Abstract
Viruses have evolved elegant strategies to manipulate the host while the host counters with defense systems including the interferon response, apoptosis, and the DNA damage response (DDR). Viruses have multiple strategies for manipulating the DDR and even the same virus can activate or inhibit the DDR at different stages of infection. Epstein-Barr virus (EBV) is a virus implicated in several human cancers including Burkitt’s lymphoma, nasopharyngeal carcinoma, post-transplant lymphoproliferative disease, and HIV associated lymphomas. Although multiple viral proteins have been implicated in EBV associated malignancies, the cellular pathways that control EBV-induced transformation and tumorigenesis remain incompletely understood. In this study, Nikitin et al demonstrate that early EBV infection induces a cellular DDR which restricts virus-mediated transformation. The EBV encoded EBNA3C protein subsequently attenuates this response to favor transformation and immortalization of host cells.
Keywords: ATM/CHK2, B cell transformation, DNA damage response, Epstein-Barr virus, tumorigenesis
Summary of methods & results
The recent article by Nikitin et al provides new insight into how the host DNA damage response (DDR) restricts Epstein-Barr virus (EBV)-induced B cell transformation and how EBV overcomes this barrier to establish latency [1]. EBV was first discovered in 1964 in cultured lymphoblasts derived from a Burkitt’s lymphoma [2]. EBV infects 90%–95% of world’s population and is now known to contribute to a variety of human diseases, including infectious mononucleosis, nasopharyngeal carcinoma, Burkitt’s lymphoma, and post-transplant lymphoproliferative disease [3]. The life cycle of EBV includes both latent and lytic cycles, which are dependent on distinct programs of viral gene expression. During the EBV latent cycle, a very limited set of viral proteins are produced. The viral genomes are maintained as circular episomes and there is no production of virions. In the latent state, viral DNA is replicated only once during S phase, in synchrony with the host chromosomal DNA[4]. Lytic cycle infection is characterized by sequential expression of the bulk of the viral genome and massive viral DNA replication with the ultimate production of infectious virions [5, 6]. It is recognized that viral latency gene products drive oncogenesis. However, recent studies have also implicated lytic EBV infection in the development of lymphomas [7, 8]. Therefore, both latent and lytic replication may contribute to the emergence of cancers.
Previous studies showed that the DDR plays an important role in the viral life cycle. EBV lytic replication elicits a DDR by triggering ATM autophosphorylation and activation. Activated ATM phosphorylates its downstream targets, such as H2AX, p53, CHK2 and RPA2. In addition, phosphorylated ATM, RPA2 and Mre11/Rad50/Nbs1 (MRN) complexes are recruited to replication compartments in nuclei during EBV lytic replication [9, 10]. In the early stages of the EBV lytic cycle, ATM mediated phosphorylation of p53 at Ser15 prevents its MDM2-dependent degradation and promotes its function in the DDR. However, during the later stages, CHK2 may contribute to p53 phosphorylation at Ser366 and Ser378, resulting in p53 degradation through the ubiquitin-proteasome pathway and blocking of p53-mediated apoptosis [11] and the EBV protein kinase BGLF4 contributes to p27 phosphorylation and its ubiquitin-proteasome dependent degradation [12]. These events lead to a prolonged pseudo S-phase environment that fosters viral DNA replication (Figure 1A). Like EBV, other viruses, such as herpes simplex virus (HSV) [13], parvovirus minute virus of mice (MVM) [14] and murine γ-herpesvirus 68 (MHV 68) [15] also trigger the DDR to benefit viral replication.
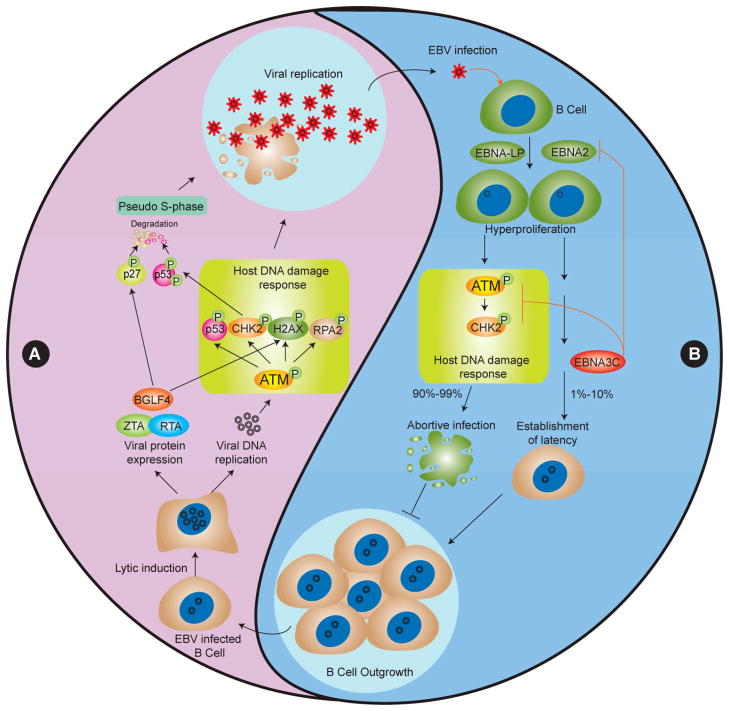
Figure 1. Differing roles of the DNA damage response in promoting viral replication and restricting latency in EBV-infected cells.
(A) The DNA damage response (DDR) is actively induced by viral protein expression and viral lytic DNA replication, as indicated by activation of ATM and its downstream targets H2AX and CHK2 phosphorylation. The DDR acts as a positive feedback to promote viral lytic replication. Simultaneously, the potential DDR inducer BGLF4 and CHK2 also contribute to p27 and p53 phoshorylation- and ubiquitin-proteasome-dependent degradation, resulting in a prolonged pseudo S-phase environment that further benefits viral DNA replication. (B) EBV infection of primary B cells induces the DDR, which in turn acts as a host anti-EBV response to restrict virus induced transformation and outgrowth of B cells. A small percentage of EBV-infected B cells bypass the host defensive DDR through EBNA3C inhibition of EBNA2 or ATM/CHK2, leading to B cell outgrowth.
To better understand the host response to viral infection that limits EBV-induced transformation, Nikitin et al. performed a detailed study using EBV infection of primary B cells [1]. First, they found that EBV infection of these cells elicits the DDR, and EBNA2 and latency III gene products are necessary to induce the response. Unexpectedly, the EBV-induced DDR is independent of the presence of nuclear viral genomes and of lytic replication while it is associated with a transient hyperproliferation of the host cell. Further, mRNA microarray analysis revealed that genes induced during this transient hyperproliferation period are enriched in “Cell proliferation” and “Response to DNA damage stimulus” categories, and are also consistent with ATM-dependent p53 target gene expression. The activated genes were subsequently repressed in the transition from the transient hyperproliferation stage to the indefinitely proliferating stage (lymphoblastoid cell line [LCL] outgrowth). The transition in cell gene expression correlates with the shift of viral gene expression from early Cp promoter usage and high EBNA2 expression in proliferating cells to Wp promoter usage and the reduced EBNA2 expression in established LCLs. Cells in early hyperproliferating divisions are more prone to growth arrest and cell death than those in later divisions, supporting the hypothesis that the EBV-induced DDR is growth suppressive.
Since ATM and its downstream effector kinase CHK2 are critical kinases involved in the DDR, blocking these kinases by inhibitors was predicted to remove the DDR restriction on B cell proliferation. Indeed, Nikitin et al. found that the efficiency of EBV-mediated transformation of peripheral blood mononuclear cells increased with treatment by small molecule inhibitors of either ATM or CHK2. The inhibitor treatment is most effective during the hyperproliferation period, suggesting that the EBV induced ATM- and CHK2-dependent growth suppressive signaling pathway restricts transformation efficiency. In order to establish latency, EBV must evolve a strategy to overcome the inhibiting effects of the host DDR. In this study, Nikitin et al. further demonstrate that EBNA3C is required to attenuate the DDR and facilitate immortalization.
Previously published data had emphasized EBV utilization of the host DDR to promote viral replication. Nikitin et al. however demonstrate that EBV-induced DDR is a robust host anti-viral strategy, consistent with the important role of the DDR as a tumor suppressor pathway during oncogenesis [16, 17]. Therefore, the DDR is differentially regulated during different stages of EBV’s life cycle, illustrating the complexity of the EBV-host interaction. Other viruses have similarly evolved complex relationships with the host DDR [18]. Kaposi’s sarcoma associated herpesvirus (KSHV) encodes vIRF1 that targets ATM and p53 to counteract the DDR and facilitate viral replication [19]. The DDR induced by KSHV v-cyclin also functions as an anti-cancer barrier in KSHV-induced cancers [20]. Human papillomavirus E6 binds to XRCC1 and inhibits its function in DNA repair and genomic stability [21], and E6 also destabilizes TIP60, the upstream regulator of ATM and the DDR [22, 23], which allows HPV to promote cell proliferation and cell survival [24]. MHV68 activates the DDR through H2AX phosphroylation to foster viral lytic replication [15], while H2AX knockdown also reduces establishment of latency [25].
Conclusion & future perspective
The study by Nikitin et al. emphasizes the EBV-induced DDR as a robust host anti-viral strategy and implicates the DNA damage induced by primary EBV infection in creating errors in the cellular genome that can act as cofactors for EBV associated tumorigenesis. Indeed, the EBNA1, LMP1 and EBNA3C latency proteins have been implicated in EBV-induced cell transformation through promoting genomic instability, inducing DNA damage, inhibiting DNA repair and inactivating cell cycle checkpoints [26, 27]. In addition, because early lytic viral genes trigger the DDR, abortive lytic infection may also have a role in the development of lymphomas [8]. The present study also provides a molecular basis for ATM and CHK2 inhibitors as useful tools for efficiently establishing LCLs for research and clinical studies. Conversely, specifically triggering the DDR in virus infected tumor cells might enhance treatments of viral-associated cancer. The authors further indicated that EBNA3C attenuates the DDR to favor transformation and immortalization of B cells. The detailed molecular mechanisms employed by EBNA2 and other latency genes in promoting the DDR and by EBNA3C in attenuating the DDR remain to be determined.
Executive summary.
Objectives
To investigate the host response that limits establishment of latent EBV infection.
Methods
EBV infection and transformation of purified B cells and peripheral blood mononuclear cells.
Immunofluorescence and in situ hybridization assays.
Flow cytometry analysis and B cell proliferation assays.
Microarray-based cellular gene expression analysis during different EBV infection stages.
Quantitative real-time PCR analysis of EBV gene expression during different EBV infection stages.
Results
Latent EBV infection of primary B cells elicits a host DNA damage response (DDR).
The DDR is associated with EBV-infected hyperproliferating B cells.
Inhibition of the DDR kinases ATM or CHK2 promotes EBV B cell transformation early in infection.
The latent viral protein EBNA3C inhibits the host DDR and positively contributes to B cell outgrowth.
Conclusion
Latent EBV infection of primary B cells induces a cellular DDR which restricts virus-mediated transformation while EBNA3C attenuates this response to favor transformation and immortalization of B cells.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1.Nikitin PA, Yan CM, Forte E, et al. An ATM/Chk2-mediated DNA damage-responsive signaling pathway suppresses Epstein-Barr virus transformation of primary human B cells. Cell Host Microbe. 2010;8(6):510–522. doi: 10.1016/j.chom.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2*.Epstein MA, Achong BG, Barr YM. Virus Particles in Cultured Lymphoblasts from Burkitt’s Lymphoma. Lancet. 1964;1(7335):702–703. doi: 10.1016/s0140-6736(64)91524-7. Describes the discovery ofEpstein-Barr virus (EBV) [DOI] [PubMed] [Google Scholar]
- 3**.Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4(10):757–768. doi: 10.1038/nrc1452. Comprehensive overview on EBV and associated cancers. [DOI] [PubMed] [Google Scholar]
- 4.Adams A. Replication of latent Epstein-Barr virus genomes in Raji cells. J Virol. 1987;61(5):1743–1746. doi: 10.1128/jvi.61.5.1743-1746.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsurumi T, Fujita M, Kudoh A. Latent and lytic Epstein-Barr virus replication strategies. Rev Med Virol. 2005;15(1):3–15. doi: 10.1002/rmv.441. [DOI] [PubMed] [Google Scholar]
- 6.Amon W, Farrell PJ. Reactivation of Epstein-Barr virus from latency. Rev Med Virol. 2005;15(3):149–156. doi: 10.1002/rmv.456. [DOI] [PubMed] [Google Scholar]
- 7.Feng WH, Cohen JI, Fischer S, et al. Reactivation of latent Epstein-Barr virus by methotrexate: a potential contributor to methotrexate-associated lymphomas. J Natl Cancer Inst. 2004;96(22):1691–1702. doi: 10.1093/jnci/djh313. [DOI] [PubMed] [Google Scholar]
- 8*.Ma SD, Hegde S, Young KH, et al. A new model of Epstein-Barr virus infection reveals an important role for early lytic viral protein expression in the development of lymphomas. J Virol. 2011;85(1):165–177. doi: 10.1128/JVI.01512-10. Demonstratesthecontributionof lytic infection to EBV-induced lymphomas in ahumanized mouse model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kudoh A, Fujita M, Zhang L, et al. Epstein-Barr virus lytic replication elicits ATM checkpoint signal transduction while providing an S-phase-like cellular environment. J Biol Chem. 2005;280(9):8156–8163. doi: 10.1074/jbc.M411405200. [DOI] [PubMed] [Google Scholar]
- 10.Kudoh A, Iwahori S, Sato Y, et al. Homologous recombinational repair factors are recruited and loaded onto the viral DNA genome in Epstein-Barr virus replication compartments. J Virol. 2009;83(13):6641–6651. doi: 10.1128/JVI.00049-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato Y, Kamura T, Shirata N, et al. Degradation of phosphorylated p53 by viral protein-ECS E3 ligase complex. PLoS Pathog. 2009;5(7):e1000530. doi: 10.1371/journal.ppat.1000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwahori S, Murata T, Kudoh A, et al. Phosphorylation of p27Kip1 by Epstein-Barr virus protein kinase induces its degradation through SCFSkp2 ubiquitin ligase actions during viral lytic replication. J Biol Chem. 2009;284(28):18923–18931. doi: 10.1074/jbc.M109.015123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shirata N, Kudoh A, Daikoku T, et al. Activation of ataxia telangiectasia-mutated DNA damage checkpoint signal transduction elicited by herpes simplex virus infection. J Biol Chem. 2005;280(34):30336–30341. doi: 10.1074/jbc.M500976200. [DOI] [PubMed] [Google Scholar]
- 14.Adeyemi RO, Landry S, Davis ME, Weitzman MD, Pintel DJ. Parvovirus minute virus of mice induces a DNA damage response that facilitates viral replication. PLoS Pathog. 2010;6(10) doi: 10.1371/journal.ppat.1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tarakanova VL, Leung-Pineda V, Hwang S, et al. Gamma-herpesvirus kinase actively initiates a DNA damage response by inducing phosphorylation of H2AX to foster viral replication. Cell Host Microbe. 2007;1(4):275–286. doi: 10.1016/j.chom.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Bartkova J, Horejsi Z, Koed K, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434(7035):864–870. doi: 10.1038/nature03482. Describes the DNA damage reponse as a potential anti-cancer barrier. [DOI] [PubMed] [Google Scholar]
- 17*.Gorgoulis VG, Vassiliou LV, Karakaidos P, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434(7035):907–913. doi: 10.1038/nature03485. Demonstrates the DNA damage reponse and genomic instability in precancerous lesions. [DOI] [PubMed] [Google Scholar]
- 18**.Lilley CE, Schwartz RA, Weitzman MD. Using or abusing: viruses and the cellular DNA damage response. Trends Microbiol. 2007;15(3):119–126. doi: 10.1016/j.tim.2007.01.003. Comprehensive summary of the relationship between viruses and the DNA damage response. [DOI] [PubMed] [Google Scholar]
- 19.Shin YC, Nakamura H, Liang X, et al. Inhibition of the ATM/p53 signal transduction pathway by Kaposi’s sarcoma-associated herpesvirus interferon regulatory factor 1. J Virol. 2006;80(5):2257–2266. doi: 10.1128/JVI.80.5.2257-2266.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koopal S, Furuhjelm JH, Jarviluoma A, et al. Viral oncogene-induced DNA damage response is activated in Kaposi sarcoma tumorigenesis. PLoS Pathog. 2007;3(9):1348–1360. doi: 10.1371/journal.ppat.0030140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iftner T, Elbel M, Schopp B, et al. Interference of papillomavirus E6 protein with single-strand break repair by interaction with XRCC1. Embo J. 2002;21(17):4741–4748. doi: 10.1093/emboj/cdf443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun Y, Jiang X, Chen S, Fernandes N, Price BD. A role for the Tip60 histone acetyltransferase in the acetylation and activation of ATM. Proc Natl Acad Sci U S A. 2005;102(37):13182–13187. doi: 10.1073/pnas.0504211102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun Y, Xu Y, Roy K, Price BD. DNA damage-induced acetylation of lysine 3016 of ATM activates ATM kinase activity. Mol Cell Biol. 2007;27(24):8502–8509. doi: 10.1128/MCB.01382-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jha S, Vande Pol S, Banerjee NS, Dutta AB, Chow LT, Dutta A. Destabilization of TIP60 by human papillomavirus E6 results in attenuation of TIP60-dependent transcriptional regulation and apoptotic pathway. Mol Cell. 2010;38(5):700–711. doi: 10.1016/j.molcel.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tarakanova VL, Stanitsa E, Leonardo SM, Bigley TM, Gauld SB. Conserved gammaherpesvirus kinase and histone variant H2AX facilitate gammaherpesvirus latency in vivo. Virology. 2010;405(1):50–61. doi: 10.1016/j.virol.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 26.Gruhne B, Sompallae R, Masucci MG. Three Epstein-Barr virus latency proteins independently promote genomic instability by inducing DNA damage, inhibiting DNA repair and inactivating cell cycle checkpoints. Oncogene. 2009;28(45):3997–4008. doi: 10.1038/onc.2009.258. [DOI] [PubMed] [Google Scholar]
- 27.Gruhne B, Kamranvar SA, Masucci MG, Sompallae R. EBV and genomic instability--a new look at the role of the virus in the pathogenesis of Burkitt’s lymphoma. Semin Cancer Biol. 2009;19(6):394–400. doi: 10.1016/j.semcancer.2009.07.005. [DOI] [PubMed] [Google Scholar]