Abstract
Normophosphatemic familial tumoral calcinosis (NFTC) is an autosomal recessive disorder characterized by calcium deposition in skin and mucosae and associated with unremitting pain and life-threatening skin infections. A homozygous missense mutation (p.K1495E), resulting in SAMD9 protein degradation, was recently shown to cause NFTC in five families of Jewish-Yemenite origin. In this study, we evaluated another Jewish-Yemenite NFTC kindred. All patients were compound heterozygous for two mutations in SAMD9: K1495E and a previously unreported nonsense mutation, R344X, predicted to result in a markedly truncated molecule. Screening of unaffected population-matched controls revealed heterozygosity for K1495E and R344X only in individuals of Jewish-Yemenite ancestry, but not in more than 700 control samples of other origins, including 93 non-Jewish Yemenite. These data may be suggestive of positive selection, considering the rarity of NFTC and the small size of the Jewish-Yemenite population; alternatively, they may reflect genetic drift or the effect of a population-specific modifier trait. Calcifications in NFTC generally develop over areas subjected to repeated trauma and are associated with marked inflammatory manifestations, indicating that SAMD9 may play a role in the inflammatory response to tissue injury. We therefore assessed the effect of cellular stress and tumor necrosis factor-α (TNF-α), a potent pro-inflammatory cytokine, on SAMD9 gene expression. Whereas exogenous hydrogen peroxide and heat shock did not affect SAMD9 transcription, osmotic shock was found to markedly upregulate SAMD9 expression. In addition, incubation of endothelial cells with TNF-α caused a dose-related, p38-dependant increase in SAMD9 expression. These data link NFTC and SAMD9 to the TNF-α signaling pathway, suggesting a role for this system in the regulation of extra-osseous calcification.
INTRODUCTION
Abnormal deposition of calcium salts within skin tissues is known as calcinosis cutis (Touart and Sau, 1998). Two major types of acquired calcinosis cutis have been recognized. Metastatic calcinosis cutis refers to calcium deposition in association with elevated circulating levels of phosphate and/or calcium, as seen in chronic renal failure or hyperparathyroidism. In contrast, dystrophic calcinosis cutis is usually secondary to some form of physical insult to the skin, as seen in autoimmune diseases and skin cancer (Touart and Sau, 1998).
Inherited calcinosis cutis is known as familial tumoral calcinosis. Two forms of familial tumoral calcinosis have been reported in the literature (Smack et al., 1996), which recapitulate many features of the two major types of acquired calcinosis cutis. Hyperphosphatemic familial tumoral calcinosis (MIM211900) is caused by increased renal reabsorption of phosphate due to mutations in two genes: FGF23 (Araya et al., 2005; Benet-Pages et al., 2005; Chefetz et al., 2005; Larsson et al., 2005a, b), coding for a potent phosphaturic protein (Fukumoto and Yamashita, 2007), and GALNT3, coding for a glycosyltransferase (Topaz et al., 2004; Ichikawa et al., 2005, 2007; Specktor et al., 2006), responsible for FGF23 O-glycosylation (Kato et al., 2006; Frishberg et al., 2007). Mutations in either gene result in elevated levels of circulating phosphate and formation of periarticular subcutaneous calcified masses, sometimes associated with bone and tooth defects (Frishberg et al., 2005; Specktor et al., 2006). In contrast, calcified tumor formation in normophosphatemic familial tumoral calcinosis (NFTC; MIM610455) is generally preceded by a vasculitis-like rash and is associated with inflammatory manifestations mostly evident in mucosal tissues (Metzker et al., 1988). Recently, a mutation in SAMD9 was shown to underlie NFTC in five families of Jewish-Yemenite origin (Topaz et al., 2006). SAMD9 encodes a very large protein, thought to be involved in the regulation of cancer cell proliferation and apoptosis (Li et al., 2007).
In this report, we present data confirming the pathogenic role of SAMD9 mutations in NFTC, suggesting that survival of SAMD9 mutations may have occurred in the Jewish-Yemenite population as a result of positive selection and indicating that tumor necrosis factor-α (TNF-α) is a major regulator of SAMD9 gene expression.
RESULTS
Identification of a novel mutation in SAMD9
We assessed a family comprising two affected children (Figure 1a). Their maternal grandparents are of Jewish-Yemenite origin as is their paternal grandmother. The paternal grandfather is of Jewish-Moroccan extraction. The two patients were dizygotic twins, born after 26 weeks of gestation. Psychomotor development was normal. Family history was unremarkable. The male patient was affected by retinopathy of prematurity. At age 6, the two patients were first assessed because of the formation of periarticular calcified masses. No preceding rash had been observed. On examination, the patients displayed subcutaneous mobile calcified masses above the knees and elbows (Figure 1b) and showed edema, erythema, and minute calcified nodules over the sides of their feet (not shown). In addition, both patients displayed marked edema and redness of the gingivae (Figure 1c). Complete blood count and routine chemistry (including phosphate levels) were normal.
Figure 1. Identification of a new mutation in SAMD9.
(a) Pedigree and haplotype analysis of an NFTC family and four healthy unrelated control individuals (C1–C4) carrying mutations p.K1495E and p.R344X. The p.K1495E- and p.R344X-associated haplotypes are boxed in red and blue, respectively. Filled symbols represent affected individuals. (b) Subepidermal calcified nodule over the left elbow of the male patient. (c) Severe gingivitis displayed by the female patient. (d) Sequence analysis revealed two distinct mutations in the patients: an A>G transition at cDNA position 4483, which is predicted to result in missense mutation p.K1495E (right upper panel); and a C>T transition at cDNA position 1030, resulting in premature termination codon p.R344X (left upper panel). The corresponding wild-type sequences are given in lower panels. (e) PCR–restriction fragment length polymorphism analysis confirms segregation of the mutations in the family. PCR amplification was performed as described in the text. Mutation p.K1495E creates a novel recognition site for MboII. Thus, heterozygous carriers display a 93 bp fragment and healthy homozygous individuals show only a 115 bp fragment; mutation p.R344X abolishes a recognition site for endonuclease BSh1236I. Thus healthy individuals display a 170 bp fragment, whereas carriers of the mutation also display a 200 bp fragment.
To confirm NFTC at the molecular level, we sequenced the entire coding region of the SAMD9 gene (including exon–intron boundaries) (Figure 1d). Both patients were found to be compound heterozygous for two distinct mutations: an A>G transition at cDNA position 4483, which is predicted to result in missense mutation p.K1495E previously reported in families affected with NFTC (Topaz et al., 2006); and a C>T transition at cDNA position 1030, resulting in the substitution of a stop codon for an arginine residue at amino-acid position 344 (p.R344X). p.K1495E has previously been shown to result in protein degradation (Topaz et al., 2006); p.R344X is predicted to result in significant truncation of the native 1590 amino-acid long SAMD9 molecule.
Using PCR–restriction fragment length polymorphism assays, we confirmed segregation of the two mutations in the family (Figure 1e).
Mutational screening
To assess the carrier rate for both mutations in the general population, we screened a cohort of healthy unrelated individuals of Jewish-Yemenite, Jewish-non-Yemenite, and Yemenite-non-Jewish origins. Both mutations were exclusively found among controls of Jewish-Yemenite extraction (Table 1). These data were suggestive of two separate founder events in the Jewish-Yemenite population. To assess this possibility, we used microsatellite markers spanning the SAMD9 locus to genotype all family members and four controls shown to carry a heterozygous mutation in SAMD9. As shown in Figure 1a, patients and carrier controls were shown to share the same mutation-associated hemihaplotype, demonstrating that p.K1495E and p.R344X arose in the Jewish-Yemenite population from two independent events.
Table 1.
Population screening of unrelated healthy individuals
| Mutation | Yemenite Jews | Non-Yemenite | Non-Jewish Yemenite |
|---|---|---|---|
| K1495E | 1 (n=154) | 0 (n=612) | 0 (n=93) |
| R344X | 5 (n=154) | 0 (n=183) | 0 (n=93) |
Regulation of SAMD9 gene expression
The physiological function of SAMD9 is currently unknown. Recent data suggest that SAMD9 may inhibit proliferation and promote apoptosis in cancer cells (Li et al., 2007). In addition, SAMD9 mutations are associated with prominent inflammatory manifestations in NFTC (Metzker et al., 1988; Topaz et al, 2006). We therefore hypothesized that SAMD9 may be under the regulation of TNF-α, a major pro-inflammatory cytokine and inducer of apoptosis, which has been suggested to be involved in the pathogenesis of extra-osseous calcification (Dellegrottaglie et al., 2006).
We initially assessed the effect of increasing concentrations of TNF-α on SAMD9 expression in endothelial cells, where SAMD9 expression is known to be the highest (Topaz et al., 2006). We observed a maximal 4.5-fold increase in SAMD9 expression upon exposure of the cells to 10 ng ml−1 TNF-α (Figure 2a). At this concentration, a maximal increase in mRNA levels was observed 6 hours post-exposure (Figure 2b). This effect could be blocked by methylprednisolone (Figure 2c) but not by doxycycline (not shown), another known inhibitor of TNF-α signaling (Festoff et al., 2006; Shen et al., 2006).
Figure 2. Effect of TNF-α on SAMD9 gene expression.

(a) EaHy cells were cultured in the presence of increasing concentrations of TNF-α, and SAMD9 gene expression was measured as described in Materials and Methods; (b) EaHy cells were exposed for increasing periods of time to 10 ng ml−1 of recombinant TNF-α, and SAMD9 gene expression was quantified by quantitative real-time PCR; (c) EaHy cells were cultured for 6 hours in the presence of 0 (C), 10 ng ml−1 of recombinant TNF-α (T), or 10 ng ml−1 of recombinant TNF-α and 0.5mM of methylprednisolone (T+M), and SAMD9 gene expression was assessed by quantitative real-time PCR.
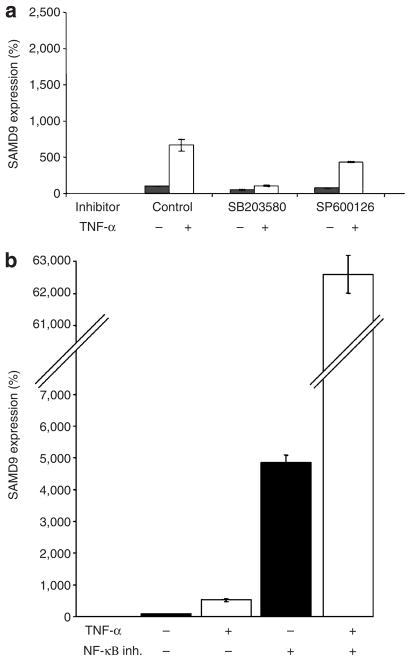
TNF-α has been shown to act through a number of cellular pathways (Holtmann and Neurath, 2004; Liu, 2005). To identify the molecular mechanisms underlying TNF-α-mediated increase in SAMD9, we assessed SAMD9 expression in the presence of TNF-α and known inhibitors of p38, c-Jun N-terminal kinase, and NF-κB. Down-regulation of c-Jun N-terminal kinase did not significantly affect the extent of SAMD9 induction (Figure 3a). In contrast, inhibition of p38 almost completely abrogated TNF-α-induced SAMD9 transcription (Figure 3a). p38 is known to mostly mediate TNF-α pro-inflammatory and pro-apoptotic signals (Saklatvala, 2004; Schieven, 2005), which is in line with recent data indicating that SAMD9 promotes apoptosis (Li et al., 2007). In contrast, TNF-α-mediated NF-κB activation is known to counteract pro-apoptotic signals (Papa et al., 2006). Accordingly, inhibition of NF-κB translocation to the nucleus was found to markedly upregulate SAMD9 transcription and to further augment the response of SAMD9 expression to TNF-α stimulation (Figure 3b).
Figure 3. TNF-α-mediated SAMD9 induction.
(a) EaHy cells were cultured in the presence (+) or absence (−) of 10ngml−1 TNF-α, p38 inhibitor (SB203580), or c-Jun N-terminal kinase inhibitor (SP600126), and SAMD9 gene expression was measured after 6 hours; (b) EaHy cells were cultured in the presence (+) or absence (−) of 10ngml−1 TNF-α and NF-κB inhibitor JSH-23, and SAMD9 gene expression was assessed as described above.
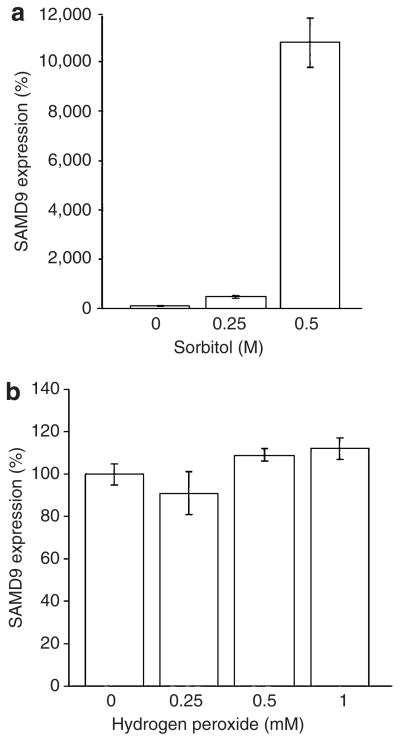
Because dystrophic calcinosis is known to occur following cellular damage and as TNF-α and cellular stresses have been shown to induce similar cellular responses (Alpert et al., 1999), we assessed the effect of a number of cellular stresses on SAMD9 expression (Figure 4). Whereas both hydrogen peroxide (Figure 4b), inducing oxidative stress, and heat stress (not shown) did not cause increased SAMD9 expression, hyperosmotic shock led to a concentration-dependant increase in SAMD9 expression (Figure 4a), with sorbitol 0.5 M inducing SAMD9 expression by more than 100-fold.
Figure 4. Effect of cellular stress on SAMD9 gene expression.
EaHy cells were cultured in the presence of increasing concentrations of (a) sorbitol or (b) hydrogen peroxide, and SAMD9 gene expression was monitored using quantitative real-time PCR.
DISCUSSION
Calcification, defined as the precipitation of calcium salts, is a process known to occur mainly in bone tissues, under physiological conditions. A growing body of evidence points to extra-osseous calcification as a major cause of morbidity and mortality in humans (Atzeni et al., 2006; Shao et al., 2006; Stoll and Bendszus, 2006; Sharma et al., 2007; Uitto and Jiang, 2007). More importantly, interventions aimed at attenuating extra-osseous calcification may increase survival among susceptible individuals (Schmitt et al., 2006). These data emphasize the need for a better understanding of the pathogenesis of extra-osseous calcification. Rare monogenic disorders offer a unique setting for the discovery of novel physiological functions. Indeed, animal models do not always faithfully replicate human pathologies and, despite recent technological advances, the study of complex traits still poses formidable conceptual and technical challenges (Antonarakis and Beckmann, 2006). Hyperphosphatemic familial tumoral calcinosis and NFTC adequately model the two major forms of acquired calcinosis cutis (Sprecher, 2007). Hyperphosphatemic familial tumoral calcinosis very much resembles metastatic calcinosis, and indeed the discovery of mutations in GALNT3 in hyperphosphatemic familial tumoral calcinosis led to the delineation of its role in the regulation of FGF23, a key protein involved in the pathogenesis of renal failure-associated hyperphosphatemia, which in turn is causally related to metastatic calcinosis and calciphylaxis in kidney disease (White et al., 2006).
In contrast, NFTC is thought to replicate many aspects of dystrophic calcinosis, a common form of extra-osseous calcification, occurring in various tissues including skin and often associated with inflammation and tissue injury as observed in acne, autoimmune diseases, and cancer (Touart and Sau, 1998).
Although the current report of a second SAMD9 mutation in NFTC definitely establishes the molecular etiology of this disorder, the physiological function of SAMD9 remains to be fully elucidated. Recent data have suggested that SAMD9 may promote cell apoptosis in cancer cells and thereby function as a tumor suppressor gene (Li et al., 2007). Given the prominent inflammatory manifestations associated with NFTC caused by SAMD9 deficiency (Metzker et al., 1988), our observations in human patients suggest a role for SAMD9 in the regulation of pro-inflammatory signals. TNF-α, a key cytokine playing an important role in homeostatic maintenance of epithelial tissues, links inflammation to the apoptotic machinery (Holtmann and Neurath, 2004). Our observations indicate that SAMD9 may be a downstream target of TNF-α signaling; whether it mediates pro-apoptotic signals, activates counter-regulatory anti-inflammatory activities, or both remains to be determined. Interestingly, hyperosmotic sorbitol, which, like TNF-α, signals through p38 (Figure 3a; Cheng et al., 2002), induced SAMD9 expression, suggesting a common regulatory pathway for SAMD9, involving inducers of cellular stress and inflammatory cell response.
The present data also shed new light on the molecular genetics of NFTC. The identification of two founder events causing an exceedingly rare disease in the Jewish Yemenite population amounting to approximately 174,600 individuals (www.cbs.gov.il) is surprising. Although recessive diseases are often thought to persist throughout evolution because of the fact that heterozygous mutations confer a selective advantage to carriers, this conjecture has only rarely been formally proven (Allison, 2004). In the present case, given the lack of information regarding the physiological function of SAMD9, we can only speculate that the occurrence of two rare mutations in a very small population is suggestive of positive selection, although random drift cannot be excluded. Owing to various historical circumstances, Yemenite Jews have been living in closed communities since the 10th century. Consequently, numerous specific genetic variants have emerged and have been maintained within this closed and endogamous population as attested by the existence of unique mutations (Avigad et al., 1990) and genetic conditions highly prevalent among Yemenite Jews (Ostrer, 2001). It could be envisaged that excessive tissue inflammation associated with deleterious mutations in SAMD9 may have conferred a survival advantage to Yemenite Jews otherwise exposed to potentially lethal transmissible diseases, a major cause of mortality and morbidity in the Jewish-Yemenite community until its immigration to Israel in the late 1940s (Borkan, 1993). Furthermore, the state of isolation of the Jewish community in Yemen may have contributed to the local adaptation, amplification, and confinement of SAMD9 mutations to this small ethnic group. On the other hand, neighboring populations must have been, at least in part, exposed to similar conditions, suggesting the possible existence of modifier traits specific to the Jewish-Yemenite population.
In summary, we have identified a novel mutation in SAMD9 underlying NFTC, definitively establishing mutations in SAMD9 as a cause of NFTC, and suggesting random drift, a selection effect in the Jewish-Yemenite population, and/or the effect of population-specific modifier traits. In addition, given the role of SAMD9 in NFTC and the fact that SAMD9 expression was found to be regulated by TNF-α, the present data indicate that further studies of the role of TNF-α in the regulation of extra-osseous calcification in the skin are warranted.
MATERIALS AND METHODS
Patients
All affected and healthy control participants or their legal guardian provided written and informed consent according to a protocol approved by local institutional review boards in adherence to the Declaration of Helsinki Principles. DNA was extracted according to standard procedures. Fifteen milliliters of blood were drawn from each participant, and DNA was extracted using a salt/chloroform extraction method.
Microsatellite analysis
Polymorphic microsatellite markers spanning the SAMD9 locus were selected from the GDB database (http://www.gdb.org), and genotypes were established by PCR amplification of genomic DNA using Supertherm Taq polymerase (Eisenberg Bro Co., Givat Schmuel, Israel) and fluorescently labeled primer pairs (Research Genetics, Invitrogen, Carlsbad, CA) according to the manufacturer’s recommendations. PCR conditions were 5 minutes at 95°C followed by 35 cycles for 30 seconds at 95°C, 30 seconds at 56°C, 30 seconds at 72°C, and a final extension step at 72°C for 5 minutes. PCR products were separated by PAGE on an ABI 310 sequencer system, and allele sizes were determined with Genescan 3.1 and Genotyper 2.0 software. Parsimonious haplotypes were subsequently established for each individual.
DNA sequencing
SAMD9 was PCR-amplified using oligonucleotide primer pairs listed in Table S1, Taq polymerase and Q solution (Qiagen, Valencia, CA) using the following cycling conditions: 94°C for 5 minutes followed by 35 cycles at 94°C for 1 minute, 59°C (unless otherwise indicated in Table S1) for 1 minute, 72°C for 1 minute 30 seconds. Gel-purified (QIAquick gel extraction kit, Qiagen) amplicons were subjected to bi-directional DNA sequencing using the BigDye terminator system on an ABI Prism 3100 sequencer (PE Applied Biosystems, Foster City, CA).
Mutation screening
Mutations were screened in the family using PCR–restriction fragment length polymorphism assays. To screen for p.R344X, a 200-bp fragment was PCR-amplified, using allele-specific reverse primer 5′-CCTGTCAATAATTTAACCAACTTTGGTCCC-3′and forward primer 5′-GAACAAAGTAAAAAATTCTCACTATTTGCG-3′; the former creates a recognition site for BSh1236I, which is abolished in the presence of p.R344X. After incubation at 37°C for 4 hours, digested PCR products were electrophoresed in a 2.5% agarose gel and visualized under UV light. p.K1495E was identified using a PCR–restriction fragment length polymorphism assay described previously (Topaz et al., 2006).
Mutations were screened in ethnically and geographically related healthy individuals using TaqMan technology (Applied Biosciences, Warrington, UK). Reactions were performed on 384-well microplates in accordance with the manufacturer’s recommended protocol and analyzed using ABI TaqMan 7900HT software. Primers and MGB probes are detailed in Table S1.
Cell cultures and reagents
EaHy cells, a human umbilical vein endothelial cells-derived transformed endothelial cell line, were maintained in high-glucose DMEM medium (Beit-Ha-Emek, Beit-Ha-Emek, Israel) supplemented with 10% (v/v) fetal calf serum (Beit-Ha-Emek) and 2mm l-glutamine (Beit-Ha-Emek).
Cells were treated with a number of molecular reagents: TNF-α (PeproTech, Rocky Hill, NJ), sorbitol (Sigma, St Louis, MO), hydrogen peroxide (Sigma), SB203580 (3 μm; Calbiochem), SP600125 (25 μm; Calbiochem), and NF-κB activation inhibitor-II JSH-23 (10 μgml−1; Calbiochem).
Quantitative real-time PCR
For quantitative real-time PCR, cDNA was synthesized from 1 μg of total RNA using the Reverse-iT first strand synthesis kit (Abgene, Epson, UK) and random hexamers. cDNA PCR amplification was carried out using the SYBR Green JumpStart Taq ReadyMix (Sigma) on a Mx3000p/5p multifilter system (Stratagene, Cedar Creek, TX) with gene-specific intron-crossing oligonucleotide pairs listed in Table S1. To ensure the specificity of the reaction conditions, at the end of the individual runs, the melting temperature (Tm) of the amplified products was measured to confirm its homogeneity. Cycling conditions were as follows: 95°C for 10 minutes, 95°C for 10 seconds, 62°C for 25 seconds, and 72°C for 15 seconds for a total of 40 cycles. Each sample was analyzed in triplicate. For quantification, standard curves were obtained using serially diluted cDNA amplified in the same real-time PCR run. Results were normalized to ACTB mRNA levels. After the quantification procedure, the products were resolved by 2.5% agarose gel electrophoresis to confirm that the reaction had amplified the correct DNA fragments of known size.
Acknowledgments
We are grateful to the family members for their enthusiastic and generous participation in our study. We wish to thank Vered Friedman and Rita Fuhrer-Mor for their help with nucleic acid analysis, Uri Seligshon for providing DNA samples, and Ariel Miller and Tamar Paperna for the gift of the EaHy cells. This study was supported in part by grants provided by Israel Science Foundation, the Rappaport Institute for Research in the Medical Sciences, NIH/NIAMS Grant R01 AR052627 and the Veronique Simone and Barry and Carole Rose Funds of the Canadian Technion Society.
Abbreviations
- NFTC
normophosphatemic familial tumoral calcinosis
- TNF-α
tumor necrosis factor-α
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Allison AC. Two lessons from the interface of genetics and medicine. Genetics. 2004;166:1591–9. doi: 10.1534/genetics.166.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpert D, Schwenger P, Han J, Vilcek J. Cell stress and MKK6b-mediated p38 MAP kinase activation inhibit tumor necrosis factor-induced IkappaB phosphorylation and NF-kappaB activation. J Biol Chem. 1999;274:22176–83. doi: 10.1074/jbc.274.32.22176. [DOI] [PubMed] [Google Scholar]
- Antonarakis SE, Beckmann JS. Mendelian disorders deserve more attention. Nat Rev. 2006;7:277–82. doi: 10.1038/nrg1826. [DOI] [PubMed] [Google Scholar]
- Araya K, Fukumoto S, Backenroth R, Takeuchi Y, Nakayama K, Ito N, et al. A novel mutation in fibroblast growth factor 23 gene as a cause of tumoral calcinosis. J Clin Endocrinol Metabol. 2005;90:5523–7. doi: 10.1210/jc.2005-0301. [DOI] [PubMed] [Google Scholar]
- Atzeni F, Sarzi-Puttini P, Bevilacqua M. Calcium deposition and associated chronic diseases (atherosclerosis, diffuse idiopathic skeletal hyperostosis, and others) Rheum Dis Clin North Am. 2006;32:413–26. viii. doi: 10.1016/j.rdc.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Avigad S, Cohen BE, Bauer S, Schwartz G, Frydman M, Woo SL, et al. A single origin of phenylketonuria in Yemenite Jews. Nature. 1990;344:168–70. doi: 10.1038/344168a0. [DOI] [PubMed] [Google Scholar]
- Benet-Pages A, Orlik P, Strom TM, Lorenz-Depiereux B. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet. 2005;14:385–90. doi: 10.1093/hmg/ddi034. [DOI] [PubMed] [Google Scholar]
- Borkan J. Changing health and changing culture: the Yemenite Jews in Israel. N Engl J Med. 1993;329:669–70. [Google Scholar]
- Chefetz I, Heller R, Galli-Tsinopoulou A, Richard G, Wollnik B, Indelman M, et al. A novel homozygous missense mutation in FGF23 causes familial tumoral calcinosis associated with disseminated visceral calcification. Hum Genet. 2005;118:261–6. doi: 10.1007/s00439-005-0026-8. [DOI] [PubMed] [Google Scholar]
- Cheng H, Kartenbeck J, Kabsch K, Mao X, Marques M, Alonso A. Stress kinase p38 mediates EGFR transactivation by hyperosmolar concentrations of sorbitol. J Cell Physiol. 2002;192:234–43. doi: 10.1002/jcp.10134. [DOI] [PubMed] [Google Scholar]
- Dellegrottaglie S, Sanz J, Rajagopalan S. Molecular determinants of vascular calcification: a bench to bedside view. Curr Mol Med. 2006;6:515–24. doi: 10.2174/156652406778018653. [DOI] [PubMed] [Google Scholar]
- Festoff BW, Ameenuddin S, Arnold PM, Wong A, Santacruz KS, Citron BA. Minocycline neuroprotects, reduces microgliosis, and inhibits caspase protease expression early after spinal cord injury. J Neurochem. 2006;97:1314–26. doi: 10.1111/j.1471-4159.2006.03799.x. [DOI] [PubMed] [Google Scholar]
- Frishberg Y, Ito N, Rinat C, Yamazaki Y, Feinstein S, Urakawa I, et al. Hyperostosis-hyperphosphatemia syndrome: a congenital disorder of O-glycosylation associated with augmented processing of fibroblast growth factor 23. J Bone Miner Res. 2007;22:235–42. doi: 10.1359/jbmr.061105. [DOI] [PubMed] [Google Scholar]
- Frishberg Y, Topaz O, Bergman R, Behar D, Fisher D, Gordon D, et al. Identification of a recurrent mutation in GALNT3 demonstrates that hyperostosis-hyperphosphatemia syndrome and familial tumoral calcinosis are allelic disorders. J Mol Med. 2005;83:33–8. doi: 10.1007/s00109-004-0610-8. [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Yamashita T. FGF23 is a hormone-regulating phosphate metabolism—unique biological characteristics of FGF23. Bone. 2007;40:1190–5. doi: 10.1016/j.bone.2006.12.062. [DOI] [PubMed] [Google Scholar]
- Holtmann MH, Neurath MF. Differential TNF-signaling in chronic inflammatory disorders. Curr Mol Med. 2004;4:439–44. doi: 10.2174/1566524043360636. [DOI] [PubMed] [Google Scholar]
- Ichikawa S, Guigonis V, Imel EA, Courouble M, Heissat S, Henley JD, et al. Novel GALNT3 mutations causing hyperostosis-hyperphosphatemia syndrome result in low intact fibroblast growth factor 23 concentrations. J Clin Endocrinol Metabol. 2007;92:1943–7. doi: 10.1210/jc.2006-1825. [DOI] [PubMed] [Google Scholar]
- Ichikawa S, Lyles KW, Econs MJ. A novel GALNT3 mutation in a pseudoautosomal dominant form of tumoral calcinosis: evidence that the disorder is autosomal recessive. J Clin Endocrinol Metabol. 2005;90:2420–3. doi: 10.1210/jc.2004-2302. [DOI] [PubMed] [Google Scholar]
- Kato K, Jeanneau C, Tarp MA, Benet-Pages A, Lorenz-Depiereux B, Bennett EP, et al. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J Biol Chem. 2006;281:18370–7. doi: 10.1074/jbc.M602469200. [DOI] [PubMed] [Google Scholar]
- Larsson T, Davis SI, Garringer HJ, Mooney SD, Draman MS, Cullen MJ, et al. Fibroblast growth factor-23 mutants causing familial tumoral calcinosis are differentially processed. Endocrinology. 2005a;146:3883–91. doi: 10.1210/en.2005-0431. [DOI] [PubMed] [Google Scholar]
- Larsson T, Yu X, Davis SI, Draman MS, Mooney SD, Cullen MJ, et al. A novel recessive mutation in fibroblast growth factor-23 causes familial tumoral calcinosis. J Clin Endocrinol Metabol. 2005b;90:2424–7. doi: 10.1210/jc.2004-2238. [DOI] [PubMed] [Google Scholar]
- Li CF, MacDonald JR, Wei RY, Ray J, Lau K, Kandel C, et al. Human sterile alpha motif domain 9, a novel gene identified as down-regulated in aggressive fibromatosis, is absent in the mouse. BMC Genomics. 2007;8:92. doi: 10.1186/1471-2164-8-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZG. Molecular mechanism of TNF signaling and beyond. Cell Res. 2005;15:24–7. doi: 10.1038/sj.cr.7290259. [DOI] [PubMed] [Google Scholar]
- Metzker A, Eisenstein B, Oren J, Samuel R. Tumoral calcinosis revisited—common and uncommon features. Report of ten cases and review. Eur J Pediatr. 1988;147:128–32. doi: 10.1007/BF00442209. [DOI] [PubMed] [Google Scholar]
- Ostrer H. A genetic profile of contemporary Jewish populations. Nat Rev Genet. 2001;2:891–8. doi: 10.1038/35098506. [DOI] [PubMed] [Google Scholar]
- Papa S, Bubici C, Zazzeroni F, Pham CG, Kuntzen C, Knabb JR, et al. The NF-kappaB-mediated control of the JNK cascade in the antagonism of programmed cell death in health and disease. Cell Death Differ. 2006;13:712–29. doi: 10.1038/sj.cdd.4401865. [DOI] [PubMed] [Google Scholar]
- Saklatvala J. The p38 MAP kinase pathway as a therapeutic target in inflammatory disease. Curr Opin Pharmacol. 2004;4:372–7. doi: 10.1016/j.coph.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Schieven GL. The biology of p38 kinase: a central role in inflammation. Curr Top Med Chem. 2005;5:921–8. doi: 10.2174/1568026054985902. [DOI] [PubMed] [Google Scholar]
- Schmitt CP, Odenwald T, Ritz E. Calcium, calcium regulatory hormones, and calcimimetics: impact on cardiovascular mortality. J Am Soc Nephrol. 2006;17:S78–80. doi: 10.1681/ASN.2005121338. [DOI] [PubMed] [Google Scholar]
- Shao JS, Cai J, Towler DA. Molecular mechanisms of vascular calcification: lessons learned from the aorta. Arterioscler Thromb Vasc Biol. 2006;26:1423–30. doi: 10.1161/01.ATV.0000220441.42041.20. [DOI] [PubMed] [Google Scholar]
- Sharma R, Pellerin D, Gaze DC, Mehta RL, Gregson H, Streather CP, et al. Mitral annular calcification predicts mortality and coronary artery disease in end stage renal disease. Atherosclerosis. 2007;191:348–54. doi: 10.1016/j.atherosclerosis.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Shen J, O’Brien D, Xu Y. Matrix metalloproteinase-2 contributes to tumor necrosis factor alpha induced apoptosis in cultured rat cardiac myocytes. Biochem Biophys Res Commun. 2006;347:1011–20. doi: 10.1016/j.bbrc.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Smack D, Norton SA, Fitzpatrick JE. Proposal for a pathogenesis-based classification of tumoral calcinosis. Int J Dermatol. 1996;35:265–71. doi: 10.1111/j.1365-4362.1996.tb02999.x. [DOI] [PubMed] [Google Scholar]
- Specktor P, Cooper JG, Indelman M, Sprecher E. Hyperphosphatemic familial tumoral calcinosis caused by a mutation in GALNT3 in a European kindred. J Hum Genet. 2006;51:487–90. doi: 10.1007/s10038-006-0377-6. [DOI] [PubMed] [Google Scholar]
- Sprecher E. Tumoral calcinosis: new insights for the rheumatologist into a familial crystal deposition disease. Curr Rheumatol Rep. 2007;9:237–42. doi: 10.1007/s11926-007-0038-6. [DOI] [PubMed] [Google Scholar]
- Stoll G, Bendszus M. Inflammation and atherosclerosis: novel insights into plaque formation and destabilization. Stroke. 2006;37:1923–32. doi: 10.1161/01.STR.0000226901.34927.10. [DOI] [PubMed] [Google Scholar]
- Topaz O, Indelman M, Chefetz I, Geiger D, Metzker A, Altschuler Y, et al. A deleterious mutation in SAMD9 causes normophosphatemic familial tumoral calcinosis. Am J Hum Genet. 2006;79:759–64. doi: 10.1086/508069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topaz O, Shurman DL, Bergman R, Indelman M, Ratajczak P, Mizrachi M, et al. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet. 2004;36:579–81. doi: 10.1038/ng1358. [DOI] [PubMed] [Google Scholar]
- Touart DM, Sau P. Cutaneous deposition diseases. Part II. J Am Acad Dermatol. 1998;39:527–44. doi: 10.1016/s0190-9622(98)70001-5. [DOI] [PubMed] [Google Scholar]
- Uitto J, Jiang Q. Pseudoxanthoma elasticum-like phenotypes: more diseases than one. J Invest Dermatol. 2007;127:507–10. doi: 10.1038/sj.jid.5700635. [DOI] [PubMed] [Google Scholar]
- White KE, Larsson TE, Econs MJ. The roles of specific genes implicated as circulating factors involved in normal and disordered phosphate homeostasis: frizzled related protein-4, matrix extracellular phosphoglycoprotein, and fibroblast growth factor 23. Endocrine Rev. 2006;27:221–241. doi: 10.1210/er.2005-0019. [DOI] [PubMed] [Google Scholar]