Abstract
While the mouse retina has emerged as an important genetic model for inherited retinal disease, the mouse vitreous remains to be explored. The vitreous is a highly aqueous extracellular matrix overlying the retina where intraocular as well as extraocular proteins accumulate during disease.1-3 Abnormal interactions between vitreous and retina underlie several diseases such as retinal detachment, proliferative diabetic retinopathy, uveitis, and proliferative vitreoretinopathy.1,4 The relative mouse vitreous volume is significantly smaller than the human vitreous (Figure 1), since the mouse lens occupies nearly 75% of its eye.5 This has made biochemical studies of mouse vitreous challenging. In this video article, we present a technique to dissect and isolate the mouse vitreous from the retina, which will allow use of transgenic mouse models to more clearly define the role of this extracellular matrix in the development of vitreoretinal diseases.
Keywords: Cellular Biology, Issue 50, mouse, vitreous, retina, proteomics, superoxide dismutase
Protocol
1. Anterior segment dissection.
Scleral tissue posterior to the limbus is grasped with a 0.22 forcep and the globe (eye ball) is stabilized.
A microsurgical blade is used to make a linear incision in the cornea from limbus to limbus.
A 0.12 Colibri forcep is then used to grasp the cornea at the incision.
The anterior chamber fluid is then absorbed with a Weck-Cel surgical spear.
2. Lens evisceration.
A 0.12 Colibri forcep grasping the cornea is used to stabilize the globe.
A fine curved needle holder (or curved dressing forceps) is inserted behind the lens toward the posterior aspect of the globe. The needle holder is partially closed, and pulled forward. Pressure is applied using the forceps on the external surface of the sclera, which pushes the lens forward through the corneal incision while leaving the eye wall intact. The vitreous appears as a translucent gel and is partially adherent to the lens.
The lens-vitreous tissue is then placed into a filtered centrifugation tube containing 20 microliters of protease inhibitor cocktail (Roche) dissolved in PBS.
3. Retina evisceration.
Continue to stabilize the globe with 0.12 Colibri forceps grasping the corneal incision.
A fine curved needle holder (or curved dressing forceps) is placed as far posterior to the globe as possible, near the optic nerve. The needle holder is partially closed, and pulled forward. Pressure is applied using the forceps on the external surface of the sclera, which pushes the retina forward through the corneal incision. The vitreous appears as a translucent gel adherent to the retina. The retina is visualized as a yellow, vascularized tissue. Any strips of pigmented tissue can be peeled away with forceps.
The retina-vitreous tissue is then placed into the filtered centrifuge tube containing the lens-vitreous tissue.
4. Filtered centrifugation.
The filtered centrifuge tube is placed in a benchtop centrifuge. Spin at 14,000 x G for 12 minutes.
Aspirate the eluant from the lower chamber, which is the vitreous. The retina remains in the upper chamber. These samples can be utilized for protein studies.
5. Representative Results
Vitreous samples were analyzed by SDS-PAGE (Figure 2). Samples were also used for an enzymatic activity assay (Figure 3).
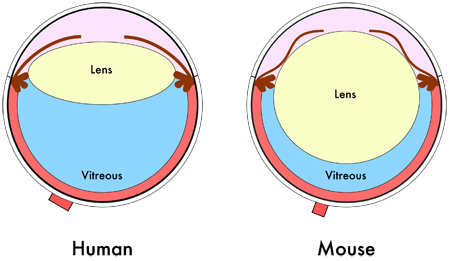 Figure 1. Mouse eye diagram. Cross-sectional schematic of the human and mouse eye shows the relative smaller vitreous volume in the mouse eye due to its larger lens.
Figure 1. Mouse eye diagram. Cross-sectional schematic of the human and mouse eye shows the relative smaller vitreous volume in the mouse eye due to its larger lens.
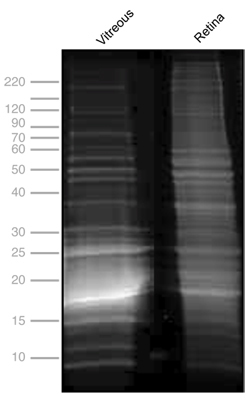 Figure 2. One-dimensional SDS-PAGE of mouse vitreous and retina. Protein profiles of mouse vitreous, 13.6 mg/mL (lane 1), and retina, 11.35 mg/mL (lane 2). Gel electrophoresis was performed at 150 kV for 45 minutes, stained with Flamingo fluorescent gel stain and visualized using a VersaDoc Imaging system (BioRad, Hercules, CA).
Figure 2. One-dimensional SDS-PAGE of mouse vitreous and retina. Protein profiles of mouse vitreous, 13.6 mg/mL (lane 1), and retina, 11.35 mg/mL (lane 2). Gel electrophoresis was performed at 150 kV for 45 minutes, stained with Flamingo fluorescent gel stain and visualized using a VersaDoc Imaging system (BioRad, Hercules, CA).
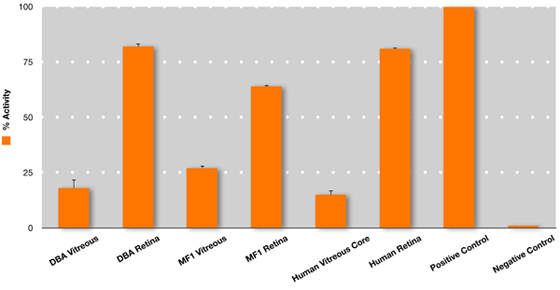 Figure 3. Superoxide dismutase (SOD) enzyme activity in the mouse and human vitreous. Measurement of total SOD activity (SOD activity colorimetric aasay kit, #AB65354, Abcam, Inc, Cambridge, MA) was performed on vitreous and retina collected by evisceration from DBA and albino mice. This was compared to SOD activity in human vitreous core and human retina collected from post-mortem human donor eyes to determine relative activity. Undiluted vitreous core samples were manually aspirated using a 23-gauge needle, 11 and retina was collected by flowering the eye and cutting the tissue away from the RPE/choroid complex. Although the assay does not distinguish between SOD isoforms, the vitreous SOD activity is likely to reflect SOD3, since it is the only extracellular isoform.12 The retina SOD activity is likely to reflect all three SOD isoforms: intracellular SOD1, mitochondrial SOD2, and extracellular SOD3. 12 For highly sensitive enzymatic assays, including this SOD assay, the possibility of cross-contamination of proteins is possible and requires further study. Error bars represent SEM.
Figure 3. Superoxide dismutase (SOD) enzyme activity in the mouse and human vitreous. Measurement of total SOD activity (SOD activity colorimetric aasay kit, #AB65354, Abcam, Inc, Cambridge, MA) was performed on vitreous and retina collected by evisceration from DBA and albino mice. This was compared to SOD activity in human vitreous core and human retina collected from post-mortem human donor eyes to determine relative activity. Undiluted vitreous core samples were manually aspirated using a 23-gauge needle, 11 and retina was collected by flowering the eye and cutting the tissue away from the RPE/choroid complex. Although the assay does not distinguish between SOD isoforms, the vitreous SOD activity is likely to reflect SOD3, since it is the only extracellular isoform.12 The retina SOD activity is likely to reflect all three SOD isoforms: intracellular SOD1, mitochondrial SOD2, and extracellular SOD3. 12 For highly sensitive enzymatic assays, including this SOD assay, the possibility of cross-contamination of proteins is possible and requires further study. Error bars represent SEM.
Discussion
Transgenic mice are an important model for investigating retinal and vitreoretinal disease.6-10 The mouse vitreous body, however, comprises a significantly smaller proportion of the eye in comparison to the human vitreous due to the large lens in the mouse eye.5 This makes it difficult to isolate and purify the mouse vitreous. Understanding the protein changes associated with the vitreous during diseases such as proliferative diabetic retinopathy, age-related macular degeneration, uveitis, and retinal detachment will give insight into the mechanisms by which they progress. This visualized experimental protocol provides a means to obtain and purify the mouse vitreous body, while maximizing the protein yield for enzymatic and proteomic analyses.
Disclosures
Experiments on animals were performed in accordance with the Association for Research and Vision in Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research.
Acknowledgments
Funding was provided by Fight for Sight and Research to Prevent Blindness.
References
- Bishop PN. Structural macromolecules and supramolecular organisation of the vitreous gel. Prog. Retin. Eye Res. 2000;19:323–344. doi: 10.1016/s1350-9462(99)00016-6. [DOI] [PubMed] [Google Scholar]
- Gao BB, Chen X, Timothy N, Aiello LP&, Feener EP. Characterization of the vitreous proteome in diabetes without diabetic retinopathy and diabetes with proliferative diabetic retinopathy. J. Proteome Res. 2008;7:2516–2525. doi: 10.1021/pr800112g. [DOI] [PubMed] [Google Scholar]
- Izuta H. Extracellular SOD and VEGF are increased in vitreous bodies from proliferative diabetic retinopathy patients. Mol. Vis. 2009;15:2663–2672. [PMC free article] [PubMed] [Google Scholar]
- Sebag J. Molecular biology of pharmacologic vitreolysis. Trans. Am. Ophthalmol. Soc. 2005;103:473–494. [PMC free article] [PubMed] [Google Scholar]
- Smith RS. Smith RS, John SWM, Nishina PM, Sundberg JP. Systematic evaluation of the mouse eye: Anatomy, pathology, and biomethods. CRC Press; 2002. pp. 161–195. [Google Scholar]
- Ihanamaki T, Metsaranta M, Rintala M, Vuorio E, Sandberg-Lall M. Ocular abnormalities in transgenic mice harboring mutations in the type II collagen gene. Eur. J. Ophthalmol. 1996;6:427–435. doi: 10.1177/112067219600600415. [DOI] [PubMed] [Google Scholar]
- Kaarniranta K. A mouse model for Stickler's syndrome: ocular phenotype of mice carrying a targeted heterozygous inactivation of type II (pro)collagen gene (Col2a1. Exp. Eye Res. 2006;83:297–303. doi: 10.1016/j.exer.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Marneros AG&, Olsen BR. Age-dependent iris abnormalities in collagen XVIII/endostatin deficient mice with similarities to human pigment dispersion syndrome. Invest. Ophthalmol. Vis. Sci. 2003;44:2367–2372. doi: 10.1167/iovs.02-1180. [DOI] [PubMed] [Google Scholar]
- Martin AC. Pathogenesis of persistent hyperplastic primary vitreous in mice lacking the arf tumor suppressor gene. Invest. Ophthalmol. Vis. Sci. 2004;45:3387–3396. doi: 10.1167/iovs.04-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song BJ, Tsang SH, Lin CS. Genetic models of retinal degeneration and targets for gene therapy. Gene. Ther. Mol. Biol. 2007;11B:229–262. [Google Scholar]
- Skeie JM, Mahajan VB. Dissection of Human Vitreous Body Elements for Proteomic Analysis. J Vis Exp. 2011. [DOI] [PMC free article] [PubMed]
- Ciechanowski K. Impaired synthesis is not the reason for decreased activity of extracellular superoxide dismutase in patients with diabetes. Arch Med Res. 2005;36:148–153. doi: 10.1016/j.arcmed.2004.11.002. [DOI] [PubMed] [Google Scholar]


