Abstract
The very late-flowering behavior of Arabidopsis winter-annual ecotypes is conferred mainly by two genes, FRIGIDA (FRI) and FLOWERING LOCUS C (FLC). A MADS-domain gene, AGAMOUS-LIKE 20 (AGL20), was identified as a dominant FRI suppressor in activation tagging mutagenesis. Overexpression of AGL20 suppresses not only the late flowering of plants that have functional FRI and FLC alleles but also the delayed phase transitions during the vegetative stages of plant development. Interestingly, AGL20 expression is positively regulated not only by the redundant vernalization and autonomous pathways of flowering but also by the photoperiod pathway. Our results indicate that AGL20 is an important integrator of three pathways controlling flowering in Arabidopsis.
Keywords: Flowering, MADS domain protein, AGL20, phase transition, activation tagging
Plants undergo several distinct phase transitions during their life cycle (Poethig 1990; Schultz and Haughn 1993; Telfer et al. 1997). The most dramatic phase change is the transition from vegetative growth to reproductive growth, or flowering. In addition, juvenile and adult phases can be distinguished during vegetative development, discernible by the distribution of trichomes on the leaf surface in Arabidopsis (Telfer et al. 1997). The transition to flowering is regulated by a complex genetic network that monitors the developmental state of the plant as well as environmental conditions. The genetic control of flowering has been extensively studied in Arabidopsis (Weigel 1995; Koornneef et al. 1998; Levy and Dean 1998). Arabidopsis is a facultative long-day plant that flowers faster under long days than short days. Genetic analyses of late-flowering mutants have identified more than 20 genes, which have been placed in at least three parallel genetic pathways based on the effect of each mutation on the response to environmental conditions. Genes such as CONSTANS (CO), GIGANTEA (GI), FT, FWA, FHA, FE, and FD have been placed in the long day-dependent pathway because mutations in them cause lateness in flowering under long days but have little effect under short days. Genes such as FCA, FPA, FVE, FY, and LUMINIDEPENDENS (LD) have been placed in the autonomous pathway because mutations in them cause delay in flowering under both long days and short days. A third pathway, which acts redundantly with the autonomous pathway, accelerates flowering upon vernalization (an extended cold treatment).
The genes defective in six late-flowering mutants, LD, CO, FCA, FHA, GI, and FT, have been isolated. LD and CO seem to encode transcription factors, whereas FCA encodes a putative RNA-binding protein that may have a role in posttranscriptional regulation (Lee et al. 1994a; Putterill et al. 1995; Macknight et al. 1997). FHA encodes a blue-light photoreceptor, CRYPTOCHROME 2, and GI encodes a protein that is regulated by a circadian clock (Guo et al. 1998; Fowler et al. 1999; Park et al. 1999), suggesting that the two genes are involved in photoperiod perception. FT, whose biochemical function is unclear, acts downstream from CO (Kardailsky et al. 1999; Kobayashi et al. 1999).
Flowering-time genes modulate the activity of floral meristem identity genes such as LEAFY (LFY) and APETALA 1 (AP1). The induction of CO activity causes the rapid expression of LFY followed by later expression of AP1 (Simon et al. 1996). Conversely, FT and FWA function to activate AP1 in parallel with LFY or downstream from LFY transcription (Ruiz-García et al. 1997). Genetic analyses have also shown that some genes such as CO, GI, FCA, and FVE affect the transcriptional induction of LFY, whereas other genes such as FWA, FE, and FT affect the response to LFY activity (Nilsson et al. 1998). Therefore, it has been suggested that flowering-time genes regulate flowering by either activating LFY expression or modulating the response to LFY. Consistently, LFY expression is activated by the transition to flowering, and constitutive expression of LFY or AP1 causes early flowering (Mandel and Yanofsky 1995; Weigel and Nilsson 1995; Blázquez et al. 1997).
Genetic analyses of naturally-occurring variations of flowering time among Arabidopsis wild-type strains or ecotypes have identified additional loci that control flowering time. Among them, two loci, FRIGIDA (FRI) and FLOWERING LOCUS C (FLC), account for most of the difference in flowering time between early- and late-flowering ecotypes (Napp-Zinn 1985; Burn et al. 1993; Lee et al. 1993; Clarke and Dean 1994; Koornneef et al. 1994). Late-flowering ecotypes have dominant alleles of FRI and FLC that act synergistically to suppress flowering, whereas early-flowering ecotypes have a recessive allele of FRI and/or weak allele of FLC (Koornneef et al. 1994; Lee et al. 1994b; Sanda and Amasino 1995; Sheldon et al. 2000). FRI and FLC confer winter annual behavior to late-flowering ecotypes such that the effect of FRI and FLC is suppressed by vernalization. FRI- and FLC-containing lines show delayed flowering under both long and short days, indicating that these two genes act in the autonomous pathway (Lee and Amasino 1995). FLC has been recently isolated and found to encode a MADS-domain protein (Michaels and Amasino 1999; Sheldon et al. 1999). Dominant, late-flowering alleles of FRI increase FLC transcript levels whereas vernalization decreases FLC levels. In addition, LD, a gene involved in the autonomous pathway, represses FLC expression. Therefore, it has been proposed that the modulation of FLC expression by the autonomous and vernalization pathways is critical to the control of flowering (Michaels and Amasino 1999; Sheldon et al. 2000).
To further elucidate the genetic control of flowering by FRI and FLC, we have screened for FRI suppressor mutants by activation-tagging mutagenesis. We have isolated a dominant FRI suppressor in which AGAMOUS-LIKE 20 (AGL20), a gene encoding a MADS-domain protein, is overexpressed. Our studies of AGL20 expression in various flowering-time mutants show that it is regulated by all three flowering-time pathways. Therefore, we propose that the level of AGL20 activity is critical to the control of flowering time and that AGL20 integrates signals from the photoperiod, vernalization, and autonomous floral induction pathways.
Results
Isolation of an FRI suppressor by activation tagging
To isolate FRI suppressor mutants, we performed activation-tagging mutagenesis (Hayashi et al. 1992; Weigel et al. 2000) in a line in which the FRI allele of the ecotype San Feliu-2 (SF2) was introgressed into Columbia (Col) through eight backcrosses (FRI-SF2; FLC-Col, referred to as FRI FLC below; Michaels and Amasino 1999). One of the primary transformants showed a very early-flowering phenotype and was designated as fsu1-1D/+ FRI FLC (fsu1 stands for FRI suppressor 1; Fig. 1A,B). T2 populations of fsu1-1D/+ FRI FLC showed an approximately 3:1 segregation ratio for the transgene marker (57 basta-resistant:20 basta-sensitive in basta selection; χ2 = 0.039, P < 0.5), as well as for early to late flowering (56 early-flowering:24 late-flowering; χ2 = 1.6, P < 0.1). The late-flowering progeny produced more than 55 rosette leaves before bolting, which was similar to the FRI FLC parent. Early-flowering T2 plants fell into two classes. The very early class produced on average five rosettes, whereas the other class produced on average 11 rosette leaves. The ratio of the two classes was roughly 1:2 (16 very early plants:40 intermediately early plants, χ2 = 0.57, P < 0.1). In addition, in the T3 population obtained from self-pollination of T2 progeny, all of the progeny from late-flowering plants were basta-sensitive (no T-DNA) and the progeny of the intermediately early class segregated 3:1 for basta resistance (hemizygous for T-DNA). The progeny of the very early class was uniformly basta-resistant (homozygous for T-DNA). The cosegregation of the T-DNA insert with the early-flowering phenotype and the simple 1:2:1 segregation ratio strongly indicated that a single T-DNA insertion was closely linked to the locus causing early flowering in fsu1-1D FRI FLC plants.
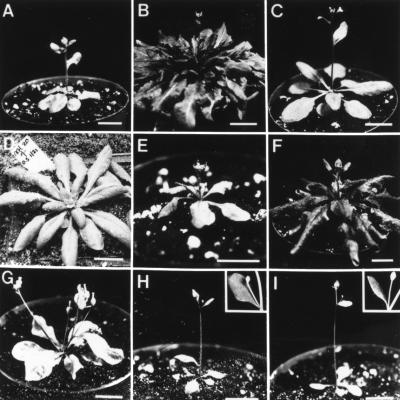
Figure 1.
Flowering phenotype of lines described in this study. (A) Homozygous fsu1-1D FRI FLC; (B) FRI FLC; (C) Columbia; (D) agl20; (E) 35S::LFY/−; (F) 35S::LFY/− FRI/−; (G) fsu1-1D/− 35S::LFY/− FRI/−; (H) fsu1-1D FRI flc-3; (I) fsu1-1D fri-Col FLC. The insets in (H) and (I) show ectopic flowers subtended by cauline leaves. All plants were grown under long days (16 h light/ 8 h dark). Bars, 1 cm.
Overexpression of AGL20 causes early flowering in FRI FLC
DNA gel blot analysis confirmed that there was only a single T-DNA insert in fsu1-1D FRI FLC mutants (data not shown). Plant DNA flanking the right border of the T-DNA insertion site was isolated by plasmid rescue (Fig. 2A)(Lee et al. 1994a). Sequence analysis of rescued plant DNA revealed that the insertion was in a part of the genome represented by bacterial artificial chromosome (BAC) clone F17K2 (GenBank accession no. AC003680). The rescued plant sequences spanned nucleotides 63,367 to 64,214 of BAC F17K2 and included sequences of the first exon of AGL20 (Fig. 2B). The four repeats of the 35S enhancer were inserted 677 base pairs upstream of the AGL20 start codon in the fsu1-1D FRI FLC mutant.
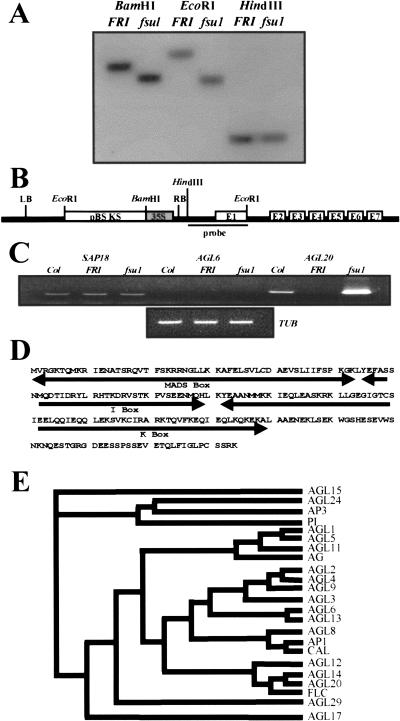
Figure 2.
Insertion of 35S enhancer in the promoter region of AGL20 causes overexpression. (A) DNA gel blot analysis showing the polymorphism between fsu1-1D FRI FLC (fsu1) and FRI FLC (FRI). (B) Diagram of genomic organization showing the insertion of 35S enhancer into the AGL20 region in fsu1-1D FRI FLC. LB and RB are left border and right border of T-DNA, respectively. A bar represents the DNA fragment obtained by plasmid rescue and used for DNA gel blot analyses. (C) RT-PCR result for SAP18, AGL6, and AGL20 in the leaves of 3-week-old Columbia (Col), FRI FLC (FRI), and fsu1-1D FRI FLC (fsu1). The β-tubulin gene (TUB) was amplified as a quantitative control. (D) The amino acid sequence of AGL20. MADS, I, and K domains are indicated. (E) Neighbor-joining phylogenetic tree for Arabidopsis MADS-domain proteins.
Reverse transcription-polymerase chain reaction (RT-PCR) of RNA isolated from the leaves of 3-week-old Columbia, FRI FLC, and the fsu1-1D FRI FLC mutant showed that AGL20 is overexpressed in fsu1-1D FRI FLC (Fig. 2C). Conversely, two putative genes near the T-DNA insertion site, AGL6 (F17K2.18) and sin3 associated polypeptide (SAP18, F17K2.17) were not overexpressed in the fsu1-1D FRI FLC mutant (Fig. 2C). This result indicates that the acceleration of flowering in fsu1-1D FRI FLC results from overexpression of AGL20. Consistent with this, when the AGL20 coding sequence under control of the CaMV 35S promoter was introduced into FRI FLC, all transgenic lines flowered earlier than FRI FLC (Table 1). Henceforth, we renamed fsu1-1D to agl20-101D.
Table 1.
Comparison of phase transitions and flowering time in the plants with different combinations of transgenics and mutations
| Genotype
|
Juvenile leavesa
|
Total rosette leaves
|
Cauline leavesb
|
n
|
|---|---|---|---|---|
| Experiment 1c
|
||||
| Columbiad | 4.8 ± 0.4 | 9.2 ± 0.4 | 2.5 ± 0.5 | 20 |
| FRI FLC | 9.3 ± 0.5 | 61.0 ± 1.4 | 12.5 ± 0.7 | 10 |
| agl20-101D FRI FLCe | 3.3 ± 0.5 | 5.2 ± 0.4 | 5.3 ± 0.9 | 25 |
| agl20-101D/− FRI FLCf | ND | 10.6 ± 1.4 | 5.4 ± 0.5 | 27 |
| 35S∷LFY/ − FRI/− FLC | ND | 16.7 ± 1.9 | 7.4 ± 1.7* | 7 |
| agl20-101D/− 35S∷LFY/ − FRI/ − FLC | ND | 7.0 ± 1.1 | 4.5 ± 1.2* | 10 |
| agl20-101D fri FLC | ND | 2.0 ± 0.4 | 3.5 ± 0.7* | 17 |
| agl20-101D FRI flc-3 | ND | 2.0 ± 0.3 | 3.3 ± 0.6* | 21 |
| 35S∷AGL20 FRI FLC S1 | ND | 14 | 6 | 1 |
| 35S∷AGL20 FRI FLC S2 | ND | 12 | 7 | 1 |
| 35S∷AGL20 FRI FLC S3 | ND | 17 | 8 | 1 |
| Experiment 2g | ||||
| Columbia, LD | ND | 11.9 ± 0.3 | 3.3 ± 0.2 | 30 |
| agl20, LD | ND | 26.2 ± 1.0 | 5.0 ± 0.6 | 5 |
| Columbia, SD | ND | 30.0 ± 1.0 | 9.0 ± 1.0 | 30 |
| agl20, SD | ND | 68.8 ± 4.9 | 15.2 ± 0.8 | 5 |
Number of juvenile leaves determined by identifying the last leaf that had trichomes only on the adaxial side. (ND) not determined.
The cauline leaves produced in plants marked with an asterisk subtend ectopic flowers instead of secondary shoots.
In experiment 1, plants were grown under long days (16 h light/8 h dark).
The genotype of Columbia is fri FLC.
Homozygous agl20-101D FRI FLC.
Heterozygous agl20-101D/+ FRI FLC.
In experiment 2, plants were grown under long days (LD) and short days (SD; 10 h light/14 h dark cycle).
AGL20 consists of seven exons and six introns (Fig. 2B). It encodes a typical MADS-domain protein that contains MADS, I (intervening), K, and C-terminal domains (Fig. 2D). Phylogenetic analysis using the M-I-K region showed that AGL20 is most similar to the MADS-domain genes AGL14 and FLC in Arabidopsis (Fig. 2E). The amino acid sequence identity in the M-I-K region is 65.4% with AGL14 and 43.6% with FLC, whereas overall amino acid sequence identity is 55.9% with AGL14 and 37.7% with FLC.
The normal role of AGL20 in the regulation of flowering was confirmed with a T-DNA insertional mutant, which flowered late in both long and short days (Fig. 1D). The agl20 mutants flowered later under short days than under long days, which indicates that the agl20 mutant is responsive to photoperiod (Table 1).
agl20-101D FRI FLC responds to photoperiod and vernalization
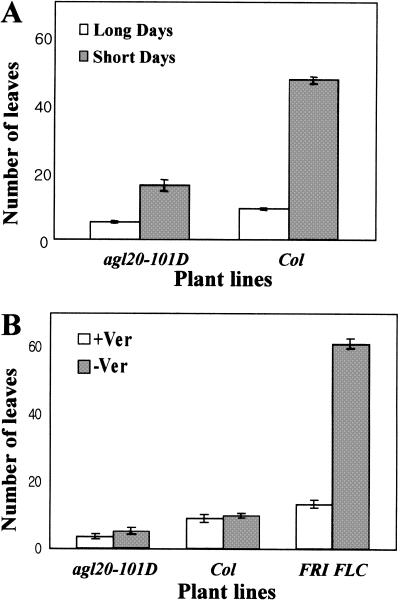
The effect of photoperiod and vernalization on the flowering time of agl20-101D FRI FLC is shown in Figure 3. Similar to Columbia, agl20-101D FRI flowered more rapidly under long days than under short days. Vernalization reduced flowering time in agl20-101D FRI FLC, but the reduction in agl20-101D FRI FLC was higher than in Columbia. Therefore, agl20-101D FRI FLC responds to both photoperiod and vernalization, indicating that agl20-101D does not abolish the sensitivity to either photoperiod or vernalization in the FRI FLC background.
Figure 3.
Effect of photoperiod and vernalization on the flowering time of agl20-101D FRI FLC. (A) The effect of photoperiod; (B) the effect of vernalization. agl20-101D FRI FLC is designated as agl20-101D. Flowering time was measured as the number of rosette leaves. agl20-101D was originally designated fsu1-1D.
agl20-101D accelerates phase transition in FRI FLC
In Arabidopsis, a marker of phase change is the distribution of trichomes on the leaf surface (Telfer et al. 1997). The leaves produced during the juvenile phase have trichomes only on their adaxial side, whereas the leaves produced during adult phase possess trichomes on both adaxial and abaxial sides. The cauline leaves produced during the reproductive phase lack trichomes on their adaxial surface. To test whether agl20-101D also affected the transition from the juvenile to adult phase, we compared the trichome distribution in different lines (Table 1). FRI FLC produced approximately twice as many juvenile leaves as Columbia, indicating that FRI FLC delays the transition from juvenile to adult phase as well as flowering. FRI FLC also produced more cauline leaves with associated secondary shoots than Columbia. The juvenile phase was found to be dramatically shortened in agl20-101D FRI FLC. However, agl20-101D FRI FLC produced significantly more cauline leaves than Columbia, although homozygous agl20-101D FRI FLC produced fewer rosette leaves than Columbia. This result suggests that AGL20 overexpression can partially rescue the delay in the transition from secondary shoots to flowers in FRI FLC.
Interestingly, the flowering phenotype of agl20-101D FRI FLC is semidominant, if flowering time is measured by rosette leaf number (Table 1). That is, homozygous agl20-101D FRI FLC plants produce fewer rosette leaves than hemizygotes, indicating that the promotion of flowering by agl20-101D is dosage-dependent and that the effects of AGL20 overexpression are not saturated in agl20-101D FRI FLC. However, the number of cauline leaves with associated secondary shoots is not distinguishable between hemizygous and homozygous agl20-101D FRI FLC (Table 1). This result indicates differential effects of agl20-101D on bolting and flowering.
FRI FLC represses LFY expression
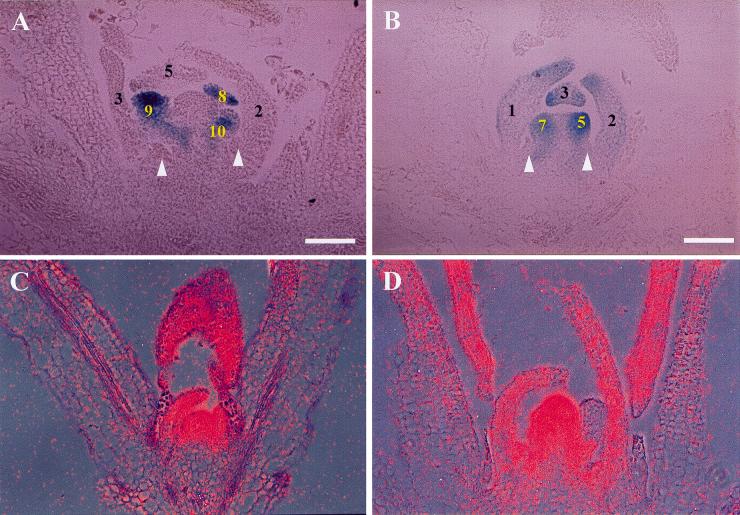
An important regulator of the transition from secondary shoots to flowers is the LFY transcription factor (Weigel and Nilsson 1995). Therefore, we monitored LFY expression levels in agl20-101D FRI FLC using a LFY::GUS fusion gene (Blázquez et al. 1997). We compared LFY promoter activity in agl20-101D/+; FRI/fri; FLC/FLC, which flowered at the same time as agl20-101D/+ FRI FLC, with fri/fri; FLC/FLC (Columbia wild type). Hemizygous agl20-101D/+ FRI FLC produced five to six nodes of cauline leaves with secondary shoots above rosette leaves, but none of these showed GUS staining (Fig. 4A). Conversely, Columbia produced two to three cauline leaves with secondary shoots above rosette leaves, followed by flowers. In the Columbia background, GUS staining was detected in the cauline leaf primordia as well as floral meristems that are produced at the same nodes where secondary shoots are produced in hemizygous agl20-101D FRI FLC plants (Fig. 4B). This result indicates that LFY expression is repressed by FRI FLC and that AGL20 overexpression cannot overcome such repression at least until after five to six secondary shoots are formed.
Figure 4.
Analysis of LFY promoter activity in agl20-101D FRI FLC and in situ localization of AGL20 RNA in Ler. Expression of a LFY::GUS transgene introduced into agl20-101D FRI FLC (A) and Columbia (B). The numbers on leaf primordia and floral meristems indicate node numbers above rosette leaves. Arrowheads indicate the position of secondary shoot primordia. In situ localization of AGL20 in the shoot apices of 8-day-old (C) and 12-day-old (D) Ler plants. agl20-101D was originally designated fsu1-1D. Bars, 50 μm.
It has been reported that 35S::LFY can partially rescue the late-flowering phenotype of FRI FLC (Nilsson et al. 1998). Reexamination of the effect of 35S::LFY on FRI FLC confirmed the previous report such that the number of rosette leaves was reduced dramatically by 35S::LFY. But the number of cauline leaves in FRI FLC was not correspondingly reduced by 35S::LFY (Fig. 1F; Table 1). However, all of the secondary shoots in the axils of cauline leaves were converted to solitary flowers by 35S::LFY. This result is in contrast to the effect of agl20-101D on FRI FLC because secondary shoot-to-flower conversions were not observed in agl20-101D FRI, even though agl20-101D FRI FLC bolted earlier than 35S::LFY in the FRI FLC background (Table 1). This result may suggest that AGL20 and LFY affect flowering through at least partially independent pathways. Consistent with this hypothesis, the effect of agl20-101D and 35S::LFY on flowering was additive such that both rosette leaf and cauline leaf numbers were reduced in agl20-101D/− 35S::LFY/− plants when compared with agl20-101D/− or 35S::LFY/− in the FRI FLC background (Fig. 1G; Table 1).
Because FRI FLC delays both flowering time and the transition from cauline leaves with secondary shoots to flowers, we investigated the phenotype of agl20-101D in the absence of FRI or FLC. We combined the fri-Col allele, which is nonfunctional, with agl20-101D as well as the flc-3 null mutant allele (Levy and Dean 1998; Michaels and Amasino 1999). Flowering time was further accelerated by the addition of fri-Col or flc-3 mutations (Fig. 1; Table 1). The rosette leaf number was reduced to two in both agl20-101D FRI flc-3 and agl20-101D fri-Col FLC lines. Interestingly, both lines showed solitary flowers in the axils of cauline leaves (Fig. 1H,I). Such a conversion of secondary shoots to flowers is also observed in 35S::LFY or 35S::AP1 plants (Mandel and Yanofsky 1995; Weigel and Nilsson 1995). This result may indicate that AGL20 overexpression can activate flower meristem identity genes such as LFY or AP1 in shoot meristems, once FRI FLC is removed.
Expression of AGL20
The expression of AGL20 RNA was determined by in situ hybridization in Landsberg erecta (Ler) wild type (Fig. 4C,D). The strong expression of AGL20 in the shoot apical meristem and slightly weaker expression in the leaf primordia were detected during vegetative growth (8 days after germination). Four days later when shoot apical meristem produced flower meristems, the expression domain of AGL20 was expanded in the shoot apical meristem, and AGL20 transcript was also detected in developing leaves. To gain further insight into the role of AGL20 in regulating phase transition and flowering, we determined AGL20 expression levels in various tissues by RT-PCR (Fig. 5). In Columbia wild type, AGL20 was expressed most strongly in leaves, but the transcript was also detected in vegetative apices, inflorescence, stems of flowering plants, and roots (Fig. 5A). Temporal changes in AGL20 expression were also determined in whole plants. In our long-day conditions, flowers in Columbia wild type initiate around 9 days after germination, as determined by first detection of AP1 expression (Fig. 5B). AGL20 expression was increased significantly around 9 days after germination (Fig. 5B).
Figure 5.
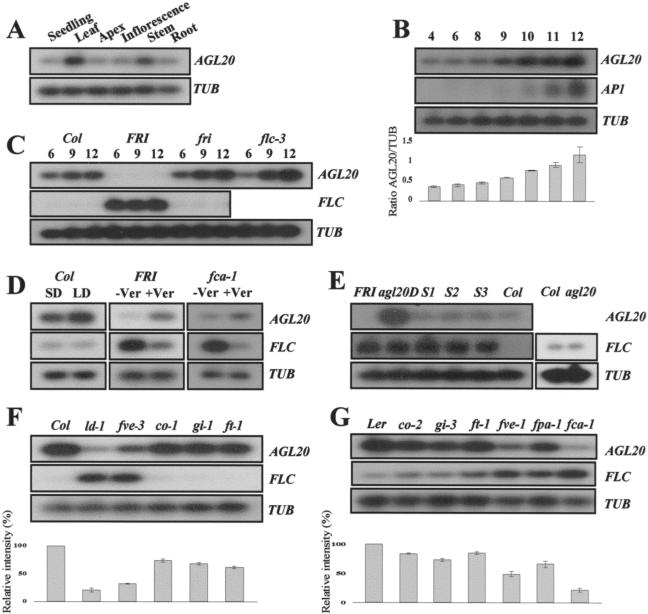
Analysis of AGL20 expression by RT-PCR. (A) Expression of AGL20 in various organs. (B) A time course of expression of AGL20, FLC, and AP1. Lane numbers indicate days after germination. (C) Comparison of expression levels of AGL20 and FLC in Columbia (Col), FRI FLC (FRI), fri (FN235) FLC (fri), and FRI flc-3 (flc-3). (D) Effect of photoperiod and vernalization on the expression of AGL20 and FLC. To detect FLC expression in Columbia grown under short days and long days, the filter was overexposed. (E) Expression of AGL20 and FLC in agl20-101D FRI FLC (agl20D), 35S::AGL20 FRI FLC (S1, S2, S3), and agl20 knockout mutant. For agl20, the filter was overexposed to detect FLC. (F) Expression of AGL20 in late-flowering mutants in Columbia background. (G) Expression of AGL20 in late-flowering mutants in Ler background. The bar graphs below panels B, F, and G represent the relative expression level of AGL20. Standard deviation obtained from three independent experiments is indicated. The β-tubulin gene (TUB) was amplified as a quantitative control.
To investigate how AGL20 expression is regulated by FRI and FLC, RT-PCR was performed using RNA extracted from whole plants of FRI FLC, FRI flc-3, and fri (FN235) FLC mutants at 6, 9, and 12 days after germination (Fig. 5C). AGL20 expression was very weak in FRI FLC, but an increased level of AGL20 expression was detected in fri (FN235) FLC or FRI flc-3 mutants. The expression level in fri (FN235) FLC or FRI flc-3 mutants was similar to that in Columbia. This result suggests that AGL20 expression is negatively regulated by FRI and FLC. Conversely, FLC expression level was increased in FRI FLC but decreased in the fri (FN235) FLC mutant, confirming that FRI positively regulates FLC, as previously reported (Fig. 5C)(Michaels and Amasino 1999).
The effect of photoperiod on AGL20 expression was also determined. For this experiment, Columbia was grown under long and short days, and plants at a similar developmental stage (when the plants showed four rosette leaves; 9 days old under long days and 14 days old under short days) were compared for AGL20 expression level. Plants grown under short days showed approximately 40% less AGL20 expression than plants grown under long days, in contrast to FLC expression, which showed no difference between long days and short days (Fig. 5D).
To determine the effect of vernalization on AGL20 expression, the FRI FLC line was used, because FRI FLC shows strong acceleration of flowering by vernalization. The FRI FLC line was subjected to 4 weeks of cold treatment and was further grown under long days until it produced four rosette leaves. As a control, the FRI FLC line was grown under long days for 9 days, at which time it produced four rosette leaves, without vernalization. AGL20 was barely detectable by RT-PCR in the FRI FLC line without vernalization. However, 4 weeks of vernalization dramatically increased the AGL20 expression level (Fig. 5D). Conversely, vernalization reduced the expression level of FLC, consistent with previous reports (Fig. 5D)(Michaels and Amasino 1999). Thus, vernalization of the FRI FLC line causes acceleration of flowering and a concomitant decrease of FLC and increase of AGL20 expression. We also determined the AGL20 expression level in vernalized fca-1, which is in the Ler background. Although Ler has only a weakly active FLC allele, flowering of fca-1 is strongly accelerated by vernalization. When vernalized, AGL20 expression increased and FLC expression decreased in fca-1 (Fig. 5D). This suggests that vernalization, which represses FLC expression, activates AGL20. In conclusion, we found that AGL20 expression levels are regulated by both photoperiod and vernalization.
AGL20 expression is regulated by other flowering-time genes
We next investigated whether genes in the autonomous pathway regulate AGL20 expression. AGL20 expression was strongly reduced in the fve-3 mutant (70% reduction compared with Columbia wild type), whereas FLC was increased (Fig. 5F). Reduced AGL20 expression and increased FLC expression were also observed in the ld-1 mutant (Fig. 5F). A simple model to account for this and the results presented above is that FLC is an upstream negative regulator of AGL20, and that genes such as LD or FVE activate AGL20 expression through repression of FLC. Alternatively, AGL20 and FLC may repress each other's expression, and the increased FLC expression in ld-1 and fve-3 leads to the repression of AGL20 expression. To distinguish the two possibilities, we compared FLC expression level in agl20-101D FRI FLC, 35S::AGL20 FRI FLC, and FRI FLC (Fig. 5E). FLC expression levels in all of the lines were very similar, although higher expression of AGL20 was detected in agl20-101D and 35S::AGL20 in FRI FLC background. In addition, FLC expression levels in agl20 loss-of-function mutants was similar to that of Columbia (Fig. 5E). These results show that AGL20 does not repress FLC expression and support the idea that FLC is an upstream negative regulator of AGL20.
We also compared how other late-flowering mutants affect the expression levels of AGL20 and FLC (Fig. 5F,G). In the Columbia background, which has a dominant FLC allele, mutants of the photoperiod pathway, such as co-1, gi-1, and ft-1 (introgressed into Columbia), showed approximately 30%–40% reduction of AGL20 expression, but FLC expression was similar to Columbia wild type (Fig. 5F). In summary, the results show that AGL20 expression is positively regulated by flowering-time genes, although it is more strongly dependent on genes in the autonomous pathway.
Similar results were obtained from late-flowering mutants in Ler, which has a weak FLC allele (Fig. 5G). All of the late-flowering mutants in Ler showed decreased levels of AGL20 expression. However, the photoperiod pathway mutants, co-2, gi-3, and ft-1, showed less reduction in AGL20 expression than the autonomous pathway mutants, fca-1, fve-1, and fpa-1, confirming that AGL20 expression is more strongly dependent on the autonomous pathway than the photoperiod pathway also in the Ler background (Fig. 5G). Interestingly, autonomous pathway mutant fpa-1 consistently showed higher expression of AGL20 than fca-1 and fve-1. It may indicate that FPA acts more or less differently with the other genes involved in the autonomous pathway. In conclusion, AGL20 expression is regulated by three floral inductive pathways, including the photoperiod, vernalization, and autonomous pathways.
Discussion
AGL20 is a floral activator
Winter-annual Arabidopsis strains such as Stockholm and SF2 flower very late in the absence of vernalization treatment. Previous genetic analyses showed that such lateness of flowering is mainly caused by the synergistic interaction of two genes, FRI and FLC (Napp-Zinn 1985; Burn et al. 1993; Lee et al. 1993; Clarke and Dean 1994; Koornneef et al. 1994; Lee et al. 1994b). To define the regulatory mechanism of FRI and FLC, a screen for downstream factor(s) repressed by FRI and FLC was pursued by the random overexpression strategy of activation tagging mutagenesis. The rationale is that if the late-flowering phenotype of the FRI FLC line is suppressed by overexpression of a certain gene, that gene may be a downstream target gene of FRI FLC repression. One of the suppressor mutants, agl20-101D FRI FLC, shows overexpression of a MADS-domain gene, AGL20. The results presented in this study show that AGL20 acts as a floral activator and is repressed by the interaction of FRI and FLC. A positive role for AGL20 is confirmed by agl20 loss-of-function mutants, which flower late.
The expression level of AGL20 correlates very well with flowering time. In the FRI FLC lines, which flower very late, AGL20 expression is very weak. The homozygous agl20-101D FRI FLC mutant overexpresses AGL20 in the FRI FLC background and flowers even earlier than Columbia. Consistently, 35S::AGL20 transformants in the FRI FLC background show accelerated flowering with increased level of AGL20 expression (Fig. 5E). Finally, fri (FN235) FLC or FRI flc-3 strains, whose flowering times are similar to that of Columbia, show similar levels of AGL20 expression as Columbia. Such a correlation between AGL20 expression level and flowering time may explain the responsiveness of agl20-101D FRI FLC to both photoperiod and vernalization. The activation of endogenous AGL20 by long days and vernalization may have an additive effect on flowering of agl20-101D FRI FLC.
AGL20 is very similar to the SaMADS A from Sinapsis alba (95.3% amino acid sequence identity; Menzel et al. 1996). SaMADS A is expressed most highly at the shoot apical meristem of plants after they have been induced to flower. Based on the expression pattern, it has been suggested that SaMADS A has an important function during the transition to flowering. Our genetic analysis of AGL20 in Arabidopsis has shown that AGL20 is indeed an important regulator of flowering. In addition, we have shown that AGL20 is expressed in vegetative tissues, consistent with a more general role of AGL20 in regulating phase transitions. AGL20 expression is gradually increased during vegetative growth. Such a gradual increase before flower initiation has also been observed in LFY and FT, two regulators of flowering (Blázquez et al. 1997; Kardailsky et al. 1999; Kobayashi et al. 1999).
AGL20 is a member of the MADS-domain family, a large family of transcription factors. Many MADS-domain proteins are involved in flower development. For example, AP1 and CAULIFLOWER function as floral meristem identity genes, and APETALA3 and PISTILLATA are involved in floral organ development (Riechmann and Meyerowitz 1997). However, AGL15 plays a role during embryogenesis and AGL12, AGL14, and AGL17 are expressed only in root tissues, suggesting that MADS-domain family members play diverse roles in plant development (Heck et al. 1995; Rounsley et al. 1995). Interestingly, the floral repressor FLC is also a member of MADS-domain family and shows the second highest amino acid sequence identity with AGL20 among Arabidopsis MADS-domain proteins.
AGL20 activates phase transition
The interaction of FRI and FLC causes not only a delay of flowering but also a delay of phase transition during all stages of plant development. Such an overall delay in plant development is observed in most late-flowering mutants (Martínez-Zapater et al. 1995; Telfer et al. 1997). agl20-101D accelerates both the transition from the juvenile to adult phase and that from vegetative to reproductive phase in the FRI FLC background. A weaker acceleration is also found in the transition from cauline leaves with secondary shoots to flowers. The different acceleration of phase transitions may result from a dual role of FRI and FLC, which are likely to regulate flowering not only through AGL20, but also through meristem identity genes such as LFY. We propose that the interaction of FRI and FLC not only inhibits expression of AGL20, but also has an independent effect on LFY, as suggested by our analysis of LFY promoter activity in agl20-101D FRI FLC plants. LFY is a key regulator of flower formation, but not of vegetative phase change (Weigel and Nilsson 1995). Thus, the repression of LFY by FRI FLC may weaken the acceleration of the transition from cauline leaves with secondary shoots to flowers in agl20-101D FRI FLC. Consistent with this hypothesis, when 35S::LFY is introduced into FRI FLC, all of the secondary shoots are converted to flowers (Nilsson et al. 1998). Although not apparent in the FRI FLC background, agl20-101Dseems to activate LFY expression once FRI FLC repression is removed. In the agl20-101D fri FLC or agl20-101D FRI flc lines, agl20-101D causes the replacement of secondary shoots by ectopic flowers as is observed in 35S::LFY. The phenotype of agl20-101D FRI FLC also supports the idea that Arabidopsis has a distinct inflorescence phase I, during which cauline leaves with associated secondary shoots are produced (Ratcliffe et al. 1998, 1999). Although homozygous agl20-101D FRI FLC lines make the transition to flowering more quickly than Columbia, these lines produce a higher number of cauline leaves than Columbia.
Floral induction pathways merge to activate AGL20
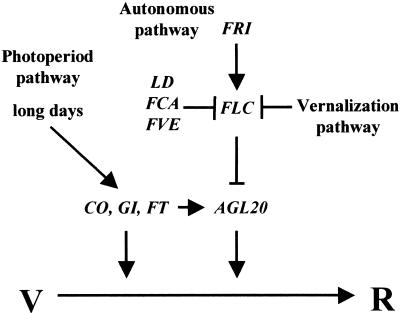
In addition to FRI and FLC, we have identified several other regulators of AGL20 expression, and a model of how three floral inductive pathways are integrated by AGL20 is presented in Figure 6. In this model, AGL20 plays a pivotal role in the control of flowering time, with the three floral inductive pathways merging to activate AGL20. Activation of AGL20 by the autonomous pathway genes, LD, FCA, and FVE, is likely mediated by repression of FLC, as is activation of AGL20 upon vernalization.
Figure 6.
Model for the integrative role of AGL20 and the interaction of flowering pathways. The horizontal line represents the vegetative (V) to reproductive (R) transition. Arrows indicate promotion, and T-bars indicate repression. In the autonomous pathway, FRI activates FLC, and FLC represses AGL20. AGL20 acts as a floral activator. Other autonomous pathway genes, such as LD, FCA, and FVE, promote flowering by activating AGL20 through the repression of FLC. Vernalization also promotes flowering by activating AGL20 expression through the repression of FLC. Photoperiod pathway genes, CO and GI, promote flowering by activating AGL20 but act also through other factor(s).
In addition to the autonomous and the vernalization pathways, the photoperiodic pathway activates AGL20. However, the reduction of AGL20 expression in co, gi, and ft mutants is relatively modest, even though at least co and gi mutants flower much later in long days than does agl20. These observations indicate that AGL20 is not the only factor downstream from the photoperiodic pathway. We have recently found that the photoperiod pathway and the autonomous pathway interact through AGL20 activity. agl20-101D can partly suppress the late-flowering phenotype of the co mutant (H. Lee and I. Lee, unpubl.), consistent with a role of AGL20 downstream from CO. Conversely, 35S::CO can compensate for the delay in flowering in fca mutants (Piñeiro and Coupland 1998). Thus, there must be some cross-talk between the autonomous and photoperiod pathway, and we propose that AGL20 is a component of this cross-talk.
A question remains which other genes integrate flowering signals. Two other candidates for integration of floral inductive signals are FT and LFY. Similar to AGL20, FT and LFY act partially downstream from CO, a gene that promotes flowering in response to long days (Simon et al. 1996; Kardailsky et al. 1999; Kobayashi et al. 1999). In addition, the expression of FT and LFY is regulated by FCA, which is involved in the autonomous pathway (Nilsson et al. 1998; J.H. Ahn and D. Weigel, pers. comm.). The analysis of agl20-101D mutants suggests that LFY acts at least in part downstream from AGL20. On the other hand, our data suggest that FT affects AGL20 expression, indicating that there is substantial cross-regulation among the three genes.
In addition to the photoperiod, vernalization, and autonomous pathways, it has been suggested that a gibberellin pathway promotes flowering (Levy and Dean 1998). A gibberellin biosynthetic mutant, ga1, does not flower under short days, and exogenous treatments of gibberellin accelerate flowering of many late-flowering mutants of both photoperiod and autonomous pathways (Wilson et al. 1992; Chandler and Dean 1994). The effect of gibberellin on AGL20 expression may lead to further understanding how the pathways controlling flowering in Arabidopsis are integrated.
Materials and methods
Plant materials and growth conditions
Plants (Arabidopsis thaliana) were grown in long days (16 h light/8 h dark) or short days (8 h light/16 h dark), under cool white fluorescent lights (100 μmole/m2/sec), at 23 ± 2°C, 60 ± 10% relative humidity. For vernalization studies, seeds were imbibed on 0.8% phytoagar containing half strength of Murashige-Skoog (MS) medium (GIBCO BRL) and incubated for 4 weeks at 4°C under short days. FRI FLC line is a Columbia near-isogenic line with FRI allele from SF2 obtained by eight backcrosses into Columbia, which was described previously (Michaels and Amasino 1999). The mutants, fri (FN235) FLC and FRI flc-3, are the lines obtained by fast neutron mutagenesis of FRI FLC. The late-flowering mutants, co-1, gi-1, fve-3, ld-1, and agl20, are in the Columbia background, but co-2, gi-3, ft-1, fd-1, fha-1, fe-1, fwa-2, fca-1, fve-1, and fpa-1 are in the Ler background. ft-1 introgressed into Columbia was provided by D. Weigel (Salk Institute).
Screening of activation-tagging mutants
FRI FLC line was transformed with pSKI015 (Weigel et al. 2000) by vacuum infiltration (Bechtold et al. 1993). For screening of activation-tagging mutants, T0 seeds were sown on soil under long days and treated with 0.1% basta (AgrEvo, USA) after 7 d. For basta segregation analysis, seeds were germinated on half-strength MS media with 0.007% basta.
Construction of 35S::AGL20
To generate a 35S::AGL20 construct, an AGL20 cDNA was amplified by RT-PCR using total RNA extracted from Columbia leaf tissues. BamH1 sites were introduced before the ATG start codon and after the TGA stop codon in the cDNA. The primers used in the PCR were 5′-CCC GGA TCC ATG GTG AGG GGC AAA ACT CAG-3′ and 5′-CCC GGA TCC TCA CTT TCT TGA AGA ACA AGG-3′. The RT-PCR product was subcloned into the pGEM-Teasy vector (Promega) to yield pHR2. The sequence of AGL20 cDNA in pHR2 was confirmed using Sequenase version 2.0 kit (U.S. Biochemical Corp.). The 645-bp insert of pHR2 was digested with BamH1 and introduced into the BamH1 site of pCGN18 vector (Jack et al. 1994). Constructs were introduced into the FRI FLC line by vacuum infiltration.
Phylogenetic analysis
Nucleotide and predicted amino acid sequences of MADS-domain proteins in Arabidopsis were obtained from GenBank. Distance matrices were calculated using the DNADIST program of PHYLIP version 3.5 (Department of Genetics, University of Washington, Seattle, WA), and the numbers of nucleotide substitutions were estimated using the two-parameter method of Kimura (1980). Distance trees were constructed using the neighbor-joining method (Saitou and Nei 1987) and implemented using the NEIGHBOR program in PHYLIP.
RT-PCR
Total RNA was extracted as described previously (Puissant and Houdebine 1990). One μg of total RNA from each tissue was reverse-transcribed with oligo-dT12-18 (GIBCO BRL) in a 20 μL reaction mixture using the Reverse Transcription System (Promega). After heat inactivation of the reaction mixture, PCR was performed using 1 μL of the first-stranded cDNA sample with 25 pmole of the primers in a 50 μL reaction. PCR conditions were as follows: 94°C (3 min); 25 cycles of 94°C (30 sec), 55°C (1 min), 72°C (1 min), and 72°C (10 min). PCR products were electrophoresed on an agarose gel, blotted onto a NYTRAN-PLUS membrane (Schleicher & Schuell), and hybridized with the appropriate probes. RT-PCR was repeated at least three times for the samples harvested separately. The primers used for RT-PCR were as follows: For β-tubulin (TUB2), two primers, 5′-CTC AAG AGG TTC TCA GCA GTA-3′ and 5′-TCA CCT TCT TCA TCC GCA GTT-3′ were used. For AGL20, two primers, 5′-CCC CAT ATG GTG AGG GGC AAA ACT C-3′ and 5′-CCC GGA TCC TCA CTT TCT TGA AGA ACA AGG-3′ were used. For FLC, two primers, 5′-CCC CAT ATG GGA AGA AAA AAA CTA G-3′ and 5′-CCC GGA TCC CTA ATT AAG TAG TGG GAG-3′ were used. For AP1, two primers, 5′-GCA CCT GAG TCC GAC GTC-3′ and 5′-GCG GCG AAG CAG CCA AGG-3′ were used.
GUS staining
For histochemical analysis of GUS, X-gluc staining and tissue fixation were performed as described by Blázquez et al. (1997). Eight-μm sections were prepared and mounted and, after removal of paraplast with xylenes, photographed under bright field on an Olympus BX50 microscope. LFY::GUS used in this study was strain DW150-209 (Blázquez et al. 1997).
Genotyping of FRI
To identify fsu1-1D with the fri-Col allele, genomic DNA was amplified by PCR using two primers, 5′-CAA CGA CCA AAC ACA ACG AC-3′ and 5′-CGC GAG ACT GAA CCT CAC GG-3′ and digested with Rsa1. The FRI-SF2 allele yielded four fragments (100, 200, 300, and 350 bp), and the 300 bp fragment was replaced by a 330 bp fragment in the fri-Col allele.
Acknowledgments
We thank S. Michaels and R. Amasino for providing fri (FN235) FLC, FRI flc-3, and fve-3 seeds and the information for primers for FRI genotyping. We also thank D. Weigel for providing the pSKI015 vector and ft-1 in Columbia seeds and G. Ditta and M. Yanofsky for agl20 mutant seeds and in situ hybridization data for AGL20. Special thanks go to R. Amasino and D. Weigel for their encouragement of this study and critical reading of the manuscript. This study was supported by the Academic Research Fund (GE 1998-019-D00134) of the Korea Research Foundation. H. Lee, E. Park, and E. Cho are supported by Brain Korea 21 program.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL ilhalee@plaza.snu.ac.kr; FAX 822-872-1993.
Article and publication are at www.genesdev.org/cgi/doi/10.1101/gad.813600.
References
- Bechtold N, Ellis J, Pelletier G. In planta Agrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C R Acad Sci. 1993;316:1194–1199. [Google Scholar]
- Blázquez M, Soowal L, Lee I, Weigel D. LEAFY expression and flower initiation in Arabidopsis. Development. 1997;124:3835–3844. doi: 10.1242/dev.124.19.3835. [DOI] [PubMed] [Google Scholar]
- Blázquez MA, Green R, Nilsson O, Sussman MR, Weigel D. Gibberellins promote flowering of Arabidopsis by activating the LEAFY promoter. Plant Cell. 1998;10:791–800. doi: 10.1105/tpc.10.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn JB, Smyth DR, Peacock WJ, Dennis ES. Genes conferring late flowering in Arabidopsis thaliana. Genetica. 1993;90:147–155. [Google Scholar]
- Chandler J, Dean C. Factors influencing the vernalization response and flowering time of late flowering mutants of Arabidopsis thaliana (L.) Heynh. J Exp Bot. 1994;45:1279–1288. [Google Scholar]
- Clarke JH, Dean C. Mapping FRI, a locus controlling flowering time and vernalization response in Arabidopsis thaliana. Mol Gen Genet. 1994;242:81–89. doi: 10.1007/BF00277351. [DOI] [PubMed] [Google Scholar]
- Fowler S, Lee K, Onouchi H, Samach A, Richardson K, Morris B, Coupland G, Putterill J. GIGANTEA: A circadian clock-controlled gene that regulates photoperiodic flowering in Arabidopsis and encodes a protein with several possible membrane-spanning domains. EMBO J. 1999;18:4679–4688. doi: 10.1093/emboj/18.17.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Yang H, Mockler TC, Lin C. Regulation of flowering time by Arabidopsis photoreceptors. Science. 1998;279:1360–1363. doi: 10.1126/science.279.5355.1360. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Czaja I, Lubenow H, Schell J, Walden R. Activation of a plant gene by T-DNA tagging auxin-independent growth in vitro. Science. 1992;258:1350–1352. doi: 10.1126/science.1455228. [DOI] [PubMed] [Google Scholar]
- Heck GR, Perry SE, Nichols KW, Fernandez DE. AGL15, a MADS domain protein expressed in developing embryos. Plant Cell. 1995;7:1271–1282. doi: 10.1105/tpc.7.8.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack T, Fox GL, Meyerowitz EM. Arabidopsis homeotic gene APETALA3 ectopic expression: Transcriptional and posttranscriptional regulation determine organ identity. Cell. 1994;76:703–716. doi: 10.1016/0092-8674(94)90509-6. [DOI] [PubMed] [Google Scholar]
- Kardailsky I, Shukla VK, Ahn JH, Dagenais N, Christensen SK, Nguyen JT, Chory J, Harrison MJ, Weigel D. Activation tagging of the floral inducer FT. Science. 1999;286:1962–1965. doi: 10.1126/science.286.5446.1962. [DOI] [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T. A pair of related genes with antagonistic roles in mediating flowering signals. Science. 1999;286:1960–1962. doi: 10.1126/science.286.5446.1960. [DOI] [PubMed] [Google Scholar]
- Koornneef M, Blankestijn-de Vries H, Hanhart C, Soppe W, Peeters T. The phenotype of some late-flowering mutants is enhanced by a locus on chromosome 5 that is not effective in the Landsberg erecta phenotype. Plant J. 1994;6:911–919. [Google Scholar]
- Koornneef M, Alonso-Blanco C, Peeters AJ, Soppe W. Genetic control of flowering time in Arabidopsis. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:345–370. doi: 10.1146/annurev.arplant.49.1.345. [DOI] [PubMed] [Google Scholar]
- Lee I, Amasino RM. Effect of vernalization, photoperiod, and light quality on the flowering phenotype of Arabidopsis plants containing the FRIGIDA gene. Plant Physiol. 1995;108:157–162. doi: 10.1104/pp.108.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Bleecker A, Amasino RM. Analysis of naturally occurring late flowering in Arabidopsis thaliana. Mol Gen Genet. 1993;237:171–176. doi: 10.1007/BF00282798. [DOI] [PubMed] [Google Scholar]
- Lee I, Aukerman MJ, Gore SL, Lohman KN, Michaels SD, Weaver LM, John MC, Feldmann KA, Amasino RM. Isolation of LUMINIDEPENDENS: A gene involved in the control of flowering time in Arabidopsis. Plant Cell. 1994a;6:75–83. doi: 10.1105/tpc.6.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Michaels SD, Masshardt AS, Amasino RM. The late-flowering phenotype of FRIGIDA and mutations in LUMINIDEPENDENS is suppressed in the Landsberg erecta strain of Arabidopsis. Plant J. 1994b;6:903–909. [Google Scholar]
- Levy YY, Dean C. The transition to flowering. Plant Cell. 1998;10:1973–1989. doi: 10.1105/tpc.10.12.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macknight R, Bancroft I, Lister C, Page T, Love K, Schmidt R, Westphal L, Murphy G, Sherson S, Cobbett C, et al. FCA, a gene controlling flowering time in Arabidopsis, encodes a protein containing RNA-binding domains. Cell. 1997;89:737–745. doi: 10.1016/s0092-8674(00)80256-1. [DOI] [PubMed] [Google Scholar]
- Mandel MA, Yanofsky MF. A gene triggering flower development in Arabidopsis. Nature. 1995;377:522–524. doi: 10.1038/377522a0. [DOI] [PubMed] [Google Scholar]
- Martínez-Zapater JM, Jarillo JA, Cruz-Alvarez M, Roldán M, Salinas J. Arabidopsis late-flowering fve mutants are affected in both vegetative and reproductive development. Plant J. 1995;7:543–551. [Google Scholar]
- Menzel G, Apel K, Melzer S. Identification of two MADS box genes that are expressed in the apical meristem of the long-day plant Sinapis alba in transition to flowering. Plant J. 1996;9:399–408. doi: 10.1046/j.1365-313x.1996.09030399.x. [DOI] [PubMed] [Google Scholar]
- Michaels SD, Amasino RM. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell. 1999;11:949–956. doi: 10.1105/tpc.11.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napp-Zinn K. Arabidopsis thaliana. In: Halevy HA, editor. CRC Handbook of Flowering. Boca Raton, FL: CRC Press; 1985. pp. 492–503. [Google Scholar]
- Nilsson O, Lee I, Blázquez MA, Weigel D. Flowering-time genes modulate the response to LEAFY activity. Genetics. 1998;150:403–410. doi: 10.1093/genetics/150.1.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DH, Somers DE, Kim YS, Choy YH, Lim HK, Soh MS, Kim HJ, Kay SA, Nam HG. Control of circadian rhythms and photoperiodic flowering by the Arabidopsis GIGANTEA gene. Science. 1999;285:1579–1582. doi: 10.1126/science.285.5433.1579. [DOI] [PubMed] [Google Scholar]
- Poethig RS. Phase changes and the regulation of shoot morphogenesis in plants. Science. 1990;250:923–930. doi: 10.1126/science.250.4983.923. [DOI] [PubMed] [Google Scholar]
- Piñeiro M, Coupland G. The control of flowering times and floral identity in Arabidopsis. Plant Physiol. 1998;117:1–8. doi: 10.1104/pp.117.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puissant C, Houdebine LM. An improvement of the single-step method of RNA isolation by acid guanidinium thiocyanate phenol-chloroform extraction. Biotechniques. 1990;8:148–149. [PubMed] [Google Scholar]
- Putterill J, Robson F, Lee K, Simon R, Coupland G. The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell. 1995;80:847–857. doi: 10.1016/0092-8674(95)90288-0. [DOI] [PubMed] [Google Scholar]
- Ratcliffe OJ, Amaya I, Vincent CA, Rothstein S, Carpenter R, Coen ES, Bradley DJ. A common mechanism controls the life cycle and architecture of plants. Development. 1998;125:1609–1615. doi: 10.1242/dev.125.9.1609. [DOI] [PubMed] [Google Scholar]
- Ratcliffe OJ, Bradley DJ, Coen ES. Separation of shoot and floral identity in Arabidopsis. Development. 1999;126:1109–1120. doi: 10.1242/dev.126.6.1109. [DOI] [PubMed] [Google Scholar]
- Riechmann JL, Meyerowitz EM. Determination of floral organ identity by Arabidopsis MADS domain homeotic proteins AP1, AP3, PI, and AG is independent of their DNA-binding specificity. Mol Biol Cell. 1997;8:1243–1259. doi: 10.1091/mbc.8.7.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounsley SD, Ditta GS, Yanofsky MF. Diverse roles for MADS box genes in Arabidopsis development. Plant Cell. 1995;7:1259–1269. doi: 10.1105/tpc.7.8.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-García L, Madueño F, Wilkinson M, Haughn G, Salinas J, Martínez-Zapater JM. Different roles of flowering-time genes in the activation of floral initiation genes in Arabidopsis. Plant Cell. 1997;9:1921–1934. doi: 10.1105/tpc.9.11.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sanda SL, Amasino RM. Genetic and physiological analysis of flowering time in the C24 line of Arabidopsis thaliana. Weeds World. 1995;2:2–8. [Google Scholar]
- Schultz EA, Haughn GW. Genetic analysis of the floral initiation process (FLIP) in Arabidopsis. Development. 1993;119:745–765. [Google Scholar]
- Sheldon CC, Burn JE, Perez PP, Metzger J, Edwards JA, Peacock WJ, Dennis ES. The FLF MADS box gene: A repressor of flowering in Arabidopsis regulated by vernalization and methylation. Plant Cell. 1999;11:445–458. doi: 10.1105/tpc.11.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon CC, Rouse DT, Finnegan EJ, Peacock WJ, Dennis ES. The molecular basis of vernalization: The central role of FLOWERING LOCUS C (FLC) Proc Natl Acad Sci. 2000;97:3753–3758. doi: 10.1073/pnas.060023597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R, Igeño MI, Coupland G. Activation of floral meristem identity genes in Arabidopsis. Nature. 1996;382:59–62. doi: 10.1038/384059a0. [DOI] [PubMed] [Google Scholar]
- Telfer A, Bollman KM, Poethig RS. Phase change and the regulation of trichome distribution in Arabidopsis thaliana. Development. 1997;124:637–644. doi: 10.1242/dev.124.3.645. [DOI] [PubMed] [Google Scholar]
- Weigel D. The genetics of flower development: From floral induction to ovule morphogenesis. Annu Rev Genet. 1995;29:19–39. doi: 10.1146/annurev.ge.29.120195.000315. [DOI] [PubMed] [Google Scholar]
- Weigel D, Nilsson O. A developmental switch sufficient for flower initiation in diverse plants. Nature. 1995;377:495–500. doi: 10.1038/377495a0. [DOI] [PubMed] [Google Scholar]
- Weigel D, Ahn JH, Blazquez MA, Borevitz JO, Christensen SK, Fankhauser C, Ferrandiz C, Kardailsky I, Malanchauvil EJ, Neff MM, et al. Activation tagging in Arabidopsis. Plant Physiol. 2000;122:1003–1013. doi: 10.1104/pp.122.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RN, Heckman JW, Somerville CR. Gibberellin is required for flowering in Arabidopsis thaliana under short days. Plant Physiol. 1992;100:403–408. doi: 10.1104/pp.100.1.403. [DOI] [PMC free article] [PubMed] [Google Scholar]