scholar]
- 18.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 19.Harrell FE, Jr, Margolis PA, Gove S, Mason KE, Mulholland EK, Lehmann D, Muhe L, Gatchalian S, Eichenwald HF. Development of a clinical prediction model for an ordinal outcome: the World Health Organization Multicentre Study of Clinical Signs and Etiological agents of Pneumonia, Sepsis and Meningitis in Young Infants. WHO/ARI Young Infant Multicentre Study Group. Stat Med. 1998;17:909–44. doi: 10.1002/(sici)1097-0258(19980430)17:8<909::aid-sim753>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 20.Laupacis A, Sekar N, Stiell IG. Clinical prediction rules. A review and suggested modifications of methodological standards. JAMA. 1997;277:488–94. [PubMed] [Google Scholar]
- 21.Steyerberg EW, Eijkemans MJ, Harrell FE, Jr, Habbema JD. Prognostic modeling with logistic regression analysis: a comparison of selection and estimation methods in small data sets. Stat Med. 2000;19:1059–79. doi: 10.1002/(sici)1097-0258(20000430)19:8<1059::aid-sim412>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 22.Steyerberg EW, Harrell FE, Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–81. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 23.Estes NA, 3rd, Halperin JL, Calkins H, Ezekowitz MD, Gitman P, Go AS, McNamara RL, Messer JV, Ritchie JL, Romeo SJ, Waldo AL, Wyse DG. ACC/AHA/Physician Consortium 2008 clinical performance measures for adults with nonvalvular atrial fibrillation or atrial flutter: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and the Physician Consortium for Performance Improvement (Writing Committee to Develop Clinical Performance Measures for Atrial Fibrillation): developed in collaboration with the Heart Rhythm Society. Circulation. 2008;117:1101–20. doi: 10.1161/CIRCULATIONAHA.107.187192. [DOI] [PubMed] [Google Scholar]
- 24.Fuster V, Ryden LE, Asinger RW, Cannom DS, Crijns HJ, Frye RL, Halperin JL, Kay GN, Klein WW, Levy S, McNamara RL, Prystowsky EN, Wann LS, Wyse DG, Gibbons RJ, et al. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation): developed in Collaboration With the North American Society of Pacing and Electrophysiology. J Am Coll Cardiol. 2001;38:1231–66. doi: 10.1016/s0735-1097(01)01587-x. [DOI] [PubMed] [Google Scholar]
- 25.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation) J Am Coll Cardiol. 2006;48:854–906. doi: 10.1016/j.jacc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Gilbert EH, Lowenstein SR, Koziol-McLain J, Barta DC, Steiner J. Chart reviews in emergency medicine research: Where are the methods? Ann Emerg Med. 1996;27:305–8. doi: 10.1016/s0196-0644(96)70264-0. [DOI] [PubMed] [Google Scholar]
- 27.Steyerberg EW. Clinical Prediction Models - A practical approach to development, validation, and updating. New York: Springer; 2009. [Google Scholar]
- 28.Shigematsu Y, Hamada M, Nagai T, Nishimura K, Inoue K, Suzuki J, Ogimoto A, Higaki J. Risk for atrial fibrillation in patients with hypertrophic cardiomyopathy: Association with insulin resistance. J Cardiol. 2011 Apr 22; doi: 10.1016/j.jjcc.2011.03.001. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Lee YS, Hyun DW, Jung BC, Cho YK, Lee SH, Shin DG, Park HS, Han SW, Kim YN. Left atrial volume index as a predictor for occurrence of atrial fibrillation after ablation of typical atrial flutter. J Cardiol. 2010;56:348–53. doi: 10.1016/j.jjcc.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Maehama T, Okura H, Imai K, Saito K, Yamada R, Koyama T, Hayashida A, Neishi Y, Kawamoto T, Yoshida K. Systemic inflammation and left atrial thrombus in patients with non-rheumatic atrial fibrillation. J Cardiol. 2010;56:118–24. doi: 10.1016/j.jjcc.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Ichiki H, Orihara K, Hamasaki S, Ishida S, Oketani N, Iriki Y, Ninomiya Y, Okui H, Kuwahata S, Fujita S, Matsushita T, Yoshifuku S, Oba R, Hirai H, Nagata K, et al. The role of infection in the development of non-valvular atrial fibrillation: up-regulation of Toll-like receptor 2 expression levels on monocytes. J Cardiol. 2009;53:127–35. doi: 10.1016/j.jjcc.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Baba O, Izuhara M, Kadota S, Mitsuoka H, Shioji K, Uegaito T, Mutsuo S, Matsuda M. Determinant factors of plasma B-type natriuretic peptide levels in patients with persistent nonvalvular atrial fibrillation and preserved left ventricular systolic function. J Cardiol. 2009;4:402–8. doi: 10.1016/j.jjcc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 33.McGinn TG, Guyatt GH, Wyer PC, Naylor CD, Stiell IG, Richardson WS. Users’ guides to the medical literature: XXII: how to use articles about clinical decision rules. Evidence-Based Medicine Working Group. JAMA. 2000;284:79–84. doi: 10.1001/jama.284.1.79. [DOI] [PubMed] [Google Scholar]
- 34.Harrell FE Jr, editor. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. [Google Scholar]
- 35.Donders AR, van der Heijden GJ, Stijnen T, Moons KG. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59:1087–91. doi: 10.1016/j.jclinepi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 36.McCullagh P. Regression models for ordinal data (with discussion) J R Stat Soc, Series B. 1980;42:109–42. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


