Abstract
Purpose
To determine the role of ultrasound biomicroscopy (UBM) in the management of children affected with retinoblastoma.
Methods
A review of clinical records of children with the diagnosis of retinoblastoma at the Hospital for Sick Children from January 1995 to December 2007, for whom UBM was used to determine the extent of intraocular tumor. Clinical characteristics were compared with UBM. Pathological correlation was performed for enucleated eyes.
Results
In total, 101 eyes of 75 patients were included in the final analysis. Only 11 eyes were diagnosed on UBM to have extension of the tumor anterior to the ora serrata, and were enucleated. Histopathological examination confirmed the anterior extension in all the 11 eyes. In total, 50 eyes were enucleated because of various reasons, such as poor visual prognosis (12 eyes), unilateral group D or E (23 eyes), recurrences (8 eyes), and treatment failure (7 eyes). None of those patients were found to have anterior extension of the disease on histopathological examination. UBM did not yield any false negative (0/50) or any false positives (0/11).
Conclusions
The UBM provided a sensitive and reproducible visualization of the anterior retina, ciliary region, and anterior segment allowing a better staging of the advanced disease process. Primary assessment of the true extent of retinoblastoma is critical for the selection of an optimal management approach.
Keywords: retinoblastoma, international retinoblastoma classification, ultrasound biomicroscopy
Introduction
Current approaches to the primary treatment of retinoblastoma with the new chemotherapeutic agents associated with focal therapy have allowed the preservation of eyes that previously would have been primarily enucleated.1, 2, 3, 4, 5 The International Retinoblastoma Classification6 stages the severity of intraocular disease on the basis of the features predictive of eye survival with current management generally based on a combination of chemotherapy and focal therapy.
The safe and effective delivery of these treatments requires that the full extent of the tumor process be identified. Involvement of the anterior retina and ciliary region with tumor is associated with a poor prognosis.6 The most commonly used imaging technologies, such as indirect ophthalmoscopy, conventional 10 MHz ocular ultrasound, CT scan and magnetic resonance imaging do not provide adequate resolution of retinoblastoma anterior to the ora serrata in the ciliary region. Failure to detect anterior tumors early can compromise the chances of saving the eye and increase the risk of extraocular disease.
Ultrasound biomicroscopy (UBM) uses high-frequency transducers yielding an increased resolution (30–50 microns with a 50 Mhz transducer) of the anterior aspect of the eye. This technique allows in vivo analysis of the anterior segment of the eye at microscopic resolution.7, 8, 9 UBM has been very useful in the management of adult anterior segment diseases, such as anterior segment tumors, iris tumors, scleral and corneal abnormalities, and glaucoma.8, 9, 10, 11, 12, 13, 14, 15
We hypothesized that UBM would be useful in the management of advanced intraocular retinoblastoma by better defining the anterior extent of the tumor process.
Patients and methods
Approval for this study was obtained from the Research Ethics Board of the Hospital for Sick Children, Toronto, Canada. Informed consent was obtained from the legal guardian of each patient.
We reviewed the clinical records of children with the diagnosis of retinoblastoma at the Hospital for Sick Children from January 1995 to December 2007, for whom an UBM was used to determine the anterior extent of retinoblastoma. All UBMs were done under general anesthesia at either the staging or a follow up examination under anesthesia using the Ultrasound Biomicroscope (Zeiss Humphrey, Humphrey Instruments, San Leandro, CA, USA) with a 50 Mhz transducer. Carbomer 980 (Liposic, Bausch & Lomb, Vaughan, ON, Canada) was used as a coupling medium, and a standard scleral depressor was used to rotate the eye in different positions. Images of the posterior iris, iridocorneal angle, ciliary processes, pars plana, and the anterior retina were obtained for 360°, captured and stored on a hard drive and/or printed. UBM findings were correlated clinically and, when possible, with histopathological studies.
All variables in this study were categorical. Descriptive data for each case was entered into Microsoft Excel, then recoded to numerical format and imported into SPSS for analysis. χ2-test was applied to analyze bivariate relationships (UBM and clinical features), using the Goodman and Kruskal tau calculation to measure significance with UBM as the independent variable. Multivariate analysis (ANOVA) assessed significant differences in clinical features between subjects having/not having UBM.
Results
Evaluation of the anterior extent of retinoblastoma using the UBM was performed in 75 patients (101 eyes). In total, 40 patients were males (52.6%). The age at diagnosis for children with bilateral disease was younger (14 months average, median 9) than for children with unilateral disease (28 months average, median 24). Right and left eyes were affected equally often. In total, 49 patients (63.1%) presented with bilateral disease.
The high-resolution images were obtained of the anterior retina and ciliary region in the majority of the cases. The clinical features that suggested use of the UBM to study the anterior segment/ciliary region included glaucoma (21%), extensive tumor occluding the view of the ora serrata (22%), retinal detachment (12%), vitreous seeding (11%), and media opacity (7%).
Anterior retinoblastoma was suspected in 37 of the 101 eyes on ophthalmoscopy, but confirmed by UBM in only 7 eyes. The remaining 64 eyes were considered to be free of anterior disease following ophthalmoscopy, but UBM identified anterior segment involvement in 4 eyes.
Bivariate analysis showed that positive UBM results were significantly associated with clinical diagnosis positive for anterior disease (P=0.022), glaucoma (P=0.017), and positive histopathology (P=0.0001). UBM results were not significantly associated with the presence of cataract, retinal detachment, seeding, or extensive tumor. Multivariate analysis comparing positive UBM/negative UBM groups, showed identical results (Table 1).
Table 1. Clinical features correlating UBM findings for anterior disease.
|
Complete cohort (n) |
UBM positive (n) |
P-value |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | C | D | E | Total | B | C | D | E | Total | Bivariatea | Univariateb | |
| Glaucoma | 0 | 0 | 6 | 15 | 21 | 0 | 0 | 1 | 4 | 5 | 0.017 | 0.016 |
| Angle closure with pupillary block | 0 | 0 | 5 | 11 | 16 | 0 | 0 | 0 | 2 | 2 | ||
| Neovascular glaucoma | 0 | 0 | 1 | 3 | 4 | 0 | 0 | 1 | 2 | 3 | ||
| Open angle glaucoma | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | ||
| Cataract | 0 | 0 | 4 | 4 | 8 | 0 | 0 | 1 | 0 | 1 | 0.799 | 0.8 |
| Retinal detachment | 1 | 1 | 9 | 7 | 18 | 0 | 0 | 0 | 4 | 4 | 0.075 | 0.054 |
| Extensive tumor | 2 | 1 | 16 | 15 | 34 | 0 | 0 | 0 | 4 | 4 | 0.553 | 0.556 |
| Tumor seeding | 0 | 1 | 12 | 7 | 20 | 0 | 0 | 0 | 3 | 3 | 0.415 | 0.418 |
| Clinically suspected anterior disease | 1 | 0 | 18 | 18 | 37 | 0 | 0 | 1 | 6 | 7 | 0.022 | 0.21 |
| Total | 6 | 3 | 66 | 26 | 101 | 0 | 0 | 2 | 9 | 11 | ||
| Eyes enucleated | 1 | 2 | 31 | 27 | 61 | 0 | 0 | 2 | 9 | 11 | 0.007 | 0.007 |
| Pathology confirmed anterior disease | 0 | 0 | 2 | 9 | 11 | 0 | 0 | 2 | 9 | 11 | 0.0001 | 0.000 |
n=number of eyes.
P-value calculated using Goodman and Kruskal tau, establishing UBM as the independent variable.
Groups are subjects with positive/negative UBM.
Evaluation of the mechanism of increased intraocular pressure (n=21)
Increased intraocular pressure at diagnosis was documented in 21 eyes. UBM revealed three different mechanisms leading to increased intraocular pressure: (1) angle closure and pupillary block (16 eyes); (2) neovascular glaucoma (4 eyes); (3) tumor obstruction of the iridocorneal angle (1 eye).
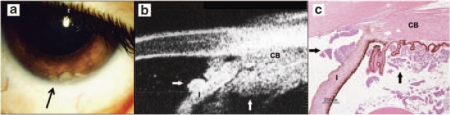
Angle closure associated with pupillary block always occurred in the presence of a large tumor volume producing anterior lens displacement. In cases of neovascular glaucoma, the iris appeared straight and rigid, although pupillary block was not present. The pupillary margin was highly reflective because of the associated ectropion uveae. The ‘clogging' of the iridocorneal angle with tumor cells was observed in one child with anterior segment disease. The eye was enucleated and histopathology confirmed the cells in the angle to be of malignant nature (Figure 1).
Figure 1.
Retinoblastoma with clinical evidence of anterior segment extension. (a) Anterior segment image showing tumor cells (arrow) in the anterior chamber. (b) UBM documentation of tumor clumps (T) in the ciliary body (CB) and anterior surface of the lens and within the anterior chamber angle (arrows). (c) Pathology showing correlation with the UBM and clinical findings.
Angle closure with pupillary block was the most frequently observed structural anomaly associated with retinoblastoma-related increased intraocular pressure, always associated with a significant tumor volume. The extensive anterior bowing of the iris was diagnostic of this condition. In cases of neovascular glaucoma the iris was usually very straight and no pupillary bloc was observed. Angle closure itself was not an indication for enucleation. However, when the intraocular pressure was raised because of tumor cells in the angle or because of neovascularization, the eye was enucleated at diagnosis (International Retinoblastoma Classification Group E).
Documentation of anterior extent of tumor (n=11 eyes)
Tumor extension anterior to the ora serrata was detected in 11 eyes using UBM. Suggestion of ciliary body involvement using indirect ophthalmoscopy was positive only in 7 eyes. In four patients, anterior involvement was not suspected using conventional methods of examination. In these patients, enucleation, which may have otherwise been delayed, was performed promptly.
Histopathology correlation
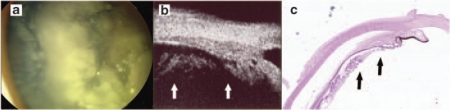
A total of 61 eyes underwent enucleation. In total, 11 eyes were enucleated after anterior segment extension was diagnosed by UBM. Histopathological examination positively correlated with UBM assessments in all cases (Figure 2). The decision to enucleate the other 50 eyes was based on: poor visual prognosis (12), unilateral RB International Retinoblastoma Classification group D or E (23), recurrence (8), and treatment failure (7). Eyes that were enucleated in the poor visual prognosis group were all bilaterally affected with eyes in Groups C, D, and E. Most of these patients developed large retinal detachment. One patient in this group was enucleated because of the parent's decision.
Figure 2.
Retinoblastoma with anterior extension. (a) Fundus photograph of an extensive tumor and apparent vitreal seeding, although the clinical examination was inconclusive for anterior segment extension. (b) UBM image showing RB involving the pars plana associated with vitreal seeding (arrows). (c) The pathology specimen confirmed the presence of anterior tumor extension (arrows).
None of those eyes were found to have anterior extension of the disease. The diagnosis of retinoblastoma was histopathologically confirmed in all cases.
Discussion
Determination of the full intraocular extent of the retinoblastoma is critical to the decision for the best therapeutic options. UBM is a very important addition as it overcomes the limitations of the other imaging tools available.7, 16, 17 We confirmed this imaging modality is a valuable adjunct in children affected with advanced retinoblastoma, particularly when anterior disease was suspected.
The UBM reflectivity of the retinoblastoma tumor is very similar to that of the iris, ciliary body, and ciliary processes. Identification of the fine line of higher reflectivity from the retina and the pigment epithelium lining the ciliary body helps to define tumor invasion beyond the retina. The anterior vitreous face is located near the anterior border of the ciliary body. This normal anterior vitreous face is generally difficult to define on UBM. It can be clearly imaged if outlined with inflammatory or tumor cells. The high internal reflectivity of the sclera and the retina is distinct from the overlying and underlying tissues, and is usually easily distinguished from retinoblastoma tumor.15
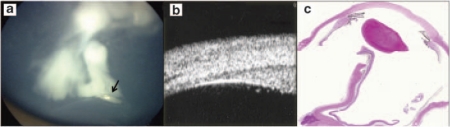
The densely arranged fibrils of the vitreous base may be hard to distinguish from retinoblastoma vitreous seeding. Retinoblastoma seeds are of moderate reflectivity, but are usually irregular in size, shape, and distribution. ‘The ability to obtain useful UBM images and their interpretation requires some experience. Serial evaluations and clinical correlations in other pathologies and otherwise healthy eyes are useful in this learning process (Figure 3).16
Figure 3.
Clinical suspicion of anterior extension. (a) Fundus photograph showing a suspicious lesion (arrow) located in the inferior retina (left). (b) UBM showing normal ciliary body and small vitreal seeds. (c) Pathology specimen showing clear ciliary body.
The size of tumors can be measured from UBM images. This information is useful for follow-up and management of patients. Children suspected to have retinoblastoma involvement of the ciliary region or anterior, should be treated by enucleation. However, when the involvement of the ciliary region is excluded on UBM, and the International Retinoblastoma Classification classification is not Group E, a chemotherapy-based approach can be safely considered despite a large tumor volume.
Cases of total retinal detachment, extensive tumor or seeding, and dense cataract challenge the clinical assessment of an anterior segment extension. In the presence of glaucoma, UBM becomes useful to assess the state of the iridocorneal angle. The UBM is known to have an important role in deciphering the structural anomalies associated with different forms of glaucoma in adults.9, 16, 18
The survival of eyes with anterior disease was previously documented with both radiotherapy and chemotherapy. This may relate to an incomplete assessment of the anterior disease process.19 To safely keep eyes with retinoblastoma, complete visualization of the tumor process is required. Earlier intervention is more likely to allow preservation of eyes, which is especially important for children with one remaining eye. UBM imaging clearly documents anterior disease and contributes to the management of children affected with retinoblastoma, with no evidence of false negative (0/50) or false positives (0/11) in our study.
In summary, UBM is a non-invasive, safe, and very efficient method of imaging the anterior segment. We show UBM to be a crucial tool in the intraocular staging of disease of children with advanced retinoblastoma. UBM provides sensitive and reproducible visualization of the anterior retina, ciliary region, and anterior segment, thereby optimizing patient management of cases with severe retinoblastoma.

The authors declare no conflict of interest.
Footnotes
Presented in part as a poster at the Canadian Ophthalmology Society meeting, Whistler, BC, Canada, June 2008.
References
- Chan HS, DeBoer G, Thiessen JJ, Budning A, Kingston JE, O'Brien JM, et al. Combining cyclosporin with chemotherapy controls intraocular retinoblastoma without requiring radiation. Clin Cancer Res. 1996;2 (9:1499–1508. [PubMed] [Google Scholar]
- Gallie BL, Budning A, DeBoer G, Thiessen JJ, Koren G, Verjee Z, et al. Chemotherapy with focal therapy can cure intraocular retinoblastoma without radiotherapy. Arch Ophthalmol. 1996;114 (11:1321–1328. doi: 10.1001/archopht.1996.01100140521001. [DOI] [PubMed] [Google Scholar]
- Kingston JE, Hungerford JL, Madreperla SA, Plowman PN. Results of combined chemotherapy and radiotherapy for advanced intraocular retinoblastoma. Arch Ophthalmol. 1996;114 (11:1339–1343. doi: 10.1001/archopht.1996.01100140539004. [DOI] [PubMed] [Google Scholar]
- Shields CL, De Potter P, Himelstein BP, Shields JA, Meadows AT, Maris JM. Chemoreduction in the initial management of intraocular retinoblastoma. Arch Ophthalmol. 1996;114 (11:1330–1338. doi: 10.1001/archopht.1996.01100140530002. [DOI] [PubMed] [Google Scholar]
- Murphree AL, Villablanca JG, Deegan WF, III, Sato JK, Malogolowkin M, Fisher A, et al. Chemotherapy plus local treatment in the management of intraocular retinoblastoma. Arch Ophthalmol. 1996;114 (11:1348–1356. doi: 10.1001/archopht.1996.01100140548005. [DOI] [PubMed] [Google Scholar]
- Linn Murphree A.Intraocular retinoblastoma: the case for a new group classification Ophthalmol Clin North Am 200518(141–53.viii. [DOI] [PubMed] [Google Scholar]
- Pavlin CJ, Sherar MD, Foster FS. Subsurface ultrasound microscopic imaging of the intact eye. Ophthalmology. 1990;97 (2:244–250. doi: 10.1016/s0161-6420(90)32598-8. [DOI] [PubMed] [Google Scholar]
- Pavlin CJ, McWhae JA, McGowan HD, Foster FS. Ultrasound biomicroscopy of anterior segment tumors. Ophthalmology. 1992;99 (8:1220–1228. doi: 10.1016/s0161-6420(92)31820-2. [DOI] [PubMed] [Google Scholar]
- Pavlin CJ, Harasiewicz K, Foster FS. Ultrasound biomicroscopy of anterior segment structures in normal and glaucomatous eyes. Am J Ophthalmol. 1992;113 (4:381–389. doi: 10.1016/s0002-9394(14)76159-8. [DOI] [PubMed] [Google Scholar]
- Vasquez LM, Pavlin CJ, McGowan H, Yucel Y, Simpson ER. Ring melanoma of the ciliary body: clinical and ultrasound biomicroscopic characteristics. Can J Ophthalmol. 2008;43 (2:229–233. doi: 10.1139/i08-025. [DOI] [PubMed] [Google Scholar]
- Zografos L, Chamot L, Bercher L, Schalenbourg A, Egger E, Gailloud C. Contribution of ultrasound biomicroscopy to conservative treatment of anterior uveal melanoma. Klin Monbl Augenheilkd. 1996;208 (5:414–417. doi: 10.1055/s-2008-1035256. [DOI] [PubMed] [Google Scholar]
- Maberly DA, Pavlin CJ, McGowan HD, Foster FS, Simpson ER. Ultrasound biomicroscopic imaging of the anterior aspect of peripheral choroidal melanomas. Am J Ophthalmol. 1997;123 (4:506–514. doi: 10.1016/s0002-9394(14)70176-x. [DOI] [PubMed] [Google Scholar]
- Weisbrod DJ, Pavlin CJ, Emara K, Mandell MA, McWhae J, Simpson ER. Small ciliary body tumors: ultrasound biomicroscopic assessment and follow-up of 42 patients. Am J Ophthalmol. 2006;141 (4:622–628. doi: 10.1016/j.ajo.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Fine N, Pavlin CJ. Primary cysts in the iridociliary sulcus: ultrasound biomicroscopic features of 210 cases. Can J Ophthalmol. 1999;34 (6:325–329. [PubMed] [Google Scholar]
- Pavlin CJ, Easterbrook M, Hurwitz JJ, Harasiewicz K, Eng P, Foster FS. Ultrasound biomicroscopy in the assessment of anterior scleral disease. Am J Ophthalmol. 1993;116 (5:628–635. doi: 10.1016/s0002-9394(14)73207-6. [DOI] [PubMed] [Google Scholar]
- Pavlin CJ, Harasiewicz K, Sherar MD, Foster FS. Clinical use of ultrasound biomicroscopy. Ophthalmology. 1991;98 (3:287–295. doi: 10.1016/s0161-6420(91)32298-x. [DOI] [PubMed] [Google Scholar]
- Pavlin CJ, Foster FS. Ultrasound biomicroscopy. High-frequency ultrasound imaging of the eye at microscopic resolution. Radiol Clin North Am. 1998;36 (6:1047–1058. doi: 10.1016/s0033-8389(05)70230-x. [DOI] [PubMed] [Google Scholar]
- Trope GE, Pavlin CJ, Bau A, Baumal CR, Foster FS. Malignant glaucoma. Clinical and ultrasound biomicroscopic features. Ophthalmology. 1994;101 (6:1030–1035. doi: 10.1016/s0161-6420(94)31222-x. [DOI] [PubMed] [Google Scholar]
- Ellsworth RM. The practical management of retinoblastoma. Trans Am Ophthalmol Soc. 1969;67:462–534. [PMC free article] [PubMed] [Google Scholar]