Abstract
Burkholderia pseudomallei is a saprophytic soil bacterium and the etiological agent that causes melioidosis. It is naturally resistant to many antibiotics and therefore is difficult to treat. Bacteriophages may provide an alternative source of treatment. We have isolated and characterised the bacteriophage ΦBp-AMP1. The phage is a member of the Podoviridae family and has a genome size of ~ 45 Kb. Molecular data based on the gene which encodes for the phage tail tubular protein suggests that the phage is distinct from known phages but related to phages which infect B. thailandensis and Ralstonia spp. The phage ΦBp-AMP1 is the first B. pseudomallei podovirus to be isolated from the environment rather than being induced from a bacterial culture. It has a broad host range within B. pseudomallei and can infect all 11 strains that we tested it on but not related Burkholderia species. It is heat stable for 8 h at 50°C but not stable at 60°C. It may potentially be a useful tool to treat or diagnose B. pseudomallei infections as it can lyse several strains of clinical relevance.
Keywords: Burkholderia pseudomallei, bacteriophage, Podoviridae
Findings
Burkholderia pseudomallei is a Gram-negative bacillus and the causative agent of melioidosis which is endemic in Southeast Asia and northern Australia [1]. The bacterium is a widely distributed environmental saprophyte commonly found in water and soil in the endemic areas. The bacterium is highly resilient to environmental stress and can survive in distilled water for months or even years [2]. It infects humans via inoculation through skin abrasions or via the inhalation route and causes either acute or chronic melioidosis. Acute infection is often manifested as septicemia, resulting in death within days of exposure. B. pseudomallei is resistant to many antibiotics including third-generation cephalosporins [3]. There is clearly a need for novel antimicrobials. One promising source of antimicrobials may come from bacteriophages which infect and kill specific bacterial cells. They are the most abundant living entities in the world, and play key roles in bacterial population dynamics and evolution [4]. Bacteriophages can be either temperate where they integrate into the host bacterial genomes, or virulent where they infect, multiply, and lyse their hosts without any initial integration period. The ability of such virulent phages to be used therapeutically has been well documented and reviewed [5,6].
Prophages have been shown to be abundant in B. pseudomallei genomes [7-9]. A recent study reported the abundance and diversity of prophages in B. pseudomallei, B. thailandensis and B. mallei genomes and suggested how they contribute to the phenotypic diversity in the Burkholderia species complex [8]. Pertinently to our study, the 37 phages identified in the Burkholderia genomes were either myoviruses, Mu-like viruses, or siphoviruses. Although one previous study reported the presence of a putative podovirus in the genome of B. pseudomallei, there are no published genetic studies to support this work [7]. Another recent study isolated six B. pseudomallei myoviruses from soil in Thailand, these were morphologically characterised to have lytic properties [10].
We report the isolation of a podovirus which is specific to B. pseudomallei. To isolate the phage, one hundred and fifty soil samples were screened. The samples were collected from rice fields within the Khon Kaen Province, Thailand. The soil samples were collected during the rainy season from a depth of around 10-20 cm. The ambient temperature in rice fields varies with season ranged from 23-35°C and in the summer time temperatures reach 40-45°C. Two grams of each soil sample was placed into 10 ml Luria-Bertani (LB) broth supplemented with 0.5 mM CaCl2. Each sample was mixed thoroughly, incubated at room temperature overnight, centrifuged at 4000 × g for 20 min and then passed through a 0.22 μm filter. Ten microlitre volumes were used in spot assays on B. pseudomallei strain K96243 grown on LB agar supplemented with 0.5 mM CaCl2. The phage was purified and kept as stock in SM-buffer with 50% glycerol at -70°C.
The isolated phage was designated Bp-AMP1 and characterised using transmission electron microscopy (TEM). This analysis revealed that it belongs to the podovirus family as it has an icosahedral capsid with a diameter of ~ 45 nm and a characteristically small podovirus tail of ~20 nm tail (Figure 1A). Pulsed field gel electrophoresis (PFGE) was carried out as described previously [11]. This revealed that the genome size was consistent with a typical podovirus size of approximately 45 Kb (Figure 1B). Phage DNA was digested using the restriction enzyme BstBI and a discrete DNA banding pattern was obtained (Figure 1C). The total size of the restriction fragments from this digestion amounted to 45.5 Kb and correlates with the PFGE estimate.
Figure 1.
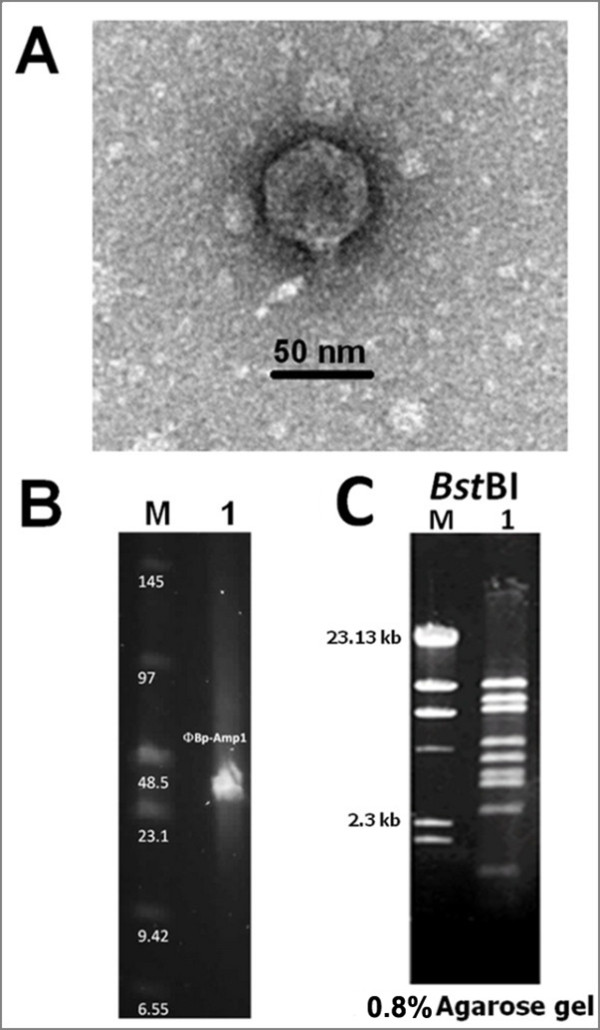
Electron microscopic and DNA analysis of ФBp-AMP1. (A) Transmission electron micrograph and ΦBp-AMP1 (B) The genome size of ΦBp-AMP1 determined by PFGE. (C) Restriction DNA pattern of ΦBp-AMP1 genomic DNA digested with BstBI (lane1). Lane M, λ HindIII DNA marker.
To investigate the host range of ΦBp-AMP1, plaque assays were performed on 10 B. pseudomallei strains isolated from distinct geographic locations, 6 B. thailandensis strains, 4 other Burkholderia spp., Pseudomonas aeruginosa and Escherichia coli strains (Table 1).
Table 1.
Efficiency of ΦBp-AMP1 to form plaques on 10 B. pseudomallei and on other bacterial strains.
| Bacteria | Strain | Source and location | Titres (pfu/ml) |
|---|---|---|---|
| B. pseudomallei | 3073A | Clinical isolation, Thailand | (3.00 ± 0.41) × 107 |
| 576 | Clinical isolation, Thailand | (2.70 ± 0.37) × 107 | |
| 1710a | Clinical isolation, Thailand | (1.50 ± 0.15) × 106 | |
| BA18 | Clinical isolation, Australia | (3.10 ± 0.33) × 105 | |
| MSHR42 | Clinical isolation, Australia | (4.00 ± 0.44) × 105 | |
| MSHR287 | Clinical isolation, Australia | (2.35 ± 0.24) × 106 | |
| MSHR668 | Clinical isolation, Australia | 10 ± 1.66 | |
| E8 | Soil, Thailand | (2.50 ± 0.20) × 104 | |
| E412 | Soil, Thailand | (1.35 ± 0.11) × 106 | |
| MSHR491 | Water, Australia | (5.00 ± 0.44) × 105 | |
| B.thailandensis | D1 | Soil, Thailand | - |
| E28 | Soil, Thailand | - | |
| E36 | Soil, Thailand | - | |
| E68 | Soil, Thailand | - | |
| E70 | Soil, Thailand | - | |
| E94 | Soil, Thailand | - | |
| B. multivorans | LMG 16660 | Clinical isolation, UK | - |
| B. ubonensis | DMST 866 | Soil, Thailand | - |
| B. vietnamensis | LMG 6999 | Clinical isolation, Vietnam | - |
| B. cepacia | ATCC 25416 | - | - |
| P. aeruginosa | ATCC 27853 | - | - |
| E. coli | ATCC 25922 | - | - |
B. pseudomallei K96243 was used to propagate the phages and the stock was used to infect the other strains. The stock gave 2.90 × 107 pfu/ml on K96243 and the titre on the other strains is given in the table.Value represent the mean pfu/ml ± S.E.M of triplicates, -, no plaque formation
ΦBp-AMP1 could infect all 11 B. pseudomallei strains however in general they were more efficient at infecting the Thai B. pseudomallei strains than the Australian strains. To do these efficiency assays, a known amount of phage stock derived from being propagated on K96243 was added to each host strain tested. This stock gave a titre of 2.90 × 107 pfu/ml when grown on the host strain. However, the titre was much lower on the Australian strains with the least good host being MSHR668 which only had a titre of 10 ± 1.66 pfu/ml. There are some exceptions to this and the Thai strain E8 was not an efficient host for the phage. It could not infect any of the tested 6 strains of B. thailandensis or the B. multivorans, B. ubonensis, B. cepacia, B. vietnamiensis, P. aeruginosa and E. coli. To the best of our knowledge, this is the first isolation of a Podoviridae family bacteriophage which specifically infects B. pseudomallei. Although B. pseudomallei myoviruses have been isolated previously, because myoviruses generally do not share any genes in common with podoviruses, ΦBp-AMP1 is likely to have a novel set of antimicrobial properties to those found in B. pseudomallei myoviruses.
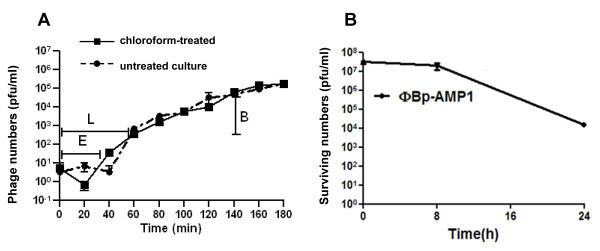
In order to characterise the physiological characteristics of this phage, one-step growth curve experiments were performed as previously described [12]. Briefly, cultures of B. pseudomallei K96243 with ca. 1 × 108 bacteria were resuspended in 1 ml LB medium and incubated with ΦBp-AMP1 at a multiplicity of infection (MOI) 0.1. The mixture was incubated at 37°C for 10 min before centrifugation and resuspension in 10 ml of pre-warmed LB broth supplemented with 0.5 mM CaCl2 and incubated at 37°C. Five hundred microlitres of culture was taken at 20 min intervals over a period of 3 h and the number of phage particles was immediately determined using plaque assays. These data showed that the eclipse, latent period and burst size of ΦBp-AMP1 on B. pseudomallei K96243 at 37°C were 40 min, 60 min and 158 ± 54, respectively (Figure 2A). By comparison, the latent times of ΦBp-AMP1 was longer and burst size was smaller than the recently described B. pseudomallei phage ST79 which had a latent period of 15 min, and a burst size of 304 [10]. Although there appears to be a population of cells that are resistant to ΦBp-AMP1, this reflects the low titre of phages added to the culture. Bacteria from this culture were isolated and were still susceptible to phage infection and when higher MOI's were used in infection experiments total lysis was observed.
Figure 2.
Growth curve and thermal stability of ФBp-AMP1. (A) One step growth curve of ФBp-AMP1 on B. pseudomallei strain K96243. Phage number (pfu/ml) in chloroform-treated (▀) and the untreated culture (●); E, eclipse period; L, latent period and B, burst size. (B) Thermal stability of ФBp-AMP1 at 50ºC. Values are the means of three independent experiments
To investigate the thermal stability of the bacteriophages, a 107 pfu/ml phage stock was incubated at 50°C and 60°C for 0, 8 and 24 h. These temperatures are significantly higher than the ambient temperature of rice fields in Thailand. At intervals, the bacteriophage stock was titred. Figure 2B showed that ΦBp-AMP1 was stable for at least 8 h at 50°C but reduced from 107 pfu/ml to 104 pfu/ml after a further period of incubation from 8 h to 24 h. However, no bacteriophages were detected after 1 h incubation at 60°C (data not shown). This data indicates that ΦBp-AMP1 may be useful even in high temperature environments. The loss of phage viability at 60°C is a common observation, as seen for example in bacteriophages which infect Lactobacillus delbrueckii [13].
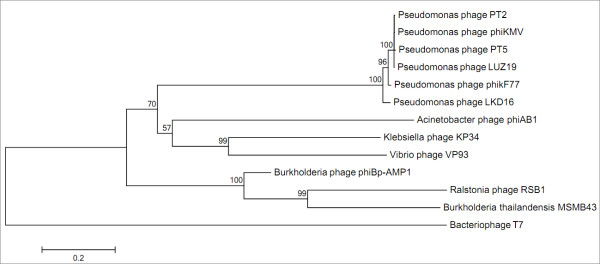
To obtain molecular data on this phage, we initially tried to amplify the DNA polymerase gene using designed primers from a subset of viruses belonging to the Podoviridae [14]. This was not successful probably due to a lack of sequence identity with known phages; we therefore randomly sequenced fragments of phage DNA. To do this, DNA was restricted using TaqαI and BstBI and cloned into the plasmid pBluescript (Stratagene, La Jolla, CA, USA). Colonies were screened to identify those that contained suitable fragment sizes. A PsiBLAST (NCBI) search from a sequenced region identified a gene (EMBL:FR850500) which had a homologue with an ORF coding for a phage tail tubular protein B (TTPB) from a temperate B. thailandensis phage (GeneBank:ZP02468145.1). Fortuitously, this gene had been used as a phylogenetic marker in previous studies [15]. Therefore, a phylogenetic analysis was performed at the amino acid level using Molecular Evolutionary Genetics Analysis (MEGA) package version 4.1 (Beta) [16,17].
The tree in Figure 3 shows the results from a Neighbor joining analysis which was performed using a maximum composite likelihood model and a bootstrap analysis with 1, 000 replicates with bacteriophage T7 as an outgroup. The tree shows that ΦBp-AMP1 is clearly separated from known phages but is most closely related to the B. thailandensis MSMB43 prophage and the T7-like Ralstonia phage RSB1 (supported by a boots trap value of 100%). This is logical because Ralstonia is closely related to soil-born Burkholderia species [18]. Interestingly, there is no clear relationship between ΦBp-AMP1 and the characterised Pseudomonas phages. Analyses were also done using Parsimony and Maximum likelihood and all showed a similar tree topology.
Figure 3.
Phylogenetic tree of ΦBp-AMP1 tail tubular protein B (TTPB) by the neighbour-joining method. Branch lengths are indicated below the branches. Bootstrap values are indicated at the nodes. The scale bar represents the proportion of amino acid compared.
In conclusion, we report the isolation of a podovirus which infects B. pseudomallei. The broad host-range and thermal stability of this phage suggests that it may have promise as a therapeutic agent. Further research is underway in our laboratories to characterise the genome of this bacteriophage and to establish its potential application for B. pseudomallei treatment.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JG, KK and NI were responsible for soil samples collection. JG performed the phage biological characterisation, data analysis and drafted the manuscript. KK participated in the co-ordination of the study in Thailand. EEG, MRJC, and SK designed the study and interpreted the data. JS and ED carried out cloning, sequencing and phylogenetic analysis. SK and MRJC critically revised the manuscript. All authors have read and approved final manuscript.
Contributor Information
Jiraporn Gatedee, Email: gatedee@hotmail.com.
Kanyanan Kritsiriwuthinan, Email: kanyanan_krit@hotmail.com.
Edouard E Galyov, Email: eg98@leicester.ac.uk.
Jinyu Shan, Email: js401@leicester.ac.uk.
Elena Dubinina, Email: ed78@leicester.ac.uk.
Narin Intarak, Email: tongtong48@hotmail.com.
Martha RJ Clokie, Email: mrjc1@leicester.ac.uk.
Sunee Korbsrisate, Email: grsks@mahidol.ac.th.
Acknowledgements
This work was supported by a Wellcome Trust, UK (Grant number 092638/Z/10/Z) to MRJC, EEG and SK. We thank the students from the Department of Medical Technology, Rangsit University for their assistances on soil sample collection, and the Wellcome Trust-Mahidol, University Oxford and Dr. H. Schweizer for providing some of the Burkholderia strains. SK is supported by "Chalermphrakiat" Grant, Faculty of Medicine Siriraj Hospital, Mahidol University. JG is supported by Rangsit University.
References
- Dance DA. Melioidosis: the trip of the ice berg. Clin Microbiol Rev. 1991;4:52–60. doi: 10.1128/cmr.4.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuthiekanun V, Smith MD, White NJ. Survival of Burkholderia pseudomallei in the absence of nutrients. Trans R Soc Trop Med Hyg. 1995;89:491. doi: 10.1016/0035-9203(95)90080-2. [DOI] [PubMed] [Google Scholar]
- Cheng AC, Currie BJ. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev. 2005;18:383–416. doi: 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clokie MRJ, Millard AD, Leterov AV, Heaphy S. Phages in nature. Bacteriophage. 2011;1:31–45. doi: 10.4161/bact.1.1.14942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski A, Miedzybrodzki R, Borysowski J, Weber-Dabrowska B, Lobocka M, Fortuna W, Letkiewicz S, Zimecki M, Filby G. Bacteriophage therapy for the treatment of infections. Curr Opin Investig Drugs. 2009;10:766–774. [PubMed] [Google Scholar]
- Kutter E, De Vos D, Gvasalia G, Alavidze Z, Gogokhia L, Kuhl S, Abedon ST. Phage therapy in clinical practice: treatment of human infections. Curr Pharm Biotechnol. 2010;11:69–86. doi: 10.2174/138920110790725401. [DOI] [PubMed] [Google Scholar]
- Sariya L, Prempracha N, Keelapan P, Chittasophon N. Bacteriophage isolated from Burkholderia pseudomallei Causes phenotypic changes in Burkholderia thailandensis. ScienceAsia. 2006;32:83–91. doi: 10.2306/scienceasia1513-1874.2006.32.083. [DOI] [Google Scholar]
- Ronning CM, Losada L, Brinkac L, Inman J, Ulrich RL, Schell M, Nierman WC, DeShazer D. Genetic and phenotypic diversity in Burkholderia: contributions by prophage and phage-like elements. BMC Microbiol. 2010;10:202. doi: 10.1186/1471-2180-10-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeShazer D. Genomic diversity of Burkholderia pseudomallei clinical isolates: subtractive hybridization reveals a Burkholderia mallei-specific prophage in B. pseudomallei 1026b. J Bacteriol. 2004;186:3938–3950. doi: 10.1128/JB.186.12.3938-3950.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordpratum U, Tattawasart U, Wongratanacheewin S, Sermswan RW. Novel lytic bacteriophages from soil that lyse Burkholderia pseudomallei. FEMS Microbiol Lett. 2010;314:81–88. doi: 10.1111/j.1574-6968.2010.02150.x. [DOI] [PubMed] [Google Scholar]
- Lingohr E, Frost F, Johnson RP. In: Bacteriophages: Methods and Protocols. Clokie MRJ, Kropinski AM, editor. Vol. 2. Humana Press; 2009. Determination of bacteriophage genome size by pulsed-field gel electrophoresis; pp. 19–25. [DOI] [PubMed] [Google Scholar]
- Pajunen M, Kiljunen S, Skurnik M. Bacteriophage phiYeO3-12, specific for Yersinia enterocolitica serotype O:3, is related to coliphages T3 and T7. J Bacteriol. 2000;182:5114–5120. doi: 10.1128/JB.182.18.5114-5120.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiberoni A, Guglielmotti DM, Reinheimer JA. Inactivation of Lactobacillus delbrueckii bacteriophages by heat and biocides. Int J Food Microbiol. 2003;84:51–62. doi: 10.1016/S0168-1605(02)00394-X. [DOI] [PubMed] [Google Scholar]
- Labonte JM, Reid KE, Suttle CA. Phylogenetic analysis indicates evolutionary diversity and environmental segregation of marine podovirus DNA polymerase gene sequences. Appl Environ Microbiol. 2009;75:3634–3640. doi: 10.1128/AEM.02317-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Y, Xiong H, Ma X, Cheng L, Huang G, Zhang ZR. Molecular characterization, structural analysis and determination of host range of a novel bacteriophage LSB-1. Virol J. p. 255. [DOI] [PMC free article] [PubMed]
- Kumar S, Nei M, Dudley J, Tamura K. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinform. 2008;9:299–306. doi: 10.1093/bib/bbn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Yabuuchi E, Kosako Y, Oyaizu H, Yano I, Hotta H, Hashimoto Y, Ezaki T, Arakawa M. Proposal of Burkholderia gen. nov. and transfer of seven species of the genus Pseudomonas homology group II to the new genus, with the type species Burkholderia cepacia (Palleroni and Holmes 1981) comb. nov. Microbiol Immunol. 1992;36:1251–1275. doi: 10.1111/j.1348-0421.1992.tb02129.x. [DOI] [PubMed] [Google Scholar]