Abstract
Versican is an abundant proteoglycan in the blood vessel wall that is increased after vascular injury and accumulates in advanced atherosclerotic plaques. Versican is a large molecule with domains that mediate binding to cytokines, enzymes, lipoproteins, other extracellular matrix molecules, and signaling receptors. There is evidence that versican exists in the normal, as well as the diseased, vessel wall as discrete fragments, which represent these functional domains. We review the literature on versican degradation in vascular tissue and the function of versican domains, all of which suggest that proteolytic modification of versican may have physiologic as well as pathologic implications for the vascular system.
Versican1 is an extracellular matrix proteoglycan expressed throughout the body, including the vasculature. In the 16 years since Zimmermann and Ruoslahti (1989) sequenced and named this gene product, over 500 papers related to versican have been published. Recent reviews have focused on the role of versican generally and, more specifically, in vascular disease (Wight 2002, Wight and Merrilees 2004). For example, synthesis and deposition of versican in the extracellular matrix are acutely increased in the neointima after arterial injury caused by angioplasty, stents, or grafting (Nikkari et al. 1994, Finn et al. 2002, Kenagy et al. 2005, Chung et al. 2002, Farb et al. 2004, Wight et al. 1997, Matsuura et al. 1996). In addition, there is selective accumulation of versican at the plaque thrombus interface in eroded human atherosclerotic plaques, suggesting a role for this proteoglycan in thrombosis (Kolodgie et al. 2004). However, little attention has been paid to versican catabolism. Given the numerous examples of gain of function after cleavage of a molecule, there is a strong likelihood that breakdown products of versican will have biologic activity. In this review, we focus on what is known about versican catabolism and possible effects of versican breakdown based on the current knowledge of versican biology.
The Nature of Versican in the Blood Vessel
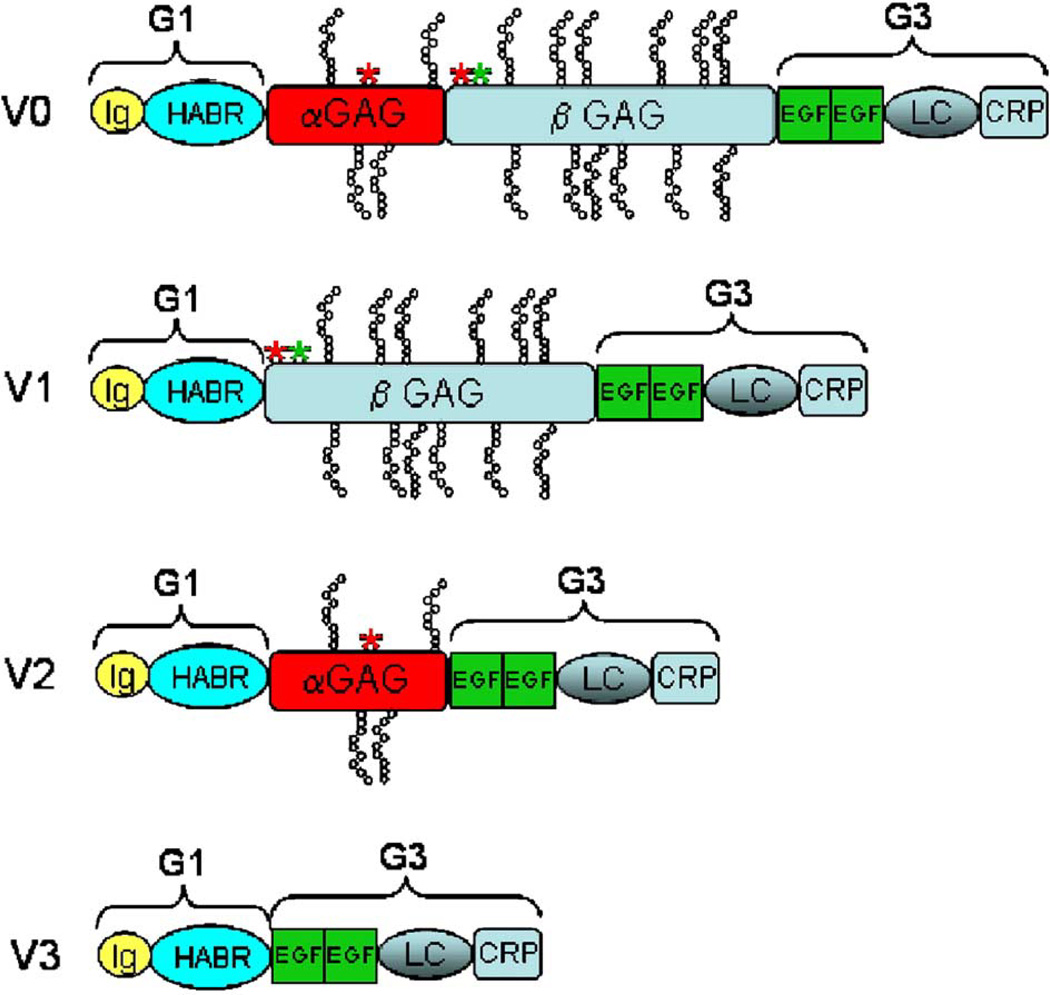
Differential RNA splicing gives rise to four isoforms of versican (V0, V1, V2, and V3), which vary by the presence or absence of two glycosaminoglycan (GAG) binding domains named αGAG and βGAG (Figure 1). All forms of versican share the remaining domains that include the amino-terminal globular domain (G1; which contains the hyaluronan-binding link modules) and the carboxy-terminal G3 domain (which contains two EGF-like repeats, a complement-regulatory protein-like repeat [CRP], and a C-type lectin domain [LC]). In the blood vessel wall, in which versican is a major proteoglycan, V0 (with both αGAG and βGAG domains), V1 (only the βGAG domain), V2 (only the αGAG domain), and V3 (neither GAG domain) have all been reported to be expressed (Lemire et al. 1999, Cattaruzza et al. 2002).
Figure 1.
Isoforms of versican. G1, N-terminal globular domain; G3, C-terminal globular domain (named after domains in aggrecan, which has a third intermediate G2 domain); Ig, Immunoglobulin-like domain; HABR, hyaluronan binding region; αGAG, α glycosaminoglycan binding domain; βGAG, β glycosaminoglycan binding domain; EGF, EGF-like domain; LC, C-type lectin domain; CRP, complement regulatory protein-like domain. *Documented (green asterisk) or potential (red asterisk) ADAMTS-mediated cleavage sites.
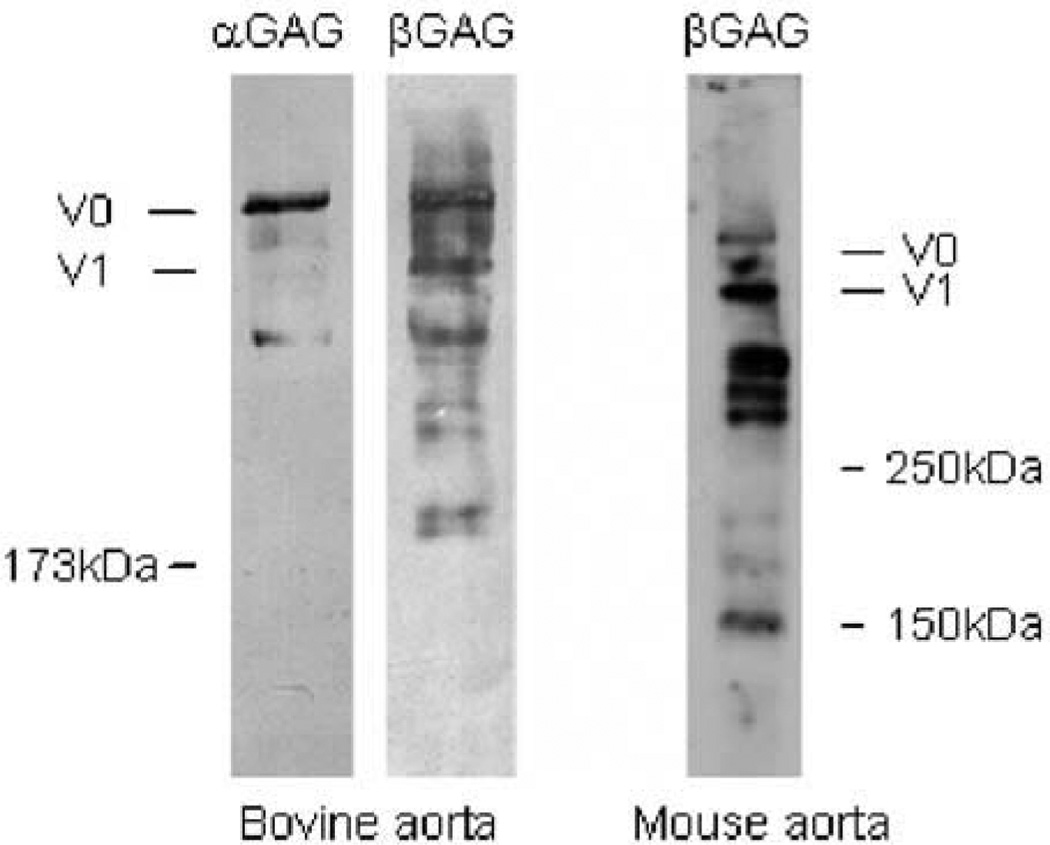
Although most attention has been paid to the synthesis and function of full-length versican and glycovariants, there is also evidence for the accumulation of proteolytic fragments of versican in the vessel wall. For example, in addition to the full-length core protein (at >350 kDa), we observed strongly reactive bands migrating at 160 and 300 kDa in Western blots of deglycosylated extracts of normal human aorta (Sandy et al. 2001). Such lower molecular weight species were also observed in conditioned medium of cultured human smooth muscle cells (SMCs) (Sandy et al. 2001) and in human atherosclerotic plaques (Formato et al. 2003) and aneurysms (Theocharis et al. 2003). Moreover, distinct fragments of versican were detected in normal bovine and mouse aortas (Figure 2). These observations underline the importance of investigating the pathways involved in versican catabolism in vascular tissue and of delineating the functional role of these proteolytic fragments in both normal and diseased blood vessels.
Figure 2.
Western blot analysis of murine and bovine aortic versican. Total proteoglycans from murine and bovine aorta were purified by DEAE Sephacel chromatography. The eluted proteins were digested with chondroitin ABC lyase, subjected to gradient SDS polyacrylamide gel electrophoresis, and transferred to nitrocellulose membranes. The transferred bovine and murine proteins were detected with antibodies specific for bovine versican αGAG and βGAG (from Dr. Dieter Zimmermann, Zurich, Switzerland) and mouse versican βGAG (Chemicon International, Temecula, CA), respectively.
Proteinases Capable of Cleaving Versican
Several proteinase families are capable of producing the fragments of versican observed in vivo in the arterial wall. For example, matrix metalloproteinase (MMP)-1 (Perides et al. 1995), -2 (Passi et al. 1999), -3 (Perides et al. 1995, Halpert et al. 1996), -7 (Halpert et al. 1996), and -9 (Passi et al. 1999) have been shown to degrade native, purified versican in vitro. Whereas MMP-8 cleaves aggrecan, the activity of this MMP against versican has not been studied. Plasmin has been shown to degrade native versican in vitro (Kenagy et al. 2002). Finally, ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs)-1 (Sandy et al. 2001, Jonsson-Rylander et al. 2005), -4 (Sandy et al. 2001), -5 (unpublished observations in Cross et al.; Cross et al. 2005), and -9 (Somerville et al. 2003) have been reported to cleave either native versican or versican peptide substrates, whereas ADAMTS-8 and -15 are candidates based on sequence similarity (Nicholson et al. 2005).
Versican in Intimal Hyperplasia
As treatments for the clinical symptoms of atherosclerosis, all forms of reconstruction of small arteries, including angioplasty, atherectomy, endarterectomy, stent angioplasty, and synthetic and vein bypass grafting, fail frequently because of lumenal narrowing, resulting in reduction in blood flow and thrombosis (Kester and Waybill 2001). Lumenal narrowing (stenosis or restenosis) is the consequence of intimal hyperplasia and pathologic remodeling. Remodeling refers to a change in the lumenal dimensions of the blood vessel, not attributable to vasospasm, vasodilation, or a change in wall area. Remodeling can be favorable (compensatory, positive, or outward) or unfavorable (pathologic, negative, or inward). Both clinical and animal model studies indicate that the relative importance of remodeling and intimal hyperplasia depends on the type of reconstruction performed. Restenosis after angioplasty is largely attributable to pathologic remodeling (a form of chronic constriction) with only minimal intimal hyperplasia. In contrast, restenosis after stent angioplasty or synthetic bypass grafting is caused by intimal hyperplasia alone, as the reconstructed vessel is rigid and cannot undergo remodeling. Intimal hyperplasia, because of its central role in the failure of stented arteries and bypass grafts, is a significant issue because these operations numbered 1,541,000 in 2000 in the US alone (from the CDC at http://www.cdc.gov/nchs/fastats/pdf/ad329t8.pdf), and of these ~30% fail (Kester and Waybill 2001). Whereas the use of drug-eluting stents lowers the risk of revascularization at 12 months by ~70%, even these interventions fail at a rate of 3% to 8%.
Pharmacologic therapy to prevent lumenal stenosis or restenosis after vascular reconstruction has been directed at inhibiting intimal hyperplasia and SMC growth. An alternative approach might be to induce intimal atrophy after lumenal narrowing has developed. This approach would be particularly useful for treating stenosis in stented vessels or synthetic bypass grafts, as intimal hyperplasia is the only mechanism for lumenal narrowing. Furthermore, it would enable the physician to treat the population of patients (up to 30%) who actually develop a problem with stenosis or restenosis. Of particular interest from the perspective of this review, a consistent feature of acute intimal hyperplasia is the increased content of versican (Nikkari et al. 1994, Finn et al. 2002, Kenagy et al. 2005, Chung et al. 2002, Farb et al. 2004). Therefore, understanding the mechanisms of intimal regression and the role of versican in this process is of great interest.
Versican Metabolism in Intimal Regression
Regression of the neointima has been reported in several systems. In the rat carotid artery model of balloon catheter-mediated injury, neointimal hyperplasia occurs over a period of approximately 4 weeks and is followed by neointimal regression over the next 4 weeks (Nuthakki et al. 2004). Although data are not available on the fate of versican during the regression process in this model, it is of interest to note that immunohistochemical analysis of versican (using 2B1 against the G3 globular domain) in human arteries after balloon angioplasty showed an accumulation of versican during the first 3 months and a decrease thereafter (Imanaka-Yoshida et al. 2001, Matsuura et al. 1996). A similar pattern was also observed in nonhuman primate arteries after balloon angioplasty (Geary et al. 1998). Like angioplasty, stent placement also initially stimulates intimal hyperplasia in animal models and human arteries. Intimal atrophy occurs spontaneously in stented arteries after 6 months in humans (Asakura et al. 1998) and 2 months in rats (Finn et al. 2002, Langeveld et al. 2004). At these late times during regression, the extracellular matrix (ECM) shows a relative loss of versican and a gain of collagen compared to earlier times (Chung et al. 2002, Finn et al. 2002, Langeveld et al. 2004, Farb et al. 2004). These data suggest that versican accumulates during neointimal progression and is lost during neointimal regression in the native artery.
Polytetrafluoroethylene Graft Neointimal Regression
Polytetrafluoroethylene (PTFE; GORETEX, Teflon) vascular grafts are used for revascularization and reconstructive procedures primarily in large (>6 mm diameter) vessels, such as in aorto-iliac bypass grafting. These grafts are like stented arteries in several respects. In both forms of reconstruction, there is a fixed geometry and inability to alter lumenal dimensions by altering overall size. In addition, there is an inflammatory foreign body response in and around the graft material or stent (Berceli et al. 2002, Inoue et al. 2002). At late times, SMCs proliferate near the endothelial cells of the lumen (Berceli et al. 2002, Inoue et al. 2002). Finally, the neointima formed has a versican-rich matrix (Inoue et al. 2002, Finn et al. 2002).
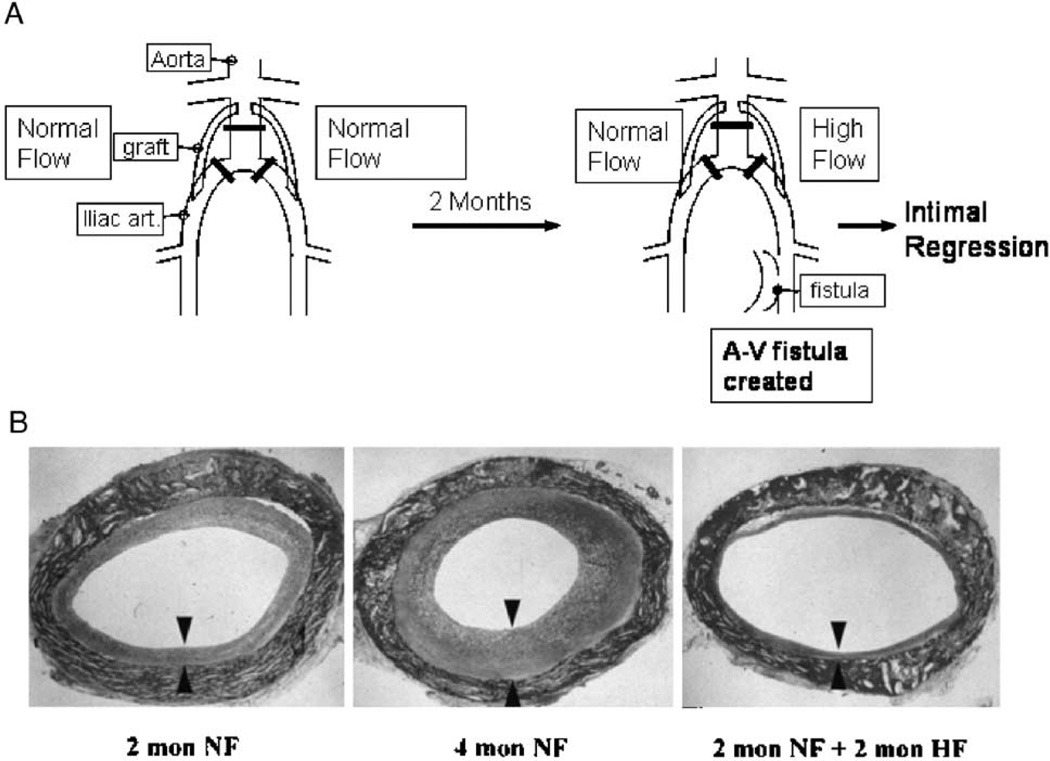
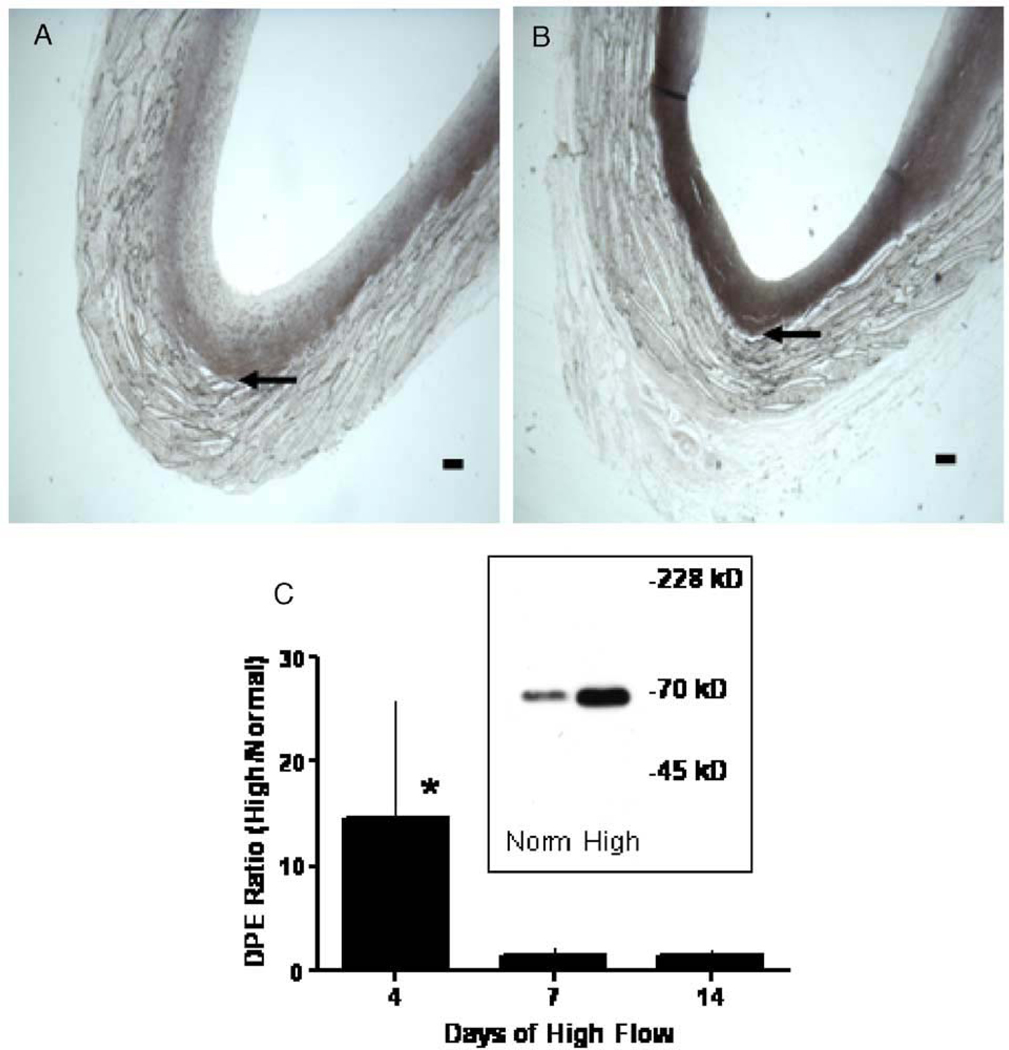
We have observed in our studies in baboon PTFE grafts that normal blood flow is associated with increased neointimal thickening and increased blood flow with decreased thickening (Mattsson et al. 1997). In 60 µm, unwrapped, PTFE grafts healing occurs by the ingrowth of capillaries along the entire graft, with a complete endothelial cell lining forming within 2 weeks and maximum neointima by 8 weeks. When this maximum neointima is subjected to high blood flow by the addition of a femoral arteriovenous fistula, the neointima begins to atrophy (Berceli et al. 2002, Mattsson et al. 1997) (Figure 3). Smooth muscle cell apoptosis increases and SMC proliferation decreases by 1 day after fistula placement (Berceli et al. 2002, Kenagy et al. 2002). There is also evidence of ECM loss. For example, by 4 days there is an increase in the amount of a 70-kDa N-terminal G1 fragment of versican V1 (Figure 4) (Kenagy et al. 2005), which is produced by ADAMTS cleavage of versican (Sandy et al. 2001). The presence of increased urokinase and plasmin could further contribute to the degradation of versican (Kenagy et al. 2002). By 14 days, there is a loss of GAG from the tissue (Figure 5), which is most likely due to loss of chondroitin sulfate/dermatan sulfate proteoglycans, including versican (Kenagy et al. 2005). Thus, there is evidence of a loss of chondroitin sulfate and of versican cleavage. Whereas staining of versican core protein with a polyclonal antibody or with a monoclonal antibody to the G3 domain (2B1) is not demonstrably altered by high flow, immunostaining with an antibody affinity-purified to the βGAG (GAG containing) domain of versican was decreased at 2 months (Kenagy et al. 2005). These immunohistochemical data suggest that versican is first cleaved to G1 and βGAG-G3 fragments, that CS chains are lost from the GAG domain, and that finally the βGAG domain is lost. One of several examples of the necessity of the sequential activity of different proteases against a substrate includes aggrecan (Arner 2002).
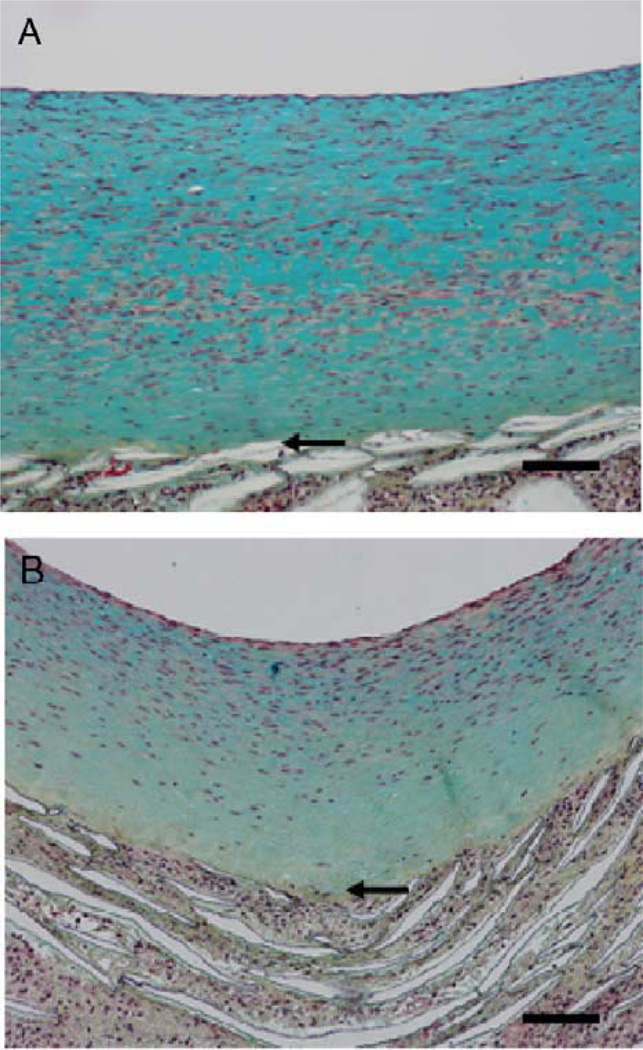
Figure 3.
(A) Schematic of the baboon bilateral aorto-iliac PTFE graft model of high blood flow-mediated intimal regression. (B) Hematoxylin–eosin-stained cross-sections of PTFE grafts after 2 months of normal blood flow (NF; left panel), 4 months of normal blood flow (center panel), and 2 months of normal flow followed by 2 months of high blood flow (HF; right panel). The arrow heads demarcate the neointima (bottom panel from Mattsson et al. 1997 with permission from Lippincott, Williams & Wilkins).
Figure 4.
Effect of high blood flow on the 70-kDa G1 DPEAAE fragment of versican in the PTFE graft neointima. Cross sections of PTFE grafts obtained 4 days after switching to high blood flow (B) or maintaining normal flow (A) were immunostained for DPEAAE. Arrows indicate the neointimal–PTFE boundary. The bars represent 0.1 mm. Western blots of the 70-kDa G1 DPEAAE fragment were developed by chemiluminescence (inset of panel C; from extracts of 4 day grafts) and quantified by scanning (C). Data are presented as the ratio of high flow values to normal flow values (DPE ratio–high/normal) (from Kenagy et al. 2005 by permission from the Histochemical Society, Inc.).
Figure 5.
Movat’s pentachrome stain of cross sections of PTFE grafts obtained 14 days after switching to high blood flow (B) or maintaining normal flow (A). The blue stain is GAG staining by Alcian blue in the Movat’s procedure. Arrows indicate the neointimal–PTFE boundary. The bars represent 0.1 mm (from Kenagy et al. 2005 by permission from the Histochemical Society, Inc.).
Possible Role of Versican Breakdown Products in Vascular Disease
Overexpression experiments suggest that versican V1 stimulates cell proliferation, whereas versican V2 inhibits proliferation (Sheng et al. 2005). The converse experiments using antisense or siRNA also indicate that endogenous versican V1 increases proliferation, including that of SMCs (Zhang et al. 1999, Huang et al. 2006) (unpublished data, Rahmani M, Wong B, Allahverdian S, Cheung C, Carthy J, Keire P, Wight T, McManus B). These data are consistent with evidence that formation of versican-hyaluronan pericellular matrix is required for SMC proliferation and migration (Evanko et al. 1999). Versican V1 may enhance proliferation via increased levels of the EGF receptor and ERK activation coupled with loss of the cell-cycle inhibitor p27 (unpublished data, Rahmani M, Wong B, Allahverdian S, Cheung C, Carthy J, Keire P, Wight T, McManus B) (Sheng et al. 2005). V1 also inhibits 3T3 cell death (Sheng et al. 2005). Whether these effects require intact versican or are mediated by cleavage fragments (matricryptins), as with ADAMTS4-mediated effects of brevican on glioma invasiveness (Matthews et al. 2000), is not known. The observations that ADAMTS-mediated cleavage of versican in the graft neointima is a regulated process and that G1 and G3 core protein fragments are retained in this, as well as in normal (Sandy et al. 2001) and diseased (Formato et al. 2003) vessels, suggest that these fragments may be functional. What evidence is there for this?
Versican Core Protein Functions
Zhang et al. (1998) have explored the functional activities of individual domains of versican by transfecting mutant forms of chicken versican into several types of cells. Of more relevance to the question of the function of cleavage fragments are the results of experiments in which these investigators added soluble recombinant versican domains to cultured cells.
The soluble G3 domain increases growth of several types of cells, including 3T3 fibroblasts and endothelial cells (Zhang et al. 1998, Zheng et al. 2004b, Wu et al. 2001). This effect requires the EGF domain, as G3 without the EGF domain can be antiproliferative (an effect duplicated by the CRP domain alone) (Wu et al. 2001). Of interest, G3 without the EGF domain also inhibits endogenous versican secretion (Zhang et al. 1999) (unpublished data; Rahmani M, Wong B, Allahverdian S, Cheung C, Carthy J, Keire P, Wight T, McManus B) and antisense and siRNA to versican inhibit proliferation. The G3 domain binds to integrin β1 (by a non-RGD mechanism), increases focal adhesion kinase activation, and protects against apoptosis (Wu et al. 2002), the latter effect also observed with native versican V1 (Sheng et al. 2005). Both the CRP and LC domains (see Figure 1) are required for β1 integrin binding and focal adhesion kinase activation. Like the G3 domain, the soluble G1 domain also increases cell proliferation, in part by decreasing the adhesion strength of cells to the matrix (Yang et al. 1999). The G3 domain also increases migration of endothelial cells and the secretion of fibronectin and vascular endothelial growth factor, both of which are chemotactic for SMCs (Zheng et al. 2004b). The G3 domain has also been implicated in macrophage aggregation, as it has been shown to bind PSGL-1 (Zheng et al. 2004a), and this activity could turn out to be critically important in promoting leukocyte accumulation during the inflammatory phases in developing lesions of atherosclerosis.
The ability of versican to bind numerous ECM proteins suggests the possibility that fragments of versican could function as competitors for such interactions. For example, the G1 domain binds to hyaluronan and link protein to form complexes, a process that may be disrupted by formation of the 70-kDa DPE G1 fragment by ADAMTS activity. Fibulin binds to versican via the EGF repeats in the G3 domain and may act to cross-link the versican–hyaluronan complexes in the intima (Olin et al. 2001). Moreover, we have demonstrated that versican V3, which lacks both GAG domains with their chondroitin sulfate chains (Figure 1), has a dramatic effect on SMC elastin synthesis and fibril formation in vitro and in vivo (Merrilees et al. 2002). Overexpression of V3 by SMCs causes increased adhesion to the substratum and decreased migration and proliferation (Lemire et al. 2002), which is the opposite observed when versican V1 is overexpressed in fibroblasts (Sheng et al. 2005). V3 may compete with V1 for hyaluronan, which would lead to versican–hyaluronan complexes lacking CS chains and a much smaller pericellular “coat” (Lemire et al. 2002). Further work will be necessary to determine the effects of specific fragments of versican on elastin fibril formation by SMCs.
Versican GAG Chain Functions
By virtue of their polyanionic nature, the chondroitin sulfate chains attached to the αGAG and βGAG domains of versican are involved in a range of functions. This includes sequestration of chemokines, such as stromal cell-derived factor-1 and MCP-1, resulting in decreased activity (Hirose et al. 2001). Binding of versican to CD44, L selectin, and P selectin is also mediated by the chondroitin sulfate chains (Kawashima et al. 2002). The chondroitin sulfate chains of versican bind large amounts of water, which is critical for maintenance of a reversibly compressible tissue. In addition, by altering the balance of free water available to proteins versican CS chains can also alter protein–protein interactions. For example, McGee and Wagner (2003) suggest that changes in CS chains can alter factor Xa–anti-thrombin–CS interactions and influence the thrombotic balance in atherosclerotic tissue (Di Cera 2003). The content of chondroitin sulfate in the tissue could be altered by proteolysis of the versican core protein with subsequent loss of fragments, a process widely studied in cartilage with regard to aggrecan. Extracellular degradation of GAG chains, such as hyaluronan and heparan sulfate, has been demonstrated with the resultant oligosaccharides exhibiting potent cell-regulatory activities (Kato et al. 1998). Whereas members of the hyaluronidase family, such as Hyal-1 and Hyal-4, exhibit specific and potent chondroitinase activities (Csoka and Frost 2001), there is no information on the presence or regulation of these enzymes after arterial injury or during graft healing. It is interesting to note that both heparan sulfate and chondroitin sulfate decrease within 6 h after arterial injury in the rabbit, which could be the result of both proteolysis and GAG hydrolysis (Bingley et al. 2001).
Future Directions
We have reviewed work that suggests that cleavage of versican into discrete fragments may occur not only as normal physiologic turnover of the vascular extracellular matrix, but also be involved in vascular pathology (Figure 6). The 70-kDa G1 fragment of versican formed by ADAMTS activity at the Glu441–Ala442 bond of V1 has been documented (Sandy et al. 2001), whereas other potential ADAMTS cleavage sites, such as Tyr423–Ile424 of V1, have been proposed based on activity against peptide substrates (Jonsson-Rylander et al. 2005). There is increasing evidence for the role of ADAMTS enzymes in the catabolic modification of versican, but further work is necessary to characterize the complete panel of proteolytic enzymes involved in versican cleavage in vascular tissue and their activation patterns in the various forms and stages of vascular pathologies. Moreover, it is important to characterize the cleavage products of versican in vascular tissue and to determine what, if any, effects these proteolytic fragments have on cellular activities during tissue remodeling, such as SMC or endothelial cell proliferation, macrophage aggregation and adhesion, apoptosis, migration or extracellular matrix deposition and resorption. Finally, identification of the catabolic network involved in versican metabolism may provide novel therapeutic targets for the prevention of chronic vascular disease.
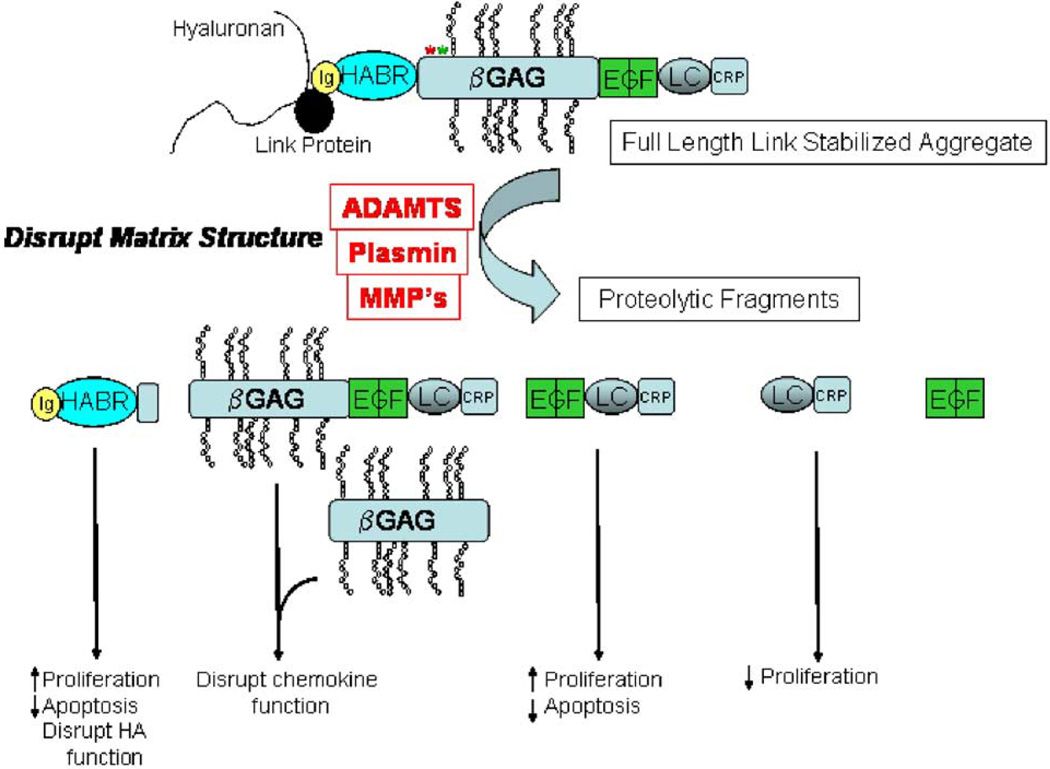
Figure 6.
Schematic of possible cleavage products of versican and possible effects of these products on vascular smooth muscle cells. Ig, Immunoglobulin-like domain; HABR, hyaluronan binding region; αGAG, α glycosaminoglycan binding domain; βGAG, β glycosaminoglycan binding domain; EGF, EGF-like domain; LC, C-type lectin domain; CRP, complement regulatory protein-like domain. The cleavage at Glu441–Ala442 (green asterisk) has been documented in vivo (Sandy et al. 2001), whereas potential cleavage by ADAMTS1 at Tyr423–Ile424 (red asterisk) has been reported (Jonsson-Rylander et al. 2005).
Acknowledgments
NIH HL18645 (TNW), HL30946 (RDK), Dianne Lynn Family Foundation (AP).
Footnotes
Gene symbol: CSPG2, chondroitin sulfate proteoglycan 2; accession number: NM_004385. Source: GeneReport: http://genome-www5.stanford.edu/cgi-bin/source/sourceResult?option=Number&criteria=NM_004385&choice=Gene).
Contributor Information
Richard D. Kenagy, Department of Surgery, Seattle, WA 98109-4714, USA.
Anna H. Plaas, Department of Internal Medicine, University of South Florida, Tampa, FL 33612, USA
Thomas N. Wight, Hope Heart Program, Benaroya Research Institute at Virginia Mason, Seattle, WA 98101-2795, USA
References
- Arner EC. Aggrecanase-mediated cartilage degradation. Curr Opin Pharmacol. 2002;2:322–329. doi: 10.1016/s1471-4892(02)00148-0. [DOI] [PubMed] [Google Scholar]
- Asakura M, Ueda Y, Nanto S, et al. Remodeling of in-stent neointima, which became thinner and transparent over 3 years—serial angiographic and angioscopic follow-up. Circulation. 1998;97:2003–2006. doi: 10.1161/01.cir.97.20.2003. [DOI] [PubMed] [Google Scholar]
- Berceli SA, Davies MG, Kenagy RD, et al. Flow-induced neointimal regression in baboon polytetrafluoroethylene grafts is associated with decreased cell proliferation and increased apoptosis. J Vasc Surg. 2002;36:1248–1255. doi: 10.1067/mva.2002.128295. [DOI] [PubMed] [Google Scholar]
- Bingley JA, Hayward IP, Campbell GR, et al. Relationship of glycosaminoglycan and matrix changes to vascular smooth muscle cell phenotype modulation in rabbit arteries after acute injury. J Vasc Surg. 2001;33:155–164. doi: 10.1067/mva.2001.109774. [DOI] [PubMed] [Google Scholar]
- Cattaruzza S, Schiappacassi M, Ljungberg R, et al. Distribution of PG-M/versican variants in human tissues and de novo expression of isoform V3 upon endothelial cell activation, migration, and neoangiogenesis in vitro. J Biol Chem. 2002;277:47626–47635. doi: 10.1074/jbc.M206521200. [DOI] [PubMed] [Google Scholar]
- Chung IM, Gold HK, Schwartz SM, et al. Enhanced extracellular matrix accumulation in restenosis of coronary arteries after stent deployment. J Am Coll Cardiol. 2002;40:2072–2081. doi: 10.1016/s0735-1097(02)02598-6. [DOI] [PubMed] [Google Scholar]
- Cross NA, Chandrasekharan S, Jokonya N, et al. The expression and regulation of ADAMTS-1, -4, -5, -9, and -15, and TIMP-3 by TGFbeta1 in prostate cells: relevance to the accumulation of versican. Prostate. 2005;63:269–275. doi: 10.1002/pros.20182. [DOI] [PubMed] [Google Scholar]
- Csoka AB, Frost GI, Stern R. The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol. 2001;20:499–508. doi: 10.1016/s0945-053x(01)00172-x. [DOI] [PubMed] [Google Scholar]
- Di Cera E. Atherosclerosis: testing the water. Arterioscler Thromb Vasc Biol. 2003;23:1713–1714. doi: 10.1161/01.ATV.0000090960.74185.DB. [DOI] [PubMed] [Google Scholar]
- Evanko SP, Angello JC, Wight TN. Formation of hyaluronan- and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:1004–1013. doi: 10.1161/01.atv.19.4.1004. [DOI] [PubMed] [Google Scholar]
- Farb A, Kolodgie FD, Hwang JY, et al. Extracellular matrix changes in stented human coronary arteries. Circulation. 2004;110:940–947. doi: 10.1161/01.CIR.0000139337.56084.30. [DOI] [PubMed] [Google Scholar]
- Finn AV, Gold HK, Tang A, et al. A novel rat model of carotid artery stenting for the understanding of restenosis in metabolic diseases. J Vasc Res. 2002;39:414–425. doi: 10.1159/000064518. [DOI] [PubMed] [Google Scholar]
- Formato M, Farina M, Spirito R, et al. Evidence for a proinflammatory and proteolytic environment in plaques from endarterectomy segments of human carotid arteries. Arterioscler Thromb Vasc Biol. 2003;24:129–135. doi: 10.1161/01.ATV.0000104013.71118.53. [DOI] [PubMed] [Google Scholar]
- Geary RL, Nikkari ST, Wagner WD, et al. Wound healing: a paradigm for lumen narrowing after arterial reconstruction. J Vasc Surg. 1998;27:96–106. doi: 10.1016/s0741-5214(98)70296-4. [DOI] [PubMed] [Google Scholar]
- Halpert I, Sires UI, Roby JD, et al. Matrilysin is expressed by lipid-laden macrophages at sites of potential rupture in atherosclerotic lesions and localizes to areas of versican deposition, a proteoglycan substrate for the enzyme. Proc Natl Acad Sci U S A. 1996;93:9748–9753. doi: 10.1073/pnas.93.18.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose J, Kawashima H, Yoshie O, et al. Versican interacts with chemokines and modulates cellular responses. J Biol Chem. 2001;276:5228–5234. doi: 10.1074/jbc.M007542200. [DOI] [PubMed] [Google Scholar]
- Huang R, Merrilees MJ, Braun K, et al. Inhibition of versican synthesis by antisense alters smooth muscle cell phenotype and induces elastic fiber formation in vitro and in neointima after vessel injury. Circ Res. 2006;98:370–377. doi: 10.1161/01.RES.0000202051.28319.c8. [DOI] [PubMed] [Google Scholar]
- Imanaka-Yoshida K, Matsuura R, Isaka N, et al. Serial extracellular matrix changes in neointimal lesions of human coronary artery after percutaneous transluminal coronary angioplasty: clinical significance of early tenascin-C expression. Virchows Arch Int J Pathol. 2001;439:185–190. doi: 10.1007/s004280000390. [DOI] [PubMed] [Google Scholar]
- Inoue S, Koyama H, Miyata T, et al. Pathogenetic heterogeneity of in-stent lesion formation in human peripheral arterial disease. J Vasc Surg. 2002;35:672–678. doi: 10.1067/mva.2002.122021. [DOI] [PubMed] [Google Scholar]
- Jonsson-Rylander AC, Nilsson T, Fritsche-Danielson R, et al. Role of ADAMTS-1 in atherosclerosis: remodeling of carotid artery, immunohistochemistry, and proteolysis of versican. Arterioscler Thromb Vasc Biol. 2005;25:180–185. doi: 10.1161/01.ATV.0000150045.27127.37. [DOI] [PubMed] [Google Scholar]
- Kato M, Wang HM, Kainulainen V, et al. Physiological degradation converts the soluble syndecan-1 ectodomain from an inhibitor to a potent activator of FGF-2. Nat Med. 1998;4:691–697. doi: 10.1038/nm0698-691. [DOI] [PubMed] [Google Scholar]
- Kawashima H, Atarashi K, Hirose M, et al. Oversulfated chondroitin/dermatan sulfates containing GlcAbeta1/IdoAalpha1–3GalNAc (4,6-O-disulfate) interact with L-and P-selectin and chemokines. J Biol Chem. 2002;277:12921–12930. doi: 10.1074/jbc.M200396200. [DOI] [PubMed] [Google Scholar]
- Kenagy RD, Fischer JW, Davies MG, et al. Increased plasmin and serine proteinase activity during flow-induced intimal atrophy in baboon PTFE grafts. Arterioscler Thromb Vasc Biol. 2002;22:400–404. doi: 10.1161/hq0302.105376. [DOI] [PubMed] [Google Scholar]
- Kenagy RD, Fischer JW, Lara S, et al. Accumulation and loss of extracellular matrix during shear stress-mediated intimal growth and regression in baboon vascular grafts. J Histochem Cytochem. 2005;53:131–140. doi: 10.1369/jhc.4A6493.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kester M, Waybill P, Kozak M. New strategies to prevent restenosis. Am J Cardiovasc Drugs. 2001;1:77–83. doi: 10.2165/00129784-200101020-00001. [DOI] [PubMed] [Google Scholar]
- Kolodgie FD, Burke AP, Wight TN, et al. The accumulation of specific types of proteoglycans in eroded plaques: a role in coronary thrombosis in the absence of rupture. Curr Opin Lipidol. 2004;15:575–582. doi: 10.1097/00041433-200410000-00012. [DOI] [PubMed] [Google Scholar]
- Langeveld B, Roks AJM, Tio RA, et al. Rat abdominal aorta stenting: a new and reliable small animal model for in-stent restenosis. J Vasc Res. 2004;41:377–386. doi: 10.1159/000080891. [DOI] [PubMed] [Google Scholar]
- Lemire JM, Braun KR, Maurel P, et al. Versican/PG-M isoforms in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:1630–1639. doi: 10.1161/01.atv.19.7.1630. [DOI] [PubMed] [Google Scholar]
- Lemire JM, Merrilees MJ, Braun KR, et al. Overexpression of the V3 variant of versican alters arterial smooth muscle cell adhesion, migration, and proliferation in vitro. J Cell Physiol. 2002;190:38–45. doi: 10.1002/jcp.10043. [DOI] [PubMed] [Google Scholar]
- Matsuura R, Isaka N, Imanaka-Yoshida K, et al. Deposition of PG-M/versican is a major cause of human coronary restenosis after percutaneous transluminal coronary angioplasty. J Pathol. 1996;180:311–316. doi: 10.1002/(SICI)1096-9896(199611)180:3<311::AID-PATH657>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Matthews RT, Gary SC, Zerillo C, et al. Brain-enriched hyaluronan binding (BEHAB)/brevican cleavage in a glioma cell line is mediated by a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) family member. J Biol Chem. 2000;275:22695–22703. doi: 10.1074/jbc.M909764199. [DOI] [PubMed] [Google Scholar]
- Mattsson EJR, Kohler TR, Vergel SM, et al. Increased blood flow induces regression of intimal hyperplasia. Arterioscler Thromb Vasc Biol. 1997;17:2245–2249. doi: 10.1161/01.atv.17.10.2245. [DOI] [PubMed] [Google Scholar]
- McGee M, Wagner WD. Chondroitin sulfate anticoagulant activity is linked to water transfer: relevance to proteoglycan structure in atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:1921–1927. doi: 10.1161/01.ATV.0000090673.96120.67. [DOI] [PubMed] [Google Scholar]
- Merrilees MJ, Lemire JM, Fischer JW, et al. Retrovirally mediated overexpression of versican V3 by arterial smooth muscle cells induces tropoelastin synthesis and elastic fiber formation in vitro and in neointima after vascular injury. Circ Res. 2002;90:481–487. doi: 10.1161/hh0402.105791. [DOI] [PubMed] [Google Scholar]
- Nicholson AC, Malik SB, Logsdon JM, et al. Functional evolution of ADAMTS genes: evidence from analyses of phylogeny and gene organization. BMC Evol Biol. 2005;5:11. doi: 10.1186/1471-2148-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikkari ST, Järveläinen HT, Wight TN, et al. Smooth muscle cell expression of extracellular matrix genes after arterial injury. Am J Pathol. 1994;144:1348–1356. [PMC free article] [PubMed] [Google Scholar]
- Nuthakki VK, Fleser PS, Malinzak LE, et al. Lysyl oxidase expression in a rat model of arterial balloon injury. J Vasc Surg. 2004;40:123–129. doi: 10.1016/j.jvs.2004.02.028. [DOI] [PubMed] [Google Scholar]
- Olin AI, Morgelin M, Sasaki T, et al. The proteoglycans aggrecan and versican form networks with fibulin-2 through their lectin domain binding. J Biol Chem. 2001;276:1253–1261. doi: 10.1074/jbc.M006783200. [DOI] [PubMed] [Google Scholar]
- Passi A, Negrini D, Albertini R, et al. The sensitivity of versican from rabbit lung to gelatinase A (MMP-2) and B (MMP-9) and its involvement in the development of hydraulic lung edema. FEBS Lett. 1999;456:93–96. doi: 10.1016/s0014-5793(99)00929-1. [DOI] [PubMed] [Google Scholar]
- Perides G, Asher RA, Lark MW, et al. Glial hyaluronate-binding protein: a product of metalloproteinase digestion of versican? Biochem J. 1995;312:377–384. doi: 10.1042/bj3120377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy JD, Westling J, Kenagy RD, et al. Versican V1 proteolysis in human aorta in vivo occurs at the Glu445-Ala446 bond a site which is cleaved by recombinant ADAMTS-1 and ADAMTS-4. J Biol Chem. 2001;276:13372–13378. doi: 10.1074/jbc.M009737200. [DOI] [PubMed] [Google Scholar]
- Sheng W, Wang GZ, Wang YL, et al. The roles of versican V1 and V2 isoforms in cell proliferation and apoptosis. Mol Biol Cell. 2005;16:1330–1340. doi: 10.1091/mbc.E04-04-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville RP, Longpre JM, Jungers KA, et al. Characterization of ADAMTS-9 and ADAMTS-20 as a distinct ADAMTS subfamily related to Caenorhabditis elegans GON-1. J Biol Chem. 2003;278:9503–9513. doi: 10.1074/jbc.M211009200. [DOI] [PubMed] [Google Scholar]
- Theocharis AD, Tsolakis I, Hjerpe A, et al. Versican undergoes specific alterations in the fine molecular structure and organization in human aneurysmal abdominal aortas. Biomed Chromatogr. 2003;17:411–416. doi: 10.1002/bmc.263. [DOI] [PubMed] [Google Scholar]
- Wight TN. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr Opin Cell Biol. 2002;14:617–623. doi: 10.1016/s0955-0674(02)00375-7. [DOI] [PubMed] [Google Scholar]
- Wight TN, Merrilees MJ. Proteoglycans in atherosclerosis and restenosis: key roles for versican. Circ Res. 2004;94:1158–1167. doi: 10.1161/01.RES.0000126921.29919.51. [DOI] [PubMed] [Google Scholar]
- Wight TN, Lara S, Riessen R, et al. Selective deposits of versican in the extracellular matrix of restenotic lesions from human peripheral arteries. Am J Pathol. 1997;151:963–973. [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Zhang Y, Cao L, et al. Identification of the motif in versican G3 domain that plays a dominant-negative effect on astrocytoma cell proliferation through inhibiting versican secretion and binding. J Biol Chem. 2001;276:14178–14186. doi: 10.1074/jbc.M100618200. [DOI] [PubMed] [Google Scholar]
- Wu Y, Chen L, Zheng PS, et al. beta 1-Integrin-mediated glioma cell adhesion and free radical-induced apoptosis are regulated by binding to a C-terminal domain of PG-M/versican. J Biol Chem. 2002;277:12294–12301. doi: 10.1074/jbc.M110748200. [DOI] [PubMed] [Google Scholar]
- Yang BL, Zhang Y, Cao L, et al. Cell adhesion and proliferation mediated through the G1 domain of versican. J Cell Biochem. 1999;72:210–220. doi: 10.1002/(sici)1097-4644(19990201)72:2<210::aid-jcb5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cao L, Kiani C, et al. Promotion of chondrocyte proliferation by versican mediated by G1 domain and EGF-like motifs. J Cell Biochem. 1999;73:445–457. [PubMed] [Google Scholar]
- Zhang Y, Cao L, Yang BL, et al. The G3 domain of versican enhances cell proliferation via epidermal growth factor-like motifs. J Biol Chem. 1998;273:21342–21351. doi: 10.1074/jbc.273.33.21342. [DOI] [PubMed] [Google Scholar]
- Zheng PS, Vais D, Lapierre D, et al. PG-M/versican binds to P-selectin glycoprotein ligand-1 and mediates leukocyte aggregation. J Cell Sci. 2004a;117:5887–5895. doi: 10.1242/jcs.01516. [DOI] [PubMed] [Google Scholar]
- Zheng PS, Wen J, Ang LC, et al. Versican/PG-M G3 domain promotes tumor growth and angiogenesis. FASEB J. 2004b;18:754–756. doi: 10.1096/fj.03-0545fje. [DOI] [PubMed] [Google Scholar]
- Zimmermann DR, Ruoslahti E. Multiple domains of the large fibroblast proteoglycan, versican. EMBO J. 1989;8:2975–2981. doi: 10.1002/j.1460-2075.1989.tb08447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]