Abstract
Cell-specific activation of transcription factor σF during sporulation in Bacillus subtilis requires the formation of the polar septum and the activity of a serine phosphatase (SpoIIE) located in the septum. The SpoIIE phosphatase indirectly activates σF by dephosphorylating a protein (SpoIIAA-P) in the pathway that controls the activity of the transcription factor. By use of a SpoIIE–GFP fusion protein in time-course and time-lapse experiments and by direct visualization of septa in living cells, we show that SpoIIE is present in the predivisional sporangium, where it often localizes near both cell poles in structures known as E-rings. We also present evidence consistent with the view that SpoIIE is present in both progeny cells after polar division. These findings are incompatible with a model for the control of σF activity in which the phosphatase is simply sequestered to one cell. Instead, we conclude that the function of SpoIIE is subject to regulation, and we present evidence that this occurs in two stages. The first stage, which involves the phosphatase function of SpoIIE, depends on the cell division protein FtsZ and could correspond to the FtsZ-dependent assembly of SpoIIE into E-rings. The second stage occurs after the dephosphorylation of SpoIIAA-P and is dependent on the later-acting, cell-division protein DivIC. Evidence based on the use of modified and mutant forms of the phosphatase protein indicates that SpoIIE blocks the capacity of unphosphorylated SpoIIAA to activate σF until formation of the polar septum is completed.
Keywords: Bacillus subtilis, SpoIIE, DivIC, FtsZ, σF, septation
An attractive system for addressing the problem of the establishment of cell fate is the process of sporulation in the bacterium Bacillus subtilis (Piggot and Coote 1976; Errington 1993; Stragier and Losick 1996). A hallmark of sporulation is the formation of an asymmetrically positioned or polar septum that partitions the sporangium into dissimilar-sized progeny cells called the forespore and the mother cell. The forespore, the smaller cell, and the mother cell transcribe different sets of genes and have different fates. Here we are concerned with a regulatory protein called SpoIIE that is located in the polar septum and is directly involved in the establishment of cell-specific transcription in the forespore.
Gene expression in the forespore is controlled by the transcription factor σF (Stragier and Losick 1996). The σF factor is synthesized shortly after the onset of sporulation in the predivisional sporangium (Gholamhoseinian and Piggot 1989). It does not become active in directing gene transcription, however, until after asymmetric division, when its activity is confined to the forespore (Margolis et al. 1991). The activity of σF is governed by a pathway consisting of the proteins SpoIIE, SpoIIAA, and SpoIIAB (Schmidt et al. 1990; Margolis et al. 1991). SpoIIAB is a dual-function protein. As an anti-σ factor, it binds to and inhibits σF in the predivisional sporangium and in the mother cell (Duncan and Losick 1993) and, as a serine protein kinase, it phosphorylates and thereby inactivates SpoIIAA (Duncan and Losick 1993; Min et al. 1993; Alper et al. 1994; Diederich et al. 1994). SpoIIAA is an anti-anti-σ factor that induces the release of σF from the SpoIIAB–σF complex (Min et al. 1993; Alper et al. 1994; Diederich et al. 1994; Duncan et al. 1996; Garsin et al. 1998). Finally, SpoIIE is a phosphatase responsible for converting the inactive, phosphorylated form of SpoIIAA (SpoIIAA-P) to the active, dephosphorylated form (Duncan et al. 1995; Arigoni et al. 1996; Feucht et al. 1996). Therefore, SpoIIE indirectly activates σF through the dephosphorylation of SpoIIAA-P.
SpoIIE is an integral membrane protein with 10 membrane-spanning segments in the amino-terminal portion of the protein and a PP2C-like phosphatase domain in its cytoplasmic tail (Adler et al. 1997; Arigoni et al. 1999). Previous work has indicated that SpoIIE progresses through three patterns of subcellular localization during the early stages of sporulation (see left-hand pathway of Fig. 1A) (Arigoni et al. 1995; Levin et al. 1997). In the predivisional sporangium, SpoIIE assembles into rings (called E-rings) near both poles of the cell. These SpoIIE rings are coincident with rings of the cell division protein FtsZ (known as Z-rings), which are also present near both ends of the predivisional sporangium (Levin and Losick 1996). Evidence shows that the bipolar localization of SpoIIE depends on Z-ring formation and that the site of FtsZ assembly dictates the position of the E-rings (Levin et al. 1997) (conversely, SpoIIE contributes to the formation of polar Z-rings; Khvorova et al. 1998). During asymmetric division, a Z-ring at one pole of the sporangium constricts at the leading edge of the invaginating septum and is eventually replaced by a polar septum into which SpoIIE is believed to be incorporated. Meanwhile, the FtsZ- and SpoIIE-containing ring at the pole distal to the septum persists. Finally, during the activation of σF in the forespore, FtsZ and SpoIIE disappear from the distal pole, resulting in a sporangium with a unipolar pattern of SpoIIE localization (Arigoni et al. 1995; Levin et al. 1997; Pogliano et al. 1997). Evidence in support of the view that the subcellular localization of SpoIIE precedes and does not depend on septum formation is the observation that SpoIIE colocalizes with FtsZ (which is absent from completed septa) and that its colocalization with FtsZ is maintained in a mutant blocked in septum formation (Levin et al. 1997).
Figure 1.
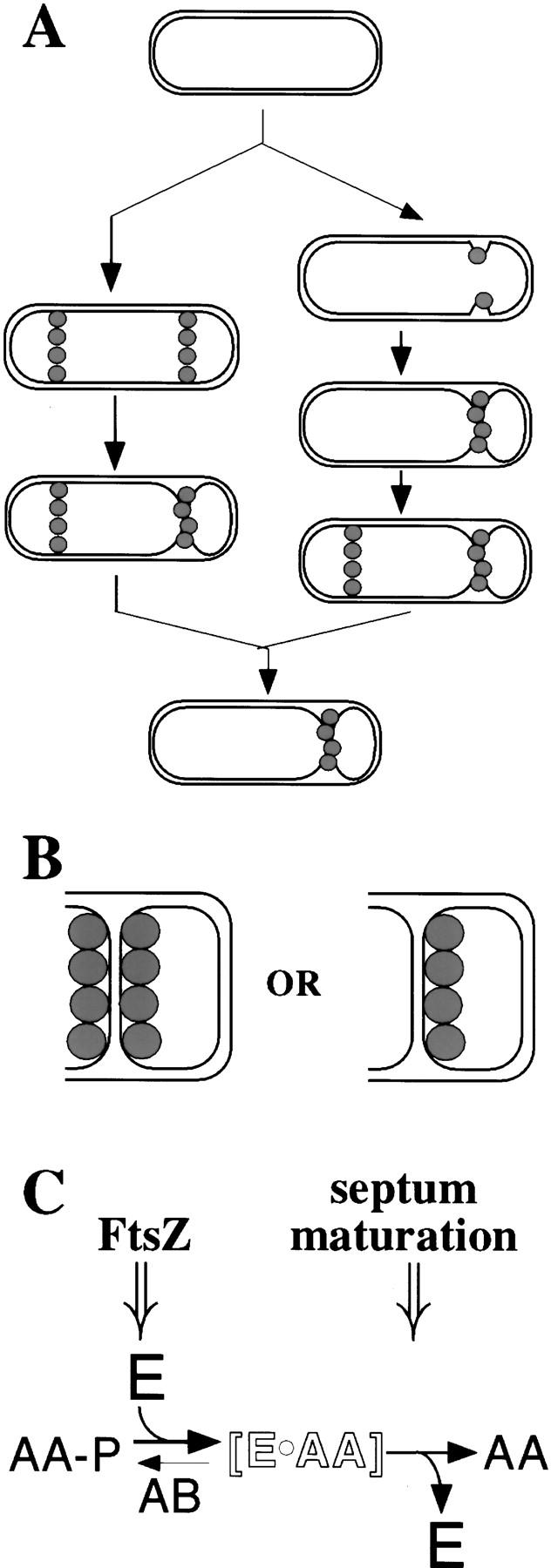
Models for the localization of SpoIIE and the regulation of σF. (A) Alternative pathways for the localization of SpoIIE. The left pathway indicates that SpoIIE (shaded circle) can assemble into E-rings at both poles of the sporangium before polar division, whereas the right pathway indicates that only a single E-ring appears up to the time of asymmetric division and that its formation occurs concomitant with (as shown) or just before the formation of the polar septum. In both models, SpoIIE at the site of cytokinesis invades and becomes part of the polar septum. (B) The left cartoon conveys the possibility that SpoIIE is displayed on both surfaces of the septum, whereas the right side conveys the possibility that SpoIIE is displayed exclusively on the forespore face of the septum. (C) A two-stage model for the role of SpoIIE in coupling σF activation to septum formation. The open lettering denotes a hypothetical intermediate in which the product (SpoIIAA; denoted AA) of SpoIIE-mediated dephosphorylation of SpoIIAA-P remains sequestered in a complex with SpoIIE (E) such that it is unable to react with SpoIIAB–σF. (See the text for details.)
The discovery that SpoIIE localizes to the polar septum revealed a direct connection between the activation of σF and the process of asymmetric division, but left unanswered the questions of how the timing and cell selectivity of σF activation are determined (Frandsen et al. 1999; Losick and Dworkin 1999). One possibility is that SpoIIE is displayed on both faces of the polar septum but because the forespore is much smaller than the mother cell, the ratio of phosphatase (SpoIIE) to kinase (SpoIIAB) would be substantially higher in the forespore than in the mother cell (Fig. 1B; Duncan et al. 1995). Other possibilities are that SpoIIE is present on both faces of the septum but only active on the forespore face (Duncan et al. 1995; Arigoni et al. 1999), that SpoIIE is sequestered entirely to the forespore (Feucht et al. 1996; Fig. 1B), or that the compartmentalization of σF activity is the product of some combination of the above mechanisms.
Wu et al. (1998) reported evidence recently in support of the view that SpoIIE is confined to the forespore face of the polar septum based on experiments in which the forespore and mother cell compartments were converted into protoplasts by lysozyme treatment. They also reported that SpoIIE initially localizes exclusively at the future septal pole of the sporangium and that this localization occurs just before, or concomitant with, septum formation (see right-hand pathway of Fig. 1A). Therefore, according to Wu et al. (1998), after asymmetric division, SpoIIE is initially present exclusively in the forespore, thereby explaining the cell-specific activation of σF. They do observe a second ring of SpoIIE at the distal pole, but they conclude that it is formed only after asymmetric division has taken place.
If, as originally proposed, however, SpoIIE is positioned near both poles before septation, then an as yet undiscovered mechanism is needed to explain the absence of σF activity before septation or in the mother cell after septation. Because of the importance of this issue to our understanding of σF regulation and the discrepancies between the conclusions of Wu et al. (1998) and previous reports, we re-examined the pattern of subcellular localization of SpoIIE during the initial stages of sporulation, taking advantage of newly developed procedures for carrying out time-lapse microscopy (Webb et al. 1998; Price and Losick 1999) and for visualizing polar septa in living sporangia (Pogliano et al. 1999). Consistent with the observations of Arigoni et al. (1995), Levin et al. (1997), and Pogliano et al. (1997), we find that SpoIIE can, and frequently does, localize near both poles of sporangia that have not yet undergone asymmetric division. In addition, we present findings inconsistent with the conclusion that SpoIIE is sequestered to the forespore.
If, as we believe, E-rings are present in the predivisional sporangium, then SpoIIE must be subject to regulation to prevent the inappropriate activation of σF before polar septation. Here we present evidence for two regulatory mechanisms that couple the activation of σF to the formation of the septum. The first mechanism acts at the level of the phosphatase activity of SpoIIE. We show that SpoIIE-dependent dephosphorylation of SpoIIAA-P is partially dependent on FtsZ, a finding consistent with the idea that efficient dephosphorylation involves the FtsZ-dependent assembly of SpoIIE into E-rings. The second regulatory mechanism acts at a step subsequent to the dephosphorylation of SpoIIAA-P. We show that in a mutant (divIC) blocked at a late stage in septum formation (that is, after the stage of Z-ring formation), unphosphorylated SpoIIAA accumulates to high levels. Yet, σF activation is blocked almost entirely. Experiments based on the use of modified and mutant forms of SpoIIE lead us to propose that SpoIIE is involved in this second regulatory step, which couples the activation of σF to the completion of septum formation. We discuss the implication of these findings for the cell-specific activation of σF.
Results and Discussion
Bipolar positioning of SpoIIE rings precedes septation
We used three approaches to investigate the issue of whether the SpoIIE phosphatase assembles into rings (henceforth referred to as E-rings) near both poles of the sporangium before or after the formation of the polar septum. In the first approach, we carried out a time-course experiment in which cells were arrested at various times after the start of sporulation by chemical fixation (see Materials and Methods). E-rings were visualized by use of a fusion to a mutant form of the green fluorescent protein (GFP) with enhanced fluorescence and speed of folding (Cormack et al. 1996; see Materials and Methods). The SpoIIE–GFP fusion protein was indistinguishable from unmodified SpoIIE in its capacity to support sporulation (see Materials and Methods). In this experiment we used DAPI staining of DNA to monitor septum formation. Because chromosome translocation into the forespore occurs after asymmetric division, sporangia exhibiting a fully translocated forespore chromosome are known to have undergone polar division. No cells sampled at the first two time points exhibited chromosome translocation (0/329 at 75 min, 0/251 at 90 min). Thereafter, the number of cells with a condensed forespore chromosome increased progressively to 15% (23/148) by 105 min, 27% (53/196) by 120 min, and 58% (46/79) by 150 min. We also confirmed that at 75 min and 90 min, 98% (177/180) and 92% (153/167) of cells, respectively, were aseptate under our conditions by staining with the membrane stain FM4-64 (Pogliano et al. 1999; see below).
At the earliest time point, 75 min, only a small fraction [14% (44/329)] of cells displayed fluorescence from the fusion protein (data not shown). At this early time, when cells were predominantly aseptate, sporangia with E-rings at both poles [6% (19/329)] were almost as common as those exhibiting a unipolar pattern [8% (25/329)]. By 90 min, when the number of fluorescing cells had increased, sporangia with two E-rings [19% (48/251)] outnumbered those with a single ring [7% (18/251)]. At 105 min, sporangia with bipolar E-rings [33% (49/148)] were observed more frequently than sporangia with a single E-ring [22% (32/148)]. Coinciding with the increase in sporangia that had undergone polar division, as determined by chromosome staining (120 and 150 min, see above), sporangia with single E-rings increased and eventually predominated. By the last time point, many sporangia had progressed to the next stage of sporulation in which the mother cell engulfs the forespore. In such sporangia, fluorescence from SpoIIE–GFP was seen to have partially migrated around or fully encircled the forespore.
The frequency of cells with two E-rings at the earliest (75 min) time point is consistent with the idea that bipolar localization can, and often does, precede septation. We also observed cells with a unipolar pattern at the early time point. Perhaps in predivisional sporangia an E-ring sometimes forms earlier at one pole than the other (but see below). Alternatively, in some cases only a single E-ring may form. It is formally possible that aseptate sporangia with bipolar E-rings can divide medially, yielding aseptate daughter cells with an E-ring at one pole. In any event, E-rings could be detected at times preceding the formation of the polar septum when they were present at one pole or both poles of the sporangium.
In a second approach to tracking the sequence of E-ring localization, we used time-lapse microscopy to monitor SpoIIE–GFP in individual, living cells over the course of the initial stages of sporulation (Fig. 2). Multiple time-lapse experiments were performed with similar results, but we focus here on a single representative field of cells for simplicity. Cells collected after 100 min of sporulation were applied to an agarose bed and viewed at 20-min intervals with a minimum of excitation light. Initially, 71% (49/69) of fluorescing cells displayed E-rings at both poles. Of these cells, 71% (35/49) lost one E-ring (developed unipolar localization) during the first 20-min interval. In contrast, none of the cells with a single, asymmetrically positioned E-ring developed a second ring over the course of the time-lapse experiment. In fact, throughout 80 min of observation by time-lapse microscopy, no cells switched from a unipolar to bipolar pattern of E-rings. In Figure 2B, three sporangia that initially have two E-rings are shown to subsequently lose an E-ring at one pole. Notice that in the initial time of observation (time 0) the E-ring that will persist (indicated with arrowhead) is somewhat thicker than the E-ring that will disappear. We know from other work in which septa are directly visualized with a membrane stain (see below) that the thickening of the E-ring corresponds to the formation of the polar septum, perhaps attributable to the stabilization of SpoIIE localization, an increase in the cross-sectional surface area available for SpoIIE localization, or the presence of SpoIIE in the two adjacent membranes of the septum. Therefore, in these time-lapse images, we believe that it is the E-ring distal to the polar septum that is disappearing.
Figure 2.
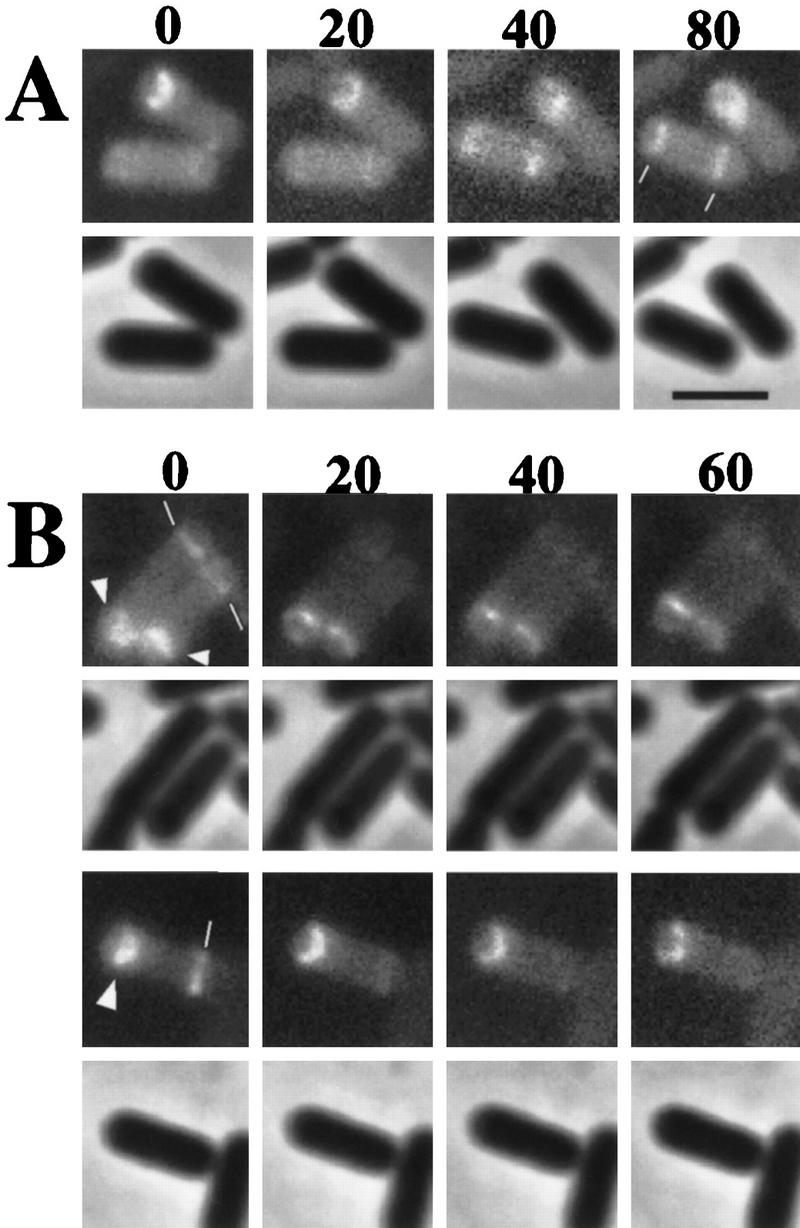
Time-lapse microscopy of SpoIIE localization in living cells. Cells from a SpoIIE–GFP-expressing strain were monitored commencing at 100 min after the start of sporulation. Time-lapse intervals are indicated in minutes from the first observation time. For each time-lapse sequence, fluorescence images are shown in a row at the top and the corresponding phase-contrast image in a row underneath. (A) A cell (the bottom cell) that initially lacked E-rings but acquired bipolar rings (marked with white lines) during the time-lapse sequence. This sporangium is judged to lack a polar septum from the thinness of the E-rings. (B) The top sequence shows sporangia that had bipolar E-rings at the start of observation. These are judged to be postdivisional sporangia from the thickness of one of the two bipolar bands of fluorescence. The septum-distal E-ring can be seen to disappear over the course of the time-lapse. The bottom sequence shows another example of a sporangium with bipolar bands of SpoIIE–GFP in which the forespore-distal ring dissipates during the time-lapse sequence. In this case, the forespore-proximal band of SpoIIE–GFP is curved, indicating that the process of engulfment has commenced. The sequence of events is slower than under normal conditions because the time-lapse experiment was carried out at room temperature. Scale bar, 2.5 μm.
We also attempted to catch cells at a very early stage of development before E-rings were present to observe the subsequent emergence of E-rings at the cell poles. Such cases were rare, but in two instances we did observe sporangia in which E-ring formation was taking place. In both cases (one of which is presented in Fig. 2A) sporangia underwent a transition in which unlocalized SpoIIE–GFP became organized into bipolar E-rings. Taken together, the results of the time-lapse experiments are consistent with the idea that E-rings can form at both poles and that the E-ring at the pole distal to the septum is disassembled subsequent to asymmetric division.
A limitation in the analysis so far is that we were not able to visualize septa in the sporangia in which fluorescence from SpoIIE–GFP was being observed. The recent application of a vital stain, FM4-64, to B. subtilis makes it possible to observe membranes and hence septa in living sporangia (Pogliano et al. 1999). Because the polar septum consists of two adjacent membranes (one from the forespore and one from the mother cell), the polar septum stands out more brightly than the cytoplasmic membrane that surrounds the sporangium.
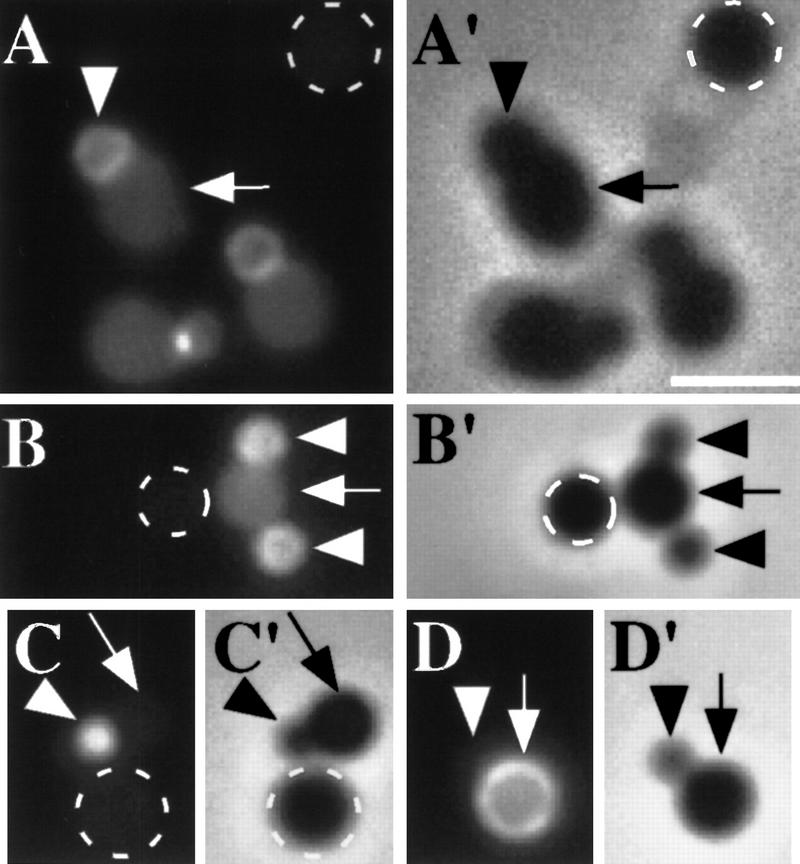
To assess directly the relationship between E-ring assembly and septum formation we observed SpoIIE–GFP fluorescence in FM4-64-stained living cells at ∼100 min after the start of sporulation. Importantly, predivisional cells were readily detected that had E-rings at one (Fig. 3A) or both poles (Fig. 3B,C). Sporangia with bipolar E-rings represented about half (11/22) of all SpoIIE-producing, aseptate cells. Notice that the fluorescence from the two polar E-rings was of comparable appearance and intensity in cells that were aseptate (Fig. 3B,C), a feature that appears to be characteristic of predivisional sporangia. The remaining aseptate cells had a single E-ring at one pole. Finally, among sporangia that were judged from membrane staining to have a polar septum, SpoIIE–GFP was positioned at both poles in only 11% (12/105) of cells and in these sporangia, fluorescence from SpoIIE–GFP associated with the septum was typically more intense than that at the opposite pole (Fig. 3D). Most postdivisional sporangia [89% (93/105)] displayed a signal from SpoIIE–GFP at only one pole, the fluorescence coinciding (as can be seen in Fig. 3E) with the position of the polar septum.
Figure 3.
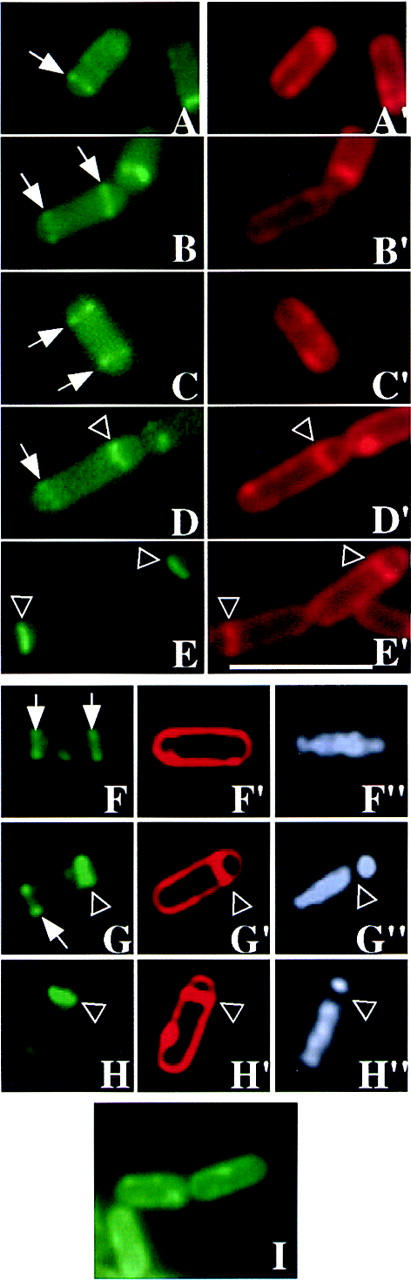
Visualization of polar septa and SpoIIE–GFP localization in living sporangia. The pattern of fluorescence (green) from SpoIIE–GFP is shown in A–H and the corresponding pattern of fluorescence (red) from the membrane stain FM4-64 is shown in A′–H′. Fluorescence from a MalF–SpoIIE–GFP producing strain is shown in I. Small arrows mark E-rings that are not associated with a septum. Bands of SpoIIE–GFP that correspond to the site of a polar septum are indicated by an open arrowhead. In the images from the DAPI-stained sporangia (F′′,G′′,H′′), the open arrowhead marks the gap separating the forespore and mother-cell chromosomes. Sporangia displayed in A–E were stained with the membrane stain, FM4-64, before inspection of GFP fluorescence. (A) Aseptate sporangium with a single E-ring. (B,C) Bipolar E-rings in aseptate sporangia. The intensity and thickness of both rings was approximately equal. (D) Postdivisional sporangium with bipolar E-rings. Note that the E-ring associated with the septum is thicker than that at the forespore-distal pole. (E) Postdivisional sporangia with single E-rings. As a further test for the presence or absence of polar septa, the sporangia of F–H were treated as follows. First, fluorescence signals from SpoIIE–GFP and from DAPI were recorded. Next, the sporangia were treated with the membrane stain FM4-64. Finally, to improve the resolution of the membrane, a series of images were collected along the Z-axis and subjected to a deconvolution algorithm, resulting in the images of panels F′–H′. Sporangia with a polar septum (G′,H′) often have a condensed forespore chromosome (G′′,H′′). The contrast in F, G, and H was heightened to more readily visualize SpoIIE–GFP localization. Note that in G, the thicker band of SpoIIE–GFP corresponds in position to the polar septum (also see Fig. 2). (I) Fluorescence from MalF–SpoIIE–GFP coincides with the cytoplasmic membrane. Scale bar, 5 μm.
That bipolar E-rings are present in predivisional sporangia can be seen even more clearly by deconvolution microscopy (see Materials and Methods; Hiraoka et al. 1990; Scalettar et al. 1996). Figure 3 (F;F′) shows deconvolved images of a predivisional sporangium stained with FM4-64 that has E-rings near both poles but is clearly lacking a polar septum. DAPI-staining of the same sporangium shows that the sporangium lacks a separated, condensed forespore chromosome (Fig. 3F“). Deconvolution was also used to visualize postdivisional sporangia harboring SpoIIE at both ends of the sporangium (Fig. 3G) or exclusively at the polar septum (Fig. 3H). In both of these postdivisional sporangia, DAPI staining reveals a separate, condensed forespore chromosome (Fig. 3G′′,H′′).
Together with the results from the time-lapse experiment, the use of a membrane stain to visualize septa supports the view that E-rings can arise at both poles of the sporangium prior to asymmetric division. Our evidence also supports the view that after asymmetric division the E-ring distal to the polar septum disappears whereas the SpoIIE associated with the septum persists until after σF is activated. Eventually SpoIIE at the septum is also eliminated (Pogliano et al. 1997).
Distribution of SpoIIE in postdivisional sporangia
A second issue central to our understanding of SpoIIE localization is whether SpoIIE is displayed on one or both faces of the sporulation septum (Fig. 1B). Direct assessment of the orientation of SpoIIE within the septum has not been feasible because immunoelectron microscopy does not provide the sensitivity, and fluorescence microscopy the resolution, necessary to address this issue. The two compartments, however, can be artificially separated as the result of lysozyme treatment of postdivisional sporangia. Degradation of the peptidoglycan layer by lysozyme allows the mother cell and forespore to swell and separate slightly, as well as freeing the membrane components to diffuse within the peripheral membrane of each compartment. The bright signal from SpoIIE–GFP in the forespore of protoplasts generated by lysozyme treatment led to the conclusion that SpoIIE is sequestered to the forespore face of the sporulation septum (Wu et al. 1998).
The difference in volume between the forespore and mother cell raises the possibility, however, that a given amount of SpoIIE–GFP packaged into the forespore would yield a more intense signal than the same amount distributed throughout the larger, mother cell compartment. To address the issue of the total relative amount of SpoIIE–GFP in the forespore as compared with the mother cell, we generated protoplasts from postdivisional sporangia harboring the spoIIE–gfp fusion. As a control for autofluorescence, protoplasts were generated from a mixture of sporangia harboring, and sporangia lacking, the spoIIE–gfp gene fusion. As observed by Wu et al. (1998), GFP fluorescence from the forespore was brighter than that in the mother cell (Fig. 4A). Nonetheless, the mother cell also exhibited substantial fluorescence. This fluorescence could be attributed to SpoIIE–GFP because the level of fluorescence in the mother-cell protoplasts was considerably greater than that observed in protoplasts of sporangia lacking SpoIIE–GFP (Fig. 4A). The total fluorescence integrated across the area of the mother cell was on average 5.9 times greater than that from a negative control (s.d. = 2.9; n = 33; see Materials and Methods). Furthermore, the total fluorescence from the forespore compared with that from the mother cell, both corrected for background, was approximately equal (integrated forespore fluorescence/integrated mother cell fluorescence = 1.2; s.d. = 0.4; n = 33). We conclude from this analysis that significant levels of SpoIIE–GFP were present in the mother-cell protoplast of the fusion-harboring cells. For comparison, fluorescence from GFP produced under the control of σF was entirely confined to the forespore protoplast (Fig. 4C), whereas fluorescence from GFP produced under the control of the mother cell transcription factor σE (Stragier and Losick 1996) was entirely restricted to the mother cell protoplast (Fig. 4D).
Figure 4.
Distribution of SpoIIE–GFP in protoplasts. Protoplasts were generated by lysozyme treatment of SpoIIE–GFP-producing sporangia of a wild-type (sporulation proficient) strain or a disporic mutant strain in which the coding sequence for a mother cell-specific transcription factor is disrupted (spoIIGB::erm). For comparison, before lysozyme treatment, GFP-producing sporangia were mixed with sporangia lacking a gene fusion. Fluorescence from SpoIIE–GFP is shown in A, B, C, and D with the corresponding phase-contrast images shown in A′, B‘, C‘, and D′. (A) SpoIIE–GFP distribution in protoplasts of wild-type sporangia. (B) SpoIIE–GFP distribution in protoplasts of disporic mutant sporangia. The highest intensity of fluorescence emanated from the forespores, which are marked with an arrowhead. Note, however, that the signal from the mother cells (arrow) is substantially higher than the background of autofluorescence observed in the protoplasts lacking SpoIIE–GFP (broken circle). (C,D) As controls, protoplasts were also generated from cells producing GFP under the control of a σF-dependent promoter (amyE::sspE(2G)–gfp; Webb et al. 1995) (C) and a mother cell-specific promoter (amyE::gfp–spoIVA; Price and Losick 1999) (D). Scale bar, 2.5 μm.
As we have seen, among postdivisional sporangia, some have SpoIIE–GFP located only at the polar septum, whereas others harbor an additional E-ring at the distal pole of the sporangium (e.g., see Fig. 3D,G). The sporangia used for generating protoplasts were harvested at 100 min after the start of sporulation, a time when most sporangia have SpoIIE–GFP located only at the polar septum (see above). Nonetheless, it was possible that in some cases the presence of a forespore-distal E-ring had contributed to the fluorescence detected in the mother cell protoplast of lysozyme-treated sporangia. To address this issue, we generated protoplasts from a SpoIIE–GFP-producing strain that harbors a mutation (spoIIGB::erm) that causes the formation of septa at both poles of the sporangium (Lewis et al. 1994; Stragier and Losick 1996). This results in a three-chamber, ‘disporic’ sporangium, which consists of two forespores, one at each end of the sporangium, and a mother cell in between. In such disporic sporangia, SpoIIE–GFP is associated with the septa at both poles (data not shown). If SpoIIE is sequestered to the forespore face of the sporulation septum, then it should be absent from the middle mother cell compartment in protoplasts of spoIIGB mutant cells. Instead, mother cell-associated fluorescence was readily observed following lysozyme treatment of spoIIGB mutant cells (Fig. 4B). That the mother cell signal was derived from SpoIIE–GFP was confirmed by comparison with the low level of autofluorescence in non-GFP-producing sporangia (Fig. 4B). Quantitating the results, we found that the total fluorescence from both forespores of a given disporic protoplast, corrected for background, was on average 2.8 times greater than that in the mother cell (s.d. = 1.6; n = 12).
The fact that the average total fluorescence from the mother cell in disporic sporangia was lower than the sum of that present in both forespores could indicate that the amount of SpoIIE displayed on both faces of the sporulation septum is not equal. The higher total level of signal in the forespores, however, could reflect the continued synthesis of SpoIIE after or during chromosome segregation; in disporic sporangia, a chromosome is incorporated into each polar forespore compartment, leaving the middle mother cell devoid of a chromosome and therefore lacking a copy of the gene fusion. In any event, the observation that fluorescence from SpoIIE–GFP could be detected readily in the mother-cell chamber of disporic sporangia is, once again, inconsistent with the contention that SpoIIE is sequestered to the forespore.
Appearance of unphosphorylated SpoIIAA during the onset of sporulation
If, as we conclude, SpoIIE is not only present in the forespore but also in the predivisional sporangium and in the mother cell of the postdivisional sporangium, then SpoIIE must somehow be subject to regulation to prevent inappropriate activation of σF. It is known that the activation of σF is dependent on the cell-division proteins FtsZ and DivIC (as confirmed in Fig. 5A,B; Gonzy-Treboul et al. 1992; Margolis 1993; Levin and Losick 1994). Therefore, an attractive hypothesis is that SpoIIE must associate with the septum to become active in dephosphorylating SpoIIAA-P. To investigate this hypothesis, we first monitored the time course of appearance of unphosphorylated SpoIIAA in wild-type cells undergoing sporulation. Lysates were prepared from cells harvested at intervals after the start of sporulation, and the lysates were subjected to isoelectric focusing (IEF), a procedure that readily resolves SpoIIAA from SpoIIAA-P. Next, SpoIIAA and SpoIIAA-P were visualized by immmunoblot analysis using anti-SpoIIAA antibodies (Garsin et al. 1998). In wild-type cells, SpoIIAA was initially present in the phosphorylated state (Fig. 6, B, top, and C). As early as 90 min after the initiation of sporulation and at later times, however, the level of unphosphorylated SpoIIAA rose to levels that were approximately equal to those of SpoIIAA-P. In agreement with previous results, the appearance of unphosphorylated SpoIIAA was entirely dependent on SpoIIE as little or no SpoIIAA was present in the unphosphorylated state in lysates from a spoIIE null mutant (Fig. 6B; Arigoni et al. 1996; Feucht et al. 1996).
Figure 5.
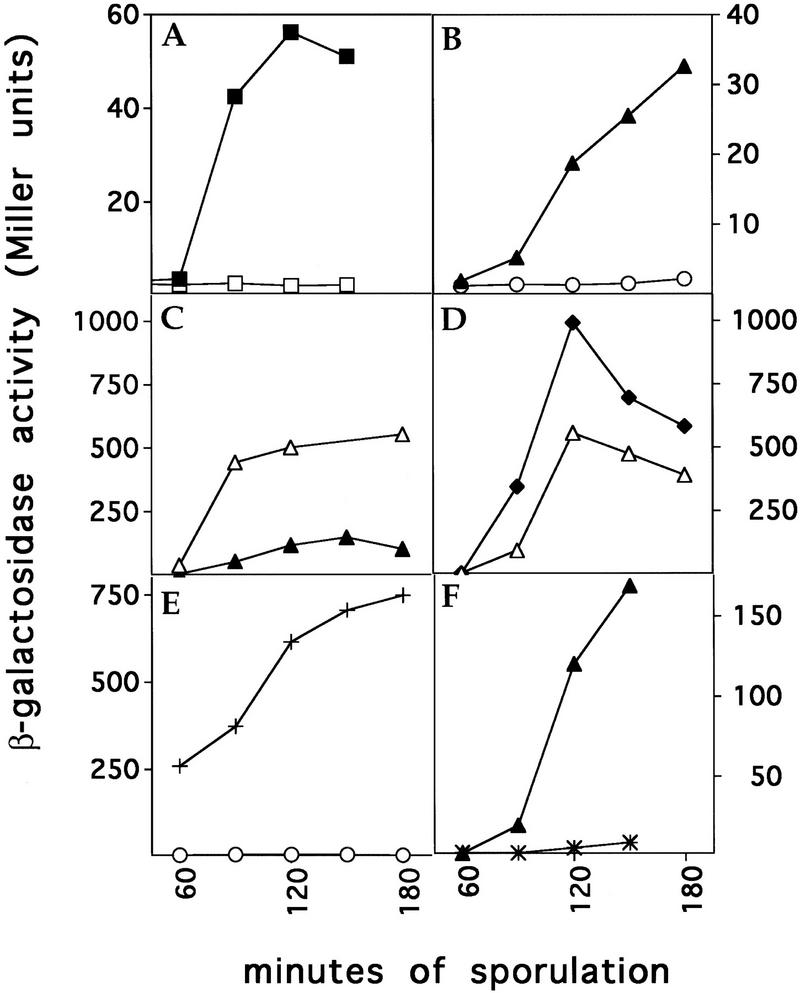
σF-Directed β-galactosidase synthesis. All strains contained a fusion of lacZ to a gene (spoIIQ) under the control of σF (Londono-Vallejo et al. 1997). Assays of β-galactosidase activity were performed as described previously (Margolis et al. 1993). (A) Effect of FtsZ depletion. A strain in which FtsZ is produced under the control of an IPTG-inducible promoter was sporulated in the presence (█) or absence (□) of inducer. (B) Effect of a divIC mutation. A strain harboring a divIC truncation (divICΩpPL27; ○) and its congenic wild-type strain (▴) were sporulated after a shift to a restrictive temperature (37°C). This experiment was performed with samples of cells from the same experiments as that described in the lower blot of Fig. 6B. (C) Effect of MalF–SpoIIE–GFP. β-galactosidase synthesis is compared between a strain that produces MalF–SpoIIE–GFP (▵) and a wild-type strain (▴). (D) Effect of MalF–SpoIIE–GFP in a divIC mutant. β-galactosidase synthesis is compared between MalF–SpoIIE–GFP-producing cells bearing wild-type divIC (▵) and MalF–SpoIIE–GFP-producing cells bearing a divIC truncation (divICΩpPL27; ♦). (E) Effect of SpoIIAA–S58A in a divIC mutant. β-galactosidase synthesis is compared between divIC mutant cells producing wild-type SpoIIAA (○) and divIC mutant cells producing SpoIIAA–S58A (+). (F) Effect of spoIIE48 mutation. β-Galactosidase synthesis is compared between cells bearing wild-type spoIIE (▴) and cells harboring spoIIE48 (*).
Figure 6.
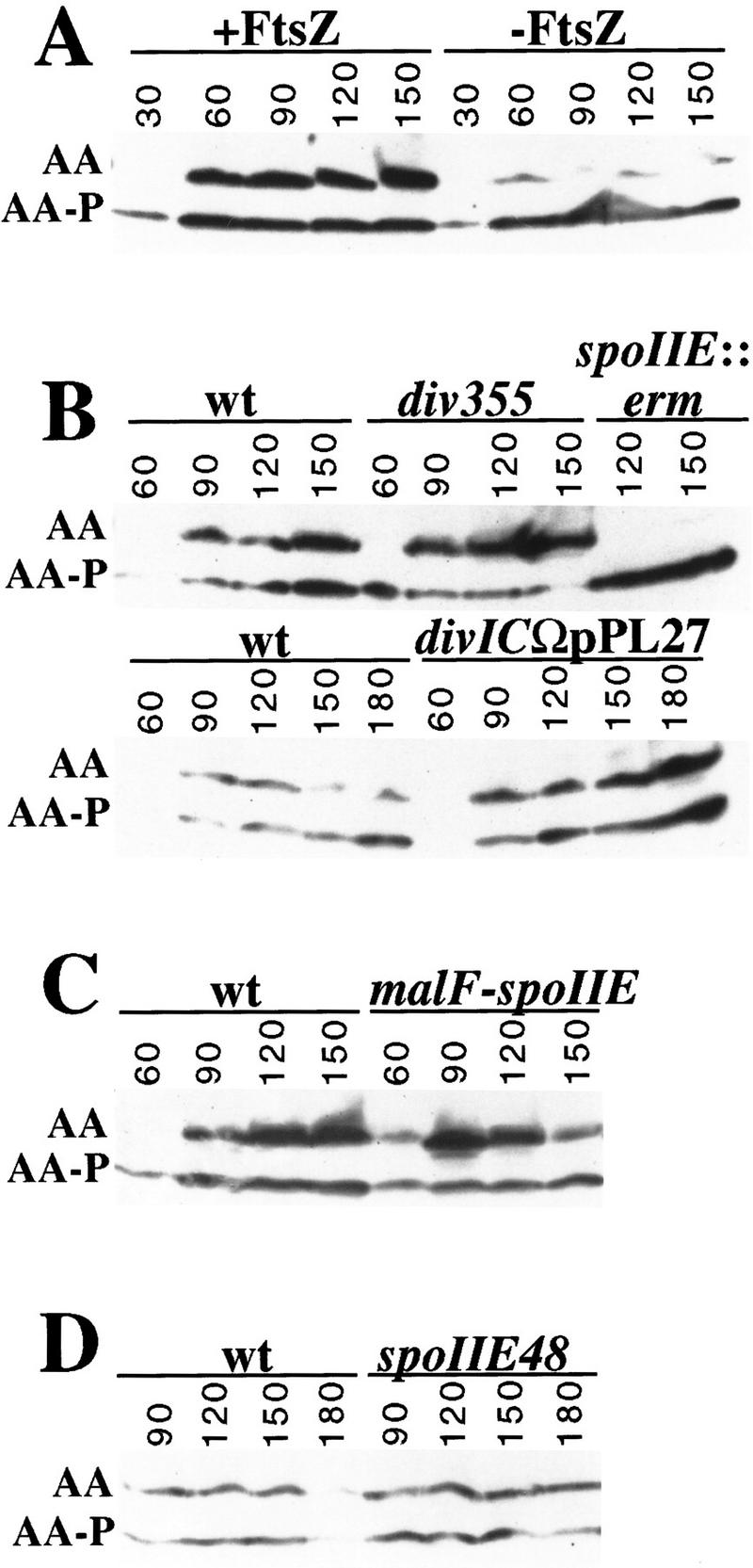
Accumulation of unphosphorylated SpoIIAA during sporulation. Lysates from cells collected at the indicated times (minutes) during sporulation were subjected to isoelectric focusing as described previously (Garsin et al. 1998). Immunoblot analysis with anti-SpoIIAA antibodies was then used to visualize SpoIIAA (AA) and SpoIIAA-P (AA-P) (Garsin et al. 1998). (A) The effect of FtsZ depletion on the accumulation of unphosphorylated SpoIIAA is shown for a strain in which the synthesis of FtsZ was under the control of an IPTG-inducible promoter (Pspac–ftsZ). (B) The upper blot compares the accumulation of unphosphorylated SpoIIAA in a divIC mutant strain (div355) with that observed in a congenic wild-type strain and spoIIE mutant strain (spoIIE::erm). The lower blot compares the accumulation of unphosphorylated SpoIIAA in cells harboring a truncation of divIC (divICΩpPL27) with that observed in a congenic wild-type strain. (C) The accumulation of unphosphorylated SpoIIAA is compared between sporulating cells of a strain that harbors malF–spoIIE–gfp and the corresponding wild-type strain. (D) The accumulation of unphosphorylated SpoIIAA is compared between wild-type sporulating cells and cells that harbor the spoIIE48 mutation.
These results are seemingly at odds with the contention that unphosphorylated SpoIIAA is concentrated in the forespore and that this is the basis for the cell-specific activation of σF (Lewis et al. 1996). As presented above, only ∼8% of the cells had formed polar septa by 90 min. Yet, about half of the SpoIIAA was in the unphosphorylated state at this time. Because total SpoIIAA (SpoIIAA and SpoIIAA-P) is evenly distributed throughout the sporangium (Lewis et al. 1996), and because the volume of the forespore is much smaller than that of the mother cell, if SpoIIAA-P were dephosphorylated exclusively in the forespore, only a small proportion (<20%) of the total SpoIIAA in the sporangium should be unphosphorylated. Therefore, the results of the time-course experiments are most easily compatible with the view that unphosphorylated SpoIIAA is present in the predivisional sporangium and the mother cell as well as in the forespore.
Dephosphorylation of SpoIIAA-P is partially dependent on the cell division protein FtsZ
To investigate a possible connection between the phosphatase activity of SpoIIE and septum formation, we examined the phosphorylation state of SpoIIAA in sporulating cells that had been partially depleted for FtsZ. We used a construct in which the transcription of ftsZ is under the control of an IPTG-inducible promoter (Pspac). In strains harboring this construct, removal of the IPTG inducer before the start of sporulation results in partial depletion of FtsZ and blockage of asymmetric division (Beall and Lutkenhaus 1991; Margolis 1993; see Materials and Methods). Figure 6A shows that the accumulation of unphosphorylated SpoIIAA was impaired in the cells that had been deprived of FtsZ. Although there was some variability from experiment to experiment (and not all experiments showed as pronounced an impairment as that of Fig. 6A), the ratio of unphosphorylated SpoIIAA to SpoIIAA-P consistently reached levels that were severalfold higher in cells that had been maintained in the presence of IPTG than in cells that had been sporulated in the absence of inducer. The reduction in the proportion of unphosphorylated SpoIIAA in response to FtsZ depletion suggests that FtsZ is required for efficient dephosphorylation of SpoIIAA-P. Impaired dephosphorylation cannot be attributed to a decrease in the level of phosphatase protein because the amount of SpoIIE in FtsZ-depleted cells is known to be equal to, or exceed, that observed in cells maintained in the presence of inducer as judged by immunoblot analysis (Levin et al. 1997). Therefore, we conclude that the phosphatase activity of SpoIIE is at least in part dependent on FtsZ.
Dephosphorylation of SpoIIAA-P does not depend on septation
Two possible explanations for the reduced levels of unphosphorylated SpoIIAA in FtsZ-depleted cells are that efficient dephosphorylation requires the FtsZ-dependent assembly of SpoIIE into E-rings (or some other kind of direct or indirect interaction of SpoIIE with FtsZ) or the subsequent invasion of SpoIIE into the sporulation septum. To distinguish between these possibilities, we determined the level of unphosphorylated SpoIIAA in cells harboring a mutation in the cell division gene divIC. Septum formation in divIC mutant cells is heat sensitive; when shifted to a restrictive temperature at the start of sporulation, polar septation is blocked and the activation of σF is prevented. Nonetheless, divIC function is not required either for Z-ring formation or the coassembly of SpoIIE into E-rings (Levin and Losick 1994; Levin et al. 1997).
The results of the immunoblot analysis showed that in cells sporulating at a restrictive temperature and harboring either of two different mutant alleles of divIC (div355 or divICΩpPL27; Fig. 6B) unphosphosphorylated SpoIIAA accumulated to levels that matched or exceeded those observed in wild-type cells. For example, whereas in wild-type cells unphosphorylated SpoIIAA reached a level comparable with that of the phosphorylated protein, in cells of the div355 mutant, unphosphorylated SpoIIAA reached levels that substantially exceeded those of the phosphorylated protein. Yet, under these conditions (in cells from the same culture of divICΩpPL27 cells used for the immunoblot analysis in Fig. 6B) the activation of σF was almost completely prevented (Fig. 5B). A similar observation has been made by J. Errington (pers. comm.) using a different cell division mutant. These are surprising results because unphosphorylated SpoIIAA is normally considered to be a potent activator of σF. Indeed, a nonphosphorylatable mutant of SpoIIAA (SpoIIAA–S58A) induces σF activity to high levels (Diederich et al. 1994). Morever, Figure 6E shows that SpoIIAA–S58A efficiently activated σF in div355 mutant cells.
We draw two conclusions from these observations. First, efficient dephosphorylation of SpoIIAA-P depends on an event closely associated with the assembly of SpoIIE into the E-ring and E-ring formation is sufficient to fully activate the phosphatase activity of SpoIIE. Because E-rings are formed before polar septation, this finding explains the observation noted above that in wild-type cells, dephosphorylation commences before asymmetric division and reaches higher levels than would be expected if dephosphorylation were simply confined to the forespore. Second, dephosphorylation of SpoIIAA-P is not sufficient to cause the activation of σF. Rather, an additional event that is dependent on cytokinesis (or some other divIC-dependent activity) is needed to switch on the activity of the forespore transcription factor. In other words, σF activation is dependent on septum formation at a step subsequent to the dephosphorylation of SpoIIAA-P.
Uncoupling σF activation from septum formation by use of modified SpoIIE
A clue as to how the morphological event of septation contributes to σF activation was provided by studies with cells harboring a modified form of SpoIIE–GFP in which the amino-terminal region, which contains 10 membrane-spanning segments (Adler et al. 1997; Arigoni et al. 1999), was replaced with the first two membrane-spanning segments of the Escherichia coli MalF protein (Boyd et al. 1987). Rather than associating with Z-rings or the sporulation septum, the chimeric MalF–SpoIIE–GFP protein exhibited a pattern of localization that coincided with the cytoplasmic membrane (Fig. 3I). Therefore, the amino-terminal region of SpoIIE contributes to the proper localization of SpoIIE or the presence of the membrane-spanning segments of MalF override localization signals located elsewhere in the protein. Importantly, in sporulating cells producing MalF–SpoIIE–GFP both the accumulation of unphosphorylated SpoIIAA and the synthesis of β-galactosidase under the direction of σF commenced earlier, and reached levels higher, than those observed in wild-type cells or in cells harboring spoIIE–gfp (Figs. 5C and 6C). This could not be attributed to enhanced levels of the fusion protein because the amount of MalF–SpoIIE–GFP was similar to that of SpoIIE–GFP as judged by immunoblot analysis (data not shown).
The high level of β-galactosidase synthesis from a σF-dependent promoter observed as early as 90 min after the start of sporulation suggested that σF activation might precede, and therefore be independent of, polar septum formation in cells harboring malF–spoIIE–gfp. Indeed, immunofluorescence microscopy experiments revealed no sporangia with compartmentalized β-galactosidase (data not shown). Instead, we observed many cells in which the entire sporangium was fluorescent. Uncompartmentalized activation of σF in cells producing MalF–SpoIIE–GFP was associated with a 106-fold reduction in spore formation relative to cells producing SpoIIE–GFP (data not shown). Further evidence that MalF–SpoIIE–GFP was capable of activating σF in a manner that did not depend on septum formation came from the use of cells harboring a divIC mutation. Under conditions in which septation was prevented because of incubation of a divIC mutant at a restrictive temperature, MalF–SpoIIE–GFP was capable of activating σF-directed synthesis of β-galactosidase to high levels (Fig. 5D).
A SpoIIE mutant that is blocked in σF activation but not dephosphorylation
We have discovered that cells harboring the classic spoIIE mutation spoIIE48, which causes a Ser to Phe substitution at residue 361 (Barak et al. 1996), are blocked in activation of σF (Fig. 5F) but not in the dephosphorylation of SpoIIAA-P (Fig. 6D). Therefore, SpoIIE–S361F and MalF–SpoIIE–GFP cause opposite phenotypes—both carry out the dephosphorylation of SpoIIAA-P but the former blocks the activation of σF, whereas the latter permits higher than normal levels of σF activation and in a manner that is independent of septum formation. Taken together, these results implicate SpoIIE itself in regulating the activation of σF at a step subsequent to the dephosphorylation of SpoIIAA-P.
What is the regulatory step and what is the nature of the involvement of SpoIIE? We hypothesize that following dephosphorylation of SpoIIAA-P, the resulting molecule of unphosphorylated SpoIIAA is retained by unmodified SpoIIE (or SpoIIE–GFP but not by MalF–SpoIIE–GFP), being sequestered in such a way that it is unable to react with the SpoIIAB–σF complex. Alternatively, unphosphorylated SpoIIAA could be retained by another protein in the septum. On completion of the septum, SpoIIE (or another protein in the septum sequestering SpoIIAA) undergoes a conformational change such that the SpoIIE-bound SpoIIAA either becomes accessible to SpoIIAB–σF or is simply released into the cytoplasm. According to this view, σF activation is tied to the formation of the polar septum in two stages by separate checkpoint mechanisms—one acting at the level of phosphatase activity and involving (or associated with) the FtsZ-dependent assembly of SpoIIE into E-rings and the other acting after septation and involving the dephosphorylation of SpoIIAA-P and involving the escape of the anti-anti-σ factor from an hypothesized SpoIIE–SpoIIAA complex (Fig. 1C).
Our results reinforce the view that the activation of σF is coupled to cytokinesis through the presence of the SpoIIE phosphatase in the septum. Left unresolved, however, is the issue of the basis for the cell-specific activation of σF. The discovery that the activity of σF is regulated at a step subsequent to the dephosphorylation of SpoIIAA-P raises the possibility that this newly discovered regulatory step is involved in limiting σF-directed gene expression to the forespore.
Materials and methods
Construction of spoIIE gene fusions
spoIIE–gfp was introduced into the chromosome by Campbell-like integration (Cutting and Horn 1990) of pPE1 (the gift of P. Eichenberger, Harvard University, Boston, MA) at the spoIIE locus to create strain PE1. pPE1 contained the coding sequence for the carboxy-terminal 300 residues of SpoIIE fused to a modified gfp-coding sequence that contained the codon substitions F64L (Cormack et al. 1996) and S65T (Heim et al. 1995). Sporulation by PE1 (producing ∼2.8 × 108 heat-resistant spores/ml) was indistinguishable from the congenic wild-type strain PY79. The malF–spoIIE–gfp fusion consisted of the coding sequences for residues 1–10 of SpoIIE fused to residues 5–68 of MalF (representing the first two membrane spanning domains of MalF; fusion D from Boyd et al. 1987) fused to residues 325–827 of SpoIIE, fused to the entirety of GFP(S65T). It was created by a PCR-based strategy in which restriction sites for FseI and AgeI were introduced at 29–36 and 966–971 bp of spoIIE. The malF–spoIIE–gfp fusion was inserted into the chromosome by double recombination at amyE.
Strain construction
All strains were derivatives of PY79 (Youngman et al. 1984) and were built with the following constructs: spoIIE::erm (representing a replacement of most of the spoIIE-coding sequence with erm; laboratory stock); thr::spoIIQ–lacZ (Arigoni et al. 1999); amyE::spoIIE–gus (from strain RL1739, laboratory stock); spoIIEΩpPE1(spoIIE–gfp) (see above); amyE::spoIIE–gfp (gift of C. Webb, Harvard University, Boston, MA); amyE::malF–spoIIE–gfp (see above); spoIIIG–lacZΩneo spoIIIGΔ1 (laboratory stock; Margolis 1993); spoIIGB::erm (Kenney and Moran 1987); ftsZΩpPL80 (pPL80 is a derivative of pAG58 generously provided by P. Levin, J. Quisel, and A. Grossman, pers. comm.); div355 (Levin and Losick 1994); divICΩpPL27 (Levin and Losick 1994); spoIIE48 (Errington and Mandelstam 1986; Barak et al. 1996); dacF::spoIIAA+ (Alper 1996); dacF::spoIIAA(S58A) (Duncan et al. 1996); amyE::sspE(2G)–gfp (Webb et al. 1995); amyE::gfp–spoIVA (Price and Losick 1999). Preparation of competent cells and transformation were performed as described in Cutting and Horn (1990).
Sporulation conditions
For the time-course experiments, as well as for the experiments of Figures 2, 3A–H, 4, 5C, and 6C, and D, cells were induced to sporulate by the resuspension method (Nicholson and Setlow 1990). For the experiments of Figures 3I, 4C, and 4D, the cells were sporulated by exhaustion in Difco sporulation (DS) medium at 25°C overnight (Harry et al. 1995). FtsZ depletion (Figs. 5A and 6A) was performed as described previously (Beall and Lutkenhaus 1991; Margolis 1993; Levin et al. 1997).
For studies of strains harboring mutations in divIC (Figs. 5B,D,E and 6B), cells were grown at a permissive temperature in culture medium (Nicholson and Setlow 1990) and shifted to the restrictive temperature on resuspension in sporulation salts (Nicholson and Setlow 1990; Levin et al. 1997). The strain containing the div355 missense allele and control strains were grown at 30°C and sporulated at 39°C, as described by Levin et al. (1997). Strains containing the truncated divIC gene (divICΩpPL27) and congenic control strains were grown in culture medium at 25°C to an OD600 of ∼0.5, suspended in sporulation salts, and shifted to the restrictive temperature of 37°C (Nicholson and Setlow 1990).
Microscopy and image acquisition
For Figures 2, 3A–E, and 4, an Olympus BX 60 microscope equipped as described in Angert and Losick (1998) was used for photomicroscopy. The GFP and phase-contrast images for the time-course, time-lapse (Fig. 2), and protoplast experiments (Fig. 4) were captured using a U-MWIB excitation cube unit (Olympus) with a band-pass excitation filter (460–490 nm) and a long-pass barrier filter (≥515-nm). For Figure 3, A–E, the membrane stain (FM4-64; Molecular Probes, Eugene, OR) was observed using a U-MWG excitation cube unit (Olympus) with a band-pass excitation filter (510–550 nm) and a long-pass barrier filter (≥590-nm). In this case, GFP was visualized using an U-MWIBA excitation cube unit (Olympus) with a band-pass excitation filter (460–490 nm) and a band-pass barrier filter (512–550 nm) to minimize emission spectra overlap from the membrane stain.
Time-lapse microscopy of a spoIIE–gfp strain (Fig. 2) was performed essentially as described in Price and Losick (1999), except that cells were first observed at 90–105 min of sporulation. Phase-contrast and fluorescence images were acquired every 20 min over the course of the time-lapse experiments. The time-lapse experiments were carried out at room temperature (∼23°C) and therefore changes in the localization of SpoIIE were slower than that observed during the time-course experiment.
Sporulation time-course experiments
At various times following the initiation of sporulation by resuspension (see above), 500 μl of a SpoIIE–GFP-producing strain was fixed in a total of 0.013% glutaraldehyde and 2.6% paraformaldehyde in 30 mm NaPO4 buffer (pH 7.4) for 15 min on ice and 30 min at room temperature. After washing, cells were applied to a poly-l-lysine (Sigma)-treated multiwell microscope slide (ICN Biochemicals). Finally, chromosomes were visualized by staining the cells for 5 min with 0.2 μg/ml DAPI in PBS/glycerol. By fixing the cells, we were able to arrest sporulation and observe large numbers of sporangia.
Detection of SpoIIE–GFP in membrane-stained cells
To minimize crossover of fluroescence from FM4-64 into the green channel in the experiment of Figure 3 (A–E), we used a narrow spectrum excitation cube (U-MWIBA) and the minimum amount (0.075–0.3 μg/ml) of FM4-64 that would give a signal. To visualize both GFP and FM 4-64 in the deconvolution experiment of panels F–H of Figure 4, we first collected GFP and DAPI images from several focal planes, then added FM4-64 (2 μg/ml) and collected the images of the same field stained with FM4-64. SpoIIE–GFP-producing cells were stained with DAPI (at 0.5 μg/ml) and applied to poly-l-lysine-treated open dishes designed for use with inverted microscopes (Delta TC3 Dish, 0.15-mm thick; Bioptechs, Inc., Butler, PA). Between 10 and 20 images of GFP and DAPI, spaced 0.1–0.2 μm through the specimen, were collected using standard filter sets for DAPI (360–400 nm excitation and 457–507 nm emission), and GFP (490–510 nm excitation and 528–566 nm emission). FM4-64 was added into the dish precisely above the position of the microscope lens. To prevent cell lysis or displacement, images were collected immediately after addition of the membrane stain using a Rhodamine filter (555–583 nm excitation and 617–690 nm emission). These images were deconvolved by 15 iterations of the Delta Vision deconvolution software (Applied Precision, Inc.), which is a constrained iterative deconvolution program.
Protoplasting and integration of fluorescence
Protoplasts were prepared as described by Wu et al. (1998). We used the MetaMorph image-processing package to calculate the relative amounts of SpoIIE–GFP in forespore and mother-cell protoplasts. Regions corresponding to forespores and mother-cell protoplasts were individually selected using the Region Tools option. The sum of all intensity values for all the pixels (in the range of hundreds) in each of the selected regions was integrated. A background was determined by integrating signal obtained over an equivalent sized region of the field in which no cells were located, and this value was subtracted from the integrated signal obtained from the protoplasts. Finally, a similar procedure was used to determine the additional background caused by cell autofluorescence by reference to protoplasts in the same field that had been derived from cells lacking spoIIE–gfp. This too was subtracted from the integrated value obtained for the GFP-containing protoplasts.
Acknowledgments
We thank P. Levin, P. Piggot, L. Shapiro, and members of the Losick laboratory for helpful discussions and advice on the manuscript. We are grateful to D. Weiss and J. Beckwith for their advice on the construction of the MalF–SpoIIE–GFP fusion. We thank P. Eichenberger, F. Gueiros, P. Levin, and C. Webb for unpublished strains, and E. Angert and D. Garsin for technical advice. We thank J. Errington for communicating results before publication. N.K. was a predoctoral fellow of the National Science Foundation. This work was supported by a Beckman Young Investigator award to K.P. and National Institutes of Health grant GM18568 to R.L.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked ‘advertisement’ in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL losick@biosun.harvard.edu; FAX (617) 496-4642.
References
- Adler E, Donella-Deana A, Arigoni F, Pinna LA, Stragier P. Structural relationship between a bacterial developmental protein and eukaryotic PP2C protein phosphatases. Mol Microbiol. 1997;23:57–62. doi: 10.1046/j.1365-2958.1997.1801552.x. [DOI] [PubMed] [Google Scholar]
- Alper S. A partner switching mechanism for the control of the Bacillus subtilis transcription factors σF and σB. Ph. D. thesis, ‘Molecular and Cellular Biology.’. Cambridge, MA: Harvard University; 1996. [Google Scholar]
- Alper S, Duncan L, Losick R. An adenosine nucleotide switch controlling the activity of a cell type-specific transcription factor in B. subtilis. Cell. 1994;77:195–205. doi: 10.1016/0092-8674(94)90312-3. [DOI] [PubMed] [Google Scholar]
- Angert ER, Losick RM. Propagation by sporulation in the guinea pig symbiont Metabacterium polyspora. Proc Natl Acad Sci. 1998;95:10218–10223. doi: 10.1073/pnas.95.17.10218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arigoni F, Duncan L, Alper S, Losick R, Stragier P. SpoIIE governs the phosphorylation state of a protein regulating transcription factor σF during sporulation in Bacillus subtilis. Proc Natl Acad Sci. 1996;93:3238–3242. doi: 10.1073/pnas.93.8.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arigoni F, Guerout-Fleury A-M, Barak I, Stragier P. The SpoIIE phosphatase, the sporulation septum, and the establisment of forespore-specific transcription in Bacillus subilis: A reassessment. Mol Microbiol. 1999;31:1407–1416. doi: 10.1046/j.1365-2958.1999.01282.x. [DOI] [PubMed] [Google Scholar]
- Arigoni F, Pogliano K, Webb CD, Stragier P, Losick R. Localization of protein implicated in establishment of cell type to sites of asymmetric division. Science. 1995;270:637–640. doi: 10.1126/science.270.5236.637. [DOI] [PubMed] [Google Scholar]
- Barak I, Behari J, Olmedo G, Guzman P, Brown DP, Castro E, Walker D, Westpheling J, Youngman P. Structure and function of the Bacillus SpoIIE protein and its localization to sites of sporulation septum assembly. Mol Microbiol. 1996;19:1047–1060. doi: 10.1046/j.1365-2958.1996.433963.x. [DOI] [PubMed] [Google Scholar]
- Beall B, Lutkenhaus J. FtsZ in Bacillus subtilis is required for vegetative septation and for asymmetric septation during sporulation. Genes & Dev. 1991;5:447–455. doi: 10.1101/gad.5.3.447. [DOI] [PubMed] [Google Scholar]
- Bork P, Brown NP, Hegyi H, Schultz J. The protein phosphatase 2C (PP2C) superfamily: Detection of bacterial homologues. Protein Sci. 1996;5:1421–1425. doi: 10.1002/pro.5560050720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd D, Manoil C, Beckwith J. Determinants of membrane protein topology. Proc Natl Acad Sci. 1987;84:8525–8529. doi: 10.1073/pnas.84.23.8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormack BP, Valdivia RH, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- Cutting SM, Horn PBV. Genetic analysis. In: Harwood CR, Cutting SM, editors. Molecular biological methods for Bacillus. Chichester, UK: John Wiley; 1990. pp. 27–60. [Google Scholar]
- Diederich B, Wilkinson JF, Magnin T, Najafi M, Errington J, Yudkin MD. Role of interactions between SpoIIAA and SpoIIAB in regulating cell-specific transcription factor σF of Bacillus subtilis. Genes & Dev. 1994;8:2653–2663. doi: 10.1101/gad.8.21.2653. [DOI] [PubMed] [Google Scholar]
- Duncan L, Losick R. SpoIIAB is an anti-sigma factor that binds to and inhibits transcription by regulatory protein sigma F from Bacillus subtilis. Proc Natl Acad Sci. 1993;90:2325–2329. doi: 10.1073/pnas.90.6.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan L, Alper S, Arigoni F, Losick R, Stragier P. Activation of cell-specific transcription by a serine phosphatase at the site of asymmetric division. Science. 1995;270:641–644. doi: 10.1126/science.270.5236.641. [DOI] [PubMed] [Google Scholar]
- Duncan L, Alper S, Losick R. SpoIIAA governs the release of the cell-type specific transcription factor sigma F from its anti-sigma factor SpoIIAB. J Mol Biol. 1996;260:147–164. doi: 10.1006/jmbi.1996.0389. [DOI] [PubMed] [Google Scholar]
- Errington J. Bacillus subtilis sporulation: Regulation of gene expression and control of morphogenesis. Microbiol Rev. 1993;57:1–33. doi: 10.1128/mr.57.1.1-33.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington J, Mandelstam J. Use of a lacZ gene fusion to determine the dependence pattern and the spore compartment expression of sporulation operon spoVA in spo mutants of Bacillus subtilis. J Gen Microbiol. 1986;132:2977–2985. doi: 10.1099/00221287-132-11-2977. [DOI] [PubMed] [Google Scholar]
- Feucht A, Magnin T, Yudkin MD, Errington J. Bifunctional protein required for asymmetric cell division and cell-specific transcription in Bacillus subtilis. Genes & Dev. 1996;10:794–803. doi: 10.1101/gad.10.7.794. [DOI] [PubMed] [Google Scholar]
- Frandsen N, Barak I, Karmazyn-Campelli C, Stragier P. Transient gene asymmetry during sporulation and establishment of cell specificity in Bacillus subtilis. Genes & Dev. 1999;13:394–399. doi: 10.1101/gad.13.4.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garsin DA, Duncan L, Paskowitz DM, Losick R. The kinase activity of the antisigma factor SpoIIAB is required for activation as well as inhibition of transcription factor σF during sporulation in Bacillus subtilis. J Mol Biol. 1998;284:569–578. doi: 10.1006/jmbi.1998.2202. [DOI] [PubMed] [Google Scholar]
- Gholamhoseinian A, Piggot PJ. Timing of spoII gene expression relative to septum formation during sporulation of Bacillus subtilis. J Bacteriol. 1989;171:5747–5749. doi: 10.1128/jb.171.10.5747-5749.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzy-Treboul G, Karmazyn-Campelli C, Stragier P. Developmental regulation of transcription of the Bacillus subtilis ftsAZ operon. J Mol Biol. 1992;224:967–979. doi: 10.1016/0022-2836(92)90463-t. [DOI] [PubMed] [Google Scholar]
- Harry EJ, Pogliano K, Losick R. Use of immunofluorescence to visualize cell-specific gene expression during sporulation in Bacillus subtilis. J Bacteriol. 1995;177:3386–3393. doi: 10.1128/jb.177.12.3386-3393.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim R, Cubitt AB, Tsien RY. Improved green fluorescence. Nature. 1995;373:663–664. doi: 10.1038/373663b0. [DOI] [PubMed] [Google Scholar]
- Hiraoka Y, Sedat JW, Agard DA. Determination of three-dimensional imaging properties of a light microscope system. Partial confocal behavior in epifluorescence microscopy. Biophys J. 1990;57:325–333. doi: 10.1016/S0006-3495(90)82534-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney TJ, Moran CP., Jr Organization and regulation of an operon that encodes a sporulation-essential sigma factor in Bacillus subtilis. J Bacteriol. 1987;169:3329–3339. doi: 10.1128/jb.169.7.3329-3339.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khvorova A, Zhang L, Higgins ML, Piggot PJ. The spoIIE locus is involved in the Spo0A-dependent switch in the location of FtsZ rings in Bacillus subtilis. J Bacteriol. 1998;180:1256–1260. doi: 10.1128/jb.180.5.1256-1260.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin PA, Losick R. Characterization of a cell division gene from Bacillus subtilis that is required for vegetative and sporulation septum formation. J Bacteriol. 1994;176:1451–1459. doi: 10.1128/jb.176.5.1451-1459.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Transcription factor Spo0A switches the localization of the cell division protein FtsZ from a medial to a bipolar pattern in Bacillus subtilis. Genes & Dev. 1996;10:478–488. doi: 10.1101/gad.10.4.478. [DOI] [PubMed] [Google Scholar]
- Levin PA, Losick R, Stragier P, Arigoni F. Localization of the sporulation protein SpoIIE in Bacillus subtilis is dependent upon the cell division protein FtsZ. Mol Microbiol. 1997;25:839–846. doi: 10.1111/j.1365-2958.1997.mmi505.x. [DOI] [PubMed] [Google Scholar]
- Lewis PJ, Partridge SR, Errington J. Sigma factors, asymmetry, and the determination of cell fate in Bacillus subtilis. Proc Natl Acad Sci. 1994;91:3849–3853. doi: 10.1073/pnas.91.9.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PJ, Magnin T, Errington J. Compartmentalized distribution of the proteins controlling the prespore-specific transcription factor sigmaF of Bacillus subtilis. Genes Cells. 1996;1:881–894. doi: 10.1046/j.1365-2443.1996.750275.x. [DOI] [PubMed] [Google Scholar]
- Londono-Vallejo JA, Frehel C, Stragier P. SpoIIQ, a forespore-expressed gene required for engulfment in Bacillus subtilis. Mol Microbiol. 1997;24:29–39. doi: 10.1046/j.1365-2958.1997.3181680.x. [DOI] [PubMed] [Google Scholar]
- Losick R, Dworkin J. Linking asymmetric division to cell fate: Teaching an old microbe new tricks. Genes & Dev. 1999;13:377–381. doi: 10.1101/gad.13.4.377. [DOI] [PubMed] [Google Scholar]
- Margolis P. Establishment of cell type during sporulation in Bacillus subtilis. Ph. D. thesis, ‘Molecular and cellular biology.’. Cambridge, MA: Harvard University; 1993. [Google Scholar]
- Margolis P, Driks A, Losick R. Establishment of cell type by compartmentalized activation of a transcription factor. Science. 1991;254:562–565. doi: 10.1126/science.1948031. [DOI] [PubMed] [Google Scholar]
- ————— Sporulation gene spoIIB from Bacillus subtilis. J Bacteriol. 1993;175:528–540. doi: 10.1128/jb.175.2.528-540.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min KT, Hilditch CM, Diederich B, Errington J, Yudkin MD. Sigma F, the first compartment-specific transcription factor of B. subtilis, is regulated by an anti-sigma factor that is also a protein kinase. Cell. 1993;74:735–742. doi: 10.1016/0092-8674(93)90520-z. [DOI] [PubMed] [Google Scholar]
- Nicholson WL, Setlow P. Sporulation, germination and outgrowth. In: Harwood CR, Cutting SM, editors. Molecular biological methods for Bacillus. Chichester, UK: John Wiley; 1990. pp. 391–450. [Google Scholar]
- Piggot PJ, Coote JG. Genetic aspects of bacterial endospore formation. Bacteriol Rev. 1976;40:908–962. doi: 10.1128/br.40.4.908-962.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogliano J, Osborne N, Sharp MD, Mello AA-d, Perez A, Sun Y-L, Pogliano K. A vital stain for studying membrane dynamics in bacteria: A novel mechanism controlling septation during Bacillus subtilis sporulation. Mol Microbiol. 1999;31:1149–1160. doi: 10.1046/j.1365-2958.1999.01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogliano K, Hofmeister AE, Losick R. Disappearance of the sigma E transcription factor from the forespore and the SpoIIE phosphatase from the mother cell contributes to establishment of cell-specific gene expression during sporulation in Bacillus subtilis. J Bacteriol. 1997;179:3331–3341. doi: 10.1128/jb.179.10.3331-3341.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price KD, Losick R. A four-dimensional view of assembly of a morphogenetic protein during sporulation in Bacillus subtilis. J Bacteriol. 1999;181:781–790. doi: 10.1128/jb.181.3.781-790.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalettar BA, Swedlow JR, Sedat JW, Agard DA. Dispersion, aberration and deconvolution in multi-wavelength fluorescence images. J Microsc. 1996;182:50–60. doi: 10.1046/j.1365-2818.1996.122402.x. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Margolis P, Duncan L, Coppolecchia R, Moran CP, Jr, Losick R. Control of developmental transcription factor sigma F by sporulation regulatory proteins SpoIIAA and SpoIIAB in Bacillus subtilis. Proc Natl Acad Sci. 1990;87:9221–9225. doi: 10.1073/pnas.87.23.9221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stragier P, Losick R. Molecular genetics of sporulation in Bacillus subtilis. Annu Rev Genet. 1996;30:297–241. doi: 10.1146/annurev.genet.30.1.297. [DOI] [PubMed] [Google Scholar]
- Webb CD, Decatur A, Teleman A, Losick R. Use of green fluorescent protein for visualization of cell-specific gene expression and subcellular protein localization during sporulation in Bacillus subtilis. J Bacteriol. 1995;177:5906–5911. doi: 10.1128/jb.177.20.5906-5911.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb CD, Graumann PL, Kahana JA, Teleman AA, Silver PA, Losick R. Use of time-lapse microscopy to visualize rapid movement of the replication origin region of the chromosome during the cell cycle in Bacillus subtilis. Mol Microbiol. 1998;28:883–892. doi: 10.1046/j.1365-2958.1998.00808.x. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Feucht A, Errington J. Prespore-specific gene expression in Bacillus subtilis is driven by sequestration of SpoIIE phosphatase to the prespore side of the asymmetric septum. Genes & Dev. 1998;12:1371–1380. doi: 10.1101/gad.12.9.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngman P, Perkins JB, Losick R. Construction of a cloning site near one end of Tn917 into which foreign DNA may be inserted without affecting transposition in Bacillus subtilis or expression of the transposon-borne erm gene. Plasmid. 1984;12:1–9. doi: 10.1016/0147-619x(84)90061-1. [DOI] [PubMed] [Google Scholar]