Abstract
The heterogeneous nature of cell types in the testis and the absence of meiotic cell culture models have been significant hurdles to the study of the unique differentiation programs that are manifest during meiosis. Two principal methods have been developed to purify, to varying degrees, various meiotic fractions from both adult and immature animals: elutriation or Staput (sedimentation) using BSA and/or percoll gradients. Both of these methods rely on cell size and density to separate meiotic cells1-5. Overall, except for few cell populations6, these protocols fail to yield sufficient purity of the numerous meiotic cell populations that are necessary for detailed molecular analyses. Moreover, with such methods usually one type of meiotic cells can be purified at a given time, which adds an extra level of complexity regarding the reproducibility and homogeneity when comparing meiotic cell samples.
Here, we describe a refined method that allows one to easily visualize, identify, and purify meiotic cells, from germ cells to round spermatids, using FACS combined with Hoechst 33342 staining7,8. This method provides an overall snapshot of the entire meiotic process and allows one to highly purify viable cells from most stage of meiosis. These purified cells can then be analyzed in detail for molecular changes that accompany progression through meiosis, for example changes in gene expression9,10and the dynamics of nucleosome occupancy at hotspots of meiotic recombination11.
Protocol
This protocol can be separated in two major steps: (1) the dissociation and Hoechst 33342 staining of mouse testis cells followed by, if necessary, (2) FACS sorting of the relevant meiotic fractions, including all stages of meiosis, from germ cells to round spermatids. Once collected, these highly enriched meiotic populations can be used for a wide range of analysis. This protocol describes the dissociation of one adult testis; volumes can be adapted accordingly for juveniles or for additional testes.
1. Testis Dissociation
Place one decapsulated testis in a 15-ml tube.
Add 3-ml of Gey's Balance Salt Solution (GBSS) containing 120 U/ml of Collagenase type I.
Add 10 μl of DNAse I (1mg/ml stock solution in 50% glycerol) and shake it vigorously by hand until you see testicular tubules starting to dissociate.
Agitate horizontally at a maximum of 120 rpm for 15 min at 33°C.
Decant for 1 min vertically at room temperature and discard supernatant.
Repeat steps 1.2 to 1.5.
Add 2.5 ml of GBSS containing Collagenase type I, 50 μl of a 50mg/ml Trypsin stock solution resuspended in 1mM HCl solution, and 10 μl of DNAse I (1mg/ml), and invert the tube several times.
Agitate horizontally at a maximum of 120 rpm for 15 min at 33°C.
Using plastic disposable Pasteur pipet with wide orifice, pipette gently up and down for 3 min.No clumps should be visible at this point.
Add 30 μl of trypsin, 10 μl of DNAse I, 40 μl of Hoechst 33342 resuspended in DMSO (10 mg/ml), and invert the tube several times.
Agitate horizontally at a maximum of 120 rpm for 15 min at 33°C.
Add 400-μl of fetal calf serum (FCS) and mix by inverting to inactivate trypsin.
Final staining is performed by adding 50 μl of Hoechst 33342 resuspended in DMSO (10 mg/ml), and 10 μl of DNAse I (1 mg/ml).
Agitate horizontally at a maximum of 120 rpm for 15 min at 33°C.
The dissociated testis sample is then passed through two 40-μm GBSS pre-wetted disposable filters over a 50-ml conical tube, then 5 μl of propidium iodide (PI) solution is added and sample is gently mixed by pipetting several times with disposable Pasteur pipette.
Sample is transferred to a 5-ml plastic syringe through an 18-gauge needle. The latter is replaced by a 22-gauge needle for sample delivery. The syringe is stored on ice and protected from the light until ready for FACS processing.
2. FACS Setup and Purification
Sorting was performed on a Becton-Dickinson Aria IIu cell sorter. The conditions described should therefore be used as a starting point especially when using different equipment. We used conditions previously described7,8.
Since the Aria IIu does not have a UV laser, we adapted the protocol for detecting Hoescht staining using a 405nm violet laser in addition we used a 488nm blue laser for forward and side scatter detection. Furthermore, some filters were modified in order to limit some red laser leakiness resulting in improved scattered plot sharpness.
The violet laser was configured with a 450/40nm band pass for detection of Hoescht Blue emission and a 585/42nm band pass for Hoescht Red emission. A 502nm long pass was used to separate blue from red fluorescence.
A 100 μm nozzle was used with a drop drive frequency of 28,000 drops/second. The sample threshold rate was approximately 4000 events/second. The temperature control option was used to maintain sample and collection tubes at 4°C the entire duration of sorting. Additionally, the sample agitation feature was used at 200 rpm to prevent the sample from sedimenting throughout the sort.
Usually, two to three testes were processed per 6 hours sort allowing the collection of 0.5- 2.0x106cells for each population (see representative section).
The sample was sorted in aliquots of approximately 750μl dispensed from the syringe. Meanwhile, during these pauses the collection tubes were kept at 4°C, protected from the light, and gently mixed prior to resuming sort.
The sorted samples were collected into a 12x75mm glass borosilicated collection tube containing 250μl DMEM supplemented with 10% FCS.
3. Representative Results:
A typical FACS profile is shown in Figure 1. All of the major steps of meiosis are identified and are indicated in the legend. If the Hoechst 33342 stain is not optimal, the global profile will appear much more compact and less defined. A common problem regards not adding sufficient DNAse, which will induce rapid cell clumping. Addition of extra DNAse at all indicted steps usually solves this problem. Freeze thawing of the DNAse stock (stored at -20°C)should be kept to a minimum, as this results in rapid loss of enzyme activity.
Spo11 is the meiotic endonuclease that directs double-strand breaks at sites of meiotic recombination hotspots12.Figure 2 shows typical profiles of a Spo11-null testis where double-strand break are not formed, resulting in an abortive meiosis12, as well as a pre-puberous profile of a 13-days old mouse, which reveals asynchronous nature of this first wave of meiosis. Standard cytogenetic analyses using the combination of synaptonemal complex protein 3 (SCP3) and phosphorylated histone H2AX antibodies stage-specific meiosis markers should be initially used to confirmed the purity and nature of the sorted meiotic cells, as described elswhere11.
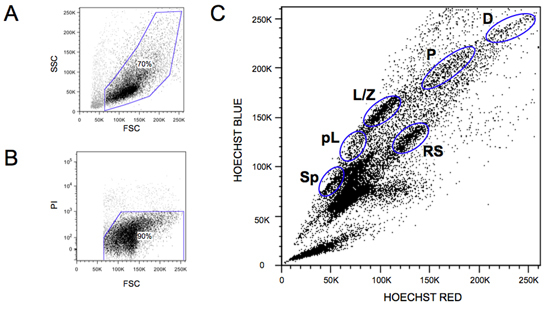 Figure 1. Wild-type meiosis FACS profiles. A) Representative scatter plot is shown. The gated cells are shown. Note the large amount of debris present in adult testis containing mostly empty membranes and spermatid tails. B) A representative PI plot shows the very limited amount of PI positive cells present in a sample. The typical gate is shown. C) A representative wild-type Hoechst 33342 FACS profile is shown. The various meiotic cell populations that can be purified using this method for further study are indicated: spermatogonia (Sp), pre-leptotene (pL), leptotene-zygotene (L/Z), early-Pachytene (eP), middle-Pachytene (mP), late-Pachytene (lP), diplotene (D), and round spermatids (RS).
Figure 1. Wild-type meiosis FACS profiles. A) Representative scatter plot is shown. The gated cells are shown. Note the large amount of debris present in adult testis containing mostly empty membranes and spermatid tails. B) A representative PI plot shows the very limited amount of PI positive cells present in a sample. The typical gate is shown. C) A representative wild-type Hoechst 33342 FACS profile is shown. The various meiotic cell populations that can be purified using this method for further study are indicated: spermatogonia (Sp), pre-leptotene (pL), leptotene-zygotene (L/Z), early-Pachytene (eP), middle-Pachytene (mP), late-Pachytene (lP), diplotene (D), and round spermatids (RS).
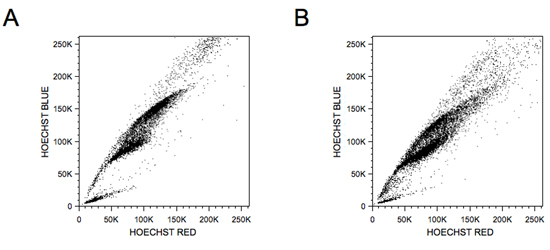 Figure 2.
Spo11-null and wild-type day 13 meiotic FACS profiles. A) A typical Spo11-null profile is shown with a clear meiotic failure, where no stage past the leptotene/zygotene early-pachytene can be detected. B) The profile of a 13-day-old wild-type male mouse shows the high concentration of leptotene/zygotene cells. This first wave of meiosis is rather asynchronous, as clearly visualized using this methodology.
Figure 2.
Spo11-null and wild-type day 13 meiotic FACS profiles. A) A typical Spo11-null profile is shown with a clear meiotic failure, where no stage past the leptotene/zygotene early-pachytene can be detected. B) The profile of a 13-day-old wild-type male mouse shows the high concentration of leptotene/zygotene cells. This first wave of meiosis is rather asynchronous, as clearly visualized using this methodology.
Discussion
The protocol presented herein allows one to simultaneously purify from adult male mice the entire range of the meiotic stage cells with very high purity, allowing investigators to study the dynamics of this fundamental process. Purified cells can be used for numerous applications ranging from RNA extraction9,10, nucleosome mapping11, recombinant molecule detection, protein analyses, and many more. However, detection methods have to be adapted to the amount of meiotic cells purified. Moreover, with the high reproducibility of this method, it is possible to pool multiple independent sorts of the same fraction performed on different days to increase the amount of material required for a particular experiment. Also, this method provides investigators with a unique visual representation of the entire meiotic process, which allows one to rapidly assess the effects of genetic knockout, developmental progression aberration, or the effect of any particular treatment aimed at affecting meiosis (e.g., irradiation, or treatment with small molecule inhibitors).
Critical steps in this protocol are (i) the use of sufficient DNAse, which greatly facilitates cell sorting by avoiding cell clumps and aggregates, and (ii) consistent Hoechst 33342 staining that is necessary to efficiently distinguish the various populations of meiotic stage cells. In addition, FACS operator should familiarize themselves with the specifics and peculiarities of meiotic cell sorting in order to optimally setup their instrument to obtain the profiles shown in Figure 1 and detailed in the video. A possible modification could be to substitute the Hoechst 33342 with the new Vybrant DyeCycle Violet Stain (Invitrogen) that has been optimized for the violet laser. However, the overall profile would not be expected to change drastically. Finally, it is also important to note that due to the nature of the tissue used, a typical procedure will take 1 ½ hr to prepare the meiotic cells and 4 to 6-hr for the cell sort, plus additional post-sort manipulations.
Disclosures
No conflicts of interest declared.
Acknowledgments
This project was supported in part by monies from the State of Florida to Scripps and award numbers R01GM085079 and R21HD061304 from the National Institute of General Medical Sciences and the National Institute of Child Health and Human Development, respectively. This is manuscript number 20917 of The Scripps Research Institute.
References
- Meistrich ML, Bruce WR, Clermont Y. Cellular composition of fractions of mouse testis cells following velocity sedimentation separation. Exp. Cell Res. 1973;79:213–227. [PubMed] [Google Scholar]
- Grabske RJ, Lake S, Gledhill BL, Meistrich ML. Centrifugal elutriation: separation of spermatogenic cells on the basis of sedimentation velocity. J. Cell. Physiol. 1975;86:177–189. doi: 10.1002/jcp.1040860119. [DOI] [PubMed] [Google Scholar]
- Purification AR. culture, and fractionation of spermatogenic cells. Methods Enzymol. 1993;225:84–113. doi: 10.1016/0076-6879(93)25009-q. [DOI] [PubMed] [Google Scholar]
- Romrell LJ, Bellve AR, Fawcett DW. Separation of mouse spermatogenic cells by sedimentation velocity. A morphological characterization. Dev. Biol. 1976;49:119–131. doi: 10.1016/0012-1606(76)90262-1. [DOI] [PubMed] [Google Scholar]
- Meistrich ML, Trostle PK. Separation of mouse testis cells by equilibrium density centrifugation in renografin gradients. Exp. Cell Res. 1975;92:231–244. doi: 10.1016/0014-4827(75)90656-4. [DOI] [PubMed] [Google Scholar]
- Namekawa SH. Postmeiotic sex chromatin in the male germline of mice. Curr. Biol. 2006;16:660–667. doi: 10.1016/j.cub.2006.01.066. [DOI] [PubMed] [Google Scholar]
- Lassalle B. Side Population' cells in adult mouse testis express Bcrp1 gene and are enriched in spermatogonia and germinal stem cells. Development. 2004;131:479–487. doi: 10.1242/dev.00918. [DOI] [PubMed] [Google Scholar]
- Bastos H. Flow cytometric characterization of viable meiotic and postmeiotic cells by Hoechst 33342 in mouse spermatogenesis. Cytometry A. 2005;65:40–49. doi: 10.1002/cyto.a.20129. [DOI] [PubMed] [Google Scholar]
- Chowdhury R, Bois PR, Feingold E, Sherman SL, Cheung VG. Genetic analysis of variation in human meiotic recombination. PLoS Genet. 2009;5:e1000648–e1000648. doi: 10.1371/journal.pgen.1000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roig I. Mouse TRIP13/PCH2 is required for recombination and normal higher-order chromosome structure during meiosis. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getun IV, Wu ZK, Khalil AM, Bois PR. Nucleosome occupancy landscape and dynamics at mouse recombination hotspots. EMBO Rep. 2010;11:555–560. doi: 10.1038/embor.2010.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanienko PJ, Camerini-Otero RD. The mouse Spo11 gene is required for meiotic chromosome synapsis. Mol. Cell. 2000;6:975–987. doi: 10.1016/s1097-2765(00)00097-6. [DOI] [PubMed] [Google Scholar]


