Abstract
Purpose
After a large-scale radiological event, there will be a pressing need to assess, within a few days, the radiation doses received by tens or hundreds of thousands of individuals. This is for triage, to prevent treatment locations from being overwhelmed, in what is sure to be a resource limited scenario, as well as to facilitate dose-dependent treatment decisions. In addition there are psychosocial considerations, in that active reassurance of minimal exposure is a potentially effective antidote to mass panic, as well as long-term considerations, to facilitate later studies of cancer and other long-term disease risks.
Materials and Methods
As described elsewhere in this issue, we are developing a Rapid Automated Biodosimetry Tool (RABiT). The RABiT allows high throughput analysis of thousands of blood samples per day, providing a dose estimate that can be used to support clinical triage and treatment decisions.
Results
Development of the RABiT has motivated us to consider the logistics of incorporating such a system into the existing emergency response scenarios of a large metropolitan area. We present here a view of how one or more centralized biodosimetry readout devices might be incorporated into an infrastructure in which fingerstick blood samples are taken at many distributed locations within an affected city or region and transported to centralized locations.
Conclusions
High throughput biodosimetry systems offer the opportunity to perform biodosimetric assessments on a large number of persons. As such systems reach a high level of maturity, emergency response scenarios will need to be tweaked to make use of these powerful tools. This can be done relatively easily within the framework of current scenarios.
Keywords: Disaster management, Radiation Triage, High Throughput Biodosimetry, Accidents-radiation
Introduction
The need for biodosimetry after a large scale radiological event is now well established (Rea et al. 2010). In terms of the need for medical intervention, the best estimate for the median lethal dose (LD50) at 60 days in humans is in the 3 to 4.5 Gy range (Anno et al. 2003), but this value can be roughly doubled by the use of antibiotics, platelet and cytokine treatment (Anno et al. 2003), so it is crucial that individuals who actually received whole-body doses above, say, 2 Gy are identified. It would be undesirable to give these treatments to “all comers” irrespective of radiation exposure, not least because there is some evidence of long-term toxicity with cytokine treatments (Nifontova et al. 2008; Beaupain et al. 2009).
Some individuals exposed in the 2 to 5 Gy dose range will be identifiable through early nausea, vomiting, and acute fatigue, but by no means all. For example, worker ‘C’ at the 1999 radiation accident at Tokai-mura received a best-estimate whole-body equivalent dose of more than 3 Gy (Hayata et al. 2001; Ishigure et al. 2001), was initially almost entirely asymptomatic, yet developed acute bone marrow failure (Hirama et al. 2003). Thus accurate biodosimetry is crucial in this dose range.
At higher doses, there is only a quite narrow dose window (approximately 7–10 Gy (Hall 2001)) in which bone-marrow transplantation is a useful option (below 7 Gy, survival rates are good solely with medication and above 10 Gy patients will generally have lethal gastrointestinal damage). Thus it is critical to ascertain whether a patient’s dose is within this dose window, such that a bone-marrow transplant would be a useful option.
The need for very high throughput biodosimetry is well illustrated by the 1987 radiation incident in Goiânia, Brazil, a city with about the same population as Manhattan. In the first few days after the incident became known, about 130,000 people (roughly 10% of the population) came for screening, of whom 20 required treatment (International Atomic Energy Agency 1988). Mass radiological triage will thus be critical after a large-scale event because of the need to identify, at an early stage, those individuals who will benefit from medical intervention, and those who will not. Eliminating and reassuring those patients who do not need medical intervention will be equally crucial in what will be a highly resource-limited scenario, as well as potentially to reduce the number of individuals unnecessarily fleeing an RDD (Radiological Dispersal Device) event (Brandao-Mello et al. 1991; Erikson 1994; Health Protection Agency 2009).
Thus it is likely that large cities could face the need to rapidly screen hundreds of thousands of individuals. What emerges (Pellmar & Rockwell 2005) is the need for very high throughput biodosimetry – analysis of tens or hundreds of thousands of samples per day. Using standard approaches, the highest throughput that can be achieved by a single lab is < 500 samples/week (Martin et al. 2007; Vaurijoux et al. 2009), and even national and international laboratory networks are expected to have throughputs of less than 3,000 samples/week (International Atomic Energy Agency 2006; Miller et al. 2007; Blakely et al. 2009).
Recent work in this arena has resulted in the development of Rapid Automated Biodosimetry Technology (RABiT) that has the potential to overcome the throughput limitations noted above (Garty et al. 2010; Garty et al. 2011). The RABiT system is designed to rapidly analyze fingerstick blood samples (i.e. single drops of blood in a capillary tube) obtained by many minimally-trained collectors at multiple distributed locations, which are then brought to one or more centralized ultra high throughput readout devices (RABiT systems). Once this system is fully operational a large city should be able to process up to one million biodosimetry samples in less than a week using a relatively small number of such machines. We discuss here the logistical and infrastructure considerations that would be necessary for such systems might be put to use in the event of a large-scale radiological or nuclear incident.
We begin by describing current thinking regarding distributed community reception centers – multiple centers responsible for surveying the population for radioactive contamination and entering them into a long-term tracking database. We briefly discuss the RABiT high throughput biodosimetry system, focusing on the requirements for distributed fingerstick sample collection. The RABiT is described in great detail elsewhere (Garty et al. 2010; Garty et al. 2011) and serves here as an example of an automated biodosimetry system. Finally we discuss how centralized biodosimetry reader devices such as the RABiT system might be deployed in the event of a radiological or nuclear emergency.
It should be noted that the discussions here generally do not reflect current emergency response scenarios. The associated high throughput biodosimetry technology is not yet sufficiently mature to have yet been incorporated into current planning, and this work represents a first effort in that direction.
Community Reception Centers
Terrorist attacks produce large numbers of victims – the 1995 Oklahoma City attack killed 168 and injured at least 600 (Frykberg 2002) and it took place in a city with a relatively low population density compared to many others in the United States. It is entirely plausible to consider that a similar attack in a densely populated city such as New York City, Washington DC, or Chicago might send many thousands of victims to the emergency rooms. Such numbers will leave medical facilities incapable of also performing radiological screening for contamination, uptake of radioactive materials, and decontamination. Accordingly, current plans call for establishing Community Reception Centers (CRC) to perform radiological screening and decontamination as well as other necessary activities as discussed in this section.
The Centers for Disease Control and Prevention (CDC) suggests establishing CRC locations as quickly as possible in the aftermath of a large-scale radiological or nuclear incidents (Centers for Disease Control 2007), after which the public will be provided with information about CRC locations and will be provided with instructions regarding who should report to which CRC. The CRC itself will include several distinct areas (as shown in Figure 1, a conceptual drawing developed by CDC) in which persons will be screened for external contamination, decontaminated if necessary, entered into the long-term tracking system, and discharged with appropriate instructions. For members of the public meeting certain conditions (e.g. presence of extensive contamination, confirmed contamination around the nose and mouth, continued elevated radiation readings following decontamination and in the absence of external contamination) it may be appropriate to include the capability for either conducting scans for internal gamma-emitting radionuclides, to obtain urine samples that can be screened for evidence of radionuclide uptake, to provide instructions for obtaining further screening at another location, or to distribute prescriptions for medical countermeasures to those who have an obvious radiological uptake. To these tasks it may be necessary to add an additional step of obtaining a biodosimetric sample for those who were directly involved in the incident due to physical proximity or injury, those with high levels of external or internal contamination, or others who meet criteria (yet to be developed) calling for such an assessment.
Figure 1.
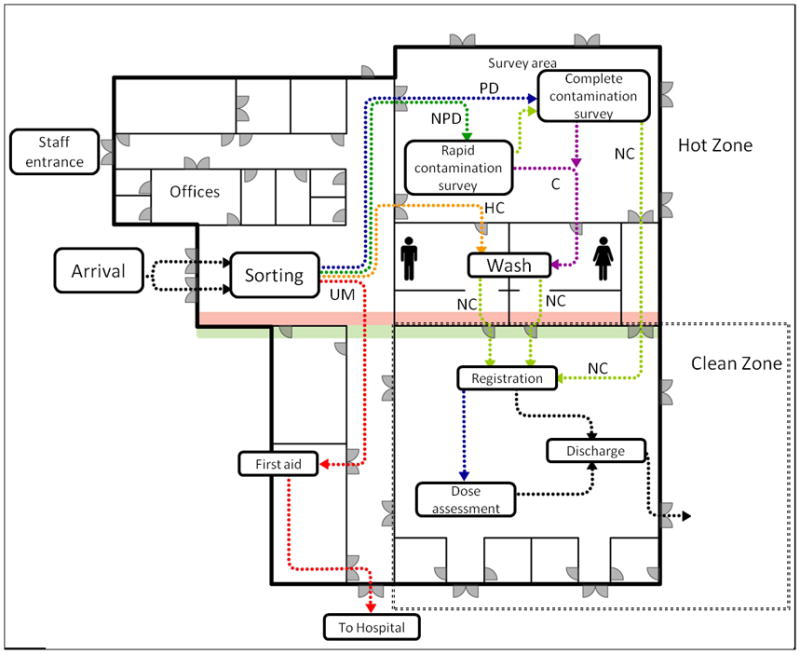
Layout of the CRC as per CDC guideline. People are initially sorted to: Urgent Medical (UM, red) who are given stabilizing first aid and transported to a medical facility; Previously Decontaminated (PD, blue), who are given a thorough contamination survey and either sent for decontamination or to the clean zone; Not Previously Decontaminated (NPD, green) who are surveyed. Highly Contaminated individuals (HC, orange) are sent immediately to decontamination. Non-contaminated individuals (after the thorough contamination survey, NC, yellow) can go directly to the clean zone while those who are contaminated (C, purple) are decontaminated at the wash area. Once in the clean zone, individuals are registered, a dose assessment is performed and they are discharged. Figure adapted from CDC web page: http://emergency.cdc.gov/radiation/crc/vcrc.asp
There has been, to date, no need to establish CRC locations for radiological purposes; thus there are no firm data on actual CRC performance. It is likely that eventual plans will assume a nominal throughput of 1000 people per hour and will base staffing and supply needs on this nominal value; actual throughput will depend, of course, on the extent of the actual incident the availability of resources (personnel, equipment, facilities, etc.), and less tangible factors such as staff experience and fatigue. These plans can then be adjusted and scaled as necessary to account for the realities of the actual situation with which a city is confronted.
An operating CRC will have several goals:
Quickly identify persons requiring decontamination, treatment for minor medical injuries, and referral for further radiological assessment
Identify (if possible) those with an uptake of radioactivity in excess of 1 Clinical Decision Guidance Level (National Council on Radiation Protection & Measurements 2010) or those for whom bioassay is advised (e.g. those with extensive contamination around the face, those with embedded radioactive fragments, etc.)
Provide screening, information, and mental health counseling for those who are worried about their radiological status or that of their families
Refer participants to hospitals or pharmacies for further treatment (for medical problems or for medical countermeasures such as Prussian blue) when appropriate
Although it may not be possible to organize a CRC as shown in Figure 1, this figure is not unreasonable – for a CRC to fulfill its mission it must accomplish the tasks noted above, necessitating the stations shown here, even if space may not permit this same layout. Those reporting to the CRC are expected to proceed through the various stations in a manner similar to that shown here.
At present there are no plans for using the CRC to participate in biodosimetry assessment because there are no available systems, capable of processing the large number of samples that would be collected. When, however, the RABiT or a similar system becomes widely available, it may prove possible to have a few devices scattered at central locations in the city (e.g. in hospitals), with samples from multiple CRC locations in the area transported to each device. Having several independent stations scattered throughout a city might be less efficient than a single large station, but the latter would leave the city vulnerable to an attack involving the facility itself. Scattering biodosimetry facilities in multiple locations helps to ensure that a city will maintain at least partial capabilities under all circumstances. This approach, in effect, adds the capabilities of the RABiT system to the existing radiological emergency response paradigm. It is tempting, however, to suggest how this paradigm might evolve to incorporate the capabilities of the RABiT system.
The chief advantage of the RABiT system is its ability to process tens of thousands of samples daily at each station. If we assume that a single CRC is able to process 12,000 people daily (making allowance for shift changes, fatigue, meals, and not operating around the clock) and that even a large city is unlikely to be able to fully staff more than 5 or 6 CRC locations then it is clear that all of a city’s CRC locations can be adequately served by only 2 RABiT devices. Furthermore, it is likely that the majority of persons requesting biodosimetry will not be contaminated and may not need to report to a CRC for processing. In fact, sending those who are almost certainly not contaminated to a CRC may hinder the CRC in attending to those who do require the high level of service that a CRC can provide. By sending those who require (or request) only biodosimetric measurements to facilities other than CRC locations we can help to reduce the overall workload at the CRC locations, enabling them to devote more time to those contaminated in a radiological emergency. Those members of the public who are almost certainly not contaminated, then, would be directed to report to local fire stations, school, community centers, or other assembly points where they would submit samples for RABiT analysis. In effect, it should be possible to sort the population into three groups, as shown in Table I.
Table I.
Possible method to sort the public into groups according to the level of attention required and the facility to which they should be sent
| Group | Injuries? | Number affected | Contaminated? | Disposition | Sample collection and priority |
|---|---|---|---|---|---|
| 1. Adjacent to site of event | Minor to severe | 100 to 1000 | Yes | Hospital | Draw at hospital, High priority samples |
| 2. Within sight or earshot of event (i.e. can see, hear, or smell smoke from explosion) | None to minor | 1000 to 10,000 | Probably | CRC | Draw at CRC Prioritize according to level of contamination or likelihood of high dose |
| 3. All others | None | 10,000 to millions | Probably not | RABiT collection center | Draw at satellite RABiT sample collection facility Analyze after high-priority samples are run |
In the event of a radiological emergency, those in Group 1 will require medical attention and, for the most seriously injured, the need for prompt medical attention will be more urgent than the need for either decontamination or radiological assessment. The ability of hospitals to provide this medical care may be compromised if they are simultaneously swamped with those with lesser injuries that do not require hospital-level attention. Those in Group 2 are unlikely to require medical care but will be close enough to the scene of the emergency that they will have a high likelihood of internal and/or external contamination and they will require the full attention of a CRC for radiological evaluation and possible decontamination. Similarly, the ability of CRC locations to provide this level of radiological care may be compromised if they are swamped with those who do not require radiological assessment or decontamination. In addition, there are likely to be a very limited number of hospitals and CRC locations in any city.
By implementing a disseminated sample collection paradigm for those in Group 3, a city can not only reduce the workload of hospitals and CRC locations but can also better serve the large numbers of the public who wish to receive a radiological assessment but who may not be able to easily reach a CRC or hospital. In other words, utilizing the RABiT system in this manner may make it possible to better serve all of those affected in a radiological emergency.
Requirements of an ultra high throughput biodosimetry assay
There a number of requirements for an ideal high throughput biodosimetry system that can be incorporated into the emergency response framework. These include
Minimal invasiveness of the sample collection
Sample collection by minimally trained personnel
Sensitivity/specificity
Processing time
Signal stability
Efficient sample tracking
Multi-use technology
FDA (Food and Drug Administration) approval
Thus, for example blood sample collection through venipuncture is not optimal both because the procedure is not minimally invasive, and therefore comparatively slow, and also because trained and certified collection personnel are required. Since public health or other agencies are not likely to have enough trained staff or volunteers to effectively respond to such an event (Aakko et al. 2008) sample collection should ideally not require highly trained personnel. Fingerstick-based blood collection on the other hand can be done rapidly and does not require extensive training and certification.
Clearly sensitivity and specificity are critical to any successful biodosimetry device. Sensitivity requirements may vary according to the circumstance: for example the goal may be simply an under/over decision (e.g. over or under 2 Gy), or the goal may be an actual dose estimate. Specificity is also critical: For example, a biodosimetric endpoint which is mimicked by, for example, trauma injury or inflammatory response, will be of limited utility. Explicit calibrations for other confounders such as age, gender, or smoking status may also be needed.
Ideally, of course, processing time should be as short as possible. However several endpoints have processing times as long as 3 days (typical lymphocyte culturing times) which is not necessarily a “showstopper” attribute, as long as high throughput can be maintained, because few treatment decisions need to be taken before one to two weeks post exposure (Hall 2001).
Likewise, post-exposure assay signal lifetime should ideally be long – ideally months or years. By contrast an assay signal which has a lifetime of, say, less than 24 hours post exposure would certainly be showstopper in that one would not expect the infrastructure for collecting biodosimetry samples to be fully in place for at least that long.
Since assay processing is unlikely to yield an instantaneous result, patient/sample tracking is critical. Patient information needs to be tracked and correlated with the samples so that when the results of the bioassay are obtained (e.g. within a few hours or up to a few days), the individual can be easily located, in person or through some other means. This is also crucial for later follow up and epidemiological studies.
The issue of multiuse technology is important for any technologically-based radiation biodosimetry system. Specifically a system designed only for biodosimetry after a radiological event is at high risk of being non-functional if it is not routinely used for long periods of time.
Finally, in order to obtain FDA approval, the technology needs to undergo extensive testing to demonstrate that it is equivalent to the “accepted” method of radiation dose assessment, namely manual processing of the micronucleus or dicentric assay (McNamee et al. 2009). If the technology uses a novel assay (e.g. gene expression, metabolic signature) it is likely that studies using in-vivo irradiated animals will be required. If on the other hand, the technology is based on a well established assay (e.g. the micronucleus assay), the FDA is likely to accept studies performed in ex-vivo irradiated blood from human volunteers. Additionally, biodosimetry technology based on blood/urine samples is considered sufficiently non-invasive to be classified as an in-vitro diagnostic (IVD) (Gutman et al. 1998), and has a shorter path to licensing compared with other, more invasive, medical devices.
The RABiT high throughput system
To date no biodosimetric system or assay optimally meets all of the requirements discussed above. We will use the RABiT system (Garty et al. 2010; Garty et al. 2011) with which we are intimately familiar as a case study for all such systems. We expect the RABiT to be typical of any automated, centralized biodosimetry system using blood-based assays, particularly when considering sample collection logistics. The RABiT approach is to use well established biodosimetry assays which are currently performed manually, and fully automate them using robotic technology, a multi-well plate platform, and advanced imaging approaches. Using ‘mature’ assays has considerable advantages in that 1) the assays are already well characterized, and 2) there is a more direct regulatory route to deployment, as the assays are already in use.
The RABiT analyzes fingerstick-derived blood samples (~30 μl,), either to estimate past radiation dose, or to identify individuals exposed above/below a cutoff dose. The RABiT fully automates two mature, but formerly manual, biodosimetry assays (Cytokinesis Block Micronucleus assay (CBMN) (International Atomic Energy Agency 2001; Fenech et al. 2003) and phosphorylation of the histone H2AX (γ-H2AX) (Nakamura et al. 2006; Turner et al. 2011)), thus converting them to ultra high throughput. Recent reliability and performance testing (Garty et al. 2011) indicated a maximum throughput of 30,000 samples per RABiT machine per day is achievable. We are currently partnered with Northrop Grumman Security Systems (Linthicum, MD, USA), to develop a field-deployable system based on the RABiT prototype. As part of this effort we will be conducting large scale testing to demonstrate that the high-throughput system can achieve equivalent dose estimates to those obtained by manual processing.
The RABiT was designed as a flexible robotically-based system, to potentially allow routine multi-use applications in a hospital or clinic setting. Examples are cytogenetic assays such as amniocentesis, or potentially for multiplex immunoassays, such as screening for multiple cytokines. A simple adaptation of the RABiT technology would allow rapid screening for individual radiosensitivity, with potential applications both for radiation oncology and radiology. These alternate uses are currently under preliminary study.
The two current RABiT assays were chosen both for their maturity and for their potential to be fully automated. Both have advantages and disadvantages (Amundson et al. 2001). Specifically, the γ-H2AX assay is rapid (<2 h) but the signal lasts only a few days post exposure (Redon et al. 2010). By contrast, the micronucleus signal is stable for many months, but the current micronucleus assay, though now high-throughput, takes ~70 hours to generate the dose estimate.
To achieve very high throughputs, the RABiT contains the following key technological innovations:
Use of small volumes of blood (~30 μl) from a standard lancet fingerstick; this is a minimally invasive, and thus potentially high throughput, approach – conventional venipuncture is not compatible with ultra high throughput.
Complete robotically-based automation of the biology, with biological processing and imaging performed in situ in multi-well plates. This allows rapid processing of multiple simultaneous samples. The use of filter bottomed multiwell plates prevents loss of lymphocytes during fluid removal steps.
Innovations in high-speed imaging allow rapid analysis following biological processing.
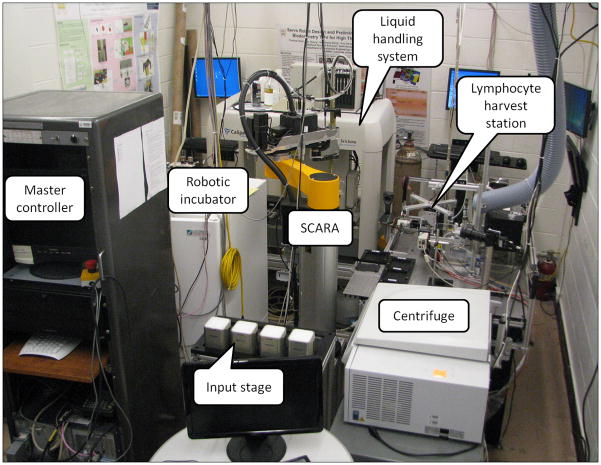
The RABiT prototype, under testing at Columbia University is shown in Figure 2. The RABiT consists of 7 stations arranged around a SCARA (Selective Compliant Articulated Robot Arm) that transfers samples from station to station:
Figure 2.
Breadboard prototype of the RABiT system.
Blood samples arriving from the field are placed into centrifuge buckets on the input stage. A centrifuge is used for separating lymphocytes from red blood cells. At each centrifugation cycle 384 capillaries are spun simultaneously. The lymphocyte harvest station (Garty et al. 2011) transfers the lymphocyte band from the capillaries to a 96-well plate for further processing. The biological assays are performed in an automated liquid handling system where reagents can be added or removed as needed. A robotically controlled incubator is used for lymphocyte culturing in the CBMN assay.
After the lymphocytes have been fixed and stained the plates are moved to a transfer to substrate system (Chen et al. 2010) where the filter bottoms are removed from the multiwell plates and sealed between two layers of transparent tape. Finally the lymphocytes are imaged using a custom built imaging system.
By modifying the number of cells scored per sample, throughput and sensitivity can be adjusted. During the initial triage, only a small number of cells may be scored to obtain a crude dose estimate (e.g. above/below 2 Gy). At a later stage, after all samples were triaged. They can be re-imaged and analyzed at higher statistics (and therefore lower throughput) to achieve a more precise dose, as would be required for long-term follow up. As described in (Garty et al. 2011; Turner et al. 2011), the γ-H2AX assay, as implemented in the RABiT, is sensitive between 1 Gy and 8 Gy, with higher sensitivity achievable through a collection of higher statistics. The micronucleus assay has similar accuracy (McNamee et al. 2009). This is well matched to the dose range required for triage following a radiological event, see below.
The RABiT system imposes several requirements on the sample collection process:
Samples will be collected in the field and will need to be transported to the RABiT with no spillage and no cross contamination.
The RABiT is designed to isolate lymphocytes, by centrifugation, from small volumes of whole blood, in heparin-coated capillaries. To ensure separation of lymphocytes out of whole blood samples, the blood needs to be layered above separation medium with no mixing.
The lymphocytes in the collected blood need to be kept viable as the micronucleus assay requires them to be cultured to division.
During transport, the blood may need to be kept chilled to prevent γ-H2AX foci repair (see Figure 4a in (Moroni et al. 2008)).
These requirements are not specific to the RABiT and would need to be maintained for almost any automated biodosimetry system, implementing a blood-based biodosimetry assay.
RABiT sample collection
Sample collection for the RABiT, as described below addresses the concerns noted above. As it does not require highly trained personnel, it can be easily merged into the emergency response scenario detailed above. Sample collectors, at the CRC or elsewhere, will draw the blood, by fingerstick and verify the contact information. Individuals with other injuries (e.g. trauma) will be triaged by a medical professional and can be evacuated to a hospital. Those who appear healthy, other than the possible radiation exposure, will be sent home after the sample collection. Samples will then be packed and transported to the RABiT (which may be across the hall or in a different state, but most likely, at the nearest large medical center).
Sample collection kit
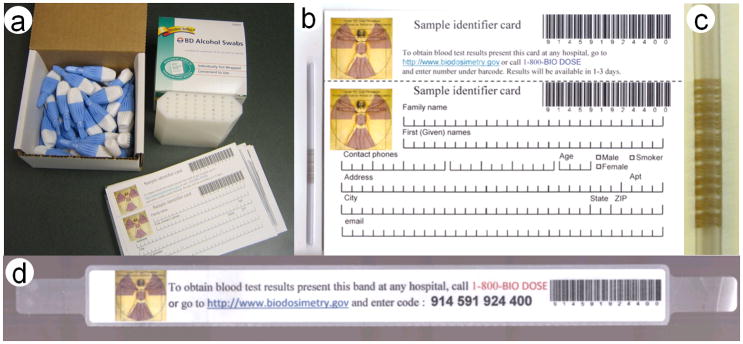
In order to facilitate blood collection, by minimally trained individuals, we have developed a sample collection kit (Figure 3a), consisting of lancets, bar-coded, heparin coated, capillary tubes with matched personal data cards and patient tracking wristbands, alcohol wipes and sample holders for filled capillaries. The kit is designed to match the 32 samples that can be collected over a 2–3 hour collection period by one sampler. We envision a few hundred such collection kits would be kept at local emergency response stores, as part of the CRC Go-kit (May et al. 2007) and would be ready to be used immediately. A much larger number of kits can then be stored at the Strategic National Stockpile (SNS), as part of the “12-hour push package” (Esbitt 2003), and will arrive at the CRC within 12 hours of a request by local authorities.
Figure 3.
(a) Sample collection kit, (b) data collection card with capillary, (c) close-up of bar-coded capillary, and (d) wristband.
Data collection card
On entering the CRC, Individuals will be handed a data collection card (Figure 3b), where they are to enter personal and contact information. In addition to the contact details, processing in the RABiT may require knowing the age, gender and smoking status so that this information is also included. The card has a printed barcode which is matched to the barcode etched on a heparinized PVC (Poly Vinyl Chloride) capillary (Figure 3b, c), attached to the card, and a detachable human readable version of the same code, with instructions on how to obtain the results of the blood test, is also provided. Alternatively, the card can contain an integrated self laminating wristband (Figure 3d) which is detached and applied to the individual. The wristband contains information allowing the individual, or their medical caregiver to obtain the results of the blood test 1–3 days following the sample collection.
Lancet
Since the RABiT requires 30 μl of blood and since multiple fingersticks would reduce the processing throughput, the reliability of the lancet in producing large blood volumes is critical. Although standard “diabetic” lancets are not sufficient for this purpose, as they are required to provide less than 5 μl of blood (Yum & Roe 1999), other, commercially available, lancets have larger blades which penetrate deeper into the skin and typically result in 50μl of blood or more (Fruhstorfer 2000; Garty et al. 2010). Care should be made, to select this class of lancet for the sample collection kit.
Fingerstick sampling procedure
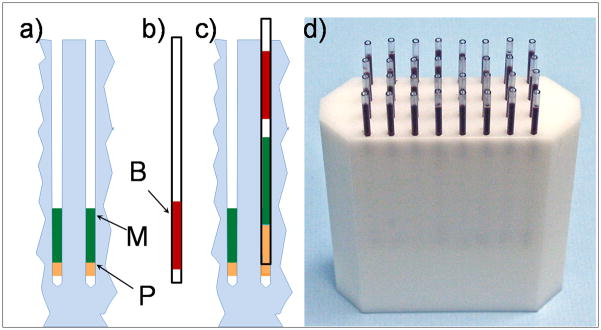
After loading about 30 μl of blood into the capillary, the sample collector then seals the top of the capillary with their (gloved) thumb, begins inserting it into the holder, which is preloaded with separation medium and sealing putty (Figure 4a), while releasing their thumb to allow trapped air to escape from the capillary.
Figure 4.
Scheme of the sample collection. (a) Sample holder with sealing putty (P) and separation medium (M); (b) blood in a capillary (B); (c) capillary loaded into sample holder layering blood above the separation medium without mixing; and (d) photograph of filled capillary holder.
As the blood in the capillary (Figure 4b) does not reach its edge, when the capillary is inserted into the holder, an air bubble is trapped between the blood and separation medium, preventing their mixing during shipping (up to 24 hours).
The sealing putty is compressed into and around the capillary ensuring a seal (Figure 4c), requiring a small force to extract the capillary from the holder. This prevents the capillary from falling out even if the holder is inverted and vigorously shaken, but still allows the RABiT robotics to extract the capillary from the holder (Garty et al. 2011). As the bottom of the capillary is sealed, the blood and separation medium cannot leak out. This procedure allows the sample to be collected by an individual with minimal training, while maintaining the required layering of the blood and separation medium and preventing contaminations. We have seen that the technique for this can be learned in a few minutes.
Transporting samples to the RABiT
After the capillary holder is filled with 32 capillaries, the top of the capillaries is sealed with a foam rubber mat, to prevent cross-contamination of the samples, and the capillary holder can be wrapped and shipped to the RABiT.
As the γ-H2AX assay, which does not require culturing the lymphocytes, provides a much faster processing (a few hours compared to 3 days for the micronucleus assay), it is the assay of choice for rapid triage. To reduce γ-H2AX signal decay during shipping, the samples need to be chilled to 4–10 °C. This can be done by adding ice packs in with the samples for shipping (see for example (Kendal et al. 1997)).
No such cooling needs to be done for the micronucleus assay. Indeed, we have verified that capillaries stored at room temperature for 24 to 48 hours, still contain a sufficient quantity of viable lymphocytes, which undergo mitosis when stimulated in the RABiT.
Adding RABiT to existing radiological and nuclear emergency response planning
Any terrorist attack is likely to cause injuries; an attack that includes high-activity radioactive sources may expose members of the public to high doses of radiation as well. Sufficiently high doses of radiation might prove to be clinically significant and must be considered (if possible) while treating the patient. Unfortunately, barring radiation dosimeters (which members of the public are not likely to be using), determining radiation exposure is a difficult matter and can take from a few to several days to accomplish. This time lag may hinder attempts to properly assess the full range of risks facing patients. If the affected population numbers in the thousands then this problem becomes even more difficult. As noted earlier, demands for biodosimetry may well outstrip existing capabilities. This mismatch between demand and capabilities might hamper both short-term and intermediate-term dose assessment as well as long-term dose reconstruction efforts.
In the immediate aftermath of a radiological or nuclear emergency it is important to be able to quickly sort patients into a few groups:
Those who have received radiation doses that are clinically insignificant (<1 Gy)
Those who have received significant doses who are expected to survive even in the absence of medical treatment (< 4 Gy)
Those for whom prompt and appropriate medical treatment is likely to prove life-saving (< 8 Gy)
Those who have received radiation dose that is likely to prove fatal under any circumstances (> 8 Gy)
One goal of radiological triage is to quickly sort patients into appropriate groups so that medical resources can be properly apportioned for the greatest overall benefit to the community (Hook & Vetter 2003). A high-throughput biodosimetry system such as the one described here, if properly utilized during a radiological or nuclear emergency can help perform this radiological triage in a clinically significant time frame. In addition, the use of the RABiT system beyond the emergency phase can help assess both the intermediate-term threat from radioactive materials uptake and the long-term risks of cancer – it may be necessary to evaluate the risk of these effects (and the need to administer medical countermeasures to help decorporate internal radionuclides) for tens or hundreds of thousands members of the affected public.
When automated biodosimetry systems are available for routine use, it should prove possible to incorporate this new capability into existing CRC plans and procedure. Administering a fingerstick to those meeting specified criteria (e.g. proximity to the event or high levels of external contamination) will add only a few minutes to the registration process. To this could be added priority coding to identify those samples requiring the most urgent processing to help identify those receiving medically significant radiation doses as rapidly as possible.
Another need during a large-scale radiological or nuclear response will be to rapidly assess radiation dose to those responding to the emergency. While many (perhaps most) emergency responders are likely to be issued radiation dosimeters, it is reasonable to use confirmatory biodosimetry assessments to aid in dose assessment under specific circumstances such as to confirm high dosimeter readings, for those known to have worked in areas with high radiation levels, or to assess radiation dose to those lacking dosimeters.
These are a few ways that a high-throughput biodosimetry system might help to augment existing capabilities and, in so doing, to help assess health risks to the public and to emergency responders; it is likely that further uses will manifest themselves as the system becomes more widely used and better-known.
When incorporating a biodosimetry system into emergency response procedures, several key factors need to be considered:
Location of the system
It is expected that a large metropolitan area would require multiple systems positioning of these systems should take into account the fact that there may be a significant infrastructure impact of a radiological/nuclear event. A system that is too close to a nuclear explosion, for example would likely be incapacitated, due to possible physical damage, contamination and injury/death of the people trained in its operation.
However, the cities that are considered most likely to be experience large-scale radiological emergencies (e.g. New York City, Los Angeles, Washington DC, and other large cities) have large metropolitan areas. Even a nuclear explosion, while catastrophic, is likely to leave much of the city’s infrastructure intact - particularly in parts of the city that are upwind of and geographically distant from the site of the explosion. A nuclear attack in, for example, Manhattan is not likely to affect the infrastructure in much of Queens, Brooklyn, Staten Island, and the Bronx and even less so in the surrounding suburbs. A radiological attack is likely to affect an even smaller fraction of the city. Thus, we anticipate that a city with multiple devices, placed intelligently at various locations, will be able to utilize some, if not all of them in the aftermath of a large-scale radiological or nuclear emergency.
Sample transport
Although transportation in the immediate aftermath of an emergency might be difficult, the movement of emergency response equipment to and from the scene of an emergency is a vitally important part of every major city’s emergency response planning. We expect that those cities that elect to incorporate use of the RABiT system into their emergency response plans will also include sample transportation into their emergency response and recovery planning, as has been the case with moving ambulances, fire trucks, and other emergency response vehicles. It should be noted that the problem of sample transport is actually reduced, when using a central location in the same city as compared to shipping samples to distant analysis labs, as is the current practice (Miller et al. 2007).
It is expected that the logistics of sample transportation from the collection sites to the RABiT may delay full utilization of the RABiT system in the first hours after a large-scale radiological or nuclear emergency; in the aftermath of any large-scale emergency the city experiencing the event will require some time to establish mass screening centers (e.g. Community Reception Centers). Thus, there is likely to be a time lag of up to a day between the time of an attack (for example) and the arrival of the first samples at a RABiT system. This time lag will make it possible to configure the RABiT system(s) for radiological emergencies.
Sample collectors and RABiT operators
Although simple, the procedures for collecting samples, in such a way that they would be usable, will need to be formalized, and a training program set up. Ideally, this will be incorporated into the training materials for operating a CRC, and rehearsed during periodic radiological training exercises. However, due to the simplicity of the procedures, it is reasonable to expect that some of the sample collectors can be trained “in real time” as part of the CRC setup.
The operators of the biodosimetry system, however, would need to be trained ahead of time. To this end biodosimetry systems that have a secondary medical use are preferable, as they will naturally have a pool of trained operators available.
Finally, we note that even sub-optimal access to the automated biodosimetry is a great improvement over existing radiation biodosimetry capabilities. As such it is important to begin to incorporate systems into the nation’s radiological and nuclear emergency infrastructure both as an improvement over current technology as well as to begin the process of learning to make full use of the system under challenging circumstances. In other words, it is better than what we have now, and we have to start the process somewhere if it is ever to live up to its potential.
Conclusions
Newly developed high throughput biodosimetry systems, such as the RABiT offer the opportunity to perform biodosimetric assessments on a large number of persons – both members of the public and emergency responders – in the event of a large-scale radiological or nuclear incident that could affect as many as a million people. This can revolutionize the ability to perform short-term radiological triage as well as long-term tracking of populations following such an attack. However care should be taken to incorporate sample collection, transport and processing into the local emergency response plan in an intelligent way, in order to fully benefit from these systems.
Acknowledgments
Development of the RABiT prototype and collection kit was supported by grant number U19 AI067773, the Columbia Center for High-Throughput Minimally Invasive Radiation Biodosimetry, the National Institute of Allergy and Infectious Diseases/National Institutes of Health.
Footnotes
Declaration of Interest
The authors report no conflicts of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of National Institute of Allergy and Infectious Diseases, the National Institutes of Health or the New York City Department of Health and Mental Hygiene. The concepts discussed here do not necessarily reflect current planning for emergency response at local or national levels.
References
- Aakko E, Weed N, Konrad R, Wiesman J. Rethinking volunteer management using a centralized volunteer staging and training area. Disaster Medicine & Public Health Preparedness. 2008;2:127–129. doi: 10.1097/DMP.0b013e31816476a2. [DOI] [PubMed] [Google Scholar]
- Amundson SA, Bittner M, Meltzer P, Trent J, Fornace AJJ. Biological indicators for the identification of ionizing radiation exposure in humans. Expert Review of Molecular Diagnostics. 2001;1:211–219. doi: 10.1586/14737159.1.2.211. [DOI] [PubMed] [Google Scholar]
- Anno GH, Young RW, Bloom RM, Mercier JR. Dose response relationships for acute ionizing-radiation lethality. Health Physics. 2003;84:565–575. doi: 10.1097/00004032-200305000-00001. [DOI] [PubMed] [Google Scholar]
- Beaupain B, Leblanc T, Reman O, Hermine O, Vannier JP, Suarez F, Lutz P, Bordigoni P, Jourdain A, Schoenvald M, Ouachee M, Francois S, Kohser F, Jardin F, Devouassoux G, Bertrand Y, Nove-Josserand R, Donadieu J. Is pegfilgrastim safe and effective in congenital neutropenia? An analysis of the french severe chronic neutropenia registry. Pediatric Blood Cancer. 2009;53:1068–1073. doi: 10.1002/pbc.22147. [DOI] [PubMed] [Google Scholar]
- Blakely WF, Carr Z, Chu MC, Dayal-Drager R, Fujimoto K, Hopmeir M, Kulka U, Lillis-Hearne P, Livingston G, Lloyd DC, Maznyk N, Perez Mdel R, Romm H, Takashima Y, Voisin P, Wilkins RC, Yoshida MA. Who 1st consultation on the development of a global biodosimetry laboratories network for radiation emergencies (BioDoseNet) Radiation Research. 2009;171:127–139. doi: 10.1667/RR1549.1. [DOI] [PubMed] [Google Scholar]
- Brandao-Mello CE, Oliveira AR, Valverde NJ, Farina R, Cordeiro JM. Clinical and hematological aspects of 137cs: The Goiania radiation accident. Health Physics. 1991;60:31–39. [PubMed] [Google Scholar]
- Centers for Disease Control. Population monitoring in radiation emergencies: A guide for state and local public health planners. 2007:CDC75. [cited Feb. 25, 2011] Available from http://emergency.cdc.gov/radiation/pdf/population-monitoring-guide.pdf.
- Chen Y, Zhang J, Wang H, Garty G, Xu Y, Lyulko OV, Turner HC, Randers-Pehrson G, Simaan N, Yao YL, Brenner DJ. Development of a robotically-based automated biodosimetry tool for high-throughput radiological triage. International Journal of Biomechatronics and Biomedical Robotics. 2010;1:115–125. [Google Scholar]
- Erikson K. A new species in trouble: Explorations in disaster, trauma, and community. New York: W W Norton; 1994. [Google Scholar]
- Esbitt D. The strategic national stockpile: Roles and responsibilities of health care professionals for receiving the stockpile assets. Disaster Management & Response. 2003;1:68–70. doi: 10.1016/s1540-2487(03)00044-0. [DOI] [PubMed] [Google Scholar]
- Fenech M, Chang WP, Kirsch-Volders M, Holland N, Bonassi S, Zeiger E. HUMN project: Detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutation Research. 2003;534:65–75. doi: 10.1016/s1383-5718(02)00249-8. [DOI] [PubMed] [Google Scholar]
- Fruhstorfer H. Capillary blood sampling: The pain of single-use lancing devices. European Journal of Pain-London. 2000;4:301–305. doi: 10.1053/eujp.2000.0179. [DOI] [PubMed] [Google Scholar]
- Frykberg ER. Medical management of disasters and mass casualties from terrorist bombings: How can we cope? Journal of Trauma-Injury Infection and Critical Care. 2002;53:201–212. doi: 10.1097/00005373-200208000-00001. [DOI] [PubMed] [Google Scholar]
- Garty G, Chen Y, Turner H, Zhang J, Lyulko OV, Bertucci A, Xu Y, Wang H, Simaan N, Randers-Pehrson G, Yao YL, Brenner DJ. The RABiT: A rapid automated biodosimetry tool for radiological triage II. Technological developments. Int J Radiat Biol. 2011 August;87(8):776–790. doi: 10.3109/09553002.2011.573612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garty G, Chen Y, Salerno A, Turner H, Zhang J, Lyulko OV, Xu Y, Wang H, Simaan N, Randers-Pehrson G, Yao YL, Amundson SA, Brenner DJ. The RABiT: A rapid automated biodosimetry tool for radiological triage. Health Physics. 2010;98:209–217. doi: 10.1097/HP.0b013e3181ab3cb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman S, Richter K, Alpert S. Update on fda regulation of in vitro diagnostic devices. Jama-Journal of the American Medical Association. 1998;280:190–192. doi: 10.1001/jama.280.2.190. [DOI] [PubMed] [Google Scholar]
- Hall EJ. Radiobiology for the radiologist. 5. Philadelphia: Lippincott Williams & Wilkins; 2001. p. xi.p. 588. [Google Scholar]
- Hayata I, Kanda R, Minamihisamatsu M, Furukawa M, Sasaki MS. Cytogenetical dose estimation for 3 severely exposed patients in the jco criticality accident in tokai-mura. Journal of Radiation Research. 2001;42(Supplement):S149–155. doi: 10.1269/jrr.42.s149. [DOI] [PubMed] [Google Scholar]
- Health Protection Agency. High dose radiation effects and tissue injury 2009 [Google Scholar]
- Hirama T, Tanosaki S, Kandatsu S, Kuroiwa N, Kamada T, Tsuji H, Yamada S, Katoh H, Yamamoto N, Tsujii H, Suzuki G, Akashi M. Initial medical management of patients severely irradiated in the tokai-mura criticality accident. British Journal of Radiology. 2003;76:246–253. doi: 10.1259/bjr/82373369. [DOI] [PubMed] [Google Scholar]
- Hook C, Vetter R. The shifting ethical responsibilities of heathcare delivery in an age of terror. 36th Annual Midyear Meeting of the Health Physics Society; San Antonio, TX. 2003. [Google Scholar]
- International Atomic Energy Agency. The radiological accident in goiânia. Vienna: IAEA; 1988. p. 132. [Google Scholar]
- International Atomic Energy Agency. Cytogenetic analysis for radiation dose assessment: A manual. Vienna: IAEA; 2001. p. 127. [Google Scholar]
- International Atomic Energy Agency. IAEA response assistance network incident and emergency center: EPR-RANET technical document. 2006. [Google Scholar]
- Ishigure N, Endo A, Yamaguchi Y, Kawachi K. Calculation of the absorbed dose for the overexposed patients at the jco criticality accident in tokai-mura. Journal of Radiation Research. 2001;42(Suppl):S137–148. doi: 10.1269/jrr.42.s137. [DOI] [PubMed] [Google Scholar]
- Kendal AP, Snyder R, Garrison PJ. Validation of cold chain procedures suitable for distribution of vaccines by public health programs in the USA. Vaccine. 1997;15:1459–1465. doi: 10.1016/s0264-410x(97)00060-1. [DOI] [PubMed] [Google Scholar]
- Martin PR, Berdychevski RE, Subramanian U, Blakely WF, Prasanna PGS. Sample tracking in an automated cytogenetic biodosimetry laboratory for radiation mass casualties. Radiation Measurements. 2007;42:1119–1124. doi: 10.1016/j.radmeas.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May L, Cote T, Hardeman B, Gonzalez GR, Adams SB, Blair RK, Pane G. A model “go-kit” for use at strategic national stockpile points of dispensing. Journal of Public Health Management & Practice January/February. 2007;13:23–30. doi: 10.1097/00124784-200701000-00005. [DOI] [PubMed] [Google Scholar]
- McNamee JP, Flegal FN, Greene HB, Marro L, Wilkins RC. Validation of the cytokinesis-block micronucleus (cbmn) assay for use as a triage biological dosimetry tool. Radiation Protection Dosimetry. 2009;135:232–242. doi: 10.1093/rpd/ncp119. [DOI] [PubMed] [Google Scholar]
- Miller SM, Ferrarotto CL, Vlahovich S, Wilkins RC, Boreham DR, Dolling JA. Canadian cytogenetic emergency network (cen) for biological dosimetry following radiological/nuclear accidents. International Journal of Radiation Biology. 2007;83:471–477. doi: 10.1080/09553000701370860. [DOI] [PubMed] [Google Scholar]
- Moroni MM, Krasnopolsky K, Subramanian U, Martin PR, Doherty KM, Prasanna PGS. Does cell culture type and blood transport temperature affect dicentric yield and radiation dose assessment? Journal of Medical, Chemical, Biological and Radiological Defense. 2008;6 [cited 2011 February 14] Available from http://www.jmedcbr.org.
- Nakamura A, Sedelnikova OA, Redon C, Pilch DR, Sinogeeva NI, Shroff R, Lichten M, Bonner WM. Techniques for gamma-H2AX detection. DNA repair, pt b. San Diego: Elsevier Academic Press Inc; 2006. p. 236. [DOI] [PubMed] [Google Scholar]
- National Council on Radiation Protection & Measurements. Report #161: Management of persons contaminated with radionuclides, vol. 1 (handbook) National council on radiation protection and measurements; 2010. pp. 158–176. [Google Scholar]
- Nifontova IN, Svinareva DA, Chertkov IL, Drize NI, Savchenko VG. Delayed effects of long-term administration of granulocyte colony-stimulating factor to mice. Bulletin of experimental biology and medicine. 2008;145:629–633. doi: 10.1007/s10517-008-0166-7. [DOI] [PubMed] [Google Scholar]
- Pellmar TC, Rockwell S. Priority list of research areas for radiological nuclear threat countermeasures. Radiation Research. 2005;163:115–123. doi: 10.1667/rr3283. [DOI] [PubMed] [Google Scholar]
- Rea ME, Gougelet RM, Nicolalde RJ, Geiling JA, Swartz HM. Proposed triage categories for large-scale radiation incidents using high-accuracy biodosimetry methods. Health Physics. 2010;98:136–144. doi: 10.1097/HP.0b013e3181b2840b. [DOI] [PubMed] [Google Scholar]
- Redon CE, Nakamura AJ, Gouliaeva K, Rahman A, Blakely WF, Bonner WM. The use of gamma-H2AX as a biodosimeter for total-body radiation exposure in non-human primates. PLoS ONE. 2010;5:e15544. doi: 10.1371/journal.pone.0015544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner HC, Brenner DJ, Chen Y, Bertucci A, Zhang J, Wang H, Lyulko OV, Xu Y, Schaefer J, Simaan N, Randers-Pehrson G, Yao YL, Garty G. Adapting the γ-H2AX assay for automated processing in human lymphocytes. 1. Technological aspects. Radiation Research. 2011;175:282–290. doi: 10.1667/RR2125.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaurijoux A, Gruel G, Pouzoulet F, Gregoire E, Martin C, Roch-Lefevre S, Voisin P, Roy L. Strategy for population triage based on dicentric analysis. Radiation Research. 2009;171:541–548. doi: 10.1667/RR1664.1. [DOI] [PubMed] [Google Scholar]
- Yum SI, Roe J. Capillary blood sampling for self-monitoring of blood glucose. Diabetes Technology & Therapeutics. 1999;1:39–40. doi: 10.1089/152091599317549. [DOI] [PubMed] [Google Scholar]