Abstract
Background
Previous studies have demonstrated, and replicated, an association between single nucleotide polymorphisms (SNPs) within the GABRA2 gene and risk for alcohol dependence. The present study examines the association of a GABRA2 SNP with another definition of alcohol involvement and with the effects of psychosocial treatment.
Methods
European-American subjects (n=812, 73.4% male) provided DNA samples for the analysis. All were participants in Project MATCH, a multi-center randomized clinical trial evaluating the efficacy of three types of psychosocial treatment for alcoholism: Cognitive Behavioral Therapy (CBT), Motivational Enhancement Therapy (MET), or Twelve Step Facilitation (TSF). The daily probabilities of drinking and heavy drinking were estimated during the 12-week treatment and 12-month post-treatment periods.
Results
Subjects homozygous for the allele associated with low risk for alcohol dependence in previous studies had lower daily probabilities of drinking and heavy drinking in the present study. This low-risk allele was also associated with a greater difference in drinking outcomes between the treatments. In addition, it enhanced the relative superiority of TSF over CBT and MET. Population stratification was excluded as a confound using ancestry informative marker analysis.
Conclusions
The assessment of genetic vulnerability may be relevant to studies of the efficacy of psychosocial treatment: GABRA2 genotype modifies the variance in drinking and can therefore moderate power for resolving differences between treatments.
Keywords: GABA(A) receptor, GABRA2, alcohol dependence, alcoholism treatment
INTRODUCTION
Alcohol dependence (AD), a common disorder associated with substantial adverse medical and psychosocial consequences (Caetano and Cunradi 2002; Grant et al., 2004), has been shown to be largely (i.e., 52–64%) heritable (Kendler 2001). Efforts to identify genes that increase risk for the disorder have included genome-wide linkage scans, which implicated a region on chromosome 4p12. The region contains a cluster of genes encoding subunits of the GABA-A receptor (Long et al., 1998; Reich et al., 1998,). Edenberg et al. (Edenberg et al., 2004) mapped this gene cluster in detail in multiplex alcoholism families and found significant associations for multiple markers in the gene encoding the GABAAα-2 subunit [GABRA2 (MIM 137140)] and for a single marker in the adjacent GABRG1 (MIM 137166) gene, which encodes the GABAAγ-1 subunit. The association of GABRA2 markers with AD was subsequently replicated wholly or in part in five independent studies (Covault et al., 2004; Enoch et al., 2006; Fehr et al., 2006; Lappalainen et al., 2005); in most of these studies, an association of AD with a haplotype block spanning the central and 3′-regions of GABRA2 was observed.
The present study examined a representative marker of the AD-associated haplotype block in GABRA2 using DNA samples from alcohol-dependent patients who participated in Project MATCH, a psychotherapy study conducted at 10 sites around the United States (Project MATCH Research Group 1997; Project MATCH Research Group 1997). The project included two parallel, but independent, clinical arms in which patients were recruited from outpatient settings (n = 952; 72% male) or aftercare settings following inpatient or day hospital treatment (n = 774; 80% male). Each of the two arms involved random assignment to one of three manual-guided, individually delivered treatments: Motivational Enhancement Therapy (MET), Cognitive Behavioral Therapy (CBT), or Twelve-Step Facilitation (TSF). For the primary analyses (Project MATCH Research Group 1997; Project MATCH Research Group 1997), alcohol consumption during each of the prior 90 days was assessed at baseline, at the end of the 12-week treatment period, and quarterly over a 1-year post-treatment period using the Form 90 Interview (Tonigan et al., 1997).
One reason for examining the GABRA2 gene in Project MATCH patients was to determine if its predictive validity generalized beyond alcohol dependence to other measures of drinking behavior. More specifically, the present analysis tested the association of genotype with the daily probabilities of drinking and heavy drinking (men: ≥5 drinks/day; women: ≥4 drinks/day).
A second reason for examining variation in GABRA2 in these patients was to determine if it would predict treatment outcome. Although GABRA2 has not yet been studied as a treatment moderator, the existing literature provides indirect support for the hypothesis. GABRA2 has, for example, been associated with an excess amount of high frequency, fast β activity in the spontaneous electroencephalogram (Edenberg et al., 2004). Fast β EEG activity has, in other studies, been linked to poor outcome in alcohol- and drug-dependent individuals (Bauer 1994; Bauer 2001; Prichep et al., 1999; Saletu-Zyhlarz et al., 2004; Winterer et al., 1998). In addition, GABRA2 genotype has been associated with Conduct Disorder problems (Dick et al., 2006,) which have, in turn, also been linked to poor outcome (Myers et al., 1995). Thus, the potential exists for GABRA2 genotype to join the long list of clinical [e.g., severity of dependence (Bottlender and Soyka 2005), comorbid psychopathology (Ciraulo et al., 2003; Kushner et al., 2005; Pettinati et al., 1999)], psychological [e.g., self-efficacy, coping skills (Ciraulo et al., 2003; Maisto et al., 2000)], or biological [e.g., fast β EEG activity, sleep disturbance (Bauer 1994; Bauer 2001; Prichep et al., 1999; Saletu-Zyhlarz et al., 2004; Winterer et al., 1998; Brower et al., 1998; Brower et al., 2001; Clark et al., 1998; Drummond et al., 1998; Gillin et al., 1994)] variables for which a relationship to substance abuse treatment outcome has been demonstrated. If an association with GABRA2 could be demonstrated, then the field may benefit from an expansion of the theoretical framework for interpreting the causes of treatment failure. As a result, new models for treatment matching may emerge.
PATIENTS AND METHODS
Patients
Eight hundred twelve self-identified European-American patients from Project MATCH provided blood for DNA extraction and were included in the analysis. Five hundred forty patients were recruited from outpatient settings and 272 from aftercare settings. The patient sample can be generally described (Table 1) as 73.4% male and averaging 40.8 years of age. They completed, on average, 13.5 yrs of education.
Table 1.
Background characteristics of patients as a function of GABRA2 genotype and treatment assignment.
| High-Risk Genotype, CBT |
High-Risk Genotype, MET |
High-Risk Genotype, TSF |
Low-Risk Genotype, CBT |
Low-Risk Genotype, MET |
Low-Risk Genotype, TSF |
|
|---|---|---|---|---|---|---|
| Demographic and Alcohol-Related Features | N=171 | N=185 | N=191 | N=97 | N=75 | N=93 |
| Age, y (SD) | 41.2 (11.9) | 40.6 (11.7) | 41.2 (11.4) | 41.2 (11.9) | 40.0 (10.1) | 39.84 (10.9) |
| Education, y (SD) | 13.3 (1.9) | 13.7 (1.9) | 13.3 (2.1) | 13.3 (2.1) | 13.8 (2.2) | 13.3 (2.1) |
| Gender, % female | 33.3 | 24.9 | 27.2 | 21.6 | 25.3 | 22.6 |
| Days Abstinent, % (SD) | 29 (29) | 28 (28) | 29 (30) | 29 (28) | 34 (31) | 32 (31) |
| Drinks/Drinking Day, # (SD) | 14.4 (9.2) | 14.8 (8.0) | 15.5 (9.7) | 15.7 (9.9) | 15.7 (9.8) | 15.2 (8.1) |
| Current Alcohol Dependence Symptoms from SCID, # (SD) | 5.9 (2.0) | 6.1 (1.8) | 6.1 (2.0) | 6.3 (1.9) | 5.9 (1.9) | 6.1 (1.9) |
| Comorbid Psychiatric Features | N=156 | N=172 | N=173 | N=91 | N=73 | N=86 |
| Antisocial Personality Disorder, % | 6.9 | 10.4 | 12.6 | 13.7 | 16.9 | 11.3 |
| ASI Psychiatric Severity (SD) | .19 (.20) | .20 (.18) | .23 (.19) | .20 (.19) | .19 (.18) | .22 (.19) |
| Beck Depression Inventory (SD) | 9.4 (7.1) | 10.5 (8.2) | 11.0 (8.6) | 10.9 (9.7) | 9.5 (6.8) | 11.7 (8.6) |
Pre-treatment alcohol consumption was high. Patients consumed an average of 15.1 drinks per drinking day and were abstinent on only 30.1% of all days during the 90-day pre-treatment period. The typical patient met 6 of the 9 DSM-III-R (American Psychiatric Association 1987) criteria for a diagnosis of alcohol dependence.
Despite their relatively high level of dependence on alcohol, patients had relatively few comorbid psychiatric disorders. For example, 10% of patients met DSM-III-R criteria (American Psychiatric Association, 1987) for a diagnosis of Antisocial Personality Disorder. In addition, the average level of psychiatric severity (i.e., measuring not addiction, per se, but other psychiatric problems in the context of substance abuse; McLellan et al. 1983) on the Addiction Severity Index was in lower third of the subscale score range of 0.0–1.0. Similarly, the mean score (10.5) on the Beck Depression Inventory (Beck et al., 1961) indicated a low level of depressive symptoms.
Genotyping
DNA was extracted from archived samples of peripheral blood using a commercial kit (PureGene™ Gentra, Minneapolis, MN). A synonymous, exonic SNP (rs279858) representative of and located in the middle of the GABRA2 haplotype block, previously shown to be associated with AD (Covault et al., 2004) and with subjective responses to alcohol (Pierucci-Lagha et al., 2005), was genotyped using a closed-tube fluorescent TaqMan 5′-nuclease allelic discrimination assay using MGB-probes (Vic-TGAGCTACTGATTTCTTCCCAT and 6FAM-TGAGCTACTGATTTTTTCCCAT; chromosome 4 plus strand sequences) and primers (GAAGCAACTTATTTGGCATTGTCA and TCTGGACTCCAGATACCTTTTTTCA) designed using Primer Express v2.0 software [Applied Biosystems Inc. (ABI) Foster City, CA]. Fluorescence plate reads and genotype calls were made using ABI 7700 and 7500 Sequence Detection Systems. Ten ng of genomic DNA was PCR amplified in 96-well plates using a 10 μl reaction volume for 40 cycles at 94°C for 15s followed by 60°C for 60s. Repeat genotyping was carried out for 12% of samples with no genotype discrepancies. PCR amplifications failed or provided ambiguous genotype results from 4 subjects (excluded from analysis).
To evaluate population stratification as a potential bias in the association analyses, we also genotyped a panel of 34 short tandem repeat ancestry informative markers (AIMs), as described previously (Yang et al., 2005; Covault et al., 2007).
Statistical Analysis
Despite a substantial sample size, the N was unfortunately not sufficient to support an analysis wherein genotype could be stratified by three levels, and further stratified by three levels of treatment assignment and two levels of gender. Instead, genotype was stratified by two levels: homozygotes for the low alcohol dependence risk A-allele (which comprises the low-risk genotype group) versus carriers of the AD-associated G-allele (which comprises the high-risk genotype group). This strategy is consistent with the grouping scheme employed in our prior studies (Covault et al., 2004; Lappalainen et al., 2005) which found an association of the G-allele with risk of alcohol dependence.
The first stage of the analysis included tests of the effects of GABRA2 genotype, treatment arm, and treatment assignment on demographic and psychological variables. In addition, differences in substance use during the 90-day pre-treatment period were tested. Pearson’s χ2 test was used to evaluate group equivalence on categorical variables. Three-factor ANOVAs (genotype by treatment assignment by treatment arm) were used to evaluate equivalence on continuous variables.
In the second analysis stage, the SAS® statistical software (Version 9.1.3) procedure GENMOD employed maximum likelihood estimation to fit log-linear models to longitudinal binary response data. Instances of incomplete data were rare. Across the combined total of 365,400 days of assessment, <1% of data were missing.
We used the software program STRUCTURE v2.1 (Pritchard et al., 2000; Falush et al., 2003) and genotype results from the AIMs, we generated estimates of the proportion of genetic ancestry for each subject (inferred to be primarily African or European based on subject self-report). The sample for this analysis included 750 of the subjects from the present study, together with AIM data from more than 1,000 additional European-American or African-American subjects to yield reliable estimates of ancestry proportions (see Covault et al. 2007). Simulations used 100,000 burn-ins followed by 500,000 runs and a population parameter K=2.
In separate analyses, we used SAS, Proc GENMOD to estimate the probabilities of any drinking and heavy drinking (i.e., ≥4 drinks in a day for women and ≥5 drinks in a day for men) on each of the 450 days from the beginning of treatment through the one-year follow-up period. Both analyses included terms to extract variance associated with Gender, Treatment Arm (outpatient versus aftercare), and a linear trend over Time. The models also included terms permitting tests of the main and interaction effects of GABRA2 Genotype, Treatment Day, and Treatment Assignment (CBT, MET, TSF). Odds ratios (ORs) and their respective likelihood statistics (χ2) were calculated to permit a formal test of the significance of these main and interaction effects.
RESULTS
GABRA2 Genotype and Allele Frequencies
The sample included 136 G-allele homozygotes (16.7%), 411 heterozygotes (50.6%) and 265 A-allele homozygotes (32.6%), a distribution that is consistent with Hardy-Weinberg equilibrium expectations (p = .55). The frequencies of the G- and A-alleles were .421 and .579, respectively.
Effects of GABRA2 genotype on pre-treatment characteristics
Analyses of pre-treatment characteristics revealed more drinks per drinking day (F=60.6, p<.001) and a lower percentage of days abstinent (F=6.2, p<.02) among patients receiving treatment in the aftercare versus outpatient arms of the project. The analyses, however, yielded no other significant effects of treatment arm on pre-treatment characteristics. Importantly, the analyses also revealed no significant differences among patients as a function of the treatment assignment, and no significant effects of genotype or interactions of genotype with either treatment assignment (Table 1) or treatment arm. Further, the ancestry proportion of the subjects was 98.5% European (SD = 2.6%), with no main or interactive effects of sex, genotype group, treatment arm (aftercare vs. outpatient), or treatment assignment (CBT vs. MET vs. TSF). Thus, it was deemed unnecessary to enter pre-treatment variables or ancestry proportion as covariates for analyses focusing on the interactive effects of genotype with treatment assignment on outcome.
Effects of GABRA2 genotype on treatment outcomes
Any drinking (OR=1.002, CI95%=1.0015–1.0024, χ2=63.9, p<.0001) and heavy drinking (OR=1.003, CI95%=1.0025–1.0035, χ2=130.9, p<.0001) were significantly predicted by the linear effect of time. The predicted probabilities of drinking and heavy drinking were lowest at the beginning of treatment and increased thereafter. Also, as expected, drinking (OR=0.839, CI95%=0.824–0.855, χ2=353.5, p<.0001) and heavy drinking (OR=0.886, CI95%=0.868–0.903, χ2=147.8, p<.0001) were lower in women than men. In addition, these probabilities varied significantly across treatment arms (drinking: OR=0.497, CI95%=0.488–0.506, χ2=5688.7, p<.0001; heavy drinking: OR=0.566, CI95%=0.556–0.578, χ2=3302.9, p<.0001), with both measures of drinking being greater among patients recruited from outpatient compared with aftercare settings. Treatment assignment was also a significant predictor of outcomes (drinking: OR=1.198, CI95%=1.108–1.296, χ2=20.5, p<.0001; heavy drinking: OR=1.494, CI95%=1.373–1.626, χ2=86.5, p<.0001). Both drinking outcomes were, in general, least probable among patients assigned to TSF, more probable among those assigned to MET, and most probable among those assigned to CBT. As shown in Figures 1 and 2, the linear change in drinking over time varied with the type of treatment (drinking: OR=0.9997, CI95%=0.9995–0.9999, χ2=8.1, p<.0044; heavy drinking: OR=0.9993, CI95%=0.9991–0.9995, χ2=31.8, p<.0001) in a complex pattern.
Figure 1.
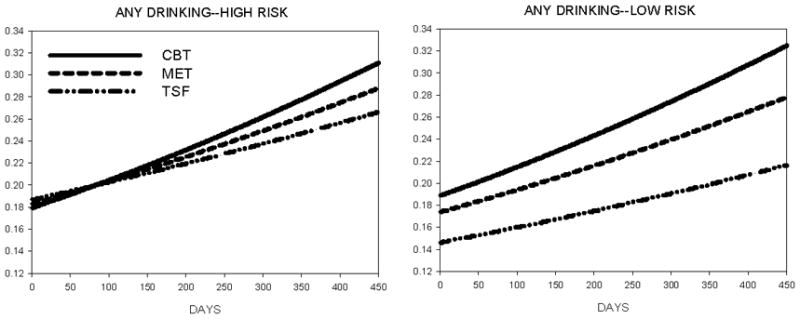
Daily probability of drinking during and after the 12-week treatment period as a function of genotype (high-risk = A/G or G/G; low-risk = A/A) and treatment assignment (CBT = Cognitive-Behavioral Treatment, MET = Motivational Enhancement Therapy, TSF = Twelve-Step Facilitation).
Figure 2.
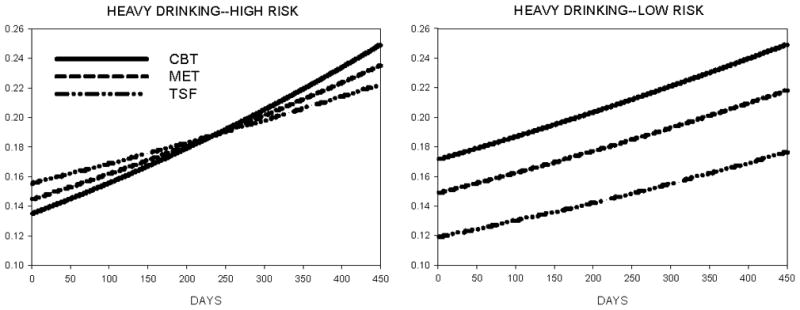
Daily probability of heavy drinking (men: > 5; women: > 4) during and after treatment as a function of genotype (high-risk = A/G or G/G; low-risk = A/A) and treatment assignment (CBT = Cognitive-Behavioral Treatment, MET = Motivational Enhancement Therapy, TSF = Twelve-Step Facilitation).
Importantly, GABRA2 genotype affected the average likelihood of drinking (OR=1.262, CI95%=1.122–1.428, χ2=15.0, p<.0001 and heavy drinking (OR=1.944, CI95%=1.714–2.206, χ2=106.8, p<.0001), with an elevated probability of both occurring among patients with the high-risk genotype. Genotype also altered the effects of treatment, albeit in a complex manner, for both drinking (OR=0.855, CI95%=0.809–0.904, χ2=30.0, p<.0001) and heavy drinking (OR=0.734, CI95%=0.692–0.779, χ2=102.3, p<.0001) outcomes. Figures 1 and 2 illustrate an interesting relation wherein greater differentiation of treatment type is seen in patients possessing the low-risk (i.e., A/A) GABRA2 genotype.
DISCUSSION
Poor outcomes following treatment for alcohol dependence are common, and efforts to improve the efficacy of treatment have become an integral part of treatment design (Daley and Marlatt 1997; Larimer et al., 1999). In outcome studies of alcoholics, for example, approximately 65–70% of patients relapse within one year of treatment, with the majority of relapses occurring within the first three months (Miller et al., 2001,). Project MATCH (Babor and Del Boca, 2003) was inspired by these concerns, and asked whether the outcomes from three specific types of treatment, viz. CBT, TSF, and MET, would vary as a function of the post hoc matching of their unique elements (i.e., cognitive restructuring with relapse prevention skills in CBT vs. a strong abstinence message encouraged by group fellowship in TSF vs. a non-confrontational motivational message in MET) with specific patient characteristics.
The patient variables chosen for inclusion in the Project MATCH analyses were drawn from a longer list of clinical and psychological variables that had predicted treatment outcome in studies published prior to 1993 [(Project MATCH Research Group 1993); see (Ciraulo et al., 2003) for a more recent review]. The list included, for example, a high level of alcohol involvement or dependence (Booth et al., 1991; Haver and Gjestad 2005; Langenbucher et al., 1996; McLellan et al., 1994; Powell et al., 1998; Salloum et al., 2005; Winterer et al., 1998; Woody et al., 1984). Alcohol involvement is typically quantified with scores on alcohol problem scales, DSM symptom counts, duration of drinking, or the number of previous treatments.
The list also included a select subset of comorbid psychiatric disorders. Of particular interest, because of its strong association with poor treatment outcome, was Antisocial Personality Disorder (ASPD), in which severe childhood conduct problems and adult antisocial behaviors are conjointly present (Pettinati et al., 1999; Powell et al., 1998). Comorbid anxiety or depressive disorders were not directly examined, for their association with poor outcome (Hatsukami and Pickens 1982; Haver and Gjestad 2005; Kushner et al., 2005; Magura et al., 2005; Willinger et al., 2002) appeared, at the time, to be highly variable and complicated by other factors, including the gender and age composition of the patient sample, as well as the preferred drug of abuse (Bobo et al., 1998; Brown et al., 1998; Hesselbrock 1991; Hodgins et al., 1999; Kranzler et al., 1996; Rounsaville et al., 1986; Sellman and Joyce 1996; Ziedonis and Kosten, 1991). Instead, a summary measure of general psychiatric severity was employed in the analysis.
Unfortunately, tests of these and many other treatment matching characteristics showed no significant interactions between the characteristic and type of treatment, during the active treatment phase (Project MATCH Research Group, 1998). During the one-year post-treatment phase, one primary matching variable, psychiatric severity, interacted significantly with treatment. In the outpatient study, patients low on psychiatric severity had more abstinent days after TSF than after CBT (Project MATCH Research Group, 1997). During the post-treatment phase, two secondary matching variables were also significant: outpatients who were high in anger and treated with MET had better post-treatment drinking outcomes than those receiving CBT; and aftercare patients high in alcohol dependence severity had better post-treatment outcomes after receiving TSF, while patients with low severity of dependence did better with CBT (Project MATCH Research Group, 1997).
A question never asked in the outcome prediction literature before or during the era of Project MATCH was whether genotype could be used as an objective predictor of risk. The failure to consider genetic factors was understandable at the time, because until 2004 (Covault et al., 2004; Edenberg et al., 2004), the only candidate genes that had been reliably associated with either the emergence or re-emergence of alcohol dependence were those encoding proteins involved in alcohol metabolism, variation in which is most evident in populations in the Far East (Gelernter and Kranzler, in press), although it is now known to be very important in other populations as well (e.g., Luo et al., 2006). In addition, there was no direct evidence suggesting that relapse risk was a stable trait, other than anecdotal reports of so-called “revolving-door” alcoholics or recidivist DUI offenders, for whom treatment entry followed by rapid treatment failure can become a stable pattern (Tait et al., 2002). Yet, one might have discerned an involvement of genetic factors from the evidence available associating poor treatment outcome with ASPD. The high heritability of ASPD has been recognized for many years (e.g., Cadoret, 1978; Gelhorn et al., 2005; Grove et al., 1990; Hicks et al., 2004; Slutske, 2001).
To date, only one published study has associated a candidate gene with alcohol treatment outcome. Wojnar and colleagues (2006) analyzed genotype frequencies of the HTR1A serotonin receptor gene and discovered an excess prevalence of the G/G versus C/C genotype among patients who would later relapse. Although their results are intriguing, the sample size was small (N=90). Furthermore, HTR1A has not yet been associated with other known and heritable risk factors for relapse other than suicidality (Wojnar et al., 2006,).
In the present study, we focused our analysis on the GABRA2 gene. Several reasons justified the decision. GABRA2 has, for example, been associated with risk for alcohol dependence in six independent studies (Covault et al., 2004; Edenberg et al., 2004; Enoch et al., 2006; Fehr et al., 2006; Lappalainen et al., 2005; Soyka et al., 2007). In addition, it has been reliably associated with both Conduct Disorder problems (Dick et al., 2006) and the amount of fast β activity (Edenberg et al., 2004,) in the electroencephalogram. Both of these variables have been associated with an elevated risk for poor treatment outcome (Bauer, 1994; Bauer, 2001; Pettinati et al., 1999; Powell et al., 1998; Saletu-Zyhlarz et al., 2004; Winterer et al., 1998).
The results of our analysis of GABRA2 yielded several findings. A principal finding was a significantly elevated probability of drinking and heavy drinking among patients with the high-risk genotype. This finding was not evident in the analysis of percent days abstinent and drinks per drinking day measured during the pre-treatment period (Table 1). The discrepancy can be explained by the limited validity of self-reported (Wish et al., 1997; Williams and Nowatzki, 2005) substance use at the time of treatment entry. In Project MATCH and other treatment studies, retrospective recall of drinking behavior during the pretreatment period may, therefore, be less accurate than recall during the treatment and post-treatment periods, which occur after the patient has been asked to carefully monitor his/her drinking behavior. In short, the daily probabilities of drinking and heavy drinking may exhibit less error over time and may therefore be more powerful metrics. The significant main effect of GABRA2 genotype and of the other independent variables revealed by our analyses must be interpreted cautiously, because they are embedded within a series of statistically significant interactions. In addition, several of these main and interaction effects, though statistically significant, were associated with ORs that indicated modest effects. Therefore, their clinical significance may not be as robust as their statistical significance. We must also be cautious in comparing the present significant effects to the original, generally non-significant effects demonstrated by Project MATCH. It is important to be mindful that the present analysis is based on a homogenous sub-sample of Project MATCH patients, i.e., European-Americans that provided DNA. Also, the original Project MATCH analyses focused on percent days abstinent over a 90-day period as the primary outcome measure whereas these analyses focused on daily estimates of any drinking and heavy drinking over a much longer time period.
Figures 1 and 2 illustrate the complex pattern of results. The simplest description of the results is that the high-risk allele blunts the variability in outcome associated with the three different psychosocial treatments, whereas the low-risk allele permits better differentiation of the effects of psychosocial treatments and, particularly, of TSF. The explanation for this pattern of results is less clear and presently speculative. The root cause may involve an enhancing effect of the high-risk allele on anxiety level (Enoch et al., 2006) or conduct problems (Dick et al., 2006), which may, in turn, distract patients from attending to the specific aspects of a treatment that differentiate it from other treatments. Alternatively, the interaction may suggest that enhanced anxiety or conduct problems in these patients selectively compromise the beneficial effects of TSF. Because TSF emphasizes total alcohol abstinence, patients with greater anxiety or conduct problems may be more likely to fail TSF than other treatments as a result of their greater stress reactivity and impulsivity. Or, patients with higher levels of anxiety or conduct problems may benefit less from the group affiliation and peer support that are integral and unique to implementation of the goals of TSF.
In summary, the present results offer an interesting insight and suggest a potential role for genetic vulnerability not only in promoting the emergence of problem drinking (Covault et al., 2004; Edenberg et al., 2004; Enoch et al., 2006; Fehr et al., 2006; Lappalainen et al., 2005; Soyka et al., 2007), but also in promoting continued drinking after psychosocial treatment. In addition, genetic vulnerability diminished the relative superiority of TSF over MET and CBT in suppressing alcohol use. The implications of these findings for future treatment matching studies should be considered. For example, the findings suggest that patients possessing the low-risk GABRA2 genotype, though drinking at a lower average level, will show more variability in drinking outcomes and may therefore be better candidates for studies of psychosocial treatments that address their psychosocial issues. In contrast, patients possessing high-risk GABRA2 genotypes may be better candidates for other (or additional) forms of treatment, e.g., medications such as naltrexone, acamprosate, or topiramate, which could address their neurobiological differences.
Footnotes
FINANCIAL DISCLOSURE
This study was supported by NIH grants P50 AA03510 (University of Connecticut Alcohol Research Center), M01 RR06192 (University of Connecticut General Clinical Research Center), K24 AA13736, R01 DA017666, R01 AA11330, and R01 AA015606. The authors have no other financial disclosures that are relevant to this work. Some investigators who participated in Project MATCH, a collaborative clinical trial that was sponsored by the National Institute on Alcohol Abuse and Alcoholism, provided phenotypic data and blood samples for DNA extraction and genotyping.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3. Washington, D. C: American Psychiatric Press; 1987. Revised. [Google Scholar]
- Babor TF, Del Boca FK. Treatment Matching in Alcoholism. Cambridge, UK: University Press; 2003. [Google Scholar]
- Bauer LO. Electroencephalographic and autonomic predictors of relapse in alcohol-dependent patients. Alcohol Clin Exp Res. 1994;18:755–760. doi: 10.1111/j.1530-0277.1994.tb00942.x. [DOI] [PubMed] [Google Scholar]
- Bauer LO. Predicting relapse to alcohol and drug abuse via quantitative electroencephalography. Neuropsychopharmacology. 2001;25:332–340. doi: 10.1016/S0893-133X(01)00236-6. [DOI] [PubMed] [Google Scholar]
- Beck A, Ward C, Mendelson M, Mock J, Erbaugh J. An Inventory for Measuring Depression. Arch Gen Psychiatry. 1961;4:561–568. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bobo JK, McIlvain HE, Leed-Kelly A. Depression screening scores during residential drug treatment and risk of drug use after discharge. Psychiatr Serv. 1998;49:693–695. doi: 10.1176/ps.49.5.693. [DOI] [PubMed] [Google Scholar]
- Booth BM, Yates WR, Petty F, Brown K. Patient factors predicting early alcohol-related readmissions for alcoholics: role of alcoholism severity and psychiatric co-morbidity. J Stud Alcohol. 1991;52:37–43. doi: 10.15288/jsa.1991.52.37. [DOI] [PubMed] [Google Scholar]
- Bottlender M, Soyka M. Outpatient alcoholism treatment: predictors of outcome after 3 years. Drug Alcohol Depend. 2005;80:83–89. doi: 10.1016/j.drugalcdep.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Hall JM. Polysomnographic and subjective sleep predictors of alcoholic relapse. Alcohol Clin Exp Res. 1998;22:1864–1871. [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Robinson EA, Zucker RA, Greden JF. Insomnia, self-medication, and relapse to alcoholism. Am J Psychiatry. 2001;158:399–404. doi: 10.1176/appi.ajp.158.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Monti PM, Myers MG, Martin RA, Rivinus T, Dubreuil ME, Rohsenow DJ. Depression among cocaine abusers in treatment: relation to cocaine and alcohol use and treatment outcome. Am J Psychiatry. 1998;155:220–225. doi: 10.1176/ajp.155.2.220. [DOI] [PubMed] [Google Scholar]
- Cadoret RJ. Psychopathology in adopted-away offspring of biologic parents with antisocial behavior. Arch Gen Psychiatry. 1978;35:176–184. doi: 10.1001/archpsyc.1978.01770260054005. [DOI] [PubMed] [Google Scholar]
- Caetano R, Cunradi C. Alcohol dependence: a public health perspective. Addiction. 2002;97:633–645. doi: 10.1046/j.1360-0443.2002.00184.x. [DOI] [PubMed] [Google Scholar]
- Ciraulo DA, Piechniczek-Buczek J, Iscan EN. Outcome predictors in substance use disorders. Psychiatr Clin North Am. 2003;26:381–409. doi: 10.1016/s0193-953x(02)00106-5. [DOI] [PubMed] [Google Scholar]
- Clark CP, Gillin JC, Golshan S, Demodena A, Smith TL, Danowski S, Irwin M, Schuckit M. Increased REM sleep density at admission predicts relapse by three months in primary alcoholics with a lifetime diagnosis of secondary depression. Biol Psychiatry. 1998;43:601–607. doi: 10.1016/s0006-3223(97)00457-5. [DOI] [PubMed] [Google Scholar]
- Covault J, Gelernter J, Hesselbrock V, Nellissery M, Kranzler HR. Allelic and haplotypic association of GABRA2 with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2004;129:104–109. doi: 10.1002/ajmg.b.30091. [DOI] [PubMed] [Google Scholar]
- Covault J, Gelernter J, Jensen K, Anton R, Kranzler HR. Markers in the 5′Region of GABRG1 associate to alcohol dependence and are in linkage disequilibrium with markers in the adjacent GABRA2 gene. Neuropsychopharmacology. 2007 May 16; doi: 10.1038/sj.npp.1301456. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley D, Marlatt G. Relapse prevention. In: Lowinson J, Ruiz P, Millman R, Langrod J, editors. Substance Abuse: A Comprehensive Textbook. 3. Baltimore: Williams & Wilkins Publishers; 1997. pp. 458–466. [Google Scholar]
- Dick DM, Bierut L, Hinrichs A, Fox L, Bucholz KK, Kramer J, Kuperman S, Hesselbrock V, Schuckit M, Almasy L, Tischfield J, Porjesz B, Begleiter H, Nurnberger J, Jr, Xuei X, Edenberg HJ, Foroud T. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behav Genet. 2006;36:577–590. doi: 10.1007/s10519-005-9041-8. [DOI] [PubMed] [Google Scholar]
- Drummond SP, Gillin JC, Smith TL, DeModena A. The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse. Alcohol Clin Exp Res. 1998;22:1796–1802. [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, Crowe RR, Goate A, Hesselbrock V, Jones K, Kwon J, Li TK, Nurnberger JI, Jr, O’Connor SJ, Reich T, Rice J, Schuckit MA, Porjesz B, Foroud T, Begleiter H. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Schwartz L, Albaugh B, Virkkunen M, Goldman D. Dimensional anxiety mediates linkage of GABRA2 haplotypes with alcoholism. Am J Med Genet B Neuropsychiatr Genet. 2006;141:599–607. doi: 10.1002/ajmg.b.30336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164:1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr C, Sander T, Tadic A, Lenzen KP, Anghelescu I, Klawe C, Dahmen N, Schmidt LG, Szegedi A. Confirmation of association of the GABRA2 gene with alcohol dependence by subtype-specific analysis. Psychiatr Genet. 2006;16:9–17. doi: 10.1097/01.ypg.0000185027.89816.d9. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler HR. Genetics of addiction. In: Galanter M, Kleber HD, editors. Textbook of Substance Abuse Treatment. 4. Washington, DC: American Psychiatric Press, Inc; in press. [Google Scholar]
- Gelhorn HL, Stallings MC, Young SE, Corley RP, Rhee SH, Hewitt JK. Genetic and environmental influences on conduct disorder: symptom, domain and full-scale analyses. J Child Psychol Psychiatry. 2005;46:580–591. doi: 10.1111/j.1469-7610.2004.00373.x. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Smith TL, Irwin M, Butters N, Demodena A, Schuckit M. Increased pressure for rapid eye movement sleep at time of hospital admission predicts relapse in nondepressed patients with primary alcoholism at 3-month follow-up. Arch Gen Psychiatry. 1994;51:189–197. doi: 10.1001/archpsyc.1994.03950030025003. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Grove WM, Eckert ED, Heston L, Bouchard TJ, Jr, Segal N, Lykken DT. Heritability of substance abuse and antisocial behavior: a study of monozygotic twins reared apart. Biol Psychiatry. 1990;27:1293–1304. doi: 10.1016/0006-3223(90)90500-2. [DOI] [PubMed] [Google Scholar]
- Hatsukami D, Pickens RW. Posttreatment depression in an alcohol and drug abuse population. Am J Psychiatry. 1982;139:1563–1566. doi: 10.1176/ajp.139.12.1563. [DOI] [PubMed] [Google Scholar]
- Haver B, Gjestad R. Phobic anxiety and depression as predictor variables for treatment outcome. A LISREL analysis on treated female alcoholics. Nord J Psychiatry. 2005;59:25–30. doi: 10.1080/08039480510018797. [DOI] [PubMed] [Google Scholar]
- Hesselbrock MN. Gender comparison of antisocial personality disorder and depression in alcoholism. J Subst Abuse. 1991;3:205–219. doi: 10.1016/s0899-3289(05)80037-9. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Krueger RF, Iacono WG, McGue M, Patrick CJ. Family transmission and heritability of externalizing disorders: a twin-family study. Arch Gen Psychiatry. 2004;61:922–928. doi: 10.1001/archpsyc.61.9.922. [DOI] [PubMed] [Google Scholar]
- Hodgins DC, el-Guebaly N, Armstrong S, Dufour M. Implications of depression on outcome from alcohol dependence: a 3-year prospective follow-up. Alcohol Clin Exp Res. 1999;23:151–157. [PubMed] [Google Scholar]
- Kendler KS. Twin studies of psychiatric illness: an update. Arch Gen Psychiatry. 2001;58:1005–1014. doi: 10.1001/archpsyc.58.11.1005. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Del Boca FK, Rounsaville BJ. Comorbid psychiatric diagnosis predicts three-year outcomes in alcoholics: a posttreatment natural history study. J Stud Alcohol. 1996;57:619–626. doi: 10.15288/jsa.1996.57.619. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Abrams K, Thuras P, Hanson KL, Brekke M, Sletten S. Follow-up study of anxiety disorder and alcohol dependence in comorbid alcoholism treatment patients. Alcohol Clin Exp Res. 2005;29:1432–1443. doi: 10.1097/01.alc.0000175072.17623.f8. [DOI] [PubMed] [Google Scholar]
- Langenbucher J, Sulesund D, Chung T, Morgenstern J. Illness severity and self-efficacy as course predictors of DSM-IV alcohol dependence in a multisite clinical sample. Addict Behav. 1996;21:543–553. doi: 10.1016/0306-4603(95)00085-2. [DOI] [PubMed] [Google Scholar]
- Lappalainen J, Krupitsky E, Remizov M, Pchelina S, Taraskina A, Zvartau E. Association between alcoholism and gamma-amino butyric acid alpha2 receptor subtype in a Russian population. Alcohol Clin Exp Res. 2005;29:493–498. doi: 10.1097/01.alc.0000158938.97464.90. [DOI] [PubMed] [Google Scholar]
- Larimer ME, Palmer RS, Marlatt GA. Relapse prevention. An overview of Marlatt’s cognitive-behavioral model. Alcohol Res Health. 1999;23:151–160. [PMC free article] [PubMed] [Google Scholar]
- Long JC, Knowler WC, Hanson RL, Robin RW, Urbanek M, Moore E, Bennett PH, Goldman D. Evidence for genetic linkage to alcohol dependence on chromosomes 4 and 11 from an autosome-wide scan in an American Indian population. Am J Med Genet. 1998;81:216–221. doi: 10.1002/(sici)1096-8628(19980508)81:3<216::aid-ajmg2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Luo X, Kranzler HR, Zuo L, Yang BZ, Lappalainen J, Gelernter J. ADH4 gene variation is associated with alcohol and drug dependence: results from family controlled and population-structured association studies. Pharmacogenet Genomics. 2005;15:755–768. doi: 10.1097/01.fpc.0000180141.77036.dc. [DOI] [PubMed] [Google Scholar]
- Luo X, Kranzler HR, Zuo L, Wang S, Schorck NJ, Gelernter J. Diplotype trend regression (DTR) analysis of the ADH gene cluster and ALDH2 gene: Multiple significant associations for alcohol dependence. Am J Hum Genet. 2005;78:973–987. doi: 10.1086/504113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Kranzler HR, Zuo L, Wang S, Schorck NJ, Gelernter J. Diplotype trend regression (DTR) analysis of the ADH gene cluster and ALDH2 gene: Multiple significant associations for alcohol dependence. Am J Hum Genet. 2006;78:973–987. doi: 10.1086/504113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magura S, Fong C, Staines GL, Cleland C, Foote J, Rosenblum A, Kosanke N, DeLuca A. The combined effects of treatment intensity, self-help groups and patient attributes on drinking outcomes. J Psychoactive Drugs. 2005;37:85–92. doi: 10.1080/02791072.2005.10399751. [DOI] [PubMed] [Google Scholar]
- Maisto SA, Connors GJ, Zywiak WH. Alcohol treatment, changes in coping skills, self-efficacy, and levels of alcohol use and related problems 1 year following treatment initiation. Psychol Addict Behav. 2000;14:257–266. doi: 10.1037//0893-164x.14.3.257. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O’Brien CP, Druley KA. Predicting response to alcohol and drug abuse treatments. Role of psychiatric severity. Arch Gen Psychiatry. 1983;40:620–625. doi: 10.1001/archpsyc.1983.04390010030004. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Alterman AI, Metzger DS, Grissom GR, Woody GE, Luborsky L, O’Brien CP. Similarity of outcome predictors across opiate, cocaine, and alcohol treatments: role of treatment services. J Consult Clin Psychol. 1994;62:1141–1158. doi: 10.1037//0022-006x.62.6.1141. [DOI] [PubMed] [Google Scholar]
- Miller WR, Walters ST, Bennett ME. How effective is alcoholism treatment in the United States? J Stud Alcohol. 2001;62:211–220. doi: 10.15288/jsa.2001.62.211. [DOI] [PubMed] [Google Scholar]
- Myers MG, Brown SA, Mott MA. Preadolescent conduct disorder behaviors predict relapse and progression of addiction for adolescent alcohol and drug abusers. Alcohol Clin Exp Res. 1995;19:1528–1536. doi: 10.1111/j.1530-0277.1995.tb01019.x. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Pierce JD, Jr, Belden PP, Meyers K. The relationship of Axis II personality disorders to other known predictors of addiction treatment outcome. Am J Addict. 1999;8:136–147. doi: 10.1080/105504999305947. [DOI] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, Nellissery M, Hernandez-Avila C, Oncken C, Morrow AL, Kranzler HR. GABRA2 alleles moderate the subjective effects of alcohol, which are attenuated by finasteride. Neuropsychopharmacology. 2005;30:1193–1203. doi: 10.1038/sj.npp.1300688. [DOI] [PubMed] [Google Scholar]
- Powell BJ, Landon JF, Cantrell PJ, Penick EC, Nickel EJ, Liskow BI, Coddington TM, Campbell JL, Dale TM, Vance MD, Rice AS. Prediction of drinking outcomes for male alcoholics after 10 to 14 years. Alcohol Clin Exp Res. 1998;22:559–566. doi: 10.1111/j.1530-0277.1998.tb04293.x. [DOI] [PubMed] [Google Scholar]
- Prichep LS, Alper KR, Kowalik SC, Vaysblat LS, Merkin HA, Tom M, John ER, Rosenthal MS. Prediction of treatment outcome in cocaine dependent males using quantitative EEG. Drug Alcohol Depend. 1999;54:35–43. doi: 10.1016/s0376-8716(98)00147-1. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Project MATCH Research Group. Project MATCH (Matching Alcoholism Treatment to Client Heterogeneity): rationale and methods for a multisite clinical trial matching patients to alcoholism treatment. Alcohol Clin Exp Res. 1993;17:1130–1145. doi: 10.1111/j.1530-0277.1993.tb05219.x. [DOI] [PubMed] [Google Scholar]
- Project MATCH Research Group. Matching Alcoholism Treatments to Client Heterogeneity: Project MATCH posttreatment drinking outcomes. J Stud Alcohol. 1997;58:7–29. [PubMed] [Google Scholar]
- Project MATCH Research Group. Project MATCH secondary a priori hypotheses. Project MATCH Research Group. Addiction. 1997;92:1671–1698. [PubMed] [Google Scholar]
- Project MATCH Research Group. Matching alcoholism treatments to client heterogeneity: treatment main effects and matching effects on drinking during treatment. Project MATCH Research Group. J Stud Alcohol. 1998;59:631–639. doi: 10.15288/jsa.1998.59.631. [DOI] [PubMed] [Google Scholar]
- Reich T, Edenberg HJ, Goate A, Williams JT, Rice JP, Van Eerdewegh P, Foroud T, Hesselbrock V, Schuckit MA, Bucholz K, Porjesz B, Li TK, Conneally PM, Nurnberger JI, Jr, Tischfield JA, Crowe RR, Cloninger CR, Wu W, Shears S, Carr K, Crose C, Willig C, Begleiter H. Genome-wide search for genes affecting the risk for alcohol dependence. Am J Med Genet. 1998;81:207–215. [PubMed] [Google Scholar]
- Rounsaville BJ, Kosten TR, Weissman MM, Kleber HD. Prognostic significance of psychopathology in treated opiate addicts. A 2.5-year follow-up study. Arch Gen Psychiatry. 1986;43:739–745. doi: 10.1001/archpsyc.1986.01800080025004. [DOI] [PubMed] [Google Scholar]
- Saletu-Zyhlarz GM, Arnold O, Anderer P, Oberndorfer S, Walter H, Lesch OM, Boning J, Saletu B. Differences in brain function between relapsing and abstaining alcohol-dependent patients, evaluated by EEG mapping. Alcohol Alcohol. 2004;39:233–240. doi: 10.1093/alcalc/agh041. [DOI] [PubMed] [Google Scholar]
- Salloum IM, Cornelius JR, Douaihy A, Kirisci L, Daley DC, Kelly TM. Patient characteristics and treatment implications of marijuana abuse among bipolar alcoholics: results from a double blind, placebo-controlled study. Addict Behav. 2005;30:1702–1708. doi: 10.1016/j.addbeh.2005.07.014. Epub 2005 Aug 1711. [DOI] [PubMed] [Google Scholar]
- Sellman JD, Joyce PR. Does depression predict relapse in the 6 months following treatment for men with alcohol dependence? Aust N Z J Psychiatry. 1996;30:573–578. doi: 10.3109/00048679609062652. [DOI] [PubMed] [Google Scholar]
- Slutske WS. The genetics of antisocial behavior. Curr Psychiatry Rep. 2001;3:158–162. doi: 10.1007/s11920-001-0014-1. [DOI] [PubMed] [Google Scholar]
- Soyka M, Preuss UW, Hesselbrock V, Zill P, Koller G, Bondy B. GABA-A2 receptor subunit gene (GABRA2) polymorphisms and risk for alcohol dependence. J Psychiatr Res. 2007 doi: 10.1016/j.jpsychires.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Tait RJ, Hulse GK, Robertson SI, Sprivulis P. Multiple hospital presentations by adolescents who use alcohol or other drugs. Addiction. 2002;97:1269–1275. doi: 10.1046/j.1360-0443.2002.00191.x. [DOI] [PubMed] [Google Scholar]
- Tonigan JS, Miller WR, Brown JM. The reliability of Form 90: an instrument for assessing alcohol treatment outcome. J Stud Alcohol. 1997;58:358–364. doi: 10.15288/jsa.1997.58.358. [DOI] [PubMed] [Google Scholar]
- Williams RJ, Nowatzki N. Validity of adolescent self-report of substance use. Subst Use Misuse. 2005;40:299–311. doi: 10.1081/ja-200049327. [DOI] [PubMed] [Google Scholar]
- Willinger U, Lenzinger E, Hornik K, Fischer G, Schonbeck G, Aschauer HN, Meszaros K. Anxiety as a predictor of relapse in detoxified alcohol-dependent patients. Alcohol Alcohol. 2002;37:609–612. doi: 10.1093/alcalc/37.6.609. [DOI] [PubMed] [Google Scholar]
- Winterer G, Kloppel B, Heinz A, Ziller M, Dufeu P, Schmidt LG, Herrmann WM. Quantitative EEG (QEEG) predicts relapse in patients with chronic alcoholism and points to a frontally pronounced cerebral disturbance. Psychiatry Res. 1998;78:101–113. doi: 10.1016/s0165-1781(97)00148-0. [DOI] [PubMed] [Google Scholar]
- Wish ED, Hoffman JA, Nemes S. The validity of self-reports of drug use at treatment admission and at followup: comparisons with urinalysis and hair assays. NIDA Res Monogr. 1997;167:200–226. [PubMed] [Google Scholar]
- Wojnar M, Brower KJ, Jakubczyk A, Zmigrodzka I, Burmeister M, Matsumoto H, Wozny E, Sliwerska E, Hegedus AM, Husar A, Slufarska A, Lipinski M, Zucker RA. Influence of impulsivity, suicidality and serotonin genes on treatment outcomes in alcohol dependence. Psychiatr Pol. 2006;40:985–994. [PubMed] [Google Scholar]
- Woody GE, McLellan AT, Luborsky L, O’Brien CP, Blaine J, Fox S, Herman I, Beck AT. Severity of psychiatric symptoms as a predictor of benefits from psychotherapy: the Veterans Administration-Penn study. Am J Psychiatry. 1984;141:1172–1177. doi: 10.1176/ajp.141.10.1172. [DOI] [PubMed] [Google Scholar]
- Yang BZ, Zhao H, Kranzler HR, Gelernter J. Practical population group assignment with selected informative markers: characteristics and properties of Bayesian clustering via STRUCTURE. Genet Epidemiol. 2005;28:302–312. doi: 10.1002/gepi.20070. [DOI] [PubMed] [Google Scholar]
- Ziedonis DM, Kosten TR. Depression as a prognostic factor for pharmacological treatment of cocaine dependence. Psychopharmacol Bull. 1991;27:337–343. [PubMed] [Google Scholar]


