Abstract
Nitrosative stress has recently been demonstrated as a causal in a select sporadic variant of Parkinson’s (PD) and Alzheimer’s (AD) diseases. Specifically, elevated levels of NO disrupt the redox activity of protein disulfide isomerase, a key endoplasmic reticulum-resident chaperone by S-nitroso modification of its redox-active cysteines. This leads to accumulation of misfolded AD- and PD-specific protein debris. We have recently demonstrated in vitro that polyphenolic phytochemicals, curcumin and masoprocol, can rescue S-nitroso-PDI formation by scavenging NOx. In this study, using dopaminergic SHSY-5Y cells, we have monitored the aggregation of green-fluorescent protein (GFP)-tagged synphilin-1 (a known constituent of PD Lewy neurites) as a function of rotenone-induced nitrosative stress. Importantly, we demonstrate a marked decrease in synphilin-1 aggregation when the cell line is previously incubated with 3,5-bis(2-flurobenzylidene) piperidin-4-one (EF-24), a curcumin analogue, prior to rotenone insult. Furthermore, our data also reveal that rotenone attenuates PDI expression in the same cell line, a phenomenon that can be mitigated through EF-24 intervention. Together, these results suggest that EF-24 can exert neuroprotective effects by ameliorating nitrosative stress-linked damage to PDI and the associated onset of PD and AD. Essentially, EF-24 can serve as a scaffold for the design and development of PD and AD specific prophylactics.
Keywords: protein disulfide isomerase, nitrosative stress, neurodegenerative disorders, synphilin-1, EF-24, Lewy body
1. Introduction
A hallmark event characteristic of neurogenerative disorders such as Lewy body dementia, Alzheimer’s (AD) and Parkinson’s diseases (PD) is the accumulation of aggregated proteins to often form Lewy-bodies in the cytosol of human neuronal cells [1–11] This commonality exists in spite of the fact that causals for AD and PD are different, depend upon whether familial or sporadic, and differ in the proteins constituting the aggregates and Lewy neurites. There is also debate whether specific protein aggregates representative these diseases are truly the cause of the disease or only a mechanism to save the cell upon onset of the neuronal pathology [12].
Among a plethora of known intra- and extracellular factors associated with the etiology of AD and PD including mitochondrial dysfunction, inflammation, diet, etc, recent studies have demonstrated that in a particular sporadic form of AD and PD, a key endoplasmic reticulum-resident oxidoreductase chaperone becomes chemically modified because of high levels of nitrosative stress [13–19]. A common feature observed in the neuronal cells of AD and PD victims in this sporadic variant was the attachment of nitric oxide (NO) to the redox-active cysteines of protein-disulfide isomerase (PDI) to form S-Nitroso PDI (Fig. 1A) [5, 7]. The formation of S-nitroso-PDI coupled with the pathogenesis of AD and PD making the oxidoreductase a chief target for the prevention of these two neurodegenerative disorders in the nitrosative-stress-linked variant of the diseases [5, 7].
Figure 1.
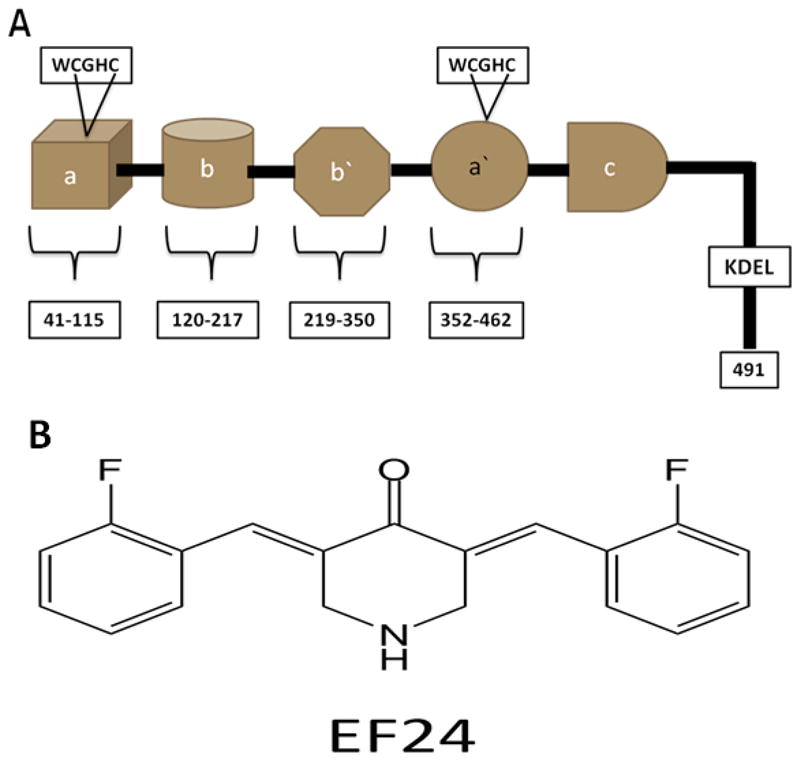
(A) Schematic representation of protein disulfide isomerase (PDI) [32]; (B) EF-24 [27]
Our laboratory recently demonstrated that the polyphenolic phytochemicals curcumin and masoprocol can scavenge NOx species from model NOx donors [20]. Additionally, these polyphenols have been shown to intervene in a variety of disease processes [21–26]. Furthermore, we have also demonstrated in vitro that they can rescue PDI from becoming chemically modified under nitrosative stress [20]. This was possible by following the oxidative regeneration of bovine pancreatic ribonuclease A (RNase A), a PDI substrate, under control and nitrosative stress conditions [20]. Our results indicated that both curcumin and masoprocol can restore PDI activity to native levels even in the presence of NOx sources. Mass spectrometric results revealed that both polyphenols scavenge NOx to form stable adducts [20]. In addition, they were found to be capable of scavenging hydroxyl radicals generated using Fenton chemistry [manuscript in preparation].
In this report we have overexpressed green fluorescent protein-tagged synphilin-1 in SHSY-5Y cells and monitored its aggregation as a biomarker for PD. Our results reveal that exposure of this cell line to rotenone, a mitochondrial reactive oxygen species elevator, lead to the aggregation of synphilin-1 as observed by fluorescence microscopy and consistent with previous reports that NO influences Lewy body formation via PDI modification [7]. Importantly, cells that were preincubated with 3,5-bis(2-flurobenzylidene) piperidin-4-one (EF-24), a bioavailable curcumin analog, prior to rotenone insult demonstrated a marked resilience to synphilin-1 aggregation (Fig. 1B) [27]. These results suggest that it may be possible to mitigate nitrosative-stress induced aggregates in cell lines using bioavailable polyphenolic phyto-analogs. Furthermore, it opens avenues for the design and development of more effective and less toxic analogs prophylactics against nitrosative-stress linked AD and PD.
2. Materials and methods
2.1. Reagent and cell line
EF-24 was synthesized in collaboration with Dr. Katja Michael (Dept. of Chemistry, UTEP) using a previously reported procedure [27]. Further purification was performed using reversed-phase HPLC (Supelco, C18 column; 0–100% acetonitrile/100 minutes) to collect the principal peak. Characterization of the principal peak by mass spectrometry revealed a molecular weight in good agreement with the expected mass of EF-24 [27]. HPLC analysis of re-purified EF-24 showed purity ≥95% by peak area analysis. Tetranitromethane (TNM) and rotenone were obtained from Sigma-Aldrich (St. Louis, MO). Other reagents were purchased as follows: Mouse monoclonal to PDI (Abcam, Cambridge, MA), and GAPDH (Glyceraldehyde 3-phosphate dehydrogenase, Cell Signaling, Danvers, MA), horseradish peroxidase (HRP)-conjugated goat anti-mouse (KPL Biomedical) and the neuroblastoma cell line SHSY-5Y (from ATCC, Manassas, VA).
2.2. Characterization of NOx adducts of EF-24
A stock solution of EF-24 (prepared by weight in acetonitrile) was diluted in to a buffer (pH 8, 100 mM Tris–HCl) to obtain concentrations ranging from 10–200 μM. Tetranitromethane was added from a stock solution (freshly prepared by weight in acetonitrile) to EF-24 so as to obtain different ratios of TNM/EF-24. The samples were separated and analyzed using reversed-phase HPLC (Supelco C18 column; 1% acetonitrile/min). Collected peaks were lyophilized and analyzed on a Q-TOF mass spectrometer (BBRC, UTEP).
2.3. Western blot analysis of PDI
Dopaminergic SHSY-5Y transfected cells were subjected to different treatments, followed by washing the cells with cold Tris-buffered saline, collected by centrifugation (3003g, 5 min at 48 °C), and lysed by sonication in a buffer containing 10 mM Tris–HCl (pH 7.4), 10 mM EDTA, 2% (w/v) SDS and protease inhibitors. Total protein concentration was measured using a bicinchonic acid kit and BSA as a standard (Pierce Biotechnology Inc., Rockford, IL). Equal volumes of protein (approximately 10 μg per lane) were subject to SDS-polyacrylamide gel electrophoresis and then transferred to polyvinyldinefluoride (PVDF) membranes. Blots were incubated in blocking buffer (5%, w/v, dried skimmed milk in Tris-buffered saline, pH 7.4, and 0.1% Tween 20) followed by incubation with anti-PDI monoclonal antibody (1:500) or anti-GAPDH (Glyceraldehyde 3-phosphate dehydrogenase, 1:1000) for 1 h and then with horseradish peroxidase (HRP)-conjugated goat anti-mouse (KPL Biomedical) for 30 min. The signal was visualized by chemiluminescence (ECL-plus or SuperSignal West Pico Chemiluminescent Substrate) according to the manufacturer’s instructions (Amersham or Pierce Biotechnology Inc.).
2.4. Preparation of EGFP- synphilin-1 fusion protein
The full-length cDNA of the human synphilin-1 (pENTR 221 from Invitrogen; Genbank accession code NP 005451) was amplified via PCR using the primer set containing the restriction sites for EcoRI (forward) and BamHI (reverse). After digestion with the corresponding enzymes, the PCR product was cloned into the pEGFP-C2 expression vector (Clontech. Palo Alto, CA). The sequence of synphilin-1 was verified by automated DNA sequencing reaction. The synphilin-1/pEGFP-C2 construct was utilized to transiently transfect neuroblastoma cells.
2.5. Cell Culture and treatment
SHSY-5Y (human neuroblastoma) cells were cultured in a 1:1 mixture of DMEM and Ham’s F12 medium supplemented with 10% fetal bovine serum, 1% Penicilin-Streptomycin. The cells were grown at 37 °C with 5% carbon dioxide. SHSY-5Y cells (1×106 cells/well) were seeded onto glass coverslips in 6-well plates and incubated for 12–16 h. Cell transfections were performed the following day with pEGFP-C2 control or the fusion protein GFP-synphilin-1, as recommended by manufacturer using effectene reagent (Qiagen, Valencia, CA). After transfection, the cells were incubated overnight to allow expression of proteins. Cells were treated with vehicle alone (DMSO) or with 1 μM EF-24 for 6 h followed by exposure to 300 nM of for 12 h. After incubation, cells were prepared for microscopy as described below.
2.6. Confocal microscopy and immunocytochemistry
Cells transfected with vector or EGFP-synphilin-1 were washed after treatment, fixed with 4% paraformaldehyde in PBS, stained with DAPI and mounted under ProLong antifade medium (Molecular Probes). To stain for PDI, cells were fixed as above, permeabilized with 0.1% (w/v) saponin in PBS, blocked with PBS plus 5% goat serum, 5% FBS and 0.1% TWEEN 20, followed by incubation with primary antibody (overnight at 4°C) and secondary rhodamine-conjugated goat anti-mouse (1:10000, KPL Biomedical), and DAPI staining. Fluorescence confocal images were captured utilizing LSM 700 confocal microscope and assisted with ZEN 2009 software (Zeiss, New York, NY).
3. Results
3.1. Ability of EF-24 to scavenge NOx
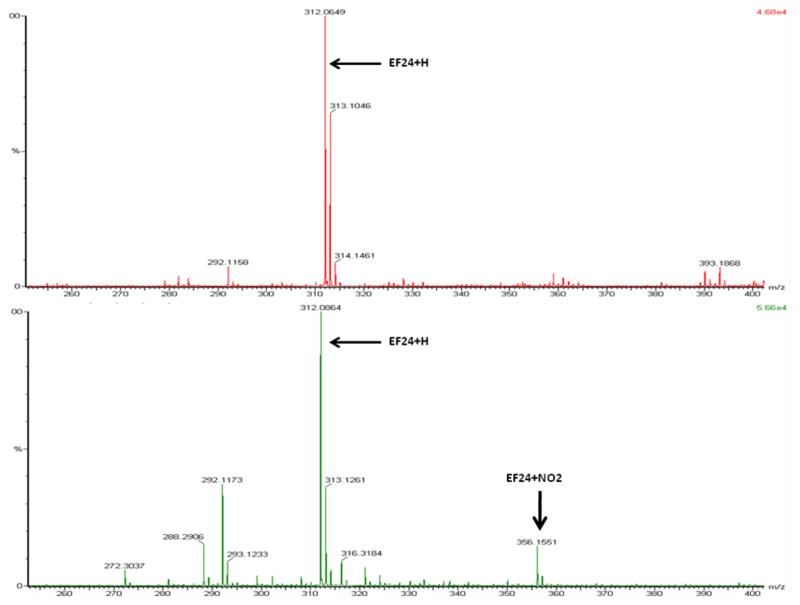
Figure 2A is a mass spectrogram of EF-24 prior to treatment with tetranitromethane (MW 312 Da is representative of protonated EF-24). A mass addition of 45 Da to EF-24 post-treatment is indicative of nitration (Fig 2B; Table.1). Note: Addition of a nitro group adds 46 Da. However, the nitrogroup replaces a hydrogen atom, resulting in the net addition of 45 Da. Further loss of a proton results in a MW of 356 Da.
Figure 2.
A) ESI–FTMS of EF-24 and B) EF-24 exposed to tetranitromethane.
Table 1.
Adduct formation between polyphenol EF-24 with NOx (pH 8, 100 mM Tris–HCl, 25 °C).
| Experimental conditions | Mass(Da) + identity | Mass (Da) + identity |
|---|---|---|
| EF-24 (control) | 312.0649 | - |
| NOx + EF-24 | 312.0664 | 356.1551 |
3.2. Impact of reactive oxygen species (ROS) on PDI expression and its attenuation by EF-24
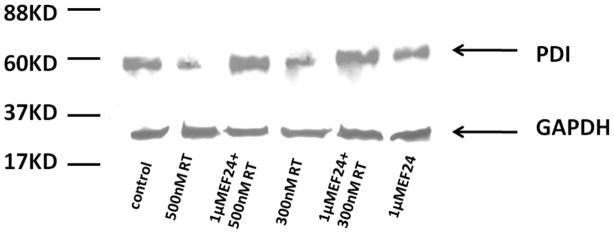
Fig 3 is a gel demonstrating the effects of rotenone and the protective role of EF-24 on the expression of PDI detected by western blotting. A dose-dependent decrease in PDI expression was observed as a function of rotenone (Lane 4 and 2) as determined by immunochemical detection. Remarkably, pretreatment of the cells with EF-24 prior to rotenone addition shows that the polyphenol is able to rescue PDI expression (Lane 3 and 5) to levels consistent with the control. The addition of EF-24 alone to the cell line did not significantly impact PDI expression levels (Lane 6).
Figure 3.
Effect of EF-24 and rotenone on PDI expression (n=1) (A) SHSY-5Y cells were treated with rotenone and EF-24 and cell lysates were analyzed by Western blot with PDI antibody. Lane 1, untreated cell lysates; Lanes 2 and 4, cells were treated with 500nm and 300nm rotenone for 12 h; Lanes 3 and 5, cells stressed with 500nm and 300nm rotenone for 12 h after pre-incubation with 1μM EF-24 for 6 h; Lane 6, cells treated with 1μM EF-24 alone. GAPDH was used as a loading control.
3.3. S-Nitrosylation of PDI mediates synphilin-1 aggregation in model cells of PD
Fig S1A shows PDI immunodetected after expression in SHSY-5Y cells. Fig S1B is a confocal microscopy image confirming that PDI is expressed at a high level within the SHSY-5Y cells and localized mostly in the endoplasmic reticulum and to a much lower level in the cytosol in SHSY-5Y cells.
Fig 4 (panels A through E) are confocal microscopy images of GFP-tagged synphilin-1 expressed in transiently transfected SHSY-5Y cells as a function of different conditions. The results clearly indicate the aggregation of synphilin-1 when exposed to 300 nM rotenone (panel A). As compared to this, pretreatment of cells with 1 μM EF-24 prior to 300 nM rotenone exposure resulted in a markedly diminished level of synphilin-1 aggregation (as evidenced through GFP fluorescence; panel B). Other experiments revealed a relatively homogenous cytosolic distribution of GFP in cells transfected with PGFP-C2 plasmid alone (panel C). In contrast, cells transfected with EGFP- synphilin-1 constructs show a punctuated (or speckled) cytosolic distribution of green signal (panel E). This observation indicates that over expression of synphilin-1 fused to EGFP protein, provided subcellular accumulation of the recombinant fusion protein, called aggregosomes. Cells, treated with the vehicle alone (DMSO; Fig. 4D) did not differ in the expression of EGFP-synphilin-1 compare to untreated cells (Fig. 4E).
Figure 4.
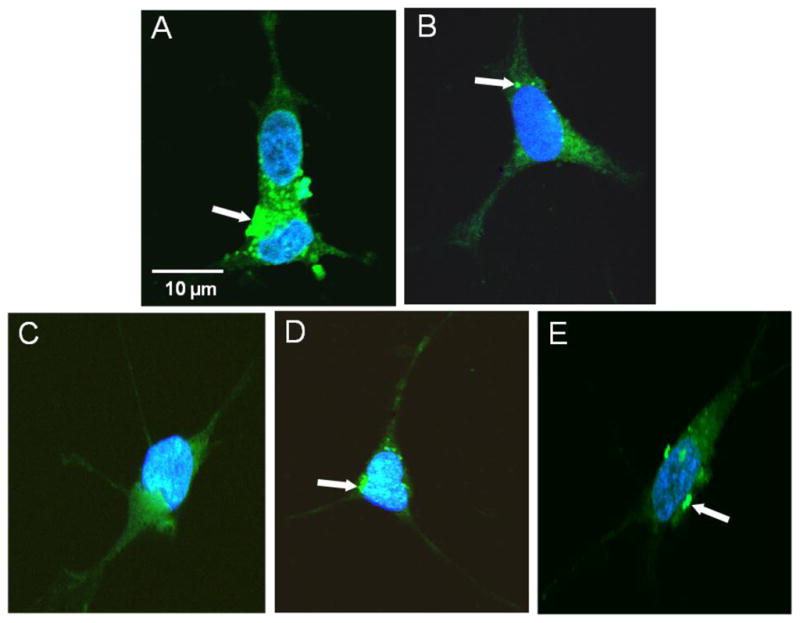
Effect of EF-24 and rotenone on synphilin-1 distribution in SHSY-5Y cell. Confocal microscopy images of SHSY5Y cells reveal the presence of intracellular aggregates in cells transfected with EGFP-tagged synphilin-1. Transfected SHSY-5Y cells were DMSO treated or stressed with rotenone and stained by immunofluorescence as detailed in the Materials and methods section. (A) Cells exposed to 300 nM rotenone for 12h; (B) Cells pretreated with 1 μM EF-24 following treatment with 300nM rotenone. Controls were (C) cells transfected with vector pEGFP-C2, and (D) cells treated with DMSO 2.5 v/v; (E) untreated cells. Nucleus was stained with DAPI, (blue color). White arrows indicate the presence of aggregates corresponding to the recombinant fusion protein.
In summary, these results showed that cells treated with rotenone apparently increased the green fluorescence by forming cytosolic inclusion bodies whereas pre-treatment with 1μM EF-24 mitigated the formation of synphilin-1 aggregation to a significant level.
4. Discussion
The endoplasmic reticulum is a specialized compartment with a redox potential designed to facilitate the (oxidative) formation of disulfide bonds in secretory or membrane-bound proteins [28–31]. This is an essential process preceding their export from the ER and is catalyzed by protein disulfide isomerase, the chief ER-resident oxidoreductase chaperone [32]. The catalytic function of PDI, executed through two redox-active cysteine-containing active sites is essential to balance the flux between incoming nascent polypeptides and outgoing biologically viable folded proteins [33–36]. Compromise or failure in the catalytic efficiency of PDI can reduce the maturative processing of nascent substrates and lead to terminal misfolding, retrotranslocation along the ERAD pathway and debris accumulation in the cytosol [37–38]. This sequence of events is perhaps the rosetta-stone for the onset of neuropathies.
Recent work has demonstrated that PDI is severely compromised by the addition of nitric oxide resulting from elevated levels of nitrosative stress [5, 7]. S-nitrosoPDI has been found in the brains of Alzheimer’s and Parkinson’s victims [7]. Upregulation of PDI on the contrary was shown to mitigate synphilin-1 aggregation, which is a biomarker in select neurodegenerative disorders [7]. These results clearly demonstrated that PDI is an attractive target in neuroprotective therapies and rescue of S-nitrosoPDI formation is a viable approach to preventing certain sporadic variants of both AD and PD [5].
Here, we hypothesized that the bioavailable curcumin analog, EF-24, can scavenge NOx and prevent covalent modification of PDI and therefore, protect against loss of oxidoreductase function. By sustaining the functional integrity of PDI, the polyphenol can potentially protects against Lewy neurite deposits, and the pathogenesis of PD and AD.
To test our hypothesis, we first exposed EF-24 to a model NOx donor under in vitro conditions. Our data indicated the formation of a nitrated product suggesting that EF-24 was capable of scavenging NOx consistent with previous results using curcumin as a free radical scavenger [20].
Next, we determined whether EF-24 was capable of rescuing PDI from nitrosative attack in cell lines and protecting against the formation of intracellular aggregates. SHSY-5Y cells previously used to demonstrate the link between nitrosative stress, PDI and Lewy body formation, was used in our study [7]. Specifically, the aggregation of GFP-tagged synphilin-1, a known cellular Lewy constituent in PD neuropathies, was monitored as a marker for PD onset [1–11].
We initially chose to examine cellular levels of expression of PDI as a function of rotenone-mediated nitrosative insult. Cellular levels of PDI expression were determined to diminish in a rotenone-dependent manner compared to a control experiment. However, preincubation of the cell lines with EF-24 prior to rotenone treatment demonstrated levels of PDI expression consistent with controls. Importantly, EF-24 alone does not increase PDI levels of expression. These results appear to suggest that nitrosative insult compromises PDI expression, though mitigation of reduced expression and return to homeostasis is possible through direct EF-24 intervention. A molecular level understanding of this phenomenon is pending.
Finally, we examined the cytosolic aggregation of synphilin-1 in the SHSY-5Y cell line under rotenone-induced nitrosative stress as previously demonstrated [7]. GFP-labeled synphilin aggregation was monitored using confocal microscopy. In comparison to control experiments, incubation of the cell line with rotenone clearly demonstrated cytosolic aggregation of synphilin-1 consistent with previous results suggesting that healthy PDI inhibits aggregation of synphilin-1 [5, 7]. As one of the controls, we tested whether unstressed cells expressing PDI could prevent synphilin-1 constituted Lewy-body-like inclusions that can be formed in the cytosol after synphilin-1 overexpression [7]. Our data revealed very limited diffused synphilin-1 localization in cytosol (panel). In contrast, rotenone treated cells showed discrete inclusions of synphilin-1 in the cytosol. These results suggest that rotenone-dependent elevation of nitric oxide attenuated the protective effect of PDI on synphilin-1 inclusions (Fig. 4A)
Next, cells were preincubated with EF-24 prior to rotenone exposure to determine whether EF-24 NOx scavenging ability demonstrated in vitro could be translated in vivo. Confocal microscopy data monitoring GFP-tagged synphilin-1 clearly indicate that unlike rotenone-induced nitrosative stressed cells (Fig. 4A), cells pre-treated with EF-24 followed by rotenone treatment showed a drastic decrease in discrete Lewy-like inclusions in cytosol (Fig. 4B). These results suggest that EF-24 can intracellularly rescue S-nitroso modification of PDI as seen under elevated levels of nitrosative stress and prevent Lewy-neurite formation in our model system.
Conclusion
Elevated levels of nitrosative stress have been linked to PDI dysfunction and downstream aggregation of misfolding-prone proteins such as synphilin-1 and α-synuclein, formation of Lewy bodies and the pathogenesis of Parkinson’s and Alzheimer’s diseases [1–11].
We demonstrate, using SH-SY5Y cell lines, that it is possible to introduce free radical scavengers that can rescue PDI from nitrosative attack. By restoring homeostasis, we show that the bioavailable curcumin analog, EF-24, protects the cell from the formation and thus accumulation of terminally misfolded debris that is usually associated with neurodegenerative disorders.
We therefore posit that curcuma-based bioavailable analogues can act as lead candidates for the development of prophylactics against select sporadic forms of AD and PD.
Supplementary Material
Highlights.
Nitrosative stress and PDI damage is known to be associated with PD/AD
EF-24, a curcumin analog can scavenge NOx
EF-24 can prevent synphilin-1 aggregation in dopaminergic SHSY-5Y cells
EF-24 restores PDI expression levels under nitrosative stress conditions
Acknowledgments
We thank Dr. Armado Varela and the staff of the Cell Culture and High Throughput Screening (HTS) Core Facility for services and facilities provided. M.N. wishes to thank Mr. Jeremiah Ramos for the synthesis and purification of EF-24 and Dr. K. Michael (UTEP) for direction. This study was supported funding from the Alzheimer’s disease Research Foundation to M.N. In addition, it was supported in part by the grant 5G12RR008124 to the Border Biomedical Research Center (BBRC)/University of Texas at El Paso) from the National Center for Research Resources (NCRR, NIH) and NIMH 5SC1MH 086070-02 to M.M.A. M.N. also wishes to acknowledge Dr. Felicia Manciu (Physics, UTEP) and NSF (DMR 0723115) for characterization of EF-24.
Abbreviations
- AD
Alzheimer’s disease
- PD
Parkinson’s disease
- PDI
protein disulfide isomerase
- EF-24 3
5-bis(2-flurobenzylidene) piperidin-4-one
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hindle JV. Ageing, neurodegeneration and Parkinson’s disease. Age Ageing. 2010;39:156–161. doi: 10.1093/ageing/afp223. [DOI] [PubMed] [Google Scholar]
- 2.Schiesling C, Kieper N, Seidel K, Kruger R. Review: Familial Parkinson’s disease--genetics, clinical phenotype and neuropathology in relation to the common sporadic form of the disease. Neuropathol Appl Neurobiol. 2008;34:255–271. doi: 10.1111/j.1365-2990.2008.00952.x. [DOI] [PubMed] [Google Scholar]
- 3.Su B, Liu H, Wang X, Chen SG, Siedlak SL, Kondo E, Choi R, Takeda A, Castellani RJ, Perry G, Smith MA, Zhu X, Lee HG. Ectopic localization of FOXO3a protein in Lewy bodies in Lewy body dementia and Parkinson’s disease. Mol Neurodegener. 2009;4:32. doi: 10.1186/1750-1326-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olanow CW. The pathogenesis of cell death in Parkinson’s disease--2007. Mov Disord. 2007;22(Suppl 17):S335–342. doi: 10.1002/mds.21675. [DOI] [PubMed] [Google Scholar]
- 5.Mattson MP. Nitro-PDI incites toxic waste accumulation. Nat Neurosci. 2006;9:865–867. doi: 10.1038/nn0706-865. [DOI] [PubMed] [Google Scholar]
- 6.Walsh DM, Selkoe DJ. Oligomers on the brain: the emerging role of soluble protein aggregates in neurodegeneration. Protein Pept Lett. 2004;11:213–228. doi: 10.2174/0929866043407174. [DOI] [PubMed] [Google Scholar]
- 7.Uehara T, Nakamura T, Yao D, Shi ZQ, Gu Z, Ma Y, Masliah E, Nomura Y, Lipton SA. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature. 2006;441:513–517. doi: 10.1038/nature04782. [DOI] [PubMed] [Google Scholar]
- 8.Uehara T. Accumulation of misfolded protein through nitrosative stress linked to neurodegenerative disorders. Antioxid Redox Signal. 2007;9:597–601. doi: 10.1089/ars.2006.1517. [DOI] [PubMed] [Google Scholar]
- 9.Wakabayashi K, Tanji K, Mori F, Takahashi H. The Lewy body in Parkinson’s disease: molecules implicated in the formation and degradation of alpha-synuclein aggregates. Neuropathology. 2007;27:494–506. doi: 10.1111/j.1440-1789.2007.00803.x. [DOI] [PubMed] [Google Scholar]
- 10.Uversky VN. Alpha-synuclein misfolding and neurodegenerative diseases. Curr Protein Pept Sci. 2008;9:507–540. doi: 10.2174/138920308785915218. [DOI] [PubMed] [Google Scholar]
- 11.Tan JM, Wong ES, Lim KL. Protein misfolding and aggregation in Parkinson’s disease. Antioxid Redox Signal. 2009;11:2119–2134. doi: 10.1089/ars.2009.2490. [DOI] [PubMed] [Google Scholar]
- 12.Gispert-Sanchez S, Auburger G. The role of protein aggregates in neuronal pathology: guilty, innocent, or just trying to help? J Neural Transm Suppl. 2006:111–117. doi: 10.1007/978-3-211-45295-0_18. [DOI] [PubMed] [Google Scholar]
- 13.Gu Z, Nakamura T, Lipton SA. Redox reactions induced by nitrosative stress mediate protein misfolding and mitochondrial dysfunction in neurodegenerative diseases. Mol Neurobiol. 2010;41:55–72. doi: 10.1007/s12035-010-8113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knott AB, Bossy-Wetzel E. Nitric oxide in health and disease of the nervous system. Antioxid Redox Signal. 2009;11:541–554. doi: 10.1089/ars.2008.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura T, Lipton SA. Emerging roles of S-nitrosylation in protein misfolding and neurodegenerative diseases. Antioxid Redox Signal. 2008;10:87–101. doi: 10.1089/ars.2007.1858. [DOI] [PubMed] [Google Scholar]
- 16.Benhar M, Forrester MT, Stamler JS. Nitrosative stress in the ER: a new role for S-nitrosylation in neurodegenerative diseases. ACS Chem Biol. 2006;1:355–358. doi: 10.1021/cb600244c. [DOI] [PubMed] [Google Scholar]
- 17.Chung KK. Say NO to neurodegeneration: role of S-nitrosylation in neurodegenerative disorders. Neurosignals. 2006;15:307–313. doi: 10.1159/000109071. [DOI] [PubMed] [Google Scholar]
- 18.Tsang AH, Chung KK. Oxidative and nitrosative stress in Parkinson’s disease. Biochim Biophys Acta. 2009;1792:643–650. doi: 10.1016/j.bbadis.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Foster MW, Hess DT, Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med. 2009;15:391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pal R, Cristan EA, Schnittker K, Narayan M. Rescue of ER oxidoreductase function through polyphenolic phytochemical intervention: implications for subcellular traffic and neurodegenerative disorders. Biochem Biophys Res Commun. 2010;392:567–571. doi: 10.1016/j.bbrc.2010.01.071. [DOI] [PubMed] [Google Scholar]
- 21.Chen Q. Nordihydroguaiaretic acid analogues: their chemical synthesis and biological activities. Curr Top Med Chem. 2009;9:1636–1659. doi: 10.2174/156802609789941915. [DOI] [PubMed] [Google Scholar]
- 22.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 23.Chauhan DP. Chemotherapeutic potential of curcumin for colorectal cancer. Curr Pharm Des. 2002;8:1695–1706. doi: 10.2174/1381612023394016. [DOI] [PubMed] [Google Scholar]
- 24.Arteaga S, Andrade-Cetto A, Cardenas R. Larrea tridentata (Creosote bush), an abundant plant of Mexican and US-American deserts and its metabolite nordihydroguaiaretic acid. J Ethnopharmacol. 2005;98:231–239. doi: 10.1016/j.jep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Bright JJ. Curcumin and autoimmune disease. Adv Exp Med Biol. 2007;595:425–451. doi: 10.1007/978-0-387-46401-5_19. [DOI] [PubMed] [Google Scholar]
- 26.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as "Curecumin": from kitchen to clinic. Biochem Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Adams BK, Ferstl EM, Davis MC, Herold M, Kurtkaya S, Camalier RF, Hollingshead MG, Kaur G, Sausville EA, Rickles FR, Snyder JP, Liotta DC, Shoji M. Synthesis and biological evaluation of novel curcumin analogs as anti-cancer and anti-angiogenesis agents. Bioorg Med Chem. 2004;12:3871–3883. doi: 10.1016/j.bmc.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Woycechowsky KJ, Raines RT. Native disulfide bond formation in proteins. Curr Opin Chem Biol. 2000;4:533–539. doi: 10.1016/s1367-5931(00)00128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tu BP, Weissman JS. Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol. 2004;164:341–346. doi: 10.1083/jcb.200311055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narayan M, Welker E, Wedemeyer WJ, Scheraga HA. Oxidative folding of proteins. Acc Chem Res. 2000;33:805–812. doi: 10.1021/ar000063m. [DOI] [PubMed] [Google Scholar]
- 31.Arolas JL, Aviles FX, Chang JY, Ventura S. Folding of small disulfide-rich proteins: clarifying the puzzle. Trends Biochem Sci. 2006;31:292–301. doi: 10.1016/j.tibs.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Wilkinson B, Gilbert HF. Protein disulfide isomerase. Biochim Biophys Acta. 2004;1699:35–44. doi: 10.1016/j.bbapap.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 33.Tian G, Xiang S, Noiva R, Lennarz WJ, Schindelin H. The crystal structure of yeast protein disulfide isomerase suggests cooperativity between its active sites. Cell. 2006;124:61–73. doi: 10.1016/j.cell.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 34.Hawkins HC, Freedman RB. The reactivities and ionization properties of the active-site dithiol groups of mammalian protein disulphide-isomerase. Biochem J. 1991;275(Pt 2):335–339. doi: 10.1042/bj2750335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weissman JS, Kim PS. Efficient catalysis of disulphide bond rearrangements by protein disulphide isomerase. Nature. 1993;365:185–188. doi: 10.1038/365185a0. [DOI] [PubMed] [Google Scholar]
- 36.Gilbert HF. Protein disulfide isomerase. Methods Enzymol. 1998;290:26–50. doi: 10.1016/s0076-6879(98)90005-2. [DOI] [PubMed] [Google Scholar]
- 37.Romisch K. A cure for traffic jams: small molecule chaperones in the endoplasmic reticulum. Traffic. 2004;5:815–820. doi: 10.1111/j.1600-0854.2004.00231.x. [DOI] [PubMed] [Google Scholar]
- 38.Romisch K. Endoplasmic reticulum-associated degradation. Annu Rev Cell Dev Biol. 2005;21:435–456. doi: 10.1146/annurev.cellbio.21.012704.133250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.