Abstract
Cell fate decisions in the early Drosophila wing disc assign cells to compartments (anterior or posterior and dorsal or ventral) and distinguish the future wing from the body wall (notum). Here we show that EGF-receptor (EGFR) signaling stimulated by its ligand, Vein, has a fundamental role in regulating two of these cell fate choices: (1) Vn/EGFR signaling directs cells to become notum by antagonizing wing development and by activating notum-specifying genes; (2) Vn/EGFR signaling directs cells to become part of the dorsal compartment by induction of apterous, the dorsal selector gene, and consequently also controls wing development, which depends on an interaction between dorsal and ventral cells.
Keywords: Drosophila, wing disc, vein, wingless, Egfr, apterous
The wing imaginal disc of Drosophila, which gives rise to the mesothoracic body wall and its appendage the wing, has been an important system for revealing the genetic mechanisms that lead to the establishment of specific cell fates. In the early wing disc, cells make three fate choices to initiate programs for future pattern formation: to be anterior (A) or posterior (P) compartment; to be dorsal (D) or ventral (V) compartment; and to be wing or body wall (notum) (Garcia-Bellido et al. 1973; Dahmann and Basler 1999). The A/P and D/V restrictions result in the establishment of organizers at the compartment boundaries that govern overall growth and patterning (Lawrence and Struhl 1996). The third choice determines whether cells ultimately differentiate wing- or notum-specific structures.
Many genes required for wing development, including those involved in both initial specification and subsequent outgrowth, have been characterized. In particular, wingless (wg), which encodes a Wnt-secreted signaling molecule, has been implicated as having a role in specifying the wing primordium in the second instar (Williams et al. 1993; Ng et al. 1996; Klein and Martinez-Arias 1998). In wg mutants, the wing is lost and an extra notum forms (Fig. 1A–D; Sharma and Chopra 1976; Morata and Lawrence 1978).
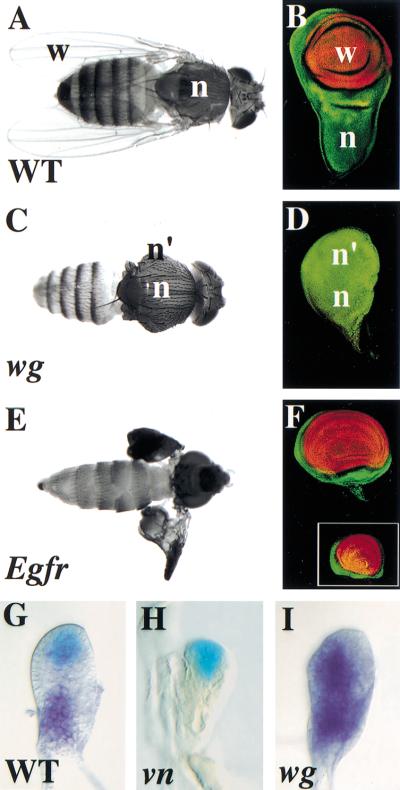
Figure 1.
Wg and Vn have complementary roles in development of the wing and notum. Late third instar wing discs (B,D,F) stained for Nubbin (Nub, wing pouch, red) and Teashirt (Tsh, notum, hinge, and ventral pleura, green). (A) Wild-type fly and (B) wing disc with a normal notum (n) and wing (w). (C) wg1/CX4 fly and (D) wing disc with normal (n) and ectopic notum (n′). The wing is lost. (E) Egfrtsla/f24 fly and (F) wing disc shifted to the restrictive temperature during the second instar. The notum is lost; the wings are present but have pattern abnormalities and failed to inflate. Inset in F is a wing disc from a vntsWB240/RGlarva raised at the restrictive temperature during the same period. (G–I) Second instar wing discs stained for β-galactosidase (β-gal) activity; wg–lacZ (blue, G,H) or vn RNA by in situ hybridization (purple, G,I). (G) In wild type, wg (blue) and vn (purple) are expressed in complementary patterns in the presumptive wing and notum, respectively. (H) wg expression is not affected in a vnγ3/γ4 mutant, which is a known molecular null (Simcox et al. 1996). (I) vn expression spreads distally in a wg1/CX4mutant.
In contrast, little is known about initial development of the notum and whether it is specified by an active gene program or results from a default pathway that operates when the wing is not specified. A candidate molecule for establishing the notum is Vein, a secreted neuregulin-like molecule that activates EGF-receptor (EGFR) signaling (Schnepp et al. 1996, 1998; Yarnitzky et al. 1997). vn is expressed in the presumptive notum in second instar wing discs and hypomorphic vn mutants lack the notum (Simcox et al. 1996). However, the role of vn is not limited to the notum as the wing primordium also fails to grow in vn null alleles and in some Egfr alleles (Clifford and Schüpbach 1989; Simcox et al. 1996). We investigated the role of Vn/EGFR signaling in wing disc development, and present evidence that the pathway is directly required for development of the notum by activating notum-specifying genes and indirectly controls wing outgrowth through regulation of apterous.
Results and Discussion
Complementary roles for Vn and Wg in notum and wing development
To determine when Vn/EGFR signaling is required for notum development, we used the temperature-sensitive alleles, Egfrtsla and vntsWB240. Inactivating Vn/EGFR activity during the second instar (a 24 hr period) caused loss of the notum (Fig. 1E,F, and inset). The wing developed but showed pattern abnormalities characteristic of vn hypomorphs (Fig. 1E; Simcox et al. 1996). Later shifts during the third instar did not cause loss of the notum (data not shown). This demonstrates Vn/EGFR activity is required for notum development in the second instar when wg is required to specify the wing (Ng et al. 1996). Thus, Vn and Wg appear to have complementary roles and we examined this relationship by following their expression in mutants.
In second instar wild-type wing discs, wg is expressed distally in a wedge of anterior ventral cells (Couso et al. 1993; Williams et al. 1993; Ng et al. 1996) and vn is expressed proximally (Simcox et al. 1996; Fig. 1G). In vn null mutants, the initiation of wg expression was normal as was expression of its target gene optomotor-blind (omb) (Grimm and Pflugfelder 1996; Fig. 1H; data not shown). In wg mutants, however, there was a dramatic and early expansion of vn expression to include distal cells (Fig. 1I), presaging the development of these cells as an extra notum.
Together these results suggest that Vn has an early role in establishing the notum and that Wg signaling is required to define a distal domain that is reduced in EGFR activity to allow wing development.
Vn/EGFR signaling is required for expression of vn and the Iroquois complex genes
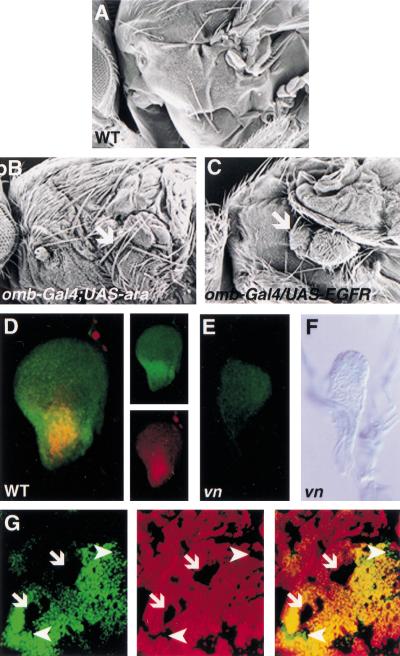
To test the role of Vn/EGFR signaling in specifying notum we examined whether the Iroquois complex (Iro-C) genes, ara and cap (Gomez-Skarmeta et al. 1996) are targets of the pathway. The Iro-C genes have been implicated in specifying notum cell fate because loss of function causes a transformation of notum to hinge (Diez del Corral et al. 1999). Furthermore, we found misexpression of ara caused loss of the wing and a duplication of notum (Fig. 2A,B). Ectopic expression of an activated form of the receptor, EGFRλtop4.2 (Queenan et al. 1997) greatly reduced the size of the wing and a small ectopic notum formed (Fig. 2C). vn is expressed in the presumptive notum in early second instar discs (Fig. 1G) and Caup/Ara are expressed in the presumptive notum at the end of the second instar (Gomez-Skarmeta et al. 1996; Diez del Corral et al. 1999). In early third instar wing discs, Caup/Ara are expressed in a domain that overlaps with vn (Fig. 2D). In vn mutants, this expression of Caup/Ara is lost (Fig. 2E) and loss of EGFR signaling, in EGFRts clones, in the medial notum resulted in a loss of Caup/Ara expression (Fig. 2G). However, clones in the lateral notum continued to express Caup/Ara (Fig. 2G), suggesting other factors regulate Iro-C gene expression in these cells at this stage.
Figure 2.
Vn/EGFR signaling is required for notum development by regulating expression of vn and the Iro-C genes. (A–C) Scanning electron micrograph of adult lateral thorax. (A) Wild type. (B) Ectopic expression of ara (omb–Gal4; UAS–ara, 29°C) causes loss of the wing and formation of an extra notum (arrow). (C) Ectopic expression of activated EGFR (omb–Gal4; EGFRλtop4.2, 25°C) causes reduction of the wing and transformation of the ventral pleura to notum (arrow). The phenotype of omb–Gal4; EGFRλtop4 flies could not be examined at 29°C as the increased activity of Gal4 caused lethality. At 25°C, omb–Gal4; EGFRλtop4 and omb–Gal4; UAS–ara and flies show a similar reduction of the wing. (D) Wild-type early third instar wing disc stained for vn–lacZ (β-gal; red) and Caup/Ara (green). Expression of vn and Caup/Ara partially overlap in the presumptive notum. Individual stains are shown on the right. (E) Expression of Caup/Ara is lost in a vnD4/L6early third instar mutant wing disc. (F) Expression of vn–lacZ (X-gal; blue) is lost in a vnL6/rF264 early third instar mutant wing disc. (G) Wing disc stained for Caup/Ara (green, left panel) and anti-β-gal (red, middle panel). The merge is shown in right-hand panel. Caup/Ara expression is lost in clones in the medial notum (arrows) lacking EGFR activity (EGFRtsla, the clones are marked by loss of β-Gal staining). Clones in the lateral notum continue to express Caup/Ara (arrowheads).
Activation of Iro-C genes could account for the requirement for EGFR activity to specify the notum at the end of the second instar as this correlates with when these genes are first expressed. However, loss of EGFR signaling at a slightly earlier time (mid-first instar to mid-second instar, see below), prior to activation of the Iro-C genes, also results in loss of the notum. A possible explanation for this comes from the finding that vn expression is lost in vn mutants (Fig. 2F). This suggests EGFR activity must be sustained, via a positive feedback loop involving transcriptional activation of vn, during the second instar, to activate the Iro-C genes and hence specify notum at the end of this period. Interestingly, the vn gene is also a target of EGFR signaling in the embryo (Golembo et al. 1999; Wessells et al. 1999).
Antagonism between Wg and Vn/EGFR signaling
We suggest the mechanisms by which wg and vn specify alternate cell fates in the early wing disc, wing, or notum are antagonistic. This is based on the observation that loss of Wg results in the spread of vn expression and the supposition that the resulting ectopic EGFR activity causes loss of the wing and a double notum phenotype (Fig. 1C,I). Further evidence that Vn/EGFR signaling represses wing development comes from the results of misexpressing a constitutive receptor, EGFRλtop4.2, in the presumptive wing. In these flies, the wing was reduced to a stump covered with sensilla characteristic of the proximal wing (hinge) region and expression of the wing specific gene vestigial (vg) (Kim et al. 1996) was repressed (Fig. 3A–D). Ectopic notal structures also formed from the ventral pleura (Fig. 2C). The ability of ectopic EGFR signaling to suppress wing development is cell autonomous because clones of cells expressing EGFRλtop4.2 lacked vg expression (Fig. 3E). In adult wings these clones produced outgrowths lacking wing characteristics but were otherwise difficult to characterize (Fig. 3F).
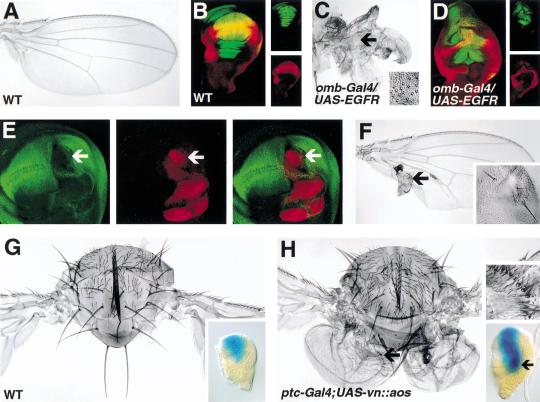
Figure 3.
Misregulation of Vn/EGFR signaling alters patterning in primordia of the wing and notum. Third instar wing discs (B,D) were examined for expression of Vg (red) and green fluorescent protein (GFP; green) (individual images are shown in panels to the right). (A) Wild-type wing and (B) wing disc. Expression of omb (omb–Gal4; UAS–GFP) and Vg overlap in the wing pouch (yellow in the merged image, B). (C) Wing and (D) wing disc following misexpression of constitutively active EGFR in the presumptive wing (omb–Gal4/UAS–EGFRλtop4.2; UAS–GFP). The wing is small (c.f. A) and partially transformed to hinge as evident from groups of ectopic sensilla characteristic of the hinge (arrow, shown at higher magnification in inset, C). Vg expression is reduced in the wing pouch (D). (E) Wing disc stained for Vg (green, left panel) and β-gal (red, middle panel). The merged images are shown in right-hand panel. Vg expression is reduced in a clone (arrow) expressing an activated form of EGFR (EGFRλtop4.2, the clone is marked by expression of β-Gal). (F) In the adult wing, EGFRλtop4.2 clones form outgrowths or vesicles (data not shown) that differentiate structures lacking wing characteristics and that sometimes include bristles (arrow, shown at higher magnification in inset). (G) Thorax of a wild-type fly. Inset, second instar wing disc showing expression of omb–lacZ in a ventral anterior domain which includes cells of the presumptive wing. (H) Four-winged fly resulting from inhibition of EGFR signaling by expression of a ligand antagonist (UAS–vn::aos–EGF) under the control of ptc–Gal4. (Similar misexpression of a dominant negative form of EGFR was embryonic lethal.) The ectopic wings appear below the normal wings in mirror symmetry and are composed mainly of posterior structures despite the fact the inhibitor was expressed in anterior cells with Ptc–Gal4. Ptc–Gal4 is expressed along the AP boundary and as the inhibitor is soluble, it could be secreted across the boundary to affect posterior cells. The ectopic wings do include some anterior wing tissue (arrow) that can be recognized as such by margin bristles (top inset) and sensilla characteristic of the third wing vein, which is anterior (data not shown). The basis for the relatively small amount of anterior tissue is not currently understood. Lower inset shows omb–lacZ expression in ptc–Gal4; UAS–vn::aos second instar wing disc. Ectopic activation of omb–lacZ in the disc indicates an ectopic wing has formed (arrow).
Although vn expression expanded in wg mutants, we did not observe a reciprocal spread of wg expression in vn mutants that would have been indicative of a double wing phenotype (Fig. 1H). However, when Vn/EGFR signaling was inhibited in the notum by expressing a ligand antagonist (Vn::Aos–EGF) (Schnepp et al. 1998) under the control of ptc–Gal4, ectopic wings were induced in ∼10% of the flies (Fig. 3G,H). This result demonstrates that presumptive notal tissue can be transformed to wing by reducing EGFR signaling. However, the transformation occurred only when EGFR signaling was reduced in a subset of cells, rather than all cells in the notum (as in a vn mutant). As described in the next section, this may reflect the indirect requirement for EGFR activity to also promote wing development.
Vn/EGFR signaling regulates expression of apterous
The loss of notum phenotype is characteristic of vn hypomorphs but in null vn alleles and some Egfr alleles both the wing and notum primordia fail to develop and the wing discs remain tiny (Clifford and Schüpbach 1989; Simcox et al. 1996). Thus, although ectopic activity of EGFR in the distal disc represses wing development, the pathway is nevertheless normally required for wing development. Using the temperature-sensitive Egfrtsla allele we found that this requirement is restricted to the period from mid-first to mid-second instar (Fig. 4A,B). Key genes involved in wing development that are active at this time include wg and apterous (ap). ap encodes a LIM-homeodomain protein (Cohen et al. 1992) that is expressed in dorsal cells and acts as a selector gene to divide the disc into dorsal and ventral compartments (Diaz-Benjumea and Cohen 1993; Blair et al. 1994). Regulation of Notch ligands by Ap leads to Notch signaling at the DV boundary and the formation of an organizer for wing outgrowth and expression of the wing-specific transcription factor vg (Kim et al. 1996; Irvine 1999).
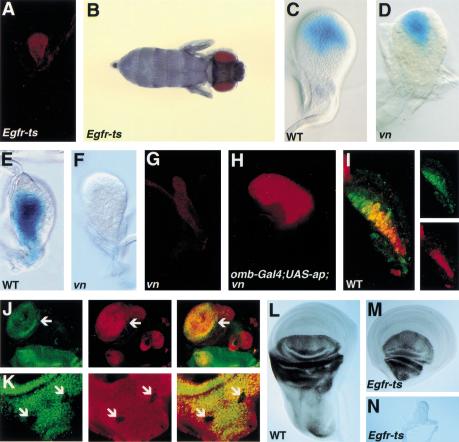
Figure 4.
Vn/EGFR signaling is required for wing development by regulating ap expression. Late third instar wing discs (A,G,H) stained for Nubbin (Nub, wing specific, red). (A) EGFRtsla/f24wing disc and (B) fly. A shift to the restrictive temperature from mid-first to mid-second results in the loss of both wing and notum and very little growth of the disc. There is no expression of Nub (A). (C) Wild-type early third instar wing disc showing expression of wg–lacZ (blue) in a symmetrical domain filling the presumptive wing pouch. (D) vnD4/L6early third instar wing disc showing wg–lacZ expression (blue) in a sector. (E) Wild-type second instar wing disc showing expression of ap–lacZ (blue) in dorsal cells. (F) vnD4/L6 second instar wing disc showing loss of ap–lacZ (blue) expression. (G) vnD4/L6 third instar wing disc. There is no expression of Nub. In second instar discs there is also no expression of Nub (data not shown). (H) omb–Gal4; UAS–ap; vnD4/L6wing disc. Expression of ap in the presumptive wing region rescues wing development in the vn mutant as evident by Nub expression. (I) Wild-type second instar disc showing expression of vn–lacZ (β-gal; red) and Ap (green). The individual stains are shown to the right. vn and Ap expression overlap in the proximal disc (presumptive notum) but Ap is also expressed more distally than vn. Discs (J,K) were stained for anti-Ap (green, left panel) and anti-β-gal (red, middle panel). The merged image is shown in right-hand panel. (J) Ap is induced in a ventral clone (arrow) expressing an activated form of EGFR (EGFRλtop4.2, the clone is marked by expression of β-Gal). (K) Ap is reduced in dorsal clones (arrows) lacking EGFR activity (EGFRtsla, the clones are marked by loss of β-Gal staining). Ap expression (black) in third instar discs (L–N). (L) Wild-type wing disc. Ap is expressed in dorsal cells. (M) EGFRtsla/f24 wing disc that was shifted to the restrictive temperature during second instar. The notum is lost but Ap is expressed in dorsal wing cells. (N) EGFRtsla/f24 wing disc that was shifted to the restrictive temperature during mid-first to mid-second instar. Ap is not expressed.
Of these two candidates, wg and ap, it seemed unlikely that wg was the key gene affected by EGFR signaling from mid-first to mid-second instar because wg expression was normal in vn mutants at mid-second instar (Fig. 1G,H). However, later in the second instar, wg expression normally expands to fill the growing wing pouch (Fig. 4C) (Couso et al. 1993; Williams et al. 1993; Ng et al. 1996) and we noted that in vn mutants, wg expression failed to undergo this expansion (Fig. 4D). A similar defect in wg expression is seen in ap mutants (Ng et al. 1996) consistent with Ap function being impaired in vn mutants. Remarkably, ap expression was completely absent in second instar vn mutant discs (Fig. 4E,F). Thus, loss of Ap can explain why there is no wing in vn mutants. This is supported by the demonstration that ectopic ap was capable of rescuing wing development in vn mutants (Fig. 4G,H).
Several additional lines of evidence demonstrate that ap is a cell autonomous target of Vn/EGFR signaling and that this relationship exists only transiently in early wing development. First, ap expression partially overlaps that of vn in the second instar (Fig. 4I). Second, ap can be induced ectopically in ventral clones misexpressing an activated form of the receptor, EGFRλtop4.2 (Fig. 4J). Third, Egfrtslamutant clones generated in the first instar show autonomous loss of ap expression (Fig. 4K), whereas clones generated in the second instar express ap normally (data not shown). Finally, loss of EGFR activity in whole discs from mid-first to mid-second instar results in complete loss of ap expression, whereas ap is still expressed in discs from larvae given a temperature shift slightly later during the second instar (Fig. 4L–N).
Early subdivision of the wing disc is under the control of Vn and Wg
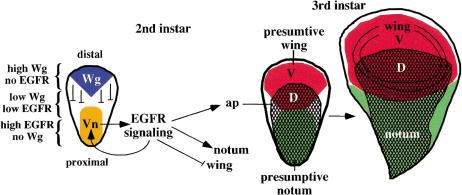
The results described here suggest that division of the early wing disc into presumptive wing and body wall regions is defined by the action of two secreted signaling molecules, Wg and Vn (Fig. 5). wg, a pro-wing gene, is required to repress vn expression, which at high levels antagonizes wing development. Antagonism between Wg and EGFR signaling has also been demonstrated in segmental patterning of the embryo (O'Keefe et al. 1997; Szuts et al. 1997) and in development of the head and third instar wing pouch (Amin et al. 1999; Wessells et al. 1999), suggesting such a relationship between these pathways may be a common theme in a number of cell fate choices. Finding that one of the main functions of Wg in early wing specification is to repress Vn/EGFR signaling in the distal region of the early disc raises the question as to whether this is the only role of Wg in wing specification and hence if wing-cell fate can be specified in the absence of both signals. This seems unlikely as nubbin, an early wing cell marker (Ng et al. 1996), is not misexpressed proximally in a vn mutant, where cells would lack both signals.
Figure 5.
Model to account for the role of EGFR signaling in wing and notum development. The wing and notum are specified by the action of two secreted signaling molecules, Wg and the EGFR ligand, Vn, which are expressed in the distal and proximal regions of the early second instar wing disc, respectively. Wg represses Vn expression restricting it to the proximal region. Wg and Vn define three domains in the early disc: (1) distal, high Wg/no EGFR signaling; (2) central, low Wg/low EGFR signaling; (3) proximal, high EGFR/no Wg. High EGFR signaling activity defines the notum by activating notum specific genes; it also functions to maintain vn expression and can repress the expression of wing specific genes. Lower EGFR activity is also sufficient to activate ap expression defining the dorsal (D) compartment. An organizer for wing patterning is established at the DV boundary; wings fail to develop in the absence of Vn/EGFR signaling because this organizer is absent.
Vn/EGFR signaling promotes development of the notum by maintaining its own activity through transcriptional activation of vn itself, and also promotes expression of ap. Thus, both vn and ap appear to be targets of EGFR signaling, but the domain of ap is clearly wider than that of vn, indicating that ap can be activated at a lower signaling threshold than vn. Vn is a secreted molecule and thus could generate a gradient of EGFR activity. This provides an explanation for how EGFR signaling can regulate both wing and notum development: vn autoregulation and notum development requires high EGFR signaling activity whilst ap expression and subsequent wing development requires lower signaling activity (Fig. 5).
Interestingly, vertebrate EGFR and its ligands are expressed in the chick limb bud in a pattern that appears to overlap with the vertebrate ap homolog Lhx2, and these factors are required for limb outgrowth in the chick (Dealy et al. 1998; Rodriguez-Esteban et al. 1998). In light of our present results it will be important to determine whether EGFR signaling controls Lhx2 expression and thus plays a role in regulating outgrowth of the vertebrate limb. Our results may also have implications for the evolution of insect wings. If the control of body wall development by EGFR signaling is ancestral, and comparative analysis of other arthropods will be required to assert this, then one of the first steps towards evolution of wings could have occurred when EGFR signaling assumed control of ap.
Materials and methods
Fly strains
The following alleles and transgenes were used: EGFRtsla, EGFRf24, vntsWB240, vnRG, vnL6, vnγ3, vnγ4, vnD4, vnrF264, wg1, wgCX4, wg–lacZ, ap–lacZ, omb–lacZ, UAS–GFP, UAS–EGFRλtop4.2, UAS–vn, UAS–vn::aos, UAS–ap, UAS–ara, omb–Gal4, and ptc–Gal4. The genes are described in detail at FlyBase (http://flybase.bio.indiana.edu).
Antibodies
Antibodies against the Iro-C protein Caupolican (rat anti-Caup) were used (J. Modolell, C.S.I.C. and Universidad Autonoma de Madrid, Spain); these crossreact with the related protein Ara but not with Mirror. Two anti-Ap antibodies were used; rat anti-Ap (J. Thomas, The Salk Institute, La Jolla, CA) and guinea-pig anti-Ap (J. Botas, Baylor College of Medicine, Houston, TX). Antibodies against Tsh (rat anti-Tsh,), Vg (rabbit anti-Vg,), and Nub (monoclonal) were from S. Kerridge, (Centre Universitaire Marseille, France), S. Carroll, (University of Wisconsin, Madison), and S. Cohen, (EMBL, Heidelberg, Germany), respectively. Monoclonal anti-β-gal antibodies were from Promega and rabbit polyclonal anti-β-gal antibodies were from Jackson ImmunoResearch. Immunostaining was performed according to standard protocols.
Clonal analysis
EGFRλtop4.2 clones were generated by heat-shocking larvae of the genotype f36a FLP1.22/UAS–EGFRλtop4.2; abx/Ubx<FRT f+ stop FRT>Gal4–LacZ (de Celis and Bray 1997) for 10 min at 37°C 48–50 hr after egg laying. Egfrtsla mutant clones were generated by heat-shocking larvae of the genotype hsflp; FRT 42 arm–lacZ M(2)60E/ FRT 42 Egfrtsla for 1 hr at 35°C during mid- to late-first or second instars and maintaining them at the restrictive temperature of 31°C. At 18°C these clones are phenotypically wild type.
Temperature shifts
To reduce EGFR activity, a temperature-sensitive allele, Egfrtsla was used; due to an additional lethal on the chromosome, this was crossed to the null allele Egfrf24. To reduce Vn activity, a temperature-sensitive allele, vnWB240 was used; this chromosome also has an additional lethal and was crossed to the null allele vnRG. Larvae of the genotype Egfrtsla/f24 or vnWB240/RGwere maintained at 18°C; to reduce activity, larvae were shifted to 30–31°C for a single 24 hr period at specific times in development and then returned to 18°C.
Acknowledgments
We thank J. Botas, S. Campuzano, S. Cohen, J. de Celis, S. Kerridge, J. Kumar, C-H. Lu, J. Modolell, K. Moses, D. O'Keefe, T. Schüpbach, J. Thomas, and the Bloomington Stock Center for reagents, our lab colleagues and R. Carthew, H. Chamberlin, and the reviewers for comments on the manuscript. This work was supported in part by the National Science Foundation (grant 97–24078 to A.S).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL camp+@pitt.edu; FAX (412) 624-4759.
E-MAIL simcox.1@osu.edu; FAX (614) 292-4466.
Article and publication are at www.genesdev.org/cgi/doi/10.1101/gad.827000.
References
- Amin A, Li Y, Finkelstein R. Hedgehog activates the EGF receptor pathway during Drosophila head development. Development. 1999;126:2623–2630. doi: 10.1242/dev.126.12.2623. [DOI] [PubMed] [Google Scholar]
- Blair SS, Brower DL, Thomas JB, Zavortink M. The role of apterous in the control of dorsoventral compartmentalization and PS integrin gene expression in the developing wing of Drosophila. Development. 1994;120:1805–1815. doi: 10.1242/dev.120.7.1805. [DOI] [PubMed] [Google Scholar]
- Clifford RT, Schüpbach T. Coordinately and differentially mutable activities of torpedo, the Drosophila melanogaster homolog of the vertebrate EGF receptor gene. Genetics. 1989;122:771–787. doi: 10.1093/genetics/123.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B, McGuffin ME, Pfeifle C, Segal D, Cohen SM. apterous, a gene required for imaginal disc development in Drosophila encodes a member of the LIM family of developmental regulatory proteins. Genes & Dev. 1992;6:715–729. doi: 10.1101/gad.6.5.715. [DOI] [PubMed] [Google Scholar]
- Couso JP, Bate M, Martinez-Arias A. A wingless-dependent polar coordinate system in Drosophila imaginal discs. Science. 1993;259:484–489. doi: 10.1126/science.8424170. [DOI] [PubMed] [Google Scholar]
- Dahmann C, Basler K. Compartment boundaries: At the edge of development. Trends Genet. 1999;15:320–326. doi: 10.1016/s0168-9525(99)01774-6. [DOI] [PubMed] [Google Scholar]
- de Celis JF, Bray S. Feed-back mechanisms affecting Notch activation at the dorsoventral boundary in the Drosophila wing. Development. 1997;124:3241–3251. doi: 10.1242/dev.124.17.3241. [DOI] [PubMed] [Google Scholar]
- Dealy CN, Scranton V, Cheng HC. Roles of transforming growth factor-α and epidermal growth factor in chick limb development. Dev Biol. 1998;202:43–55. doi: 10.1006/dbio.1998.8988. [DOI] [PubMed] [Google Scholar]
- Diaz-Benjumea FJ, Cohen SM. Interaction between dorsal and ventral cells in the imaginal disc directs wing development in Drosophila. Cell. 1993;75:741–752. doi: 10.1016/0092-8674(93)90494-b. [DOI] [PubMed] [Google Scholar]
- Diez del Corral R, Aroca P, Gómez-Skarmeta JL, Cavodeassi F, Modolell J. The Iroquois homeodomain proteins are required to specify body wall identity in Drosophila. Genes & Dev. 1999;13:1754–1761. doi: 10.1101/gad.13.13.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bellido A, Ripoll P, Morata G. Developmental compartmentalisation of the wing disk of Drosophila. Nat New Biol. 1973;245:251–253. doi: 10.1038/newbio245251a0. [DOI] [PubMed] [Google Scholar]
- Golembo M, Yarnitzky T, Volk T, Shilo BZ. Vein expression is induced by the EGF receptor pathway to provide a positive feedback loop in patterning the Drosophila embryonic ventral ectoderm. Genes & Dev. 1999;13:158–162. doi: 10.1101/gad.13.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Skarmeta JL, del Corral RD, de la Calle-Mustienes E, Ferre-Marco D, Modolell J. Araucan and caupolican, two members of the novel iroquois complex, encode homeoproteins that control proneural and vein-forming genes. Cell. 1996;85:95–105. doi: 10.1016/s0092-8674(00)81085-5. [DOI] [PubMed] [Google Scholar]
- Grimm S, Pflugfelder GO. Control of the gene optomotor-blind in Drosophila wing development by decapentaplegic and wingless. Science. 1996;271:1601–1604. doi: 10.1126/science.271.5255.1601. [DOI] [PubMed] [Google Scholar]
- Irvine KD. Fringe, Notch, and making developmental boundaries. Curr Opin Genet Dev. 1999;9:434–441. doi: 10.1016/S0959-437X(99)80066-5. [DOI] [PubMed] [Google Scholar]
- Kim J, Sebring A, Esch JJ, Kraus ME, Vorwerk K, Magee J, Carroll SB. Integration of positional signals and regulation of wing formation and identity by Drosophila vestigial gene. Nature. 1996;382:133–138. doi: 10.1038/382133a0. [DOI] [PubMed] [Google Scholar]
- Klein T, Martinez-Arias AM. Different spatial and temporal interactions between Notch, wingless, and vestigial specify proximal and distal pattern elements of the wing in Drosophila. Dev Biol. 1998;194:196–212. doi: 10.1006/dbio.1997.8829. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Struhl G. Morphogens, compartments, and pattern: Lessons from Drosophila? Cell. 1996;85:951–961. doi: 10.1016/s0092-8674(00)81297-0. [DOI] [PubMed] [Google Scholar]
- Morata G, Lawrence PA. The development of wingless, a homeotic mutation of Drosophila. Dev Biol. 1978;48:227–240. doi: 10.1016/0012-1606(77)90266-4. [DOI] [PubMed] [Google Scholar]
- Ng M, Diaz-Benjumea FJ, Vincent JP, Wu J, Cohen SM. Specification of the wing by localized expression of wingless protein. Nature. 1996;381:316–318. doi: 10.1038/381316a0. [DOI] [PubMed] [Google Scholar]
- O'Keefe L, Dougan ST, Gabay L, Raz E, Shilo BZ, DiNardo S. Spitz and Wingless, emanating from distinct borders, cooperate to establish cell fate across the Engrailed domain in the Drosophila epidermis. Development. 1997;124:4837–4845. doi: 10.1242/dev.124.23.4837. [DOI] [PubMed] [Google Scholar]
- Queenan AM, Ghabrial A, Schupbach T. Ectopic activation of torpedo/Egfr, a Drosophila receptor tyrosine kinase, dorsalizes both the eggshell and the embryo. Development. 1997;124:3871–3880. doi: 10.1242/dev.124.19.3871. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Esteban C, Schwabe JW, Pena JD, Rincon-Limas DE, Magallon J, Botas J, Belmonte JC. Lhx2, a vertebrate homologue of apterous, regulates vertebrate limb outgrowth. Development. 1998;125:3925–3934. doi: 10.1242/dev.125.20.3925. [DOI] [PubMed] [Google Scholar]
- Schnepp B, Grumbling G, Donaldson T, Simcox A. Vein is a novel component in the Drosophila epidermal growth factor receptor pathway with similarity to the neuregulins. Genes & Dev. 1996;10:2302–2313. doi: 10.1101/gad.10.18.2302. [DOI] [PubMed] [Google Scholar]
- Schnepp B, Donaldson T, Grumbling G, Ostrowski S, Schweitzer R, Shilo B-Z, Simcox A. EGF domain swap converts a Drosophila EGF-receptor activator into an inhibitor. Genes & Dev. 1998;12:908–913. doi: 10.1101/gad.12.7.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma RP, Chopra VL. Effect of the Wingless (wg1) mutation on wing and haltere development in Drosophila melanogaster. Dev Biol. 1976;48:461–465. doi: 10.1016/0012-1606(76)90108-1. [DOI] [PubMed] [Google Scholar]
- Simcox AA, Grumbling G, Schnepp B, Bennington-Mathias C, Hersperger E, Shearn A. Molecular, phenotypic, and expression analysis of vein, a gene required for growth of the Drosophila wing disc. Dev Biol. 1996;177:475–489. doi: 10.1006/dbio.1996.0179. [DOI] [PubMed] [Google Scholar]
- Szuts D, Freeman M, Bienz M. Antagonism between EGFR and Wingless signaling in the larval cuticle of Drosophila. Development. 1997;124:3209–3219. doi: 10.1242/dev.124.16.3209. [DOI] [PubMed] [Google Scholar]
- Wessells RJ, Grumbling G, Donaldson T, Wang SH, Simcox A. Tissue-specific regulation of vein/EGF receptor signaling in Drosophila. Dev Biol. 1999;216:243–259. doi: 10.1006/dbio.1999.9459. [DOI] [PubMed] [Google Scholar]
- Williams JA, Paddock SW, Carroll SB. Pattern formation in a secondary field: A hierarchy of regulatory genes subdivides the developing Drosophila wing disc into discrete subregions. Development. 1993;117:571–584. doi: 10.1242/dev.117.2.571. [DOI] [PubMed] [Google Scholar]
- Yarnitzky T, Min L, Volk T. The Drosophila neuregulin homolog Vein mediates inductive interactions between myotubes and their epidermal attachment cells. Genes & Dev. 1997;11:2691–2700. doi: 10.1101/gad.11.20.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]