Abstract
The relationship between hypertension and chronic kidney disease (CKD) is bidirectional in nature and, generally, management strategies for cardiovascular risk reduction also attenuate progression of CKD. Prevalent hypertension increases with diminishing kidney function, and the management strategy changes with level of kidney function. In this review, we will examine the evidence for management of hypertension, as a modifiable risk factor for cardiovascular disease in CKD, and the impact of this management on progression of CKD.
Key Words: ACE inhibitor, ARB, Proteinuria, RAAS, Risk reduction
Hypertension and Chronic Kidney Disease
Hypertension and chronic kidney disease (CKD) frequently exist together, though the influence of one upon the other is difficult to clarify. Hypertension is the number-two cause of end-stage renal disease (ESRD) following diabetes in the United States and is a comorbid condition in approximately 61–66% of those with an estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2[1]. Prevalent hypertension in CKD increases with diminishing eGFR with a prevalence of 35.8% in CKD stage 1 and 84.1% in CKD stages 4–5 [1]. In comparison, CKD has a prevalence of 27.5% in patients with diagnosed hypertension and 22% among those with undiagnosed hypertension [2].
Treatment of hypertension is arguably the most important CKD and cardiovascular disease (CVD) risk reduction strategy. The overall mortality rate in CKD increases from 69.1 in CKD stages 1–2 to 89.7 in stages 3–5 per 1,000 patient-years at risk [1,2,3,4]. The relationship between CVD mortality and CKD is a graded one and increases linearly with diminishing GFR [1]. In a study of 18,597 participants aged 50–80 years treated for 3.8 years with aspirin and a protocol for blood pressure control, patients had progressively increasing rates of major CVD events as well as total mortality rates with decreasing GFR. Cardiovascular event rates were increased by 70–100% for every 50% decline of eGFR [3].
The coexistence of hypertension is associated with more rapid progression of CKD [4,5,6]. Several studies indicate treating hypertension in patients with CKD and proteinuria may slow the decline in GFR and progression of CKD [7,8,9]. Despite the evidence for control of blood pressure in reducing the risk of progressive CKD, blood pressure control is generally inadequate in the CKD population [10]. In patients with CKD stages 1–2, more than one third are unaware of having hypertension, 14% are not treated, and only 11% are treated adequately [1].
Therefore, in this review, we will examine the evidence for management of hypertension, as a modifiable risk factor for CVD in CKD, and the impact of this management on progression of CKD.
Approach to Therapy
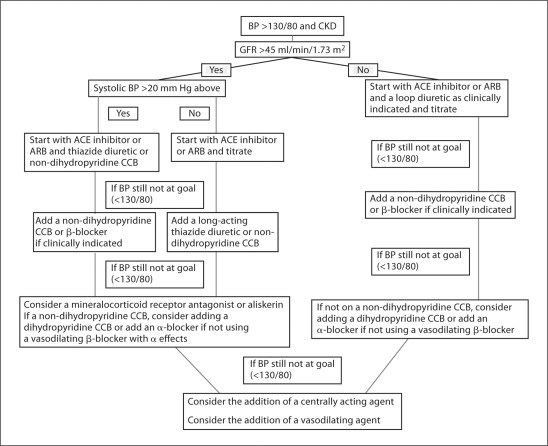
A rational approach to the treatment of hypertension in those with CKD includes both non-pharmacologic and pharmacologic approaches. Figure 1 depicts a generalized approach to the pharmacologic management of hypertension in chronic kidney disease. Although currently being reviewed by the Joint National Committee (JNC) on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure for an eighth (JNC 8) report, current guidelines based on JNC 7 advocate a blood pressure goal of <130/80 mm Hg in those with CKD [11,12]. This goal is supported by several studies suggesting a lower blood pressure may slow CKD progression. A meta-analysis in 2003 reported a lowered risk of CKD progression with a blood pressure goal of 110–129 mm Hg, and an increase in the relative risk for CKD progression at blood pressures >130 mm Hg. The beneficial results were most notable in those with proteinuria exceeding 1 g/d [13].
Fig. 1.
Pharmacologic approach to hypertension management in CKD. BP = Blood pressure; CCB = calcium channel blocker; ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker.
Lifestyle and Dietary Approaches
The evidence for non-pharmacologic intervention for hypertension in those without CKD is more compelling. The same interventions have largely been extrapolated to the CKD population. Importantly, compliance and patient interest are the biggest challenges for instituting lifestyle changes; however, they should be a component of all successful pharmacologic regimens. Lifestyle modifications recommended by the Kidney Disease Outcomes Quality Initiative (K/DOQI) and the Canadian Society of Nephrology include smoking cessation, weight reduction, exercise, and dietary sodium restriction [12,14].
Low-Sodium Diet
Dietary sodium restriction is recommended to reduce extracellular fluid volume expansion and to lower blood pressure. Sodium intake has a dose-dependent relationship with blood pressure, and a modest reduction of 100 mmol/day (6 g of salt) will significantly reduce systolic and diastolic blood pressure in both hypertensive and normotensive subjects in as little as 4 weeks [15]. Blood pressure reduction with salt restriction can be seen across non-diabetic hypertensive ethnic groups, including Blacks, Hispanics, and Asians. Reduced sodium intake can lessen the incidence of hypertension by approximately 20% [16]. Excess sodium intake leads to resistance to renin-angiotensin-aldosterone system (RAAS) blockade, and sodium restriction can be as effective as the addition of a thiazide to a therapeutic regimen containing angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARB) [17,18].
Weight Loss
Weight loss, as in the non-CKD population, can augment blood pressure reduction. In this context, obesity is an independent risk factor for the development and progression of CKD [19]. Reductions in body mass index (BMI) in obese patients with CKD with non-surgical interventions can markedly decrease systolic blood pressure and proteinuria, along with cessation of GFR loss. Surgical intervention in morbidly obese individuals with a BMI >40 has the potential for normalization of GFR and reduction of systolic blood pressure and micro-albuminuria [20]. Unfortunately, many studies represent only short-term outcomes.
The National Kidney Foundation K/DOQI extends recommendations from JNC 7 for weight loss in CKD to a BMI <25 for those overweight and maintenance of weight for those with a BMI <25 [11,12]. Avoidance of high-protein diets is advised in light of the exorbitant amounts of protein in Westernized diets and potential risk for enhancement of progression of CKD. Conversely, very restrictive diets may put CKD patients at overall health risk as these patients are already prone to protein-energy malnutrition.
Additionally, physical activity may provide a benefit. In the non-CKD population relatively modest amounts of physical activity, 30–60 min per week, lead to significant reductions in blood pressure in hypertensive subjects. The effect is graduated with increasing amounts of physical activity. Physical activity and the relationship with hypertension has not been well studied in the CKD population. Nevertheless, there appears to be a survival benefit in the CKD population that participates in regular physical activity [21].
Pharmacologic Interventions
Diuretics
Thiazide diuretics have become an integral element for treating hypertension in the early stages of CKD. While salt restriction has shown to be equivalent to thiazide diuretics for blood pressure reduction in the early stages of CKD, diuretics are essential when sodium restriction alone is unsatisfactory [22]. Thiazides induce a reduction in systolic and diastolic blood pressure between 10–15 and 5–10 mm Hg, respectively [23]. Along with chronic volume depletion, decreased systemic vascular resistance accounts for the long-term antihypertensive effects of thiazide diuretics. However, thiazide diuretics lose much of their antihypertensive effects as GFR falls below 50 ml/min/1.73 m2 and are recommended only in patients with an eGFR of >30 ml/min/1.73 m2[12]. Although hydrochlorothiazide (HCTZ) is the most widely used thiazide for treating high blood pressure, clinical studies demonstrate that chlorthalidone is more efficacious in reducing blood pressure [24,25,26,27]. Side effects of thiazide diuretics include increases in blood glucose levels, hyperuricemia, hypercalcemia, and hypokalemia, which are dose dependent.
As the GFR declines, thiazide diuretics lose their utility in promoting natriuresis. Loop diuretics can be employed for effective volume management. Directed therapy with loop diuretics for volume control and hypertension comes in 2 stages, initiation and maintenance. Once the patient has achieved a maximum diuresis without symptoms of orthostatic hypotension, cramps, fatigue or decreased renal function, then the patient should be titrated to the lowest dose necessary to maintain established dry weight [28]. The pharmacokinetics and pharmacodynamics of loop diuretics change with advancing CKD and need to be continually monitored and adjusted to maintain dry weight. In this regard, increased doses of loop diuretics are required with diminishing kidney function, and inadequately dosed furosemide administration will result in sodium retention. Due to its short-acting nature, furosemide should be dosed at least twice daily.
RAAS Inhibition
RAAS inhibition is the cornerstone of management in patients with hypertension and CKD. RAAS inhibition is considered first-line therapy by both the K/DOQI and the JNC 7 [11,12] and is achieved most commonly with ACE inhibitors and ARBs. ACE inhibitors competitively block the action of ACE, thereby reducing circulating levels of angiotensin II (Ang II). ARBs specifically block the binding of Ang II to the angiotensin type I receptor (AT1R). By intervening in the RAAS, several actions are blunted, including direct vasoconstriction, release of noradrenaline from sympathetic nerve terminals, stimulation of proximal tubular reabsorption of sodium, stimulation of aldosterone secretion, and vasopressin release. ACE inhibition not only reduces blood pressure in the CKD patient, but also reduces the progression of CKD in non-diabetic kidney disease and proteinuria [29,30,31]. Like the ACE inhibitors, ARBs also reduce blood pressure, decrease proteinuria, and limit CKD progression in diabetic kidney disease [32,33], and possibly non-diabetic kidney disease as well [34]. The utility of a combination of ARBs and ACE inhibitors becomes apparent by greater reductions in proteinuria than achieved by monotherapy; however, these comparisons are true with respect to conventional doses, and monotherapy at higher doses may be of equal benefit [34,35]. A major limitation with the use of ACE inhibitor and ARB regimens in the CKD population is the risk for hyperkalemia. Both classes of agents increase the risk of hyperkalemia and the odds of mortality increase when hyperkalemia is present [36]. The risk of hyperkalemia can be mitigated by the concomitant use of diuretics [37], dietary potassium restriction, and potassium resin binders.
Mineralocorticoid receptor (MR) antagonists offer an additional opportunity for RAAS intervention in those with CKD and resistant hypertension, as defined by three or more maximally dosed anti-hypertensives including a diuretic. Similar to ACE and ARB use, the role of MR antagonism is also limited due to a potential risk for hyperkalemia in those with advanced CKD. Spironolactone and eplerenone are the MR antagonists that potentially prevent the aldosterone escape mechanism that occurs with ACE inhibitors and ARBs [38]. Mounting evidence supports a significant reduction in blood pressure and proteinuria when these MR antagonists are added to an ACE inhibitor or ARB [39,40]. While these results would support improvements in mortality and kidney-related outcomes, data is lacking and future work is warranted. The beneficial cardiac effects of MR antagonists in patients with heart failure are well known, but have not been duplicated in patients with an eGFR <60 ml/min/1.73 m2[41]. Adverse effects associated with MR antagonists include breast tenderness, gynecomastia, hyperkalemia, prostatic hypertrophy, erectile dysfunction, and menstrual irregularities. Eplerenone has a more tolerable profile with reduced sexual side effects and gynecomastia. The incidence of hyperkalemia (>5.5 mmol/l) is approximately 5.7% in those with an eGFR <60 ml/min/1.73 m2 and was more pronounced in those with an eGFR <45 ml/min/1.73 m2[42].
Direct renin inhibition became available with introduction of aliskiren, and limited evidence supports its use in CKD patients. In patients with type 2 diabetes mellitus, hypertension, and diabetic kidney disease, combination therapy with maximal doses of losartan and aliskiren led to a 20% reduction in albuminuria compared to losartan and placebo [43]. Long-term data regarding mortality and renal outcomes are not available. Serum potassium elevations >5.5 mmol/l were more frequent with aliskiren (22.5%) versus placebo (13.6%) in stage 3 CKD [44]. All other adverse event rates were similar between treatments, irrespective of CKD stage, except for an increased rate of renal dysfunction seen in the aliskiren group in stage 3 CKD patients [44]. As additional studies and comparisons to other RAAS agents are performed, the role of aliskiren in clinical practice will be better understood. At this time, aliskiren is recommended to patients that have incomplete RAAS blockade with an eGFR >30 ml/min/1.73 m2. Aliskiren, when used in combination with ACE inhibitors or ARBs, has greater blood pressure reduction than monotherapy. Additionally, when evaluated in type 2 diabetic patients with albuminuria, the combination is more antiproteinuric than monotherapy with ARB.
Calcium Channel Blockers
Calcium channel blockers (CCBs) are well-established blood pressure reduction agents in CKD. They can be used as second-line antihypertensive agents and are a good alternative for patients intolerant of ACE inhibitors and ARBs. They are proven safe and effective at reducing CKD progression and cardiovascular events when used in combination with other agents [45]. Of the two subclasses available, the non-dihydropyridines not only lower blood pressure, but decrease glomerular pressure and reduce proteinuria. Comparatively, the dihydropyridines reduce blood pressure, but have no effect on glomerular pressure and are inconsistent with the degree of reduction in proteinuria [46]. Treatment with either class of CCB in those with proteinuric nephropathies reduces proteinuria when coupled with an ACE inhibitor or an ARB. More importantly, CCBs will preserve renal function in both diabetics and non-diabetics with proteinuria [30,43]. Synergism with other drug classes is an important feature. The ACE inhibitor trandolapril, and verapamil, a non-dihydropyridine, cause similar reductions in proteinuria. When verapamil and ramipril are combined, the level of protein reduction is nearly double when dosed for similar blood pressure reductions [46]. Thus, CCBs can be used either as monotherapy or to complement existing therapy in CKD hypertensive patients.
β-Blockers
Sympathetic over-activity in CKD contributes to the maintenance of hypertension; thus, β-blockers have a theoretical benefit in the treatment of hypertension in those with CKD. The most notable longitudinal data assessing CKD progression with a β-blocker is the African American Study of Kidney Disease (AASK) in which metoprolol was not as effective as ACE inhibitors in slowing GFR decline. However, metoprolol did have a reduced risk of ESRD and mortality benefit as compared to amlodipine [47]. Vasodilating β-blockers, labetalol and carvedilol, have been evaluated in hypertensive patients with renal impairment. While blood pressure reduction can be achieved, data are limited on the use of labetalol with regard to CKD progression and proteinuria [48]. Studies with carvedilol indicate that renal blood flow and GFR are preserved with reductions in microalbuminuria in both diabetic and non-diabetic hypertensives with microalbuminuria [49]. Carvedilol has a significant mortality benefit in ESRD patients with heart failure, but large prospective trials evaluating the use in CKD are lacking. Finally, the newest agent available, nebivolol, has proven to be safe in those with CKD stage 3 [50]. In animal models with kidney injury, nebivolol provides reductions in proteinuria and renal fibrosis [51]. Additional studies are needed to assess the role of β-blockers in CKD progression. Clearly, β-blockers play an important role in those with CVD and CKD, but they are not first-line agents and should be reserved for those with compelling indications.
Endothelin Antagonism
Endothelin has been implicated in CKD progression and podocyte injury; intervention has theoretical benefits. Two agents have been evaluated for use in hypertension: avosentan and darusentan [52]. The addition of avosentan in CKD stage 3 and 4 patients with diabetic nephropathy as a complementary agent results in a significant reduction in proteinuria independent of decreased blood pressure. These findings have also been duplicated in non-diabetic CKD [53,54]. The most notable adverse reaction is associated sodium and fluid retention that may lead to congestive heart failure; in fact, the ASCEND trial was prematurely ended for this reason. Additional studies evaluating CKD progression, mortality, and cardiovascular outcomes are needed before the role of endothelin antagonists are established.
α-Blockers
α-Blockers should not be considered as first-line therapy. However, they have a role in resistant hypertension by blocking vasoconstricting α-1 adrenoreceptors on vascular smooth muscles. Evidence supports the adjunctive role of the α-blocker doxazosin with ACE inhibitors, CCB, β-blockers, and diuretics in non-diabetic CKD patients in the Anglo-Scandinavian Cardiac Outcomes Trial–Blood Pressure Lowering Arm (ASCOT-BPLA) whose blood pressure remained above 140/90 mm Hg. There was no apparent excess of heart failure among doxazosin users in that study, and plasma lipid profiles were improved [55]. However, when compared to chlorthalidone in ALLHAT, doxazosin had higher risks of heart failure with similar rates of stroke and combined CVD [56]. The decision to employ α-1 antagonists should balance these increased risks. Common adverse events include nasal congestion and dizziness. α-Blockers do cause sodium retention, and diuretic therapy may need to be incorporated, but this may add to the orthostatic hypotensive effects of this class. In CKD patients treated with diuretics, doxazosin improves blood pressure [55].
Central Sympatholytic
Clonidine, the most widely used central sympatholytic, successfully reduces blood pressure. Clonidine stimulates both a2-adrenergic receptors and I1-imidazoline receptors in the rostral ventrolateral medulla nuclei resulting in decreased sympathetic outflow. Clonidine is typically administered orally or as a transdermal patch. Transdermal patches have several advantages that include continuous drug delivery, improved compliance, decreased rebound upon stopping, and decreased side effects including somnolence and dry mouth. Skin reactions are common with the transdermal patch. Sodium retention can occur with central sympatholytics, and diuretics may be required. Clonidine is generally considered to have neutral effects upon proteinuria.
Vasodilators
Two direct vasodilators, hydralazine and minoxidil, are available for blood pressure control. Both are considered fourth-line agents for resistant hypertension when hypertensive goals have not been reached with other agents. Despite the long history of experience with hydralazine, the effects of the agent on mortality, morbidity, and renal outcomes are poorly understood [57]. Notable reflexive tachycardia is associated with the use of hydralazine that can be controlled with β-blockers or a centrally acting α-agonist. When combined with a nitrate, significant reduction in mortality in Blacks with heart failure was seen, but this has not been specifically studied in the setting of CKD [58]. Minoxidil is another adjunctive agent in poorly responsive, severe hypertension associated with CKD. Minoxidil, while more efficacious in the degree of blood pressure reduction compared to hydralazine, has a more significant side effect profile [59]. Simultaneous administration of a diuretic with a β-blocker or a combined α/β-blocker is often required to control edema and mitigate the tachycardia associated with minoxidil use. Sodium retention following the start of minoxidil can be the cause of significant edema often resulting in temporary cessation of the medication. A loop diuretic will usually suffice, but the addition of metolazone is required in extreme cases [60].
Conclusion
Hypertension in the CKD population is an important clinical concern leading to increased risk of CKD progression and CVD mortality. Both non-pharmacologic and pharmacologic interventions are available to control and mitigate the associated increased risks. There is a wide range of therapeutic interventions clinicians can choose from, and therapy should be directed at achieving control of hypertension, keeping in mind the associated risk factors.
Disclosure Statement
There are no conflicts of interest.
References
- 1.U.S. Renal Data System, USRDS 2010 Annual Data Report . Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2010. [Google Scholar]
- 2.Crews DC, Plantinga LC, Miller ER, 3rd, Saran R, Hedgeman E, Saydah SH, Williams DE, Powe NR, Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team Prevalence of chronic kidney disease in persons with undiagnosed or prehypertension in the United States. Hypertension. 2010;55:1102–1109. doi: 10.1161/HYPERTENSIONAHA.110.150722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jardine MJ, Ninomiya T, Perkovic V, Cass A, Turnbull F, Gallagher MP, Zoungas S, Lambers Heerspink HJ, Chalmers J, Zanchetti A. Aspirin is beneficial in hypertensive patients with chronic kidney disease: a post-hoc subgroup analysis of a randomized controlled trial. J Am Coll Cardiol. 2010;56:956–965. doi: 10.1016/j.jacc.2010.02.068. [DOI] [PubMed] [Google Scholar]
- 4.Shulman NB, Ford CE, Hall WD, Blaufox MD, Simon D, Langford HG, Schneider KA. Prognostic value of serum creatinine and effect of treatment of hypertension on renal function. Results from the hypertension detection and follow-up program. The Hypertension Detection and Follow-Up Program Cooperative Group. Hypertension. 1989;13(suppl 5):I80–I93. doi: 10.1161/01.hyp.13.5_suppl.i80. [DOI] [PubMed] [Google Scholar]
- 5.Walker WG, Neaton JD, Cutler JA, Neuwirth R, Cohen JD. Renal function change in hypertensive members of the Multiple Risk Factor Intervention Trial. Racial and treatment effects. The MRFIT Research Group. J Am Med Assoc. 1992;268:3085–3091. [PubMed] [Google Scholar]
- 6.Perry HM, Jr, Miller JP, Fornoff JR, Baty JD, Sambhi MP, Rutan G, Moskowitz DW, Carmody SE. Early predictors of 15-year end-stage renal disease in hypertensive patients. Hypertension. 1995;25(4 Pt 1):587–594. doi: 10.1161/01.hyp.25.4.587. [DOI] [PubMed] [Google Scholar]
- 7.Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, de Jong PE, de Zeeuw D, Shahinfar S, Toto R, Levey AS. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med. 2003;139:244–252. doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]
- 8.Sarnak MJ, Greene T, Wang X, Beck G, Kusek JW, Collins AJ, Levey AS. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the Modification of Diet in Renal Disease Study. Ann Intern Med. 2005;142:342–351. doi: 10.7326/0003-4819-142-5-200503010-00009. [DOI] [PubMed] [Google Scholar]
- 9.Wright JT, Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG, African American Study of Kidney Disease and Hypertension Study Group Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 10.Peralta CA, Hicks LS, Chertow GM, Ayanian JZ, Vittinghoff E, Lin F, Shlipak MG. Control of hypertension in adults with chronic kidney disease in the United States. Hypertension. 2005;45:1119–1124. doi: 10.1161/01.HYP.0000164577.81087.70. [DOI] [PubMed] [Google Scholar]
- 11.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ, National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 12.Kidney Disease Outcomes Quality Initiative (K/DOQI) K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 suppl 2):S1–S290. [PubMed] [Google Scholar]
- 13.Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, de Jong PE, de Zeeuw D, Shahinfar S, Toto R, Levey AS, AIPRD Study Group Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med. 2003;139:244–252. doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]
- 14.Levin A, Hemmelgarn B, Culleton B, Tobe S, McFarlane P, Ruzicka M, Burns K, Manns B, White C, Madore F, Moist L, Klarenbach S, Barrett B, Foley R, Jindal K, Senior P, Pannu N, Shurraw S, Akbari A, Cohn A, Reslerova M, Deved V, Mendelssohn D, Nesrallah G, Kappel J, Tonelli M. Canadian Society of Nephrology: Guidelines for the management of chronic kidney disease. CMAJ. 2008;179:1154–1162. doi: 10.1503/cmaj.080351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He FJ, Marciniak M, Visagie E, Markandu ND, Anand V, Dalton RN, MacGregor GA. Effect of modest salt reduction on blood pressure, urinary albumin, and pulse wave velocity in white, black, and Asian mild hypertensives. Hypertension. 2009;54:482–488. doi: 10.1161/HYPERTENSIONAHA.109.133223. [DOI] [PubMed] [Google Scholar]
- 16.Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high normal blood pressure: the Trials of Hypertension Prevention, phase II: the Trials of Hypertension Prevention Collaborative Research Group. Arch Intern Med. 1997;157:657–667. [PubMed] [Google Scholar]
- 17.Buter H, Hemmelder MH, Navis G, de Jong PE, de Zeeuw D. The blunting of the antiproteinuric efficacy of ACE inhibition by high sodium intake can be restored by hydrochlorothiazide. Nephrol Dial Transplant. 1998;13:1682–1685. doi: 10.1093/ndt/13.7.1682. [DOI] [PubMed] [Google Scholar]
- 18.Vogt L, Waanders F, Boomsma F, de Zeeuw D, Navis G. Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J Am Soc Nephrol. 2008;19:999–1007. doi: 10.1681/ASN.2007060693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Chen X, Song Y, Caballero B, Cheskin LJ. Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int. 2008;73:19–33. doi: 10.1038/sj.ki.5002586. [DOI] [PubMed] [Google Scholar]
- 20.Navaneethan SD, Yehnert H, Moustarah F, Schreiber MJ, Schauer PR, Beddhu S. Weight loss interventions in chronic kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2009;4:1565–1574. doi: 10.2215/CJN.02250409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beddhu S, Baird BC, Zitterkoph J, Neilson J, Greene T. Physical activity and mortality in chronic kidney disease (NHANES III) Clin J Am Soc Nephrol. 2009;4:1901–1906. doi: 10.2215/CJN.01970309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buter H, Hemmelder MH, Navis G, de Jong PE, de Zeeuw D. The blunting of the antiproteinuric efficacy of ACE inhibition by high sodium intake can be restored by hydrochlorothiazide. Nephrol Dial Transplant. 1998;13:1682–1685. doi: 10.1093/ndt/13.7.1682. [DOI] [PubMed] [Google Scholar]
- 23.Ernst ME, Moser M. Use of diuretics in patients with hypertension. N Engl J Med. 2009;361:2153–2164. doi: 10.1056/NEJMra0907219. [DOI] [PubMed] [Google Scholar]
- 24.Rahman M, Pressel S, Davis BR, Nwachuku C, Wright JT, Jr, Whelton PK, Barzilay J, Batuman V, Eckfeldt JH, Farber M, Henriquez M, Kopyt N, Louis GT, Saklayen M, Stanford C, Walworth C, Ward H, Wiegmann T. Renal outcomes in high-risk hypertensive patients treated with an angiotensin-converting enzyme inhibitor or a calcium channel blocker versus a diuretic: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Arch Intern Med. 2005;165:936–946. doi: 10.1001/archinte.165.8.936. [DOI] [PubMed] [Google Scholar]
- 25.Multiple Risk Factor Intervention Trial Research Group Multiple Risk Factor Intervention Trial: risk factor changes and mortality results. JAMA. 1982;248:1465–1477. [PubMed] [Google Scholar]
- 26.Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeability. Hypertension. 2004;43:4–9. doi: 10.1161/01.HYP.0000103632.19915.0E. [DOI] [PubMed] [Google Scholar]
- 27.Ernst ME, Carter BL, Goerdt CJ, Steffensmeier JJ, Phillips BB, Zimmerman MB, Bergus GR. Comparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressure. Hypertension. 2006;47:352–358. doi: 10.1161/01.HYP.0000203309.07140.d3. [DOI] [PubMed] [Google Scholar]
- 28.Zamboli P, De Nicola L, Minutolo R, Bertino V, Catapano F, Conte G. Management of hypertension in chronic kidney disease. Curr Hypertens Rep. 2006;8:497–501. doi: 10.1007/s11906-006-0029-4. [DOI] [PubMed] [Google Scholar]
- 29.Jafar TH, Schmid CH, Landa M, Giatras I, Toto R, Remuzzi G, Maschio G, Brenner BM, Kamper A, Zucchelli P, Becker G, Himmelmann A, Bannister K, Landais P, Shahinfar S, de Jong PE, de Zeeuw D, Lau J, Levey AS. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med. 2001;135:73–87. doi: 10.7326/0003-4819-135-2-200107170-00007. [DOI] [PubMed] [Google Scholar]
- 30.The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia) Randomized placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet. 1997;349:1857–1863. [PubMed] [Google Scholar]
- 31.Barnett AH, Bain SC, Bouter P, Karlberg B, Madsbad S, Jervell J, Mustonen J, Diabetics Exposed to Telmisartan and Enalapril Study Group Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med. 2004;351:1952–1961. doi: 10.1056/NEJMoa042274. [DOI] [PubMed] [Google Scholar]
- 32.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S, RENAAL Study Investigators Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 33.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I, Collaborative Study Group Renoprotective effect of the angiotensin receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 34.Li PK, Leung CB, Chow KM, Cheng YL, Fung SK, Mak SK, Tang AW, Wong TY, Yung CY, Yung JC, Yu AW, Szeto CC, HKVIN Study Group Hong Kong study using valsartan in IgA nephropathy (HKVIN): a double-blind, randomized, placebo-controlled study. Am J Kidney Dis. 2006;47:751–760. doi: 10.1053/j.ajkd.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 35.Kunz R, Friedrich C, Wolbers M, Mann JF. Meta-analysis: effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med. 2008;148:30–48. doi: 10.7326/0003-4819-148-1-200801010-00190. [DOI] [PubMed] [Google Scholar]
- 36.Einhorn LM, Zhan M, Hsu VD, Walker LD, Moen MF, Seliger SL, Weir MR, Fink JC. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169:1156–1162. doi: 10.1001/archinternmed.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weinberg JM, Appel LJ, Bakris G, Gassman JJ, Greene T, Kendrick CA, Wang X, Lash J, Lewis JA, Pogue V, Thornley-Brown D, Phillips RA, African American Study of Hypertension and Kidney Disease Collaborative Research Group Risk of hyperkalemia in nondiabetic patients with chronic kidney disease receiving antihypertensive therapy. Arch Intern Med. 2009;169:1587–1594. doi: 10.1001/archinternmed.2009.284. [DOI] [PubMed] [Google Scholar]
- 38.Bomback AS, Klemmer PJ. The incidence and implications of aldosterone breakthrough. Nat Clin Pract Nephrol. 2007;3:486–492. doi: 10.1038/ncpneph0575. [DOI] [PubMed] [Google Scholar]
- 39.Bomback AS, Kshirsagar AV, Amamoo MA, Klemmer PJ. Change in proteinuria after adding aldosterone blockers to ACE inhibitors or angiotensin receptor blockers in CKD: a systematic review. Am J Kidney Dis. 2008;51:199–211. doi: 10.1053/j.ajkd.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 40.Navaneethan SD, Nigwekar SU, Sehgal AR, Strippoli GF. Aldosterone antagonists for preventing the progression of chronic kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2009;4:542–551. doi: 10.2215/CJN.04750908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 42.Heshka J, Ruzicka M, Hiremath S, McCormick BB. Spironolactone for difficult to control hypertension in chronic kidney disease: an analysis of safety and efficacy. J Am Soc Hypertens. 2010;4:295–301. doi: 10.1016/j.jash.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK, AVOID Study Investigators Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008;358:2433–2446. doi: 10.1056/NEJMoa0708379. [DOI] [PubMed] [Google Scholar]
- 44.Persson F, Lewis JB, Lewis EJ, Rossing P, Hollenberg NK, Parving HH, AVOID Study Investigators Impact of baseline renal function on the efficacy and safety of aliskiren added to losartan in patients with type 2 diabetes and nephropathy. Diabetes Care. 2010;33:2304–2309. doi: 10.2337/dc10-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs. diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;88:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 46.Toto RD. Management of hypertensive chronic kidney disease: role of calcium channel blockers. J Clin Hypertens (Greenwich) 2005;7(4 suppl 1):15–20. doi: 10.1111/j.1524-6175.2004.4471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wright JT, Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG, African American Study of Kidney Disease and Hypertension Study Group Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 48.Bakris GL, Hart P, Ritz E. Beta blockers in the management of chronic kidney disease. Kidney Int. 2006;70:1905–1913. doi: 10.1038/sj.ki.5001835. [DOI] [PubMed] [Google Scholar]
- 49.Bakris GL, Fonseca V, Katholi RE, McGill JB, Messerli FH, Phillips RA, Raskin P, Wright JT, Jr, Oakes R, Lukas MA, Anderson KM, Bell DS, GEMINI Investigators Metabolic effects of carvedilol vs. metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. JAMA. 2004;292:2227–2236. doi: 10.1001/jama.292.18.2227. [DOI] [PubMed] [Google Scholar]
- 50.Cohen-Solal A, Kotecha D, van Veldhuisen DJ, Babalis D, Böhm M, Coats AJ, Roughton M, Poole-Wilson P, Tavazzi L, Flather M, SENIORS Investigators Efficacy and safety of nebivolol in elderly heart failure patients with impaired renal function: insights from the SENIORS trial. Eur J Heart Fail. 2009;11:872–880. doi: 10.1093/eurjhf/hfp104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whaley-Connell A, Habibi J, Johnson M, Tilmon R, Rehmer N, Rehmer J, Wiedmeyer C, Ferrario CM, Sowers JR. Nebivolol reduces proteinuria and renal NADPH oxidase-generated reactive oxygen species in the transgenic Ren2 rat. Am J Nephrol. 2009;30:354–360. doi: 10.1159/000229305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moore R, Linas S. Endothelin antagonists and resistant hypertension in chronic kidney disease. Curr Opin Nephrol Hypertens. 2010;19:432–436. doi: 10.1097/MNH.0b013e32833a7a25. [DOI] [PubMed] [Google Scholar]
- 53.Dhaun N, Macintyre IM, Melville V, Lilitkarntakul P, Johnston NR, Goddard J, Webb DJ. Blood pressure-independent reduction in proteinuria and arterial stiffness after acute endothelin: a receptor antagonism in chronic kidney disease. Hypertension. 2009;54:113–119. doi: 10.1161/HYPERTENSIONAHA.109.132670. [DOI] [PubMed] [Google Scholar]
- 54.Mann JF, Green D, Jamerson K, Ruilope LM, Kuranoff SJ, Littke T, Viberti G, ASCEND Study Group Avosentan for overt diabetic nephropathy. J Am Soc Nephrol. 2010;21:527–535. doi: 10.1681/ASN.2009060593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mori Y, Matsubara H, Nose A, Shibasaki Y, Masaki H, Kosaki A, Okigaki M, Fujiyama S, Tanaka-Uchiyama Y, Hasegawa T, Iba O, Tateishi E, Amano K, Iwasaka T. Safety and availability of doxazosin in treating hypertensive patients with chronic renal failure. Hypertens Res. 2001;24:359–363. doi: 10.1291/hypres.24.359. [DOI] [PubMed] [Google Scholar]
- 56.Davis BR, Cutler JA, Furberg CD, Wright JT, Farber MA, Felicetta JV, Stokes JD, ALLHAT Collaborative Research Group Relationship of antihypertensive treatment regimens and change in blood pressure to risk for heart failure in hypertensive patients randomly assigned to doxazosin or chlorthalidone: further analyses from the Antihypertensive and Lipid-Lowering treatment to prevent Heart Attack Trial. Ann Intern Med. 2002;137:313–320. doi: 10.7326/0003-4819-137-5_part_1-200209030-00006. [DOI] [PubMed] [Google Scholar]
- 57.Kandler MR, Mah GT, Tejani AM, Stabler SN. Hydralazine for essential hypertension. Cochrane Database Syst Rev. 2010:CD004934. doi: 10.1002/14651858.CD004934.pub3. [DOI] [PubMed] [Google Scholar]
- 58.Taylor AL, Ziesche S, Yancy C, Carson P, D'Agostino R, Jr, Ferdinand K, Taylor M, Adams K, Sabolinski M, Worcel M, Cohn JN, African-American Heart Failure Trial Investigators Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049–2057. doi: 10.1056/NEJMoa042934. [DOI] [PubMed] [Google Scholar]
- 59.Gottlieb TB, Katz FH, Chidsey CA., 3rd Combined therapy with vasodilator drugs and beta-adrenergic blockade in hypertension. A comparative study of minoxidil and hydralazine. Circulation. 1972;45:571–582. doi: 10.1161/01.cir.45.3.571. [DOI] [PubMed] [Google Scholar]
- 60.Sica DA. Minoxidil: an underused vasodilator for resistant or severe hypertension. J Clin Hypertens (Greenwich) 2004;6:283–287. doi: 10.1111/j.1524-6175.2004.03585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]