Abstract
Retinoid acid, the bioactive metabolite of vitamin A, is a potent signaling molecule in the brains of growing and adult animals, regulates numerous gene products, and modulates neurogenesis, neuronal survival and synaptic plasticity. Vitamin A deficiency (VAD) is a global health problem, yet our knowledge of its effects on behavior and learning is still emerging. Here we review studies that have implicated retinoids in learning and memory deficits of post-embryonic and adult rodent and songbird models. Dietary vitamin A supplementation improves learning and memory in VAD rodents and can ameliorate cognitive declines associated with normal aging. Songbird studies examine the effects of retinoid signaling on vocal/auditory learning and are uniquely suited to study the behavioral effects of VAD because the neural circuitry of the song system is discrete and well understood. Similar to human speech acquisition, avian vocal learning proceeds in well-defined stages of template acquisition, rendition and maturation. Local blockade of retinoic acid production in the brain or excess dietary retinoic acid results in the failure of song maturation, yet does not affect prior song acquisition. Together these results yield significant insights into the role of vitamin A in maintaining neuronal plasticity and cognitive function in adulthood.
Keywords: Learning, Neuromodulation, Neuronal plasticity, Retinoic acid, Vitamin A
1 Introduction
Vitamin A is a fat-soluble micronutrient that is converted into retinoic acid at or near the site of activity in the body for use as a transcriptional regulator. Microarray studies have found retinoid signaling to affect a large number of genes in different tissues [1–3], and by some estimates affects ~15% of known human protein-coding genes [4], thus represents a strong candidate for neuromodulation. Vitamin A deficiency (VAD) is globally one of the most common forms of malnutrition in human populations, with ocular disorders, immunosuppression and impaired growth commonly described [5], and although considerable political efforts have been undertaken to eliminate VAD over the last half-century [5] it today remains a problem in much of the developing world [6, 7]. In light of the recent attention of micronutrients to human cognition, a lack of a strong role for vitamin A is surprising [8, 9]. However, based on evidence from animal models and associative human studies, a significant role of vitamin A is likely. For instance, the acne-control drug, Accutane (a retinoic acid analogue), was recently discovered to reduce hippocampal neurogenesis and proliferation, along with the ability to learn a maze in adult mice [10], while in humans the incidence of depression is ~10% higher among Accutane users [11]. Thus, use of therapeutic retinoids likely has effects on the maturation and maintenance of the adult brain and associated behaviors (though subtle compared to the dramatic effects on embryonic development). Supplementation of vitamin A or retinoid derivatives as therapeutic agents has been widely proposed for psychiatric pathologies including schizophrenia and Alzheimer’s disease [12], and may be used for controlling certain cancers [13, 14]. In light of animal models that show how retinoid signaling affects neuronal maintenance and behavior, we review work that has altered retinoid signaling up or down in the brains of rodents and songbirds with subsequent neurobiological effects that are tied to a specific cognitive function or complex behaviors.
2 Vitamin A and retinoic acid metabolism
The biologically active metabolite of vitamin A, retinoic acid, is the ligand of a set of receptors (retinoic acid and retinoid × receptors) that act as transcriptional regulators restricted to chordates, and it is best known as a signaling molecule during early embryogenesis [15, 16]. However, tightly controlled retinoid signaling is also important throughout adolescence and adulthood, and has been shown to play various roles in the continued formation, differentiation and maintenance of neuronal phenotypes. Owing to its low stability and low abundance in neuronal tissue, retinoic acid has proved challenging to effectively quantify by methods such as HPLC [17], but recent improvements in direct retinoic acid quantification [18] may prompt its quantification in sub-regions of the brain. Rather, retinoid signaling in the brain has often been inferred by the presence of aldehyde dehydrogenase, the terminal enzyme in the retinoic acid synthesis pathway. Three retinaldehyde-specific aldehyde dehydrogenases (RalDH) are present in high levels in the embryo and/or occur in the developing eye [19], but the isoform RalDH2 remains prevalent in post-embryonic and adult brains at lower levels. In embryos, differential expression of RalDHs sets up diffusion gradients of retinoic acid across structures such as the whole body plan, limb bud or eye. These gradients broadly determine patterns of structure formation [20, 21]. However, because adults and juveniles have greater physical structural complexity and lower levels of RalDH2 expression in their bodies than embryos, the spatial scale in which gradients of retinoic acid may act is not clear. It is likely with lower concentrations of retinoic acid present in tissues, concentration gradients must occur across a smaller space, perhaps over several cells. In addition, several gene products in addition to RalDH2, including cellular retinoic acid-binding proteins and retinal dehydrogenases/reductases, and cytochrome degradation enzymes provide additional regulatory control of retinoic acid levels [22, 23], and these regulatory gene products may change with development. Since RalDH2 is expressed by fully differentiated neurons in adults, retinoic acid may be produced in the functional cell bodies of one brain region for axonal transport to other remote regions where it may then direct neuromodulation. In either scenario, poorly understood transport processes and much smaller spatial fields of retinoid signaling are likely the norm in post-embryonic tissues, further necessitating the need for a tightly controlled system of retinoid signaling to maintain critical neuronal function.
Experiments to investigate the actions of retinoid signaling on neural properties and behaviors have either downregulated (e.g. simulated VAD) or upregulated retinoid signaling in the brain. VAD models may be achieved with the use of synthetic diets that are deficient in retinoic acid and all potential retinoic acid precursors, or the synthesis of retinoic acid may be disrupted by pharmacological blockers of retinoic acid synthesis [24]. Research with rodents has employed a transgenic knockout model for the retinoic acid receptor [25], and with the recently released songbird genome [26] in combination with development of viral transfection to generate transgenic songbird models, this may soon be a realistic technique for songbird researchers. Conversely, vitamin A and/or retinoic acid supplementation provides a model to better understand the therapeutic effects of retinoid signaling. This approach complements VAD as it results in excess retinoic acid levels in the brain. This is a significant approach as frequently both deficits of retinoid signaling and overproduction of retinoic acid can have disruptive effects, resulting in a detectable phenotypic response.
3 Rodent studies of neuromodulation by retinoic acid
Rodents models have proven informative on the effects of retinoid signaling on post-embryonic neuromodulation as retinoids affect hippocampal long-term depression (LTD) and potentiation, both measures of long-lasting synaptic plasticity, and neurogenesis [12, 27, 28]. The first clear evidence that retinoids play a role in cognitive function came from work with knockout mice that lacked either one of the retinoic acid receptors, RARβ, or one of the retinoid × receptors, RXRγ [25]. These particular receptors are uniquely expressed in hippocampal regions of the adult mouse brain that are implicated in spatial and relational memory, whereas the other retinoic acid receptors and retinoid × receptors are more uniform in their distribution. Thus, mutant mice for these receptors showed normal development and growth with no abnormal physical or neuronal morphology, yet demonstrated cognitive deficits in learning the Morris water maze and impaired motor control and balance, compared to wild-type mice. These behavioral impairments correlated with electrophysiological differences in hippocampal CA1 cells in that RARβ mutants lost long-term potentiation (LTP) and the RARβ and RXRγ mutants both lost LTD. Both of these correspond to changes in long-term synaptic efficacy that can affect learning and memory.
Following these findings, several studies have demonstrated a dietary link between retinoids and behavior or neuronal plasticity. By experimentally inducing VAD in neonatal mice, Misner et al. [29] showed that poor retinoid nutrition also affects LTP and LDP in mice, along with the more obvious physical and ocular deformities typical of VAD. These electrophysiological effects occurred without apparent physical differences in the underlying neuronal structure of the hippocampus, and in fact when proper retinoid nutrition was returned to retinoid-deprived mice, LTP and LTD returned to normal [29]. Behaviorally, dietary VAD in rodents also results in cognitive declines in memory tasks, but unlike the apparent rescue effect of supplemental retinoids for electrophysiological function, the behavioral rescue effect was not as consistent in all animals as there continue to be age-related effects on susceptibility to VAD. For instance, in a two-arm discrimination maze (baited with food) young VAD mice demonstrated persistent exploratory behaviors over a training period that is characteristic of naive animals, compared to normal mice that readily go to food after they learn the maze [30], and these effects do not go away when normal diets return, compared to older animals. Species differences are also evident: in contrast to mice, rats deprived of vitamin A at an early age showed cognitive decline that seems to improve once a regular diet is resumed [31, 32].
In parallel to the deprivation experiments, high doses of the 13-cis retinoic acid isomer administered to adult mice also results in cognitive deficits, and are correlated with reduced cell proliferation in the hippocampus and the proliferative regions of the ventricle [10]. Thus, excessively high levels of retinoic acid also have detrimental effects, suggesting that it needs to be regulated within a narrow concentration range. The maintenance of the adult olfactory bulb also requires retinoid signaling and is known for high levels of neuronal plasticity in terms of adult neurogenesis or variable profiles of gene expression [33]. Furthermore in a mouse model of Alzheimer’s disease that overexpresses genes for β-amyloid and presenilin 1, mice can be rescued from Alzheimer’s-related learning deficits by therapeutic all-trans retinoic acid (ATRA) administration [34]. ATRA-treated mice showed fewer of the neurodegenerative β-amyloid deposits in their brains, yet the possibility exists that the cognitive improvements were unrelated to the decrease in β-amyloid deposits, as aged wild-type mice that are given retinoic acid also show improvements in their cognitive abilities [35]. Indeed, therapeutic retinoid tools are promising for nervous system injuries, age-related declines in cognitive function as well as dementia-associated diseases; however, because of the multiple gene/signaling pathways and multiple aspects of neuronal plasticity known to be affected by retinoid signaling, careful research is needed.
4 Avian studies of retinoic acid neuromodulation
While rodent models best address spatial and relational memory, the songbird model addresses vocal and auditory learning, a trait shared with humans but that is not possible to test with most other mammal models, including nonhuman primates. Besides being one of the few organisms that evolved vocal learning, songbirds are powerful models for examining the neural basis of vocal learning because the brain areas that control song are composed of a series of discrete nuclei that have been well-characterized anatomically and physiologically [36, 37]. Birdsong consists of a learned complex vocal-motor sequence that is reinforced through sensorimotor learning during a critical period of high neuronal plasticity in the song system of juveniles [38]. Young zebra finches (Taeniopygia guttata) that hear song from male tutors during the Sensory Acquisition Period (20–60 days post hatch [dph]; Fig. 1) will acquire a stored template of the tutor’s song in their memory. They then enter the Sensorimotor Phase (~40–90 dph) where individuals develop their songs by matching the highly variable song that they produce to that of the tutor’s song stored in their memory. By adulthood (~90 dph) their songs mature and “crystallize” to the point that variability is minimal and the songs that they produce show a strong resemblance to that sang by their tutors [39]. In zebra finches these critical periods are well characterized [38] and this model provides a system that closely resembles the acquisition of human speech where infants first exhibit variable babbling, followed by experimentation with the sounds and pronunciation of spoken language [40].
Figure 1.
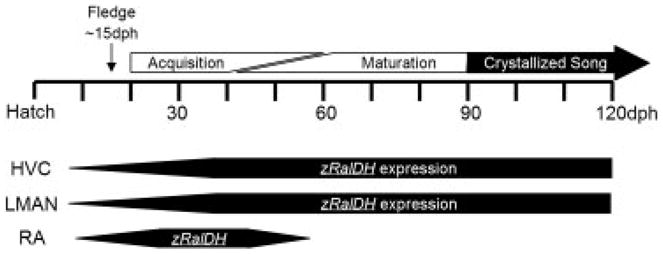
Timeline of critical periods within the zebra finch (T. guttata) song development and patterns of zRalDH expression in the song nuclei HVC, LMAN and RA (see text for proper names) of the zebra finch brain.
The avian song system consists of several well-defined song nuclei that function in two complementary circuits. Birdsong begins in the song nucleus HVC (hyperstriatum ventrale, pars caudalis), which originates the neuronal pathways that are responsible for the learning and the production of song (Fig. 2). Projection neurons from the HVC to the robust nucleus of the arcopallium (RA) begin the posterior vocal-motor pathway and provide direct control to vocal and respiratory motor neurons (Fig. 2, black arrows). The anterior pathway originates as projection neurons from HVC to area X of the medial striatum (gray arrow), and from there sequentially to the dorsal lateral nucleus of the medial thalamus and to the lateral magnocellular nucleus of the nidopallium (LMAN) and into RA. Retinoic acid production in adults seems to be tightly linked to this anterior pathway. The large neurons that originate in the HVC and project to area X (Fig. 2, shown as a gray arrow) and the large neurons within LMAN are major sources of zRalDH (the zebra finch analog of RalDH2) expression in the adult song system, while song nucleus RA shows only a transient expression pattern in juveniles that disappears by 50 dph (Fig. 1). By 4 dph zRalDH is detectable within HVC, LMAN and RA and levels gradually increase as these song nuclei grow. During this time, neuronal connections among the song nuclei are being established and new cells are migrating in from the proliferative regions along the ventricle. zRalDH expression in RA is characteristic of juvenile songbirds between ~10 and 50 dph and levels peak at ~38 dph [41] which corresponds to the invasion of HVC projection neurons into the RA [42] and the first attempts at song in the juvenile. While zRalDH in the RA is largely absent after 50 dph, its expression continues in HVC and LMAN into adulthood, indicating a persistent production of retinoic acid in the adult song system [41].
Figure 2.
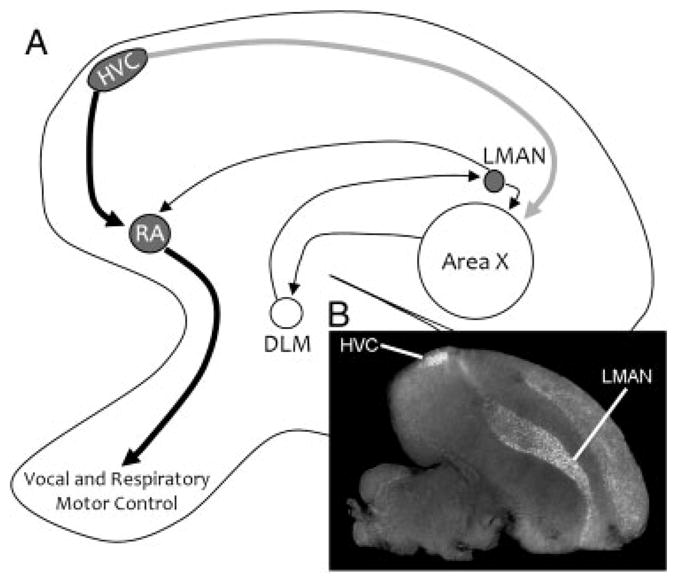
(A) Parasaggital view of direct and anterior pathways of the zebra finch (T. guttata) song system. Bold arrows show the direct motor pathway, while thin arrows delineate the anterior forebrain loop. Projections and nuclei that are known to express zRalDH are shown in gray, and nuclei with no expression are shown in white. (B) In situ hybridization of antisense riboprobe for the zRalDH shows strong expression in HVC and in the nidopallial layer of the telencephalon, including LMAN.
The anterior pathway is responsible for song learning in juveniles and for modulating plasticity in learned song in adults. For instance, ablation of LMAN during the critical period of juvenile song acquisition reduces variability in juvenile song prematurely and inhibits learning ability [43, 44]. Thus, this circuit contributes to the neuronal and behavioral plasticity that is necessary to learn a complex behavior such as song. Our lab has altered retinoid signaling in the songbird brain with the application of pharmacological blockers of retinoic acid synthesis locally over HVC [41]. Disulfiram disrupts the final step in the synthesis of retinoic acid production by inhibiting retinaldehyde-specific zRalDH, while expression of other known class 1 aldehyde dehydrogenases does not occur in the songbird brain [24]. Birds treated with disulfiram at 30–35 dph (compared to controls who received saline implants in HVC or disulfiram implants in another brain region) showed evidence of song acquisition from a tutor male, but songs remained variable into adulthood, with frequent omission or variability of song elements into adulthood, indicating that maturation and crystallization did not occur. Song of disulfiram-treated birds resembled closely the variable song of juveniles, with poor note morphology and instability of fast frequency modulations. In contrast, adult birds with disulfiram placed over their HVCs showed no detrimental effects to their already crystallized songs [41].
In a similar study, male finches were supplemented with a high daily dose of ATRA (oral administration in corn oil vehicle between 30 and 120 dph) to test for possible changes to the qualities of their song compared to controls given vehicle alone [45]. Songs were recorded at 120 dph to analyze vocal development and the brains were examined for the expression of several candidate retinoid-regulated genes. ATRA-treated birds sang a complex song that contained normal features of conspecific song, including a motif with syllables that were presumably copied from the adult songs in the aviary. However, based on the analysis of multiple consecutive renditions (minimum 10 motifs per bird), the songs of ATRA-treated birds demonstrated more variability than controls. These songs had lower consistency, linearity and stereotypy [46], indicating lower levels of syntactic stereotypy compared to controls. These effects were largely due to frequent note additions and/or omissions in comparison with the most typical motif for each bird, as well as higher variability in the duration of syllables and inter-syllabic intervals than in controls. ATRA-treated birds also had lower similarity and accuracy scores [47]. The lower scores in the ATRA-treated group reflect higher variability in several acoustic features (such as pitch, spectral continuity, entropy) across multiple song renditions for each individual. In sum, songs from ATRA-treated birds resembled the plastic song of juveniles. Thus, song maturation appears to be arrested prior to song crystallization when retinoid signaling is upregulated through dietary supplementation, similar to birds where the production of retinoic acid was locally blocked in HVC [41]. In both scenarios of increased and decreased retinoid levels, birds show elements of song acquisition, but fail to crystallize their songs.
These results raise several important questions regarding the roles of retinoid signaling on genetic and cellular processes in the song system during the juvenile song-development period and over the adult life-span. Retinoic acid drives neuronal differentiation by effects on gene expression [21, 23], thus in the context of the song system, genes with differential expression in the HVC and that are shown to be affected by changes in retinoic acid levels, are likely to have important effects on the development of the song system. Indeed, microarray studies that have examined positive or negative induction of genes in tissues such as breast carcinoma tissue, Xenopus larva and lymphoid tissue have yielded different patterns of induction among the various systems [1–3]. Thus, neuronal tissue is likely to have a unique induction of genes in response to changes in retinoic acid levels, compared to other tissues. Four genes, (neurogranin) nrgn, BMP/RA-inducible neural-specific protein brinp1 (a.k.a. dbc, deleted in bladder cancer in humans) and a retinal short-chain dehydrogenase/reductase, sdr2/scdr9, retinoic acid receptor alpha, rar-α, have shown altered expression in the brain with the previously described feeding trial [45]. Of these brinp1 and nrgn have prominent expression in HVC – nrgn is associated with synaptic development and remodeling [48, 49] and decreases in HVC in response to ATRA, while brinp1 is an inhibitor of the cell cycle, possibly through the TrkC/NT3 receptor [50] and shows broad increases in neural tissues in response to ATRA. The function of both these genes is consistent with a role in modulating different kinds of neuronal plasticity in the song system, in fact, a positive linear relationship was shown between nrgn expression levels in both LMAN and area X with the degree of song stereotypy. Retinoic acid is known to modulate neuronal proliferation, migration, differentiation and synaptic connectivity, but at this point we know little about how genetic programs directed by retinoic acid drive these cellular processes in the avian song system. In terms of the histological structure of the song system and volume of song nuclei there are no apparent effects of altered retinoid signaling [45], similar to a lack of an effect in the volume of the murine hippocampus [29]. Sdr2/scdr9 has low expression in the song system, but shows increased expression with increased levels of retinoic acid in area X and LMAN of the anterior loop of the song system, consistent with a likely role of this gene product in providing an inhibitory regulatory role in retinoic acid signaling.
5 Concluding remarks
Even though this is a relatively new area of research, the evidence strongly suggests that vitamin A, through its main metabolite retinoic acid, continues to exert important actions on brain physiology and behavior in post-embryonic and adult life. Avian and murine studies suggest that a balance of retinoic acid is required to attenuate the behavioral plasticity that is required for the storage and recovery of memory. That is, levels of retinoic acid are maintained at moderate levels by a complex of control mechanisms and feedbacks, so that too much or too little of this ligand will result in similar deficits in learned behaviors. However, it is still unclear how underlying pathways of retinoic acid signaling change with different levels of the ligand and how this differs in different neuronal systems. Retinoic acid is broadly implicated in neurogenesis, cell differentiation, synaptic connectivity and electrophysiological potentiation – all processes that affect what we commonly think of as neuronal plasticity. Furthermore, neurobiological systems may respond to retinoic acid differently at various stages of development, but additional work is needed to fully understand this issue. In terms of learned behavior, plasticity is required for neural systems to be able to adapt to broad and constantly changing environmental conditions, yet excessive plasticity may be detrimental for an animal to effectively learn and consolidate very specific patterns, particularly behaviors as complex as learned vocalizations.
The studies discussed here indicate the continued importance of vitamin A as a nutrient for the brain not only during embryonic development but also during adulthood. The study of retinoic acid effects on the song system offers a unique opportunity to identify the molecular regulators of a complex behavior such as vocal learning. With the avian song system, we have detailed knowledge on the composition and physiology of specific neuronal elements, and thus presents an opportunity to identify the retinoic acid targets at a cellular and molecular level. Overall, research in basic model organisms to further our knowledge of how vitamin A affects the brain and behavior will expand our understanding of the significance of VAD to human populations in terms of learning ability. This is significant as measuring cognitive abilities across multiple cultures is notoriously difficult.
The decline in cognitive ability is one of the classic hallmarks of human aging, and in animal models under conditions of poor vitamin A nutrition. Resumption of vitamin A and proper levels of retinoic acid signaling may have restorative effects, but again, research to identify the extent of possible recovery and the critical periods during the life stage is needed. Indeed, VAD at early stages in the life cycle of rodents seems to have irreversible effects on the brain, but adult animals may be more tolerant to episodes of retinoid malnutrition [31]. Compared to short-lived rodents, birds are very long-lived for their body sizes [51], and thus may be an appropriate model for the long-term effects of VAD in humans. Therapeutic doses of retinoic acid may ameliorate age-related dementia and psychosis, yet may also exert undesirable effects on the brain through other pathways. Further studies on animal models like rodents and songbirds are poised to further reveal the importance of vitamin A and retinoid signaling for brain function and behavior.
Acknowledgments
This research was supported by grants 2R01-DC002853 for C.V. Mello and 1F32-NS062609 for C.R. Olson. The authors’ use of animal models for research is covered under the OHSU IACUC ]0721. This review benefited from the thoughtful comments of K.L. Horback and two anonymous reviewers.
Abbreviations
- ATRA
all-trans retinoic acid
- HVC
hyperstriatum ventrale, pars caudalis
- LMAN
lateral magnocellular nucleus of the nidopallium
- LTD
long-term depression
- LTP
long-term potentiation
- RalDH
retinaldehyde-specific aldehyde dehydrogenases
- RA
robust nucleus of the arcopallium
- VAD
vitamin A deficiency
Footnotes
The authors have declared no conflict of interest.
References
- 1.Chen Y, Dokmanovic M, Stein WD, Ardecky RJ, Roninson IB. Agonist and antagonist of retinoic acid receptors cause similar changes in gene expression and induce senescence-like growth arrest in MCF-7 breast carcinoma cells. Cancer Res. 2006;66:8749–8761. doi: 10.1158/0008-5472.CAN-06-0581. [DOI] [PubMed] [Google Scholar]
- 2.Rasooly R, Schuster GU, Gregg JP, Xiao JH, et al. Retinoid × receptor agonists increase bcl2a1 expression and decrease apoptosis of naive T lymphocytes. J Immunol. 2005;175:7916–7929. doi: 10.4049/jimmunol.175.12.7916. [DOI] [PubMed] [Google Scholar]
- 3.Arima K, Shiotsugu J, Niu R, Khandpur R, et al. Global analysis of RAR-responsive genes in the Xenopus neurula using cDNA microarrays. Dev Dyn. 2005;232:414–431. doi: 10.1002/dvdy.20231. [DOI] [PubMed] [Google Scholar]
- 4.Cawley S, Bekiranov S, Ng HH, Kapranov P, et al. Unbiased mapping of transcription factor binding sites along human chromosomes 21 and 22 points to widespread regulation of noncoding RNAs. Cell. 2004;116:499–509. doi: 10.1016/s0092-8674(04)00127-8. [DOI] [PubMed] [Google Scholar]
- 5.Underwood BA, Arthur P. The contribution of vitamin A to public health. FASEB J. 1996;10:1040–1048. [PubMed] [Google Scholar]
- 6.Jiang J, Toschke AM, von Kries R, Lin L. Vitamin A status among children in China. Public Health Nutr. 2006;9:955–960. doi: 10.1017/phn2006944. [DOI] [PubMed] [Google Scholar]
- 7.Arlappa N, Laxmaiah A, Balakrishna N, Harikumar R, Brahmam GN. Clinical and sub-clinical vitamin A deficiency among rural pre-school children of Maharashtra, India. Ann Hum Biol. 2008;35:606–614. doi: 10.1080/03014460802380778. [DOI] [PubMed] [Google Scholar]
- 8.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- 9.Gómez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci. 2008;9:568–578. doi: 10.1038/nrn2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crandall J, Sakai Y, Zhang J, Koul O, et al. 13-Cis-retinoic acid suppresses hippocampal cell division and hippocampal-dependent learning in mice. Proc Natl Acad Sci USA. 2004;101:5111–5116. doi: 10.1073/pnas.0306336101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hull P, D’Arcy C. Acne, depression and suicide. Dermatol Clin. 2005;23:665–674. doi: 10.1016/j.det.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Malaspina A, Michael-Titus AT. Is the modulation of retinoid and retinoid-associated signaling a future therapeutic strategy in neurological trauma and neurodegeneration? J Neurochem. 2008;104:584–595. doi: 10.1111/j.1471-4159.2007.05071.x. [DOI] [PubMed] [Google Scholar]
- 13.Peng X, Yun D, Christov K. Breast cancer progression in MCF10A series of cell lines is associated with alterations in retinoic acid and retinoid × receptors and with differential response to retinoids. Int J Oncol. 2004;25:961–971. [PubMed] [Google Scholar]
- 14.Haugen BR, Larson LL, Pugazhenthi U, Hays WR, et al. Retinoic acid and retinoid × receptors are differentially expressed in thyroid cancer and thyroid carcinoma cell lines and predict response to treatment with retinoids. J Clin Endocrinol Metabol. 2004;89:272–280. doi: 10.1210/jc.2003-030770. [DOI] [PubMed] [Google Scholar]
- 15.Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol. 2006;66:606–630. doi: 10.1002/neu.20242. [DOI] [PubMed] [Google Scholar]
- 16.Campo-Paysaa F, Marlétaz F, Laudet V, Schubert M. Retinoic acid signaling in development: tissue-specific functions and evolutionary origins. Genesis. 2008;46:640–656. doi: 10.1002/dvg.20444. [DOI] [PubMed] [Google Scholar]
- 17.Gundersen TE. Methods for detecting and identifying retinoids in tissue. J Neurobiol. 2006;66:631–644. doi: 10.1002/neu.20243. [DOI] [PubMed] [Google Scholar]
- 18.Kane MA, Folias AE, Wang C, Napoli JL. Quantitative profiling of endogenous retinoic acid in vivo and in vitro by tandem mass spectrometry. Anal Chem. 2008;80:1702–1708. doi: 10.1021/ac702030f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duester G. Keeping an eye on retinoic acid signaling during eye development. Chem Bio Interact. 2009;178:178–181. doi: 10.1016/j.cbi.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stratford T, Horton C, Maden M. Retinoic acid is required for the initiation of outgrowth in the chick limb bud. Curr Biol. 1996;6:1124–1133. doi: 10.1016/s0960-9822(02)70679-9. [DOI] [PubMed] [Google Scholar]
- 21.McCaffery P, Drager UC. Regulation of retinoic acid signaling in the embryonic nervous system: a master differentiation factor. Cytokine Growth Factor Rev. 2000;11:233–249. doi: 10.1016/s1359-6101(00)00002-2. [DOI] [PubMed] [Google Scholar]
- 22.Napoli JL. Interactions of retinoid binding proteins and enzymes in retinoid metabolism. Biochim Biophys Acta. 1999;1440:139–162. doi: 10.1016/s1388-1981(99)00117-1. [DOI] [PubMed] [Google Scholar]
- 23.McCaffery P, Zhang J, Crandall JE. Retinoic acid signaling and function in the adult hippocampus. J Neurobiol. 2006;66:780–791. doi: 10.1002/neu.20237. [DOI] [PubMed] [Google Scholar]
- 24.Denisenko-Nehrbass NI, Mello CV. Molecular targets of disulfiram action on song maturation in zebra finches. Brain Res Mol Brain Res. 2001;87:246–250. doi: 10.1016/s0169-328x(01)00002-x. [DOI] [PubMed] [Google Scholar]
- 25.Chiang MY, Misner D, Kempermann G, Schikorski T, et al. An essential role for retinoid receptors RAR[beta] and RXR[gamma] in long-term potentiation and depression. Neuron. 1998;21:1353–1361. doi: 10.1016/s0896-6273(00)80654-6. [DOI] [PubMed] [Google Scholar]
- 26.Stapley J, Birkhead TR, Burke T, Slate J. A linkage map of the zebra finch Taeniopygia guttata provides new insights into avian genome evolution. Genetics. 2008;179:651–667. doi: 10.1534/genetics.107.086264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lane MA, Bailey SJ. Role of retinoid signaling in the adult brain. Prog Neurobiol. 2005;35:275–293. doi: 10.1016/j.pneurobio.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Luo T, Wagner E, Dräger UC. Integrating retinoic acid signaling with brain function. Dev Psychol. 2009;45:139–150. doi: 10.1037/0012-1649.45.1.139. [DOI] [PubMed] [Google Scholar]
- 29.Misner DL, Jacobs S, Shimizu Y, de Urquiza AM, et al. Vitamin A deprivation results in reversible loss of hippocampal long-term synaptic plasticity. Proc Natl Acad Sci USA. 2001;98:11714–11719. doi: 10.1073/pnas.191369798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Etchamendy N, Enderlin V, Marighetto A, Pallet V, et al. Vitamin A deficiency and relational memory deficit in adult mice: relationships with changes in brain retinoid signaling. Behav Brain Res. 2003;145:37–49. doi: 10.1016/s0166-4328(03)00099-8. [DOI] [PubMed] [Google Scholar]
- 31.Cocco S, Diaz G, Stancampiano R, Diana A, et al. Vitamin A deficiency produces spatial learning and memory impairment in rats. Neuroscience. 2002;115:475–482. doi: 10.1016/s0306-4522(02)00423-2. [DOI] [PubMed] [Google Scholar]
- 32.Bonnet E, Touyarot K, Alfos S, Pallet V, et al. Retinoic acid restores adult hippocampal neurogenesis and reverses spatial memory deficit in vitamin A deprived rats. PLoS One. 2008;3:e3487. doi: 10.1371/journal.pone.0003487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haskell GT, Maynard TM, Shatzmiller RA, LaMantia A. Retinoic acid signaling at sites of plasticity in the mature central nervous system. J Comp Neurol. 2002;452:228–241. doi: 10.1002/cne.10369. [DOI] [PubMed] [Google Scholar]
- 34.Ding Y, Qiao A, Wang Z, Goodwin JS, et al. Retinoic acid attenuates β-amyloid deposition and rescues memory deficits in an Alzheimer’s disease transgenic mouse model. J Neurosci. 2008;28:11622–11634. doi: 10.1523/JNEUROSCI.3153-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Etchamendy N, Enderlin V, Marighetto A, Vouimba RM, et al. Alleviation of a selective age-related relational memory deficit in mice by pharmacologically induced normalization of brain retinoid signaling. Behav Brain Res. 2001;21:6423–6429. doi: 10.1523/JNEUROSCI.21-16-06423.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeigler HP, Marler P, editors. Behavioral Neurobiology of Birdsong. New York Academy of Sciences; New York: 2004. [Google Scholar]
- 37.Zeigler HP, Marler P, editors. Neuroscience of Birdsong. Cambridge University Press; Cambridge: 2008. [Google Scholar]
- 38.Wilbrecht L, Kirn JR. In: Behavioral Neurobiology of Birdsong. Zeigler HP, Marler P, editors. New York Academy of Sciences; New York: 2004. [Google Scholar]
- 39.Adret P. In search of the song template. In: Zeigler HP, Marler P, editors. Behavioral Neurobiology of Birdsong. New York Academy of Sciences; New York: 2004. [DOI] [PubMed] [Google Scholar]
- 40.Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annu Rev Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- 41.Denisenko-Nehrbass NI, Jarvis E, Scharff CNF, Mello CV. Site-specific retinoic acid production in the brain of adult songbirds. Neuron. 2000;27:359–370. doi: 10.1016/s0896-6273(00)00043-x. [DOI] [PubMed] [Google Scholar]
- 42.Sakaguchi H, Saito N. Developmental changes in axon terminals visualized by immunofluorescence for the growth-associated protein, GAP-43, in the robust nucleus of the archistriatum of the zebra finch. Dev Brain Res. 1996;95:245–251. doi: 10.1016/0165-3806(96)00085-5. [DOI] [PubMed] [Google Scholar]
- 43.Kao MH, Brainard MS. Lesions of an avian basal ganglia circuit prevent context-dependent changes to song variability. J Neurophysiol. 2006;96:1441–1455. doi: 10.1152/jn.01138.2005. [DOI] [PubMed] [Google Scholar]
- 44.Ölveczky BP, Andalman AS, Fee MS. Vocal experimentation in the juvenile songbird requires a basal ganglia circuit. PLoS Biol. 2005;3:902–909. doi: 10.1371/journal.pbio.0030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wood WE, Olson CR, Lovell PV, Mello CV. Dietary retinoic acid affects song maturation and gene expression in the song system of the zebra finch. Dev Neurobiol. 2008;68:1213–1224. doi: 10.1002/dneu.20642. [DOI] [PubMed] [Google Scholar]
- 46.Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J Neurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tchernichovski O, Nottebohm F, Ho CE, Pesaran B, Mitra PP. A procedure for an automated measurement of song similarity. Anim Behav. 2000;59:1167–1176. doi: 10.1006/anbe.1999.1416. [DOI] [PubMed] [Google Scholar]
- 48.Krucker T, Siggins GR, McNamara RK, Lindsley KA, et al. Targeted disruption of RC3 reveals a calmodulin-based mechanism for regulating metaplasticity in the hippocampus. J Neurosci. 2002;22:5525–5535. doi: 10.1523/JNEUROSCI.22-13-05525.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhabotinsky AM, Camp RN, Epstein IR, Lisman JE. Role of the neurogranin concentrated in spines in the induction of long-term potentiation. J Neurosci. 2006;26:7337–7347. doi: 10.1523/JNEUROSCI.0729-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawano H, Nakatani T, Mori T, Ueno S, et al. Identification and characterization of novel developmentally regulated neural-specific proteins, BRINP family. Mol Brain Res. 2004;125:60–75. doi: 10.1016/j.molbrainres.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 51.Munshi-South J, Wilkinson GS. Bats and birds: exceptional longevity despite high metabolic rates. Ageing Res Rev. 2010;9:12–19. doi: 10.1016/j.arr.2009.07.006. [DOI] [PubMed] [Google Scholar]


