Abstract
We describe a minimally-invasive and reproducible method to measure canine pelvic limb muscle strength and muscle response to repeated eccentric contractions. The pelvic limb of an anesthetized dog is immobilized in a stereotactic frame to align the tibia at a right angle to the femur. Adhesive wrap affixes the paw to a pedal mounted on the shaft of a servomotor to measure torque. Percutaneous nerve stimulation activates pelvic limb muscles of the paw to either push (extend) or pull (flex) against the pedal to generate isometric torque. Percutaneous tibial nerve stimulation activates tibiotarsal extensor muscles. Repeated eccentric (lengthening) contractions are induced in the tibiotarsal flexor muscles by percutaneous peroneal nerve stimulation. The eccentric protocol consists of an initial isometric contraction followed by a forced stretch imposed by the servomotor. The rotation effectively lengthens the muscle while it contracts, e.g., an eccentric contraction. During stimulation flexor muscles are subjected to an 800 msec isometric and 200 msec eccentric contraction. This procedure is repeated every 5 sec. To avoid fatigue, 4 min rest follows every 10 contractions with a total of 30 contractions performed.
Protocol
1. Setting up for the experiment
Load the Dynamic Muscle Control (DMC) software.
Power up the physiology rig following manufacturer’s instructions.
Running the DMC software:
It is assumed that the hardware is connected to the computer that runs the DMC software, and that the system is calibrated. The software may be revised from time to time; therefore, instructions are provided below relevant to the current software version that we use. When the software is opened, it is already running.
The user can initially write a protocol in DMC (e.g., to stimulate at 50 Hz for 1.5 s to produce an isometric tetanus) and the protocol can then be saved for subsequent use. We use two standard protocols: (1) isometric contraction protocol (e.g., twitch or tetanus); and (2) eccentric stretch-injury protocol. We start with the isometric contraction protocol for the twitch. To optimize the twitch, the Monitor Outputs screen is used; torque generated during the twitch can be observed as the electrodes are moved. When the maximum twitch response is achieved, the data are collected by returning to the main screen (select Continue) and selecting Begin and Done. Files are saved from the Save As pop-up window. With the electrodes maintained in the same position, a tetanus (50 Hz) is then collected and saved. When all isometric contractions are completed, the eccentric stretch-injury protocol can be loaded and run. Because there are a series of contractions to be executed in this protocol (10 contractions), on the main screen the Run One button is selected to reveal Run All status. Now when Begin is selected, a Run All Protocols pop up window appears that asks for the root file name and the directory into which the files are to be saved. Then, Start Protocol Series Test and Done are selected to initiate the eccentric protocol. The files are saved automatically into the selected Directory.
2. Positioning the animal in the physiology rig
Animal care and use committee (ACUC) guidelines must be followed for all procedures involving animals.
Anesthetize dog using inhalation anesthesia (such as isoflurane) according to approved protocol. Check blink reflex to be sure that the animal is deeply anesthetized. CO2 monitoring, pulse oximetry and temperature should be closely monitored during the entire procedure. We typically use propofol for induction followed by sevoflurane for inhalation anesthesia.
Shave the hair around the lateral stifle (knee) where the stimulating electrodes will be placed (i.e., the lateral aspect of the limb beginning at the fibular head to 2-3 cm distal to the greater trochanter.
Position dog on the physiology table in dorsal recumbency with the pelvic limb positioned so that the femur and tibia make a right angle, and the tibia is parallel to the tabletop. Either the right or left limb may be tested.
Prior to attaching dog paw to force pedal, check to insure that the force pedal is clear and safe to rotate. Follow the powering on procedure for the physiology rig and activate the pedal. Note: the force pedal should be "stiff" when the user attempts to move it manually.
Gently rest the dog’s paw in the foot pedal; measure and record the length of the dog’s paw from the center axis of rotation to the end of the lateral digit.
Secure the paw in the foot pedal using elastic self-adhering wrap.
3. Isometric twitch torque (tibiotarsal flexion)
Following a safety check, connect sterile shielded monopolar 24-gauge stimulating electrodes to the stimulus isolation unit (SIU).
Set the stimulator to: continuous pulse output at 1 Hz (1 pulse per second)
Software Settings: Load the isometric contraction protocol (on the main screen select Load Protocol). The Run One button should be visible next to the Begin button. This ensures the protocol is only run one time.
With gloved hands, clean the skin with alcohol, and palpate the common peroneal nerve just distal to the lateral proximal fibula. With a finger marking the area of the nerve, carefully insert the cathode (black) electrode under the skin and just external to the periosteum. The electrode should be placed parallel to the surface of the skin at a depth of about 2cm, sufficient to prevent the electrode from moving when the hand is removed.
Place the anode (red) electrode under the skin, parallel and slightly distal to the cathode electrode. Both electrodes should now be internal to the common peroneal nerve just distal to the fibular head.
Activate the stimulator at 1 Hz. Observe the twitch responses on the monitor. Make small adjustments in the electrode positions to obtain the largest twitch amplitude possible. The electrode should be completely removed and replaced in three to four ½ cm increments parallel to the tibia to find the largest twitch response. This is a critical component of the procedure and great care must be taken to find the largest response.
Record the twitch responses by exiting the Monitor Outputs screen (select Continue), then select Begin and Done to collect the data. Save the file with the desired file name in the Save As pop up window. Turn off the stimulator.
4. Isometric tetanic torque (tibiotarsal flexion)
Without moving the electrodes, change the stimulator settings to 50 Hz for 1.5 sec and enable the external trigger function. This allows the stimulator to be activated by the software.
Software settings: The same isometric contraction protocol is used to collect the tetanic response as for the twitch. Assuming the stimulating electrodes have not been moved, Begin and Done are selected to produce the tetanus. In the isometric protocol to produce a tetanus, the stimulator is triggered by the software to maintain the correct timing. Save the tetanic response in the Save As pop up window.
5. Eccentric protocol
Without moving the electrodes, change the stimulator settings to: 50 Hz for 1 sec (100V), and enable the external trigger function. This allows the stimulator to be activated by the software.
Software settings: Load the eccentric stretch injury protocol, a command to the servomotor to rotate 29 degrees (30 degrees is the maximum). Select Run All. Select Begin; enter the root file name for the eccentric series of files and the folder into which they are to be saved. Select Start Protocol Series Test, and then Done to initiate the protocol.
In this protocol the stimulator is set to be triggered by the software to maintain the correct timing. As the response to each eccentric contraction appears on the screen, it is automatically saved.
A four minute rest period follows each set of ten eccentric contractions.
Collect a total of 30 eccentric contractions (three sets of ten contractions; each set separated by a four minute rest period).
6. Isometric twitch torque (tibiotarsal extension)
Repeat the twitch protocol above, but place the electrodes perpendicular to the skin and proximal to the original electrode placement for the flexion protocol. The tibial nerve is stimulated as it courses caudally and distally within the gastrocnemious muscle bellies. Paired stimulating and reference electrodes are placed deep within the musculature caudal and just proximal to the stifle. The approximate point of placement is along a line that bisects the 90o angle formed by the stifle (knee). Because the tibial nerve lies deeper (and caudal) to the common peroneal nerve, the electrodes should be placed in several areas to elicit the optimal twitch potentials. This is a critical component of the procedure. Note: torque tracings for tibiotarsal twitch and tetany will appear inverted relative to tibiotarsal flexion tracings.
7. Isometric tetanic torque (tibiotarsal extension)
Without moving the electrodes, change the stimulator settings to 50 Hz for 1.5 sec and enable the external trigger function. This allows the stimulator to be activated by the software.
Software settings to: 50 Hz for 1.5 sec and enable the external trigger function. This allows the stimulator to be activated by the software.
Software settings: The same isometric contraction protocol is used for the tetanus as for the twitch. Assuming the stimulating electrodes have not been moved, Begin and Done are selected to produce the tetanus.
In the isometric protocol to produce a tetanus, the stimulator is triggered by the software to maintain the correct timing. Save the tetanic response in the pop up Save File window.
8. Data analysis
Dynamic muscle analysis (DMA) program (Aurora Scientific) is used to obtain the torque and temporal information collected from twitch, tetanic and eccentric contractions.
Running the DMA program: The software may be revised from time to time; therefore, instructions are provided below relevant to the current software version that we use. When the DMA software is opened it is not running. To activate the program, select the white arrow button in the top left corner. The DMA software has a number of functions, but for our purposes, only a few relevant functions will be described. Load a file to analyze: The Reload recent data file or new data file? pop up window appears when the DMA program is started. Select New; in the next window select Labview; in the next window, select the appropriate folder and file; select Open. Select Remove Filter Plot to remove this window. Enter a filter frequency in the Cut-off Frequency box. Optimal filter frequency would have to be determined based on the specific data to be assessed. As an example, we use 30 Hz for our isometric responses and 10 Hz for the eccentric responses. Select the Use filtered data button. Under Select Plot Data, select Filtered Force. Use the two slide bars at the bottom to expand the display and move through the file.
Twitch analysis (Fig 1): Assuming the file is loaded and the above steps are completed, set the position of the cursors: There are 3 cursors: (1) blue, (2) yellow and (3) white. For isometric twitches and tetani or other isometric contractions, the blue is set at the point where torque rises from the resting torque; the yellow is placed where the torque returns to resting torque (i.e., after the relaxation phase); the white is placed to the right of the yellow cursor (not needed). The cursors are easily moved with the left button depressed on the mouse and dragging the selected cursor to its desired position. Perform the analysis: When the cursors are positioned, select Do Muscle Analysis, then Show Results. On the Analyzed Muscle Data Results pop up window, a number of variables are displayed such as Maximum Force ; Baseline Force ; ½ Relaxation Time, etc. The data are presented for the torque response that lies between cursors 1 (blue) and 2 (yellow). All of these variables or specific variables can be logged to a data file; however, that process is not described herein. If a data log is not set up, the data would have to be recorded by hand. Select Close Window to return to the main screen. Select Exit Program to close the DMA, and then select the white arrow to begin analysis of a new file.
Tetany analysis (Fig 2): The same steps as described above for the twitch.
Eccentric analysis (Fig 3): The eccentric analysis requires more steps to set up and uses all 3 cursors. If the eccentric torque response is negative (i.e., the torque tracings appear in the downward direction), it can be inverted by entering -1 in the Reference Area (sq cm) box (torque values are unchanged) and select Do not normalize; the button reverses to indicate the data have been normalized (in this case, inverted). Enter the desired Cut Off Frequency (e.g., 10 Hz) and select Use unfiltered data to reverse the button to Use filtered data, now filtered data will be used for the analysis. Position the blue cursor at the point where isometric torque begins to rise from resting torque; position the yellow cursor at the point where the eccentric stretch torque begins to rise from the isometric plateau (see figure below); position the white cursor where torque returns to resting torque. Select Do Muscle Analysis, then Show Results. On the Analyzed Muscle Data Results pop up window, you can select the data between cursors 1 (blue) and 2 (yellow) for the isometric torque data, or between cursors 2 (yellow) and 3 (white) for the eccentric torque data. All of these variables or specific variables can be logged to a data file; however, that process is not described herein. If a data log is not set up, the data would have to be recorded by hand. Select Close Window to return to the main screen. Select Exit Program to close the DMA, and then select the white arrow to begin analysis of a new file.
9. Representative Results
Twitch torque: Tibial nerve stimulation (1 Hz) activated single twitches of tibiotarsal extensor muscles (Fig 1). Note that the Y-axis indicates "force"; torque (the product of force and distance) is calculated by the user. We measure the length of the paw (from the axis of rotation to the end of the lateral digit). This length is the distance (lever arm) used to determine torque (N-m).
Isometric tetanic torque (tibiotarsal extension): tibial nerve stimulation tetanized tibiotarsal extensor muscles (1.5 sec train of 50hz pulses). We used Dynamic Muscle Control computer software (DMC, Aurora Scientific, Inc.) to control stimulation timing and to capture torque responses (Fig 2).
Eccentric protocol: The eccentric contractions were conducted in the tibiotarsal flexors. Percutaneous peroneal nerve stimulation activated an initial isometric contraction followed by joint rotation causing lengthening of the activated flexor muscles (eccentric contraction; Fig 3).6
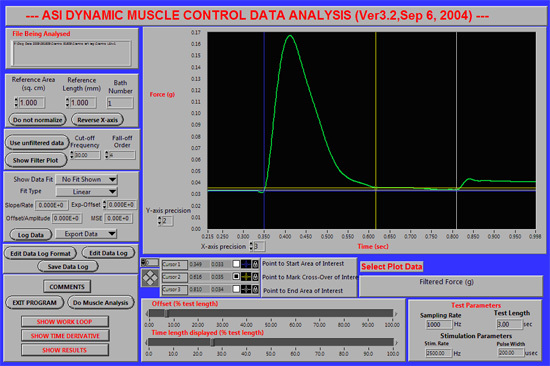 Figure 1. Placement of the blue (#1), yellow (#2) and white (#3) cursors to analyze a twitch. A representative screen shot of the DMA software is shown
for tibiotarsal extension twitch in a healthy 13 kg dog. Note that for subsequent analysis performed by the software, the data are exported to a spreadsheet, and the
force output (shown in the figure as Force (g) can be converted to torque (N-m).Torque is calculated by the product of force and lever arm distance (in this the
distance = length measured from axis of rotation to lateral digit of the paw).
Figure 1. Placement of the blue (#1), yellow (#2) and white (#3) cursors to analyze a twitch. A representative screen shot of the DMA software is shown
for tibiotarsal extension twitch in a healthy 13 kg dog. Note that for subsequent analysis performed by the software, the data are exported to a spreadsheet, and the
force output (shown in the figure as Force (g) can be converted to torque (N-m).Torque is calculated by the product of force and lever arm distance (in this the
distance = length measured from axis of rotation to lateral digit of the paw).
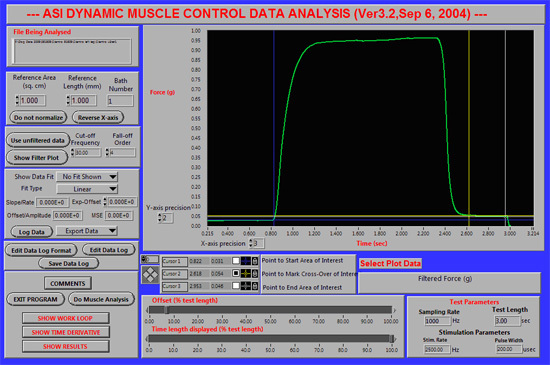 Figure 2. Placement of the blue (#1), yellow (#2) and white (#3) cursors to analyze a tetanus. A representative screen shot of the DMA software is
shown for tibiotarsal extension tetany, same animal as shown in Fig 1.
Figure 2. Placement of the blue (#1), yellow (#2) and white (#3) cursors to analyze a tetanus. A representative screen shot of the DMA software is
shown for tibiotarsal extension tetany, same animal as shown in Fig 1.
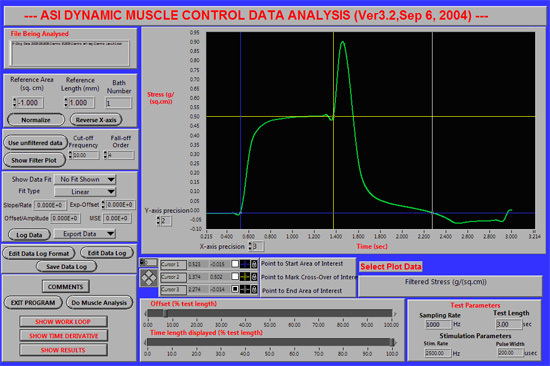 Figure 3. Placement of the blue (#1), yellow (#2) and white (#3) cursors to analyze an eccentric stretch-injury contraction. A representative screen
shot of the DMA software is shown for a single eccentric stretch-injury contraction in a healthy 13 kg dog. The initial plateau (green line) illustrates the isometric
phase of the contraction. The isometric phase is followed by an eccentric (stretch) phase as illustrated by the rapid peak in the green line. Repeated eccentric
contractions typically result in a steady decline in isometric torque.
Figure 3. Placement of the blue (#1), yellow (#2) and white (#3) cursors to analyze an eccentric stretch-injury contraction. A representative screen
shot of the DMA software is shown for a single eccentric stretch-injury contraction in a healthy 13 kg dog. The initial plateau (green line) illustrates the isometric
phase of the contraction. The isometric phase is followed by an eccentric (stretch) phase as illustrated by the rapid peak in the green line. Repeated eccentric
contractions typically result in a steady decline in isometric torque.
Discussion
This protocol is used to assess large animal muscle function in vivo to analyze, document and determine efficacy of treatment interventions or to document biological features of disease progression.1-6 Animal positioning, limb placement and electrode placement are key variables that significantly impact results. In a preliminary study, mean alveolar concentration (MAC) values for isoflurane did not significantly affect force measurements (Schueler RO, Koch H, Kornegay JN, unpublished data, 1993).Hardware and software are commercially available and methods well-established.7,1,2,8,4,9-11,6,12 It is recommended that users obtain and use the software and equipment described above when following this protocol.
Modifications: limitations of the current servomotor include body mass of about 23 kg and paw length of 20cm. Note that the torque limit is ~8 N-meters for the torque transducer (model 310LR) described here. This value may be exceeded by normal dogs at 6 months or older. Possible modifications to the current protocol include updating the software and to increase the torque range of the transducer. Aurora Scientific manufactures a limb positioning device for large animals that can be substituted for the stereotactic frame used in the current protocol. This method has successfully been applied to other animal models, including non-human primates.
Disclosures
The production of this video-article was sponsored by Aurora Scientific, Inc.
Acknowledgments
National Institutes of Health (NIH), Joshua Frase Foundation, Muscular Dystrophy Association (MDA).
References
- Childers MK, Okamura CS, Bogan DJ, Bogan JR, Petroski GF, McDonald K, Kornegay JN. Eccentric contraction injury in dystrophic canine muscle. Arch Phys Med Rehabil. 2002;83:1572–1578. doi: 10.1053/apmr.2002.35109. [DOI] [PubMed] [Google Scholar]
- Childers MK, Okamura CS, Bogan DJ, Bogan JR, Sullivan MJ, Kornegay JN. Myofiber injury and regeneration in a canine homologue of Duchenne muscular dystrophy. Am J Phys Med Rehabil. 2001;80:175–181. doi: 10.1097/00002060-200103000-00004. [DOI] [PubMed] [Google Scholar]
- MK Childers, JT Staley, JN Kornegay, KS McDonald. Skinned single fibers from normal and dystrophin-deficient dogs incur comparable stretch-induced force deficits. Muscle Nerve. 2005;31:768–771. doi: 10.1002/mus.20298. [DOI] [PubMed] [Google Scholar]
- Kornegay JN, Bogan DJ, Bogan JR, Childers MK, Cundiff DD, Petroski GF, Schueler RO. Contraction force generated by tarsal joint flexion and extension in dogs with golden retriever muscular dystrophy. J Neurol Sci. 1999;166:115–121. doi: 10.1016/s0022-510x(99)00118-5. [DOI] [PubMed] [Google Scholar]
- Liu JM, Okamura CS, Bogan DJ, Bogan JR, Childers MK, Kornegay JN. Effects of prednisone in canine muscular dystrophy. Muscle Nerve. 2004;30:767–773. doi: 10.1002/mus.20154. [DOI] [PubMed] [Google Scholar]
- Tegeler CJ, Grange RW, Bogan DJ, Markert CD, Case D, Kornegay JN, Childers MK. Eccentric contractions induce rapid isometric torque drop in dystrophin-deficient dogs. Muscle Nerve. 2010;42:130–132. doi: 10.1002/mus.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DG. Eccentric muscle damage: mechanisms of early reduction of force. Acta Physiol Scand. 2001;171:311–319. doi: 10.1046/j.1365-201x.2001.00833.x. [DOI] [PubMed] [Google Scholar]
- Friden J, Lieber RL. Eccentric exercise-induced injuries to contractile and cytoskeletal muscle fibre components. Acta Physiol Scand. 2001;171:321–326. doi: 10.1046/j.1365-201x.2001.00834.x. [DOI] [PubMed] [Google Scholar]
- Merkies IS, Faber CG. Outcome measures in Duchenne muscular dystrophy: are we ready for the new therapeutic era? Neuromuscul Disord. 2009;19:447–447. doi: 10.1016/j.nmd.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Nagaraju K, Willmann R. Developing standard procedures for murine and canine efficacy studies of DMD therapeutics: report of two expert workshops on "Pre-clinical testing for Duchenne dystrophy". Neuromuscul Disord. 2009;19:502–506. doi: 10.1016/j.nmd.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U, Allen TJ. Damage to skeletal muscle from eccentric exercise. Exerc Sport Sci Rev. 2005;33:98–104. doi: 10.1097/00003677-200504000-00007. [DOI] [PubMed] [Google Scholar]
- Warren GL, Lowe DA, Armstrong RB. Measurement tools used in the study of eccentric contraction-induced injury. Sports Med. 1999;27:43–59. doi: 10.2165/00007256-199927010-00004. [DOI] [PubMed] [Google Scholar]


