Abstract
Injury to the optic nerve can lead to axonal degeneration, followed by a gradual death of retinal ganglion cells (RGCs), which results in irreversible vision loss. Examples of such diseases in human include traumatic optic neuropathy and optic nerve degeneration in glaucoma. It is characterized by typical changes in the optic nerve head, progressive optic nerve degeneration, and loss of retinal ganglion cells, if uncontrolled, leading to vision loss and blindness.
The optic nerve crush (ONC) injury mouse model is an important experimental disease model for traumatic optic neuropathy, glaucoma, etc. In this model, the crush injury to the optic nerve leads to gradual retinal ganglion cells apoptosis. This disease model can be used to study the general processes and mechanisms of neuronal death and survival, which is essential for the development of therapeutic measures. In addition, pharmacological and molecular approaches can be used in this model to identify and test potential therapeutic reagents to treat different types of optic neuropathy.
Here, we provide a step by step demonstration of (I) Baseline retrograde labeling of retinal ganglion cells (RGCs) at day 1, (II) Optic nerve crush injury at day 4, (III) Harvest the retinae and analyze RGC survival at day 11, and (IV) Representative result.
Protocol
All equipments and reagents used are sterile. All animal experiments were approved by the Animal Care and Use Committee (ACUC) at the NEI/NIH (animal study protocol NEI-570), and were performed according to the NIH guidelines and regulations.
1. Baseline Retrograde Labeling of Retinal Ganglion Cells (RGCs) At Day 1
The purpose of this procedure is to label the retinal ganglion cells retrogradely by injecting a neural tracer dye into the superior colliculus of the mice three days before the optic nerve crush injury. The dye will be retrogradely taken up by the retinal ganglion cells and provides a marker for the living RGCs (Figure 1). This approach yields reproducible labeling of viable RGCs with little variation 1-5.
Deeply anesthetize the mouse. Clean the hair on top of the head. Place the mouse in a small stereotaxic apparatus. Protective oinment is applied to both eyes. Disinfect the head skin three times each using an iodophor and a 70% alcohol scrub.
Make an incision in the skin to expose the skull, and keep it dry and clean using 3% hydrogen peroxide.
Identify and mark the bregma. Drill a hole above the superior colliculus at 2.9 mm behind the bregma and 0.5 mm lateral to the midline of each hemisphere7. During drilling, apply saline to the site where the hole is drilled to prevent secondary heat injury.
Using a stereotaxic measuring device and a Hamilton syringe, inject the FluoroGold neural tracer dye (1 μl of 3% Fluorochrome in saline) very slowly into the superior colliculus of each hemisphere at a depth of 1.6 mm from the bony surface of the brain.
Suture the incision site. Give a subcutaneous injection of buprenorphine for analgesia.
Move the mouse to a warm and dry area and monitor it until it is able to maintain an upright posture, and return it to its home cage. For the first three days after the labeling procedure, systemic analgesics (e.g., buprenorphine) and topical antibiotic ointment are given twice daily and the mouse is closely monitored.
2. Optic Nerve Crush Injury At Day 4
In this procedure, we will apply a crush injury to the optic nerve to cause a primary damage to the axons (Figure 2), which will lead to a gradual death of the retinal ganglion cells.
Three days after the tracer dye application, the mouse is deeply anesthetized. Using a dissecting microscope, the conjunctiva of one eye is incised at about the 4 o'clock position using a pair of spring scissors.
Gently deflect the orbital muscles and put them aside. Expose the white optic nerve at its exit from the eye globe. Take great care not to damage any blood vessels.
With the aid of a pair of cross-action forceps, apply a crush injury to the optic nerve at about 2 mm from the eyeball for about 3 seconds. Take great care not to damage the ophthalmic artery to cause bleeding.
After completion of the crush injury, the incision is sutured.
Before the mouse recovers from anesthesia, give a subcutaneous injection of buprenorphine for analgesia.
Move the mouse to a warm and dry area and monitor it, until it is able to maintain an upright posture and return it to its home cage. For the first three days after the crush injury procedure, systemic analgesics (e.g., buprenorphine) and topical antibiotic ointment are given twice daily and the mouse is closely monitored.
3. Harvest the Retinae and Analyze RGC Survival At Day 11
The purpose of this procedure is to harvest the retinaeto analyze retinal ganglion cell survival.
At day 11 (day 7 after ONC), the mouse is euthanized by CO2 infusion and cervical dislocation.
Eyes are enucleated with the help of a pair of forceps by applying pressure to the orbit.
The eyes are fixed in 4% paraformaldehyde solution for two hours and washed in PBS three times. Dissect the retina. The procedure of retina dissection has already been published previously by JoVE in 2010 by Gustmann, S. et al.6, and therefore will not be described here.
Images of the surviving retinal ganglion cells are taken using a fluorescent microscope in defined regions of the retina, and the retinal ganglion cell density can be counted. A representative result of the fluorescence-labeled retinal ganglion cells with or without optic nerve crush injury is shown in Figure 3.
4. Representative Results:
To analyze retinal ganglion cell survival, seven days after optic nerve crush injury, the retinae were harvested, fixed, flattened and mounted. Retinal images were taken using a fluorescent microscope. Compared with the uninjured normal retina (left, Normal), the number of viable RGCs (green, retrogradely labeled by the FluoroGold neural tracer dye injected into the superior colliculus) is significantly lower in the retinae with an optic nerve crush injury (right, ONC) (Figure 3).
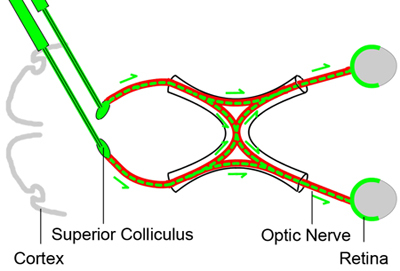 Figure 1. Baseline retrograde labeling of retinal ganglion cells
In order to count the viable retinal ganglion cells at different time points after optic nerve crush injury, the retinal ganglion cells are labeled retrogradely by injecting a neural tracer dye (green color) into the superior colliculus in the brain three days before the optic nerve (red) crush injury. Since the axons of the retinal ganglion cells reside in the superior colliculus, the tracer dye will be taken up by the RGCs retrogradely and provides a marker for the living RGCs.
Figure 1. Baseline retrograde labeling of retinal ganglion cells
In order to count the viable retinal ganglion cells at different time points after optic nerve crush injury, the retinal ganglion cells are labeled retrogradely by injecting a neural tracer dye (green color) into the superior colliculus in the brain three days before the optic nerve (red) crush injury. Since the axons of the retinal ganglion cells reside in the superior colliculus, the tracer dye will be taken up by the RGCs retrogradely and provides a marker for the living RGCs.
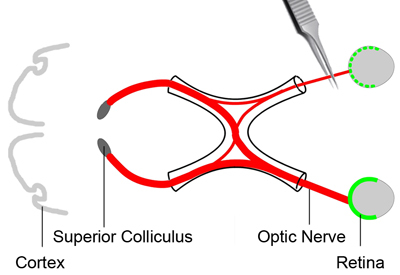 Figure 2. Optic nerve crush injury
In order to analyze the status of retinal ganglion cell survival, a crush injury is applied to the optic nerve (red) to cause a primary damage to the axons. This will lead to a gradual death of the retinal ganglion cells. Three days after the tracer dye (green) injection, the mouse is anesthetized. The conjunctiva of one eye is incised. Deflect the orbital muscles and put them aside. Expose the optic nerve at its exit from the eye globe. Apply a crush injury to the optic nerve at about 2 mm from the eyeball for about 3 seconds using a pair of cross-action forceps.
Figure 2. Optic nerve crush injury
In order to analyze the status of retinal ganglion cell survival, a crush injury is applied to the optic nerve (red) to cause a primary damage to the axons. This will lead to a gradual death of the retinal ganglion cells. Three days after the tracer dye (green) injection, the mouse is anesthetized. The conjunctiva of one eye is incised. Deflect the orbital muscles and put them aside. Expose the optic nerve at its exit from the eye globe. Apply a crush injury to the optic nerve at about 2 mm from the eyeball for about 3 seconds using a pair of cross-action forceps.
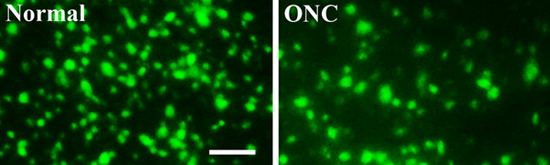 Figure 3. Fluorescence-labeled retinal ganglion cells with or without optic nerve crush injury
To analyze retinal ganglion cell survival, seven days after optic nerve crush injury, the retinae were harvested, fixed, flattened and mounted. Retinal images were taken using a fluorescent microscope. Compared with the uninjured normal retina (left, Normal), the number of viable RGCs (green, retrogradely labeled by the FluoroGold neural tracer dye injected into the superior colliculus) was significantly lower in the retinae with an optic nerve crush injury (right, ONC) (Figure 3). Scale bar: 50 μm
Figure 3. Fluorescence-labeled retinal ganglion cells with or without optic nerve crush injury
To analyze retinal ganglion cell survival, seven days after optic nerve crush injury, the retinae were harvested, fixed, flattened and mounted. Retinal images were taken using a fluorescent microscope. Compared with the uninjured normal retina (left, Normal), the number of viable RGCs (green, retrogradely labeled by the FluoroGold neural tracer dye injected into the superior colliculus) was significantly lower in the retinae with an optic nerve crush injury (right, ONC) (Figure 3). Scale bar: 50 μm
Discussion
The optic nerve crush injury murine model is useful to study the process of RGC death and survival. This model is also often used to investigate the effects of different reagents and genes on RGC apoptosis and survival. One advantage of this model is that it has a high degree of reproducibility with minimal variations.
However, special care is required in several steps in this model. First, when making the optic nerve crush injury, it is essential not to use too much force and not to crush for too long, because they may lead to damage to the ophthalmic artery and therefore subsequent retinal ischemia. In addition, when trying to expose the optic nerve, one should take great care not to damage other blood vessels surrounding the mouse eye.
Disclosures
No conflicts of interest declared.
Acknowledgments
Our research is supported by the Intramural Research Program of the NIH, National Eye Institute.
References
- Yoles E. GM1 reduces injury-induced metabolic deficits and degeneration in the rat optic nerve. Investigative ophthalmology & visual science. 1992;33:3586–3591. [PubMed] [Google Scholar]
- Fisher J. Vaccination for neuroprotection in the mouse optic nerve: implications for optic neuropathies. J Neurosci. 2001;21:136–142. doi: 10.1523/JNEUROSCI.21-01-00136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkovitch-Verbin H. RGC death in mice after optic nerve crush injury: oxidative stress and neuroprotection. Investigative ophthalmology & visual science. 2000;41:4169–4174. [PubMed] [Google Scholar]
- Li Y. VEGF-B inhibits apoptosis via VEGFR-1-mediated suppression of the expression of BH3-only protein genes in mice and rats. The Journal of clinical investigation. 2008;118:913–923. doi: 10.1172/JCI33673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z. Survival effect of PDGF-CC rescues neurons from apoptosis in both brain and retina by regulating GSK3beta phosphorylation. The Journal of experimental medicine. 2010;207:867–880. doi: 10.1084/jem.20091704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustmann S, Dunker N. In vivo-like organotypic murine retinal wholemount culture. J Vis Exp. 2010 doi: 10.3791/1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin K, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1997. [Google Scholar]


