Abstract
The bcl-6 proto-oncogene encodes a POZ/zinc finger transcriptional repressor expressed in germinal center (GC) B and T cells and required for GC formation and antibody affinity maturation. Deregulation of bcl-6 expression by chromosomal rearrangements and point mutations of the bcl-6 promoter region are implicated in the pathogenesis of B-cell lymphoma. The signals regulating bcl-6 expression are not known. Here we show that antigen receptor activation leads to BCL-6 phosphorylation by mitogen-activated protein kinase (MAPK). Phosphorylation, in turn, targets BCL-6 for rapid degradation by the ubiquitin/proteasome pathway. These findings indicate that BCL-6 expression is directly controlled by the antigen receptor via MAPK activation. This signaling pathway may be crucial for the control of B-cell differentiation and antibody response and has implications for the regulation of other POZ/zinc finger transcription factors in other tissues.
Keywords: BCL-6, BCR signaling, MAP kinase, POZ/zinc finger proteins, ubiquitin–proteasome
The bcl-6 proto-oncogene was identified by virtue of its involvement in chromosomal translocations in diffuse large cell lymphoma (DLCL), the most common form of non-Hodgkin’s lymphoma (NHL) (Baron et al. 1993; Kerckaert et al. 1993; Ye et al. 1993; Miki et al. 1994). Subsequent studies have demonstrated that rearrangements of the bcl-6 gene can be found in 30%–40% of DLCL and in a minority (5%–10%) of follicular lymphoma (FL) (Bastard et al. 1994; LoCoco et al. 1994; Otsuki et al. 1995). These rearrangements juxtapose heterologous promoters, derived from other chromosomes, to the bcl-6 coding domain, causing its deregulated expression by a mechanism called promoter substitution (Ye et al. 1995; Chen et al. 1998). The 5′ noncoding region of the bcl-6 gene can also be altered by somatic point mutations that are detectable, independent of rearrangements, in ∼70% DLCL, 45% FL, and AIDS-associated NHL (Migliazza et al. 1995; Gaidano et al. 1997). Taken together, rearrangements and mutations of the bcl-6 promoter region represent the most frequent genetic alteration in human B-cell malignancies, suggesting they may be important for tumorigenesis (Dalla-Favera et al. 1996).
The BCL-6 protein is a nuclear phosphoprotein belonging to the POZ/zinc finger (ZF) family of transcription factors (Kerckaert et al. 1993; Ye et al. 1993; Miki et al. 1994). It contains six Krüppel-type carboxy-terminal zinc finger (ZF) motifs that have been shown to recognize specific DNA sequences in vitro (Chang et al. 1996; Seyfert et al. 1996) and an amino-terminal POZ motif (Albagli et al. 1995) shared by various ZF molecules including the Drosophila developmental regulators Tramtrack and Broad-complex (Harrison and Travers 1990; DiBello et al. 1991), the human KUP (Chardin et al. 1991), ZID (Bardwell and Treisman 1994), and PLZF (Chen et al. 1993) proteins as well as by POX viruses (Koonin et al. 1992) and the actin-binding Drosophila oocyte protein Kelch (Xue and Cooley 1993). BCL-6 functions as a potent transcriptional repressor by binding to its DNA target sequence (Deweindt et al. 1995; Chang et al. 1996; Seyfert et al. 1996).
BCL-6 is an important regulator of lymphoid development and function. In the B-cell lineage, the BCL-6 protein is found only in B cells within germinal centers (GC), but not in pre-B cells or in differentiated progeny such as plasma cells. In the T-cell lineage, BCL-6 protein is detectable in cortical thymocytes and in CD4+ T cells within GC as well as scattered in the perifollicular area (Cattoretti et al. 1995; Onizuka et al. 1995; Allman et al. 1996). Mice deficient in BCL-6 display normal B-cell, T-cell, and lymphoid organ development but have a selective defect in T-cell-dependent antibody responses because of the inability of follicular B cells to proliferate and form GC (Dent et al. 1997; Ye et al. 1997). In addition, BCL-6-deficient mice develop an inflammatory response in multiple organs characterized by infiltration of eosinophils and IgE-bearing B lymphocytes typical of a Th2-mediated inflammatory response. These phenotypes may be explained by the ability of BCL-6 to bind the STAT-6 DNA-binding site and repress transcription activated by STAT-6, the main nuclear effector of IL-4 signaling (Dent et al. 1997; Ye et al. 1997).
The expression and requirement of BCL-6 during GC formation and its alteration in GC-derived lymphoma suggest that BCL-6 may be a key regulator of GC development and antibody-mediated immune response. Toward the elucidation of the signals that regulate GC expression, we report here the identification of a signaling pathway by which B-cell antigen receptor directly regulates BCL-6 stability.
Results
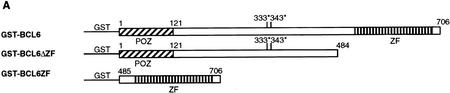
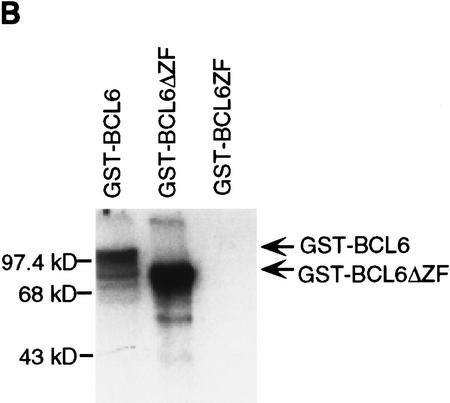
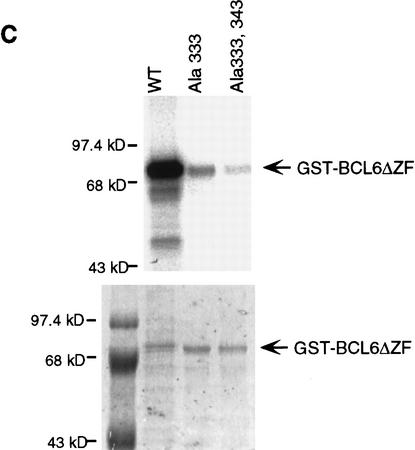
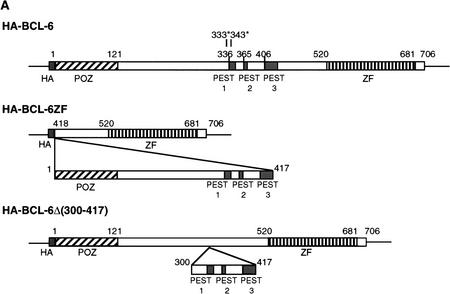
Recent studies have shown that the BCL-6 protein is phosphorylated at multiple sites by mitogen-activated protein kinases (MAPKs), ERK-1 and ERK-2, but not by Jun amino-terminal kinase (JNK) in vitro and in vivo (Moriyama et al. 1997). The results shown in Figure 1 confirm that purified recombinant MAPK (ERK-2) can phosphorylate GST–BCL-6 fusion proteins in vitro. The phosphorylation targets were mapped to the amino-terminal half of the molecule since a carboxy-terminal deletion mutant (GST-BCL-6ΔZF) could be phosphorylated at levels comparable to the wild-type molecule, whereas an amino-terminal deletion mutant (GST–BCL-6ZF) could not be phosphorylated at all (Fig. 1B). Because BCL-6 contains two perfect consensus sites (PXSP) for MAPK-mediated phosphorylation (see Fig. 1A), we generated two mutants (BCL-6Ala333 and BCL-6Ala333,343) in which one or both of these sites were altered by substituting serines with alanines. These two mutants were phosphorylated at much lower levels than wild-type BCL-6, with BCL-6Ala333,343 displaying the lowest levels (Fig. 1C). This result indicates that the Ser333 and Ser343 residues represent a significant fraction, although not all, of the BCL-6 phosphorylation target sites. The residual low level of phosphorylation is consistent with the existence of additional potential MAPK target sequences (SP) clustered within the central domain of the BCL-6 molecule.
Figure 1.
Phosphorylation of BCL-6 by ERK2 in vitro: (A) Schematic representation of wild-type and mutant GST–BCL-6 fusion proteins. (*) Serines within MAPK phosphorylation sites (PXSP). (ZF) Zinc finger domain. (B) ERK2 kinase assays using GST–BCL-6 wild-type and deletion mutants as substrates in the presence of [γ-32P]ATP. (C) (Top) ERK2 kinase assay for wild-type (WT) or mutant (Ala333; Ala333,343) GST-BCL6ΔZF proteins. (Bottom) Coomassie blue staining of the gel shown at top demonstrating comparable amounts of proteins loaded. Molecular mass markers are shown at left.
MAPK-mediated phosphorylation induces BCL-6 degradation
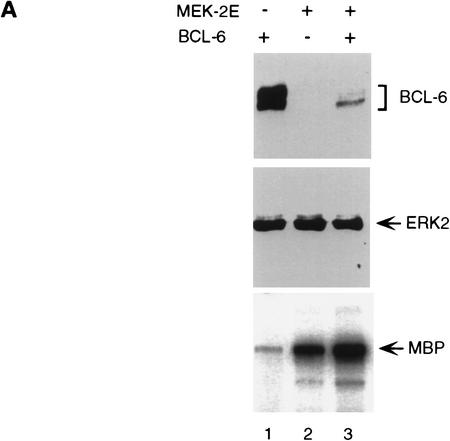
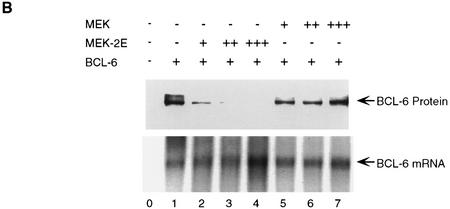
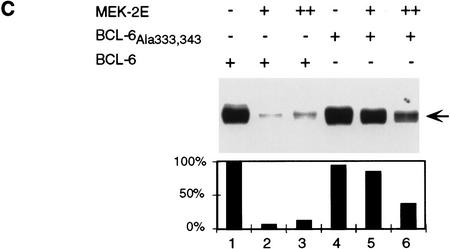
To determine the effects of MAPK-mediated phosphorylation on bcl-6 expression and function, 293T cells (which do not express endogenous BCL-6) were cotransfected with vectors expressing BCL-6, and a MEK (MAP/ERK kinase) mutant (MEK-2E) that functions as a constitutively active MAPK kinase (Yan and Templeton 1994). Western blot analysis of transfected cell extracts showed that MEK-2E expression (documented by increased ERK2 kinase activity of MEK-2E transfected cell extracts in solid-phase kinase assays in vitro; Fig. 2A, bottom) induced a dramatic reduction of BCL-6, but not ERK, levels (Fig. 2A, top and middle). The observed reduction in BCL-6 protein levels was dependent upon the phosphorylation activity of MEK-2E, since it did not occur when a vector expressing inactive MEK was cotransfected with bcl-6 (Fig. 2B). Northern blot analysis of the same transfected cells showed that the reduction in BCL-6 protein levels were not caused by decreased bcl-6 mRNA levels (Fig. 2B, bottom). Furthermore, the MEK-2E-induced decrease in BCL-6 levels was dependent on target phosphorylation, as the partial phosphorylation-resistant mutant BCL-6Ala333,343 was partially resistant to MEK-2E-mediated down-regulation (Fig. 2C). These results indicate that the MEK-2E-induced decrease in BCL-6 levels is not caused by decreased gene transcription or protein synthesis, but rather by decreased protein stability.
Figure 2.
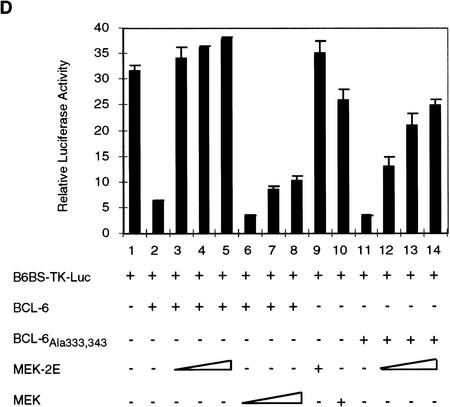
BCL-6 protein degradation induced by overexpression of MEK-2E in 293T cells. (A) Western blot analysis of 293T cells transfected with 5 μg of BCL-6 (lanes 1,3) and 5 μg of MEK-2E (lanes 2,3) using anti-BCL-6 (N-70-6; top) or anti-ERK2 (C-14; middle) antibodies. (Bottom) The results of solid-phase ERK2 kinase assay performed on cell extract from the same transfectants used in the top. (B) Western blot (top) and Northern blot (bottom) analysis of BCL-6 in 293T cells transfected with pMT2T–BCL-6 (BCL-6) (5 μg) (lanes 1–7) and various amounts of MEK-2E–CMV (MEK-2E) (lanes 2–4) or MEK–CMV (MEK) (5, 10, or 15 μg) (lanes 5–7) as indicated. (C) Western blot analysis of 293T cell extracts transfected with wild-type BCL-6 (lanes 1–3) or BCL-6Ala333,343 (lanes 4–6) in the absence (lanes 1,4) or presence (lanes 2,3,5,6) of cotransfected MEK-2E. (Bottom) The results of densitometric scanning of the autoradiography; analogous results were obtained in three independent experiments. (D) Analysis of BCL-6 transrepression activity in the presence of active MEK. 293T cells were transfected with 2.0 pmole of B6BS–TK–Luc, 0.04 pmole of pMT2T–BCL-6 (lanes 2–8) or pMT2T–BCL-6Ala333,343 (lanes 11–14) and increasing amounts (0.1, 0.2, 0.4, 0.4 pmole) of MEK-2E (lanes 3–5,9,12–14) or MEK (lanes 6–8,10) as indicated. Cells were harvested 48 hr after transfection and luciferase activities were measured by a luminometer.
Consistent with the MEK-2E-induced reduction in BCL-6 levels, a transient cotransfection assay in 293T cells showed that MEK-2E, but not MEK, could eliminate the transcriptional transrepressor activity of wild-type BCL-6 (Fig. 2D, lanes 3–5) on a reporter vector expressing the luciferase gene downstream to the BCL-6 DNA-binding site (B6BS) (Chang et al. 1996); the partial phosphorylation-resistant mutant BCL-6Ala333,343 was partially resistant to MEK-2E (Fig. 2D, lanes 12–14). Overall, these results indicate that MAPK activation leads to functional inactivation of BCL-6 by causing its accelerated degradation.
BCL-6 degradation is mediated by ubiquitin/proteasome pathway
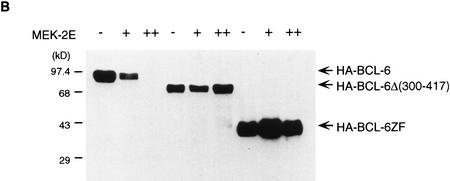
In examining the possible mechanisms for MAPK-mediated degradation of BCL-6, we noted that the cluster of MAPK putative phosphorylation sites are embedded in a region enriched in proline, glutamine, and serine, within which we identified three typical PEST sequences that score 9.4, 5.0, and 2.6, respectively (Fig. 3A; any score above zero denotes a possible PEST region; scores greater than five indicate the strongest candidates). These motifs have been demonstrated to represent targets for regulated protein degradation (Rogers et al. 1986; Rechsteiner and Rogers 1996). To determine whether MAPK-mediated BCL-6 degradation targeted these PEST sequences, we constructed vectors expressing two epitope HA-tagged bcl-6 deletion mutants (see Fig. 3A) and cotransfected them with the MEK-2E vector into 293T cells. Western blot analysis using anti-HA antibodies (Fig. 3B) showed that MEK-2E-mediated degradation targeted the amino-terminal half of the molecule and it was completely abolished in the BCL-6Δ(300–417) internal deletion mutant that lacks a small portion of the BCL-6 protein containing all three PEST sequences. These results indicate that MAPK-induced phosphorylation and degradation of BCL-6 target PEST sequences located in the same domain as the MAPK phosphorylation sites.
Figure 3.
BCL-6 contains PEST sequences that are required for phosphorylation-induced degradation. (A) Schematic representation of HA-tagged BCL-6 proteins. PEST sequences were identified by the PEST-FIND program. PEST1: AA336–AA351 (KSDCQPNSPTESCSSK), score 9.4; PEST2: AA365–AA371(KSPTDPK), score 5.0; PEST3: AA406–AA430 (RAYTAPPACQPPMEPENLDLQSPTK), score 2.6. (B) 293T cells were transfected with 5 μg of pMT2T vectors expressing HA–BCL-6 (lanes 1–3), HA–BCL-6Δ (300–417) (lanes 4–6), or HA–BCL-6ZF (lanes 7–9) in the absence of MEK-2E (lanes 1,4,7) or in the presence of increasing amount (5, 10 μg) of MEK-2E (lanes 2,5,8,3,6,9). Forty-eight hours after transfection, equal amounts of cell lysates were analyzed (after normalization for transfection efficency based on β-galactosidase activity of cotransfected plasmids) by 8% SDS-PAGE and Western blot using anti-HA (12CA5) antibodies.
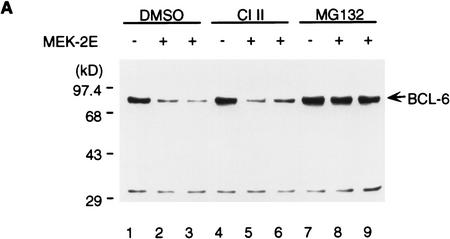
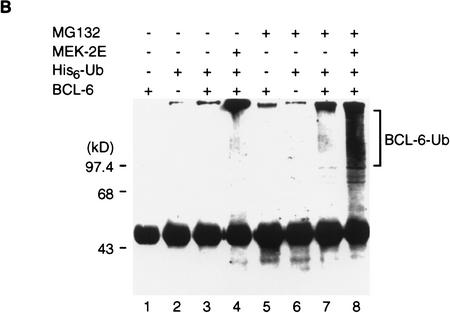
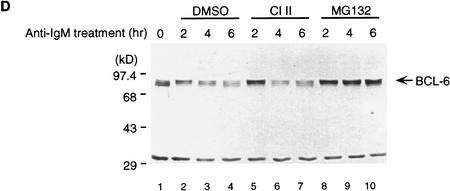
The involvement of PEST sequences suggested that MAPK-induced BCL-6 degradation could be mediated by the ubiquitin/proteasome pathway (Hochstrasser 1996). Therefore, we tested whether MEK-2E mediated degradation of BCL-6 in transfected 293T cells could be inhibited by the proteasome inhibitor Cbz-LLL (MG132) (Kim and Maniatis 1996; Palombella et al. 1994; Rock et al. 1994). Figure 4A shows that BCL-6 degradation was completely inhibited by MG132, but not by DMSO (solvent control) or calpain inhibitor II (CI II), a cysteine-protease inhibitor (Kim and Maniatis 1996; Palombella et al. 1994). Because the addition of multiple ubiquitins to the proteolysis substrate is a key step preceding target degradation by the proteasome, we then tested whether BCL-6/ubiquitin conjugates in vivo could be detected. To this end, 293T cells were transfected with vectors expressing BCL-6, MEK-2E, and epitope (His6)-tagged ubiquitin in the presence or absence of MG132. Cell lysates were subjected to immunoprecipitation with anti-BCL-6 antibodies, and the immunoprecipitates were analyzed by Western blotting using anti-ubiquitin antibodies. Figure 4B shows that in the absence of MG132, low levels of BCL-6/ubiquitin were detectable when BCL-6 and ubiquitin were coexpressed with exogenous MEK-2E (lane 4); in the presence of MG132, typical ladders representing multi-ubiquitinated forms of BCL-6 were detectable at high levels in the presence of MEK-2E (lane 8); low levels were detectable also in its absence (lane 7), suggesting that the normal turnover of BCL-6 degradation may be mediated by basal levels of endogenous MAPK activity. Based on the specific pharmacological inhibition and the detection of MEK-2E-inducible BCL-6/ubiquitin conjugates, we conclude that MAPK-induced phosphorylation induces degradation of BCL-6 via the ubiquitin/proteasome pathway.
Figure 4.
MAPK-induced BCL-6 degradation is mediated by the ubiquitin/proteasome pathway. (A) Western blot analysis of BCL-6 proteins in 293T cells transfected with BCL-6 in the absence or presence of cotransfected MEK-2E treated with 0.2% DMSO (lanes 1–3), 50 μm Calpain inhibitor II (lanes 4–6), or 50 μm MG132 (lanes 7–9) (added 8 hr after transfection). (B) 293T cells were transfected with BCL-6, His6–Ub, and MEK-2E in the absence (lanes 1–4) or presence of MG132 (lanes 5–8). Cell lysates were immunoprecipitated with anti-BCL-6 antibodies (N-70-6) and the immunoprecipitants were analyzed by 6% SDS-PAGE followed by Western blot analysis using anti-ubiquitin antibodies.
MAPK-mediated phosphorylation and degradation of BCL-6 is induced by antigen-receptor signaling in B cells
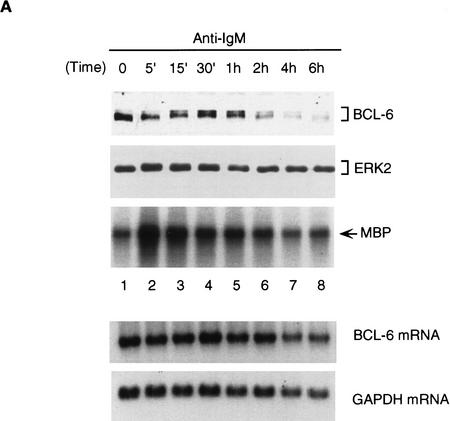
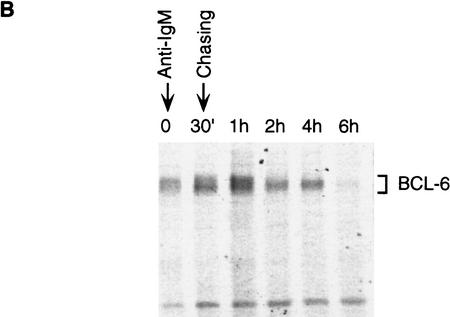
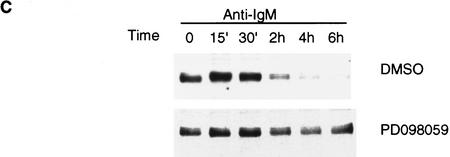
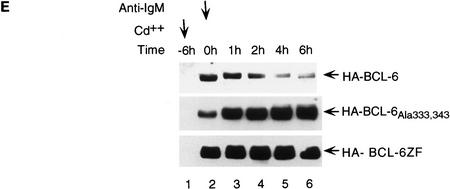
To demonstrate the physiological significance of MAPK-mediated phosphorylation/degradation of BCL-6 in B cells, we treated a B-cell lymphoma cell line (Ramos) with anti-IgM antibodies, a treatment that mimics B-cell antigen-receptor signaling and specifically activates MAPK (ERK2) (Gold et al. 1992; Sakata et al. 1995; Sutherland et al. 1996). As previously demonstrated, an in vitro assay showed that ERK2 kinase activity was rapidly increased 5 min after anti-IgM treatment (Fig. 5A); this was followed by hyperphosphorylation of ERK2 and BCL-6 (note the slow-migrating bands in Fig. 5A) and by the disappearance of BCL-6, but not ERK2. In the same experiment, Northern blot analysis showed that bcl-6 mRNA levels did not change during anti-IgM treatment of Ramos cells (Fig. 5A, bottom). To determine whether hyperphosphorylation was associated with increased BCL-6 instability, we analyzed the half-life of BCL-6 in anti-IgM-treated Ramos cells by a “pulse-chase” labeling experiment. The results (Fig. 5B) showed that the hyperphosphorylated (slow-migrating) forms of BCL-6 were significantly less stable than the hypophosphorylated (fast-migrating) forms (half life 4–6 hr). Anti-IgM-induced BCL-6 degradation was dependent on phosphorylation as it was inhibited by a specific MAPK inhibitor PD098059 (Fig. 5C) (Dudley et al. 1995; Pang et al. 1995), and was mediated by the ubiquitin/proteasome pathway since it was specifically inhibited by MG132 (Fig. 5D). Finally, anti-IgM treatment of Ramos cells stably transfected with cadmium-inducible vectors expressing HA-tagged wild-type, 333/343 mutant, or amino-terminal deleted BCL-6 proteins showed that degradation required phosphorylation of the 333 and 343 serines as well as the amino-terminal half of BCL-6 containing the PEST motifs (Fig. 5E). These results demonstrate that MAPK-mediated phosphorylation of BCL-6 and its degradation by the ubiquitin/proteasome pathway represent a physiologic pathway that can be activated by antigen-receptor signling in B cells.
Figure 5.
BCL-6 is phosphorylated and degraded by antigen-receptor signaling in B cells. Ramos cells (1 × 106/ml) were treated with anti-IgM (10 μg/ml) and harvested at different time points after treatment as indicated. (A) (Top three panels) Equal amounts of cell extracts were used for Western blot analysis using anti-BCL-6 (top), or anti-ERK2 (middle) antibodies, and for solid-phase ERK2 kinase assays (MBP, bottom). Equal amounts of RNAs (10 μg) were used for Northern blot analysis with BCL-6 or GAPDH probes (bottom). (B) Hyperphosphorylated BCL-6 proteins are more unstable. Ramos cells were pulse labeled for 1 hr with [35S]methionine and [35S]cysteine, and then treated with anti-IgM (10 μg/ml) for 30 min and subsequently incubated in the presence of an excess of nonradioactive methionine and cysteine for the indicated times (chase). Cell extracts were immunoprecipitated with anti-BCL-6 antibodies and analyzed by SDS-PAGE followed by autoradiography. (C) Anti-IgM induced BCL-6 phosphorylation and degradation is prevented by a specific MEK inhibitor. Western blot analysis of BCL-6 in Ramos cells treated with anti-IgM in the presence of 0.2% DMSO or 50 μm PD098059 (added 30 min before anti-IgM treatment). (D) Anti-IgM induced BCL-6 degradation is prevented by a specific proteasome inhibitor. Western blot analysis of BCL-6 in Ramos cells treated with anti-IgM in the presence of 0.2% DMSO (lanes 2–4), 50 μm Calpain inhibitor II (lanes 5–7), and 50 μm MG132 (lanes 8–10) (added 1 hr before the treatment). (E) Mutant BCL-6 proteins are resistant to anti-IgM-induced degradation. Ramos cells stably transfected with pHeBo–MT–HA–BCL6, pHeBo–MT–HA–BCL-6Ala333,343 and pHeBo–MT–HA–BCL6ZF were treated with 1 μm CdCl2 for 6 hr to induce exogenous BCL-6 expression. Cells were then treated wth anti-IgM (10 μg/ml) and harvested at different time points as indicated. Equal amounts of cell extracts were loaded on 7% (HA–BCL–6 or HA–BCL–6Ala333,343) or 10% SDS-PAGE (HA–BCL-6ZF) and the amount of exogenous BCL-6 proteins were analyzed by Western blot using anti-HA antibodies (12CA5).
Discussion
The present study identifies a signal transduction pathway by which the antigen receptor regulates the stability of the BCL-6 transcription factor in B cells. The results have implications for the normal mechanism regulating GC formation as well as for the role of deregulated bcl-6 expression in lymphomas deriving from GC B cells. In addition, several observations suggest that MAPK-mediated regulation of POZ/Zinc finger protein stability may represent a general, highly conserved regulatory mechanism in eukaryotic cells.
Regulation of BCL-6 stability during GC formation
The finding that antigen-receptor-induced activation of MAPK leads to BCL-6 degradation must be seen in the context of the complex network of signals modulating receptor signaling in GC B cells (Tedder et al. 1997; Cambier 1997). During GC formation, activation of this pathway is consistent with the observation that pre-GC B cells in the follicular mantle zone, the site where B cells encounter the antigen, express bcl-6 RNA, but not the BCL-6 protein (Allman et al. 1996; Cattoretti et al. 1995). Within the GC, the coexistence of antigen and bcl-6 expression implies that antigen-receptor signaling must be modulated by mechanisms that allow BCL-6 stability. These mechanisms may include down-regulation of antigen-receptor expression in centroblasts (MacLennan 1994), modulation of receptor signaling by CD22 or Fc γ receptor (Tedder et al. 1997; Cambier 1997), and the activity of de-ubiquitinases (DUB), which regulate substrate ubiquitination and are induced by cytokines acting on GC B cells (Zhu et al. 1996). During post-GC differentiation, antigen-induced degradation may serve as a rapid mechanism to down-regulate bcl-6 expression, in synergy with transcriptional down-regulation by CD40 signaling (Allman et al. 1996; Cattoretti et al. 1997). Finally, the regulation of BCL-6 stability during GC development is likely to be affected by various additional signals that activate MAPK in B cells, including various cytokines (TNF, IL-6, IL-2) (Vietor et al. 1993; Minami et al. 1994; Fukada et al. 1996). The effect of these signals on the pathway linking the antigen receptor to BCL-6 can be tested in the experimental systems used in this study.
Implication for lymphomagenesis
Most B-cell lymphoma types, FL, DLCL, and Burkitt (BL) lymphoma, are thought to derive from the GC B cells. Although rearrangements and/or mutations of the bcl-6 regulatory region are found most frequently in DLCL and FL, all GC-derived lymphomas, including those carrying a structurally normal bcl-6 gene, express the BCL-6 protein (Cattoretti et al. 1995). This implies that the BCL-6 protein is stable in tumor cells and suggests that MAPK-mediated degradation may be blocked by genetic or epigenetic alterations affecting the pathway leading to BCL-6 degradation. The observation that BCL-6 degradation can be triggered from the cell surface by activation of the antigen receptor has potential relevance for the therapy of B-cell lymphoma.
MAPK-mediated regulation of POZ/zinc finger transcription factors
MAPK is a ubiquitous, evolutionarily conserved signal transducer that is activated by heterogeneous signals that originate from the cell membrane and are transduced to MAPK via RAS proteins (Gold and Matsuuchi 1995; Alberola-Ila et al. 1997). Accordingly, POZ/zinc finger proteins represent a large family of highly conserved transcription factors including Drosophila cell fate regulators such as Tramtrack and Broad-complex, as well as human cancer-associated proteins such as BCL-6 and PLZF. These molecules have strong structural (POZ and ZF domains), as well as functional homologies being transcriptional repressors that control cell differentiation (Chen et al. 1994; Emery et al. 1994; Albagli et al. 1995). Most notably, POZ/zinc finger proteins also carry possible MAPK phosphorylation sites and PEST sequences in approximately the same position as those carried by BCL-6 (H. Niu et al., unpubl.). In Drosophila, degradation of TTK88, a POZ/zinc finger inhibitor of neural-cell differentiation, has been shown to be mediated by MAPK (Li et al. 1997; Tang et al. 1997). Thus, degradation of POZ/zinc finger transcription factors may represent a general mechanism by which the RAS/MAPK pathway controls cell function and differentiation.
Materials and methods
Reagents and plasmids
Goat anti-human IgM (μ-heavy chain specific) was obtained from Southern Biotechnology. Polyclonal anti-BCL-6 (N-70-6) antiserum was produced by using the amino-terminal peptides of BCL-6 (Cattoretti et al. 1995). Monoclonal mouse anti-ERK2 (C-14) was purchased from Santa Cruz Biothechnology (Santa Cruz, CA). Monoclonal mouse anti-ubiquitin was obtained from Zymed Laboratories (South San Francisco, CA). Monoclonal mouse anti-HA (12CA5) was purchased from Boehringer Mannheim, as was Calpain Inhibitor II. Protein A–Sepharose CL-4B and glutathione–Sepharose were purchased from Pharmacia. Myelin basic protein (MBP) and N-CBZ-Leu-Leu-Leu-AL (MG132) were obtained from Sigma. PD098059 was purchased from Calbiochem–Novabiochem (La Jolla, CA). The GST–BCL6, GST–BCL6ΔZF, GST–BCL6ZF, GST–BCL6ΔZFAla333, and GST–BCL6ΔZFAla333,343 fusion proteins were produced by pGEX-2TK-based plasmids (Pharmacia Biotech) containing full-length, deletion, or point mutants of bcl-6. The point mutations (Ala333, Ala343) were generated by PCR-based methods; the sequence of the resulting plasmids was confirmed by nucleotide sequence analysis. pMT2T–BCL-6 and B6BS–TK–LUC have been described as previous (Chang et al. 1996). pMT2T–BCL-6Ala333,343 was constructed by transferring the BclI–NcoI fragments of plasmid pGEX-2TK–BCL6ΔZFAla333,343 into the pMT2T–BCL-6 vector. MEK-2E-EE–CMV and MEK-EE–CMV for expressing of constitutively active or inactive MEK were provided by Dr. D. Templeton (Case Western Reserve University, Cleveland, OH). The pMT2T–HA–BCL-6, pMT2T–HA–BCL-6Δ (300–417), and pMT2T–HA–BCL–6ZF vectors were constructed by inserting the sequences encoding the HA epitope upstream and in frame with bcl-6 coding sequences. Deletion mutants of bcl-6 were produced by PCR-based methods and confirmed by sequencing. His6-ubiquitin–CMV was kindly provided by T. Maniatis (Harvard Medical School, Boston, MA). Episomally replicating plasmid pHeBo-MT, which carries EBV oriP, hygromycin B, and MT promoter efficiently yields hygromycin-resistant colonies.
ERK2 kinase assays
BCL-6 GST fusion proteins were purified using glutathione–Sepharose beads as suggested by the manufacturer (Pharmacia Biotech). Recombinant ERK2 (New England Biolabs) assays were performed as suggested by the manufacturer using purified wild-type and mutant GST fusion proteins as substrates. In solid-phase ERK2 kinase assays, cells were lysed in ice-cold lysis buffer (50 mm Tris at pH 7.5, 10% glycerol, 1% Triton X-100, 150 mm NaCl, 100 mm NaF, 5 μm ZnCl2, 1 mm Na3VO4, 10 mm EGTA, 2 mm PMSF, 1 μg/ml aprotinin, 1 μg/ml leupeptin, and 1 μg/ml pepstatin) and centrifuged at 100,000g for 15 min at 4°C. The supernatant (250–500 μg cellular protein) was then immunoprecipitated using anti-ERK2 antibodies (C-14) and protein A–Sepharose CL-4B. Beads were washed three times with lysis buffer and once with kinase buffer (50 mm Tris at pH 7.5, 10 mm MnCl2, 5 mm MgCl2). Reactions were initiated by adding 50 μl kinase buffer containing substrate MBP, 5 μm ATP, and 5 μCi [γ-32P]ATP. After 15 min at 37°C, reactions were terminated by adding 2× SDS-PAGE sample buffer. Samples were electrophoresed on 15% SDS–polyacrylamide gels which were then dried and analyzed by autoradiography.
Cell transfection
293T cells, grown in DMEM, 10% FBS, were transfected transiently with various DNA vectors using standard calcium phosphate precipitation methods. Ramos cells, grown in IMDM, 10% FBS, were transfected stably with the plasmid pHeBo–MT–HA–BCL-6, pHeBo–MT–HA–BCL-6Ala333,343 and the deletion-mutant construct pHeBo–MT–HA–BCL-6ZF by electroporation followed by selection in hygromycin B (400 μg/ml). HA–bcl-6 gene expression under control of the metallothionein (MT) promoter were induced by adding 1 μm of CdCl2.
Northern and Western blot analysis
Total RNA were isolated from cells by using Trizol-reagents (GIBCO-BRL) and equal amounts of RNA were separated on 1% formaldehyde-agarose gel. Northern blot analysis was performed by using standard methods with full-length bcl-6 cDNA as probes and normalized by GAPDH hybridization. Whole-cell lysates were prepared by lysing cells in RIPA buffer with 2 mm PMSF, 1 μg/ml aprotinin, 1 μg/ml of leupeptin, 1 μg/ml pepstatin, 1 mm Na3VO4, 5 mm NaF, and 10 mm β-glycerophosphate. For transient transfectants, protein amounts loaded on gel were normalized by transfection efficiency (β-gal activity). For Ramos cells and their stable transfectants (untreated or treated), equal amounts of protein were analyzed by 8% or 10% SDS-PAGE, and subsequently by Western blot analysis using anti-BCL-6 (N-70-6), anti-ERK2 (C-14), or anti-HA (12CA5) antibodies at 1:3000, 1:1000, or 1:500 dilutions. The results were visualized by ECL (Amersham).
In vivo ubiquitination assay
293T cells were transfected transiently with pMT2T–BCL-6, His6–ubiquitin–CMV, and MEK-2E–CMV vectors as indicated. MG132 (50 μm) was added 8 hr after transfection. The total amount of transfected DNA was kept constant in all experiments by adding empty vector. Twenty-four hours after transfection, cells were lysed in RIPA buffer with 10 mm N-ethylmaleimide and various protease inhibitors as described (Pagano et al. 1995). The cell lysates were then immunoprecipitated using anti-BCL-6 antibodies. The immunoprecipitates were loaded on 6% SDS-PAGE and processed for Western blot analysis using the anti-ubiquitin antibodies (Zymed) at 1:1000 dilution as described (Avantaggiati et al. 1996).
Pulse-chase labeling experiment
Ramos cells (12 × 107) were collected by centrifugation, washed in PBS, resuspended in 100 ml of DMEM without methionine and cysteine (GIBCO-BRL), and starved for 60 min. [35S]methionine and [35S]cysteine (3 mCi; ICN) were added and pulse-labeled for 60 min and then treated with anti-IgM for 30 min. Cold methionine and cysteine were then added to final concentrations of 150 μg/ml. Cells were collected and lysed in RIPA buffer with proteinase and phosphatase inhibitors. The cell extracts, adjusted for equal cpm, were immunoprecipitated with anti-BCL-6 antibodies, and analyzed by SDS-PAGE followed by autoradiography.
Acknowledgments
We thank D. Templeton for a gift of the MEK-2E-EE-CMV and MEK-EE–CMV plasmids; T.K. Kim, and T. Maniatis for the His6-ubiquitin–CMV plasmid; S.W. Rogers, P. Zhang, and L. Liao for help with the use of the PEST-FIND program; and S. Chellapan for helpful discussions. This work was supported by National Institutes of Health grant CA-37295.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL RD10@columbia.edu; FAX (212) 305-5498.
References
- Albagli O, Dhordain P, Deweindt C, Lecocq G, Leprince D. The BTB/POZ domain: A new protein-protein interaction motif common to DNA- and actin-binding proteins. Cell Growth & Differ. 1995;6:1193–1198. [PubMed] [Google Scholar]
- Alberola-Ila J, Takaki S, Kerner JD, Perlmutter RM. Differential signaling by lymphocyte antigen receptors. Annu Rev Immunol. 1997;15:125–154. doi: 10.1146/annurev.immunol.15.1.125. [DOI] [PubMed] [Google Scholar]
- Allman D, Jain A, Dent A, Maile RR, Selvaggi T, Kehry MR, Staudt LM. BCL-6 expression during B-cell activation. Blood. 1996;87:5257–5268. [PubMed] [Google Scholar]
- Avantaggiati M, Carbone M, Graessman A, Nakatani Y, Howard B, Levine A. The SV40 large T antigen and adenovirus E1A oncoproteins interact with distinct isoforms of the transcriptional co-activator p300. EMBO J. 1996;15:2236–2248. [PMC free article] [PubMed] [Google Scholar]
- Bardwell VJ, Treisman R. The POZ domain: A conserved protein–protein interaction motif. Genes & Dev. 1994;8:1664–1677. doi: 10.1101/gad.8.14.1664. [DOI] [PubMed] [Google Scholar]
- Baron BW, Nucifora G, McCabe N, Espinosa R, Le BM, McKeithan TW. Identification of the gene associated with the recurring chromosomal translocations t(3;14)(q27;q32) and t(3;22)(q27;q11) in B-cell lymphomas. Proc Natl Acad Sci. 1993;90:5262–5266. doi: 10.1073/pnas.90.11.5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard C, Deweindt C, Kerckaert JP, Lenormand B, Rossi A, Pezzella F, Fruchart C, Duval C, Monconduit M, Tilly H. LAZ3 rearrangements in non-Hodgkin’s lymphoma: Correlation with histology, immunophenotype, karyotype, and clinical outcome in 217 patients. Blood. 1994;83:2423–2427. [PubMed] [Google Scholar]
- Cambier JC. Positive and negative signal co-operativity in the immune system: The BCR, Fc gamma RIIB, CR2 paradigm. Biochem Soc Trans. 1997;25:441–445. doi: 10.1042/bst0250441. [DOI] [PubMed] [Google Scholar]
- Cattoretti G, Chang CC, Cechova K, Zhang J, Ye BH, Falini B, Louie DC, Offit K, Chaganti RS, Dalla-Favera R. BCL-6 protein is expressed in germinal-center B cells. Blood. 1995;86:45–53. [PubMed] [Google Scholar]
- Cattoretti G, Zhang J, Cleary AM, Lederman S, Gaidano G, Carbone A, Chaganti RSK, Dalla-Favera R. Downregulation of BCL-6 gene expression by CD40 and EBV latent membrane protein-1 (LMP1) and its block in lymphoma carrying BCL-6 rearrangements. (Suppl. I) Blood. 1997;90:p175a. [Google Scholar]
- Chang CC, Ye BH, Chaganti RS, Dalla-Favera R. BCL-6, a POZ/zinc-finger protein, is a sequence-specific transcriptional repressor. Proc Natl Acad Sci. 1996;93:6947–6952. doi: 10.1073/pnas.93.14.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chardin P, Courtois G, Mattei MG, Gisselbrecht S. The KUP gene, located on human chromosome 14, encodes a protein with two distant zinc fingers. Nucleic Acids Res. 1991;19:1431–1436. doi: 10.1093/nar/19.7.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Brand NJ, Chen A, Chen SJ, Tong JH, Wang ZY, Waxman S, Zelent A. Fusion between a novel Kruppel-like zinc finger gene and the retinoic acid receptor-alpha locus due to a variant t(11;17) translocation associated with acute promyelocytic leukaemia. EMBO J. 1993;12:1161–1167. doi: 10.1002/j.1460-2075.1993.tb05757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Guidez F, Rousselot P, Agadir A, Chen SJ, Wang ZY, Degos L, Zelent A, Waxman S, Chomienne C. PLZF-RAR alpha fusion proteins generated from the variant t(11;17)(q23;q21) translocation in acute promyelocytic leukemia inhibit ligand-dependent transactivation of wild-type retinoic acid receptors. Proc Natl Acad Sci. 1994;91:1178–1182. doi: 10.1073/pnas.91.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Iida S, Louie DC, Dalla-Favera R, Chaganti RSK. Heterologous promoters fused to BCL-6 by chromosomal translocations affecting band 3q27 cause its deregulated expression during B-cell differentiation. Blood. 1998;91:603–607. [PubMed] [Google Scholar]
- Dalla-Favera R, Ye BH, Cattoretti G, Lo Coco F, Chang CC, Zhang J, Migliazza A, Cechova K, Niu H, Chaganti S, Chen W, Louie DC, Offit K, Chaganti RS. BCL-6 in diffuse large-cell lymphomas. In: DeVita VT, Hellman S, Rosenberg SA, editors. Important advances in oncology. Philadelphia, PA: Lippincott–Raven Publishers; 1996. pp. 139–148. [PubMed] [Google Scholar]
- Dent AL, Shaffer AL, Yu X, Allman D, Staudt LM. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science. 1997;276:589–592. doi: 10.1126/science.276.5312.589. [DOI] [PubMed] [Google Scholar]
- Deweindt C, Albagli O, Bernardin F, Dhordain P, Quief S, Lantoine D, Kerckaert JP, Leprince D. The LAZ3/BCL6 oncogene encodes a sequence-specific transcriptional inhibitor: A novel function for the BTB/POZ domain as an autonomous repressing domain. Cell Growth & Differ. 1995;6:1495–1503. [PubMed] [Google Scholar]
- DiBello PR, Withers DA, Bayer CA, Fristrom JW, Guild GM. The Drosophila Broad-Complex encodes a family of related proteins containing zinc fingers. Genetics. 1991;129:385–397. doi: 10.1093/genetics/129.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery IF, Bedian V, Guild GM. Differential expression of Broad-Complex transcription factors may forecast tissue-specific developmental fates during Drosophila metamorphosis. Development. 1994;120:3275–3287. doi: 10.1242/dev.120.11.3275. [DOI] [PubMed] [Google Scholar]
- Fukada T, Hibi M, Yamanaka Y, Takahashi-Tezuka M, Fujitani Y, Yamaguchi T, Nakajima K, Hirano T. Two signals are necessary for cell proliferation induced by a cytokine receptor gp130: Involvement of STAT3 in anti-apoptosis. Immunity. 1996;5:449–460. doi: 10.1016/s1074-7613(00)80501-4. [DOI] [PubMed] [Google Scholar]
- Gaidano G, Carbone A, Pastore C, Capello D, Migliazza A, Gloghini A, Roncella S, Ferrarini M, Saglio G, Dalla-Favera R. Frequent mutation of the 5′ noncoding region of the BCL-6 gene in acquired immunodeficiency syndrome-related non-Hodgkin’s lymphomas. Blood. 1997;89:3755–3762. [PubMed] [Google Scholar]
- Gold MR, Matsuuchi L. Signal transduction by the antigen receptors of B and T lymphocytes. Int Rev Cytol. 1995;157:181–276. doi: 10.1016/s0074-7696(08)62159-2. [DOI] [PubMed] [Google Scholar]
- Gold MR, Sanghera JS, Stewart J, Pelech SL. Selective activation of p42 mitogen-activated protein (MAP) kinase in murine B lymphoma cell lines by membrane immunoglobulin cross-linking. Evidence for protein kinase C-independent and -dependent mechanisms of activation. Biochem J. 1992;287:269–276. doi: 10.1042/bj2870269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SD, Travers AA. The Tramtrack gene encodes a Drosophila finger protein that interacts with the ftz transcriptional regulatory region and shows a novel embryonic expression pattern. EMBO J. 1990;9:207–216. doi: 10.1002/j.1460-2075.1990.tb08097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstrasser M. Ubiquitin-dependent protein degradation. Annu Rev Genet. 1996;30:405–439. doi: 10.1146/annurev.genet.30.1.405. [DOI] [PubMed] [Google Scholar]
- Kerckaert JP, Deweindt C, Tilly H, Quief S, Lecocq G, Bastard C. LAZ3, a novel zinc-finger encoding gene, is disrupted by recurring chromosome 3q27 translocations in human lymphomas. Nature Genet. 1993;5:66–70. doi: 10.1038/ng0993-66. [DOI] [PubMed] [Google Scholar]
- Kim TK, Maniatis T. Regulation of interferon-gamma-activated STAT1 by the ubiquitin-proteasome pathway. Science. 1996;273:1717–1719. doi: 10.1126/science.273.5282.1717. [DOI] [PubMed] [Google Scholar]
- Koonin EV, Senkevich TG, Chernos VI. A family of DNA virus genes that consists of fused portions of unrelated cellular genes. Trends Biochem Sci. 1992;17:213–214. doi: 10.1016/0968-0004(92)90379-n. [DOI] [PubMed] [Google Scholar]
- Li S, Li Y, Carthew RW, Lai Z-C. Photoreceptor cell differentiation requires regulated proteolysis of the transcriptional repressor Tramtrack. Cell. 1997;90:469–478. doi: 10.1016/s0092-8674(00)80507-3. [DOI] [PubMed] [Google Scholar]
- LoCoco CF, Ye BH, Lista F, Corradini P, Offit K, Knowles DM, Chaganti RS, Dalla-Favera R. Rearrangements of the BCL6 gene in diffuse large cell non-Hodgkin’s lymphoma. Blood. 1994;83:1757–1759. [PubMed] [Google Scholar]
- MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- Migliazza A, Martinotti S, Chen W, Fusco C, Ye BH, Knowles DM, Offit K, Chaganti RS, Dalla-Favera R. Frequent somatic hypermutation of the 5′ noncoding region of the BCL6 gene in B-cell lymphoma. Proc Natl Acad Sci. 1995;92:12520–12524. doi: 10.1073/pnas.92.26.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T, Kawamata N, Hirosawa S, Aoki N. Gene involved in the 3q27 translocation associated with B-cell lymphoma, BCL5, encodes a Kruppel-like zinc-finger protein. Blood. 1994;83:26–32. [PubMed] [Google Scholar]
- Minami Y, Oishi I, Liu ZJ, Nakagawa S, Miyazaki T, Taniguchi T. Signal transduction mediated by the reconstituted IL-2 receptor. Evidence for a cell type-specific function of IL-2 receptor beta-chain. J Immunol. 1994;152:5680–5690. [PubMed] [Google Scholar]
- Moriyama M, Yamochi T, Semba K, Akiyama T, Mori S. BCL-6 is phosphorylated at multiple sites in its serine- and proline-clustered region by mitogen-activated protein kinase (MAPK) in vivo. Oncogene. 1997;14:2465–2474. doi: 10.1038/sj.onc.1201084. [DOI] [PubMed] [Google Scholar]
- Onizuka T, Moriyama M, Yamochi T, Kuroda T, Kazama A, Kanazawa N, Sato K, Kato T, Ota H, Mori S. BCL-6 gene product, a 92- to 98-kD nuclear phosphoprotein, is highly expressed in germinal center B cells and their neoplastic counterparts. Blood. 1995;86:28–37. [PubMed] [Google Scholar]
- Otsuki T, Yano T, Clark HM, Bastard C, Kerckaert JP, Jaffe ES, Raffeld M. Analysis of LAZ3 (BCL-6) status in B-cell non-Hodgkin’s lymphomas: Results of rearrangement and gene expression studies and a mutational analysis of coding region sequences. Blood. 1995;85:2877–2884. [PubMed] [Google Scholar]
- Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, Chau V, Yew PR, Draetta GF, Rolfe M. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- Palombella VJ, Rando OJ, Goldberg AL, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell. 1994;78:773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- Pang L, Sawada T, Decker SJ, Saltiel AR. Inhibition of MAP kinase kinase blocks the differentiation of PC-12 cells induced by nerve growth factor. J Biol Chem. 1995;270:13585–13588. doi: 10.1074/jbc.270.23.13585. [DOI] [PubMed] [Google Scholar]
- Rechsteiner M, Rogers SW. PEST sequences and regulation by proteolysis. Trends Biochem Sci. 1996;21:267–271. [PubMed] [Google Scholar]
- Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg AL. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- Rogers S, Wells R, Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: The PEST hypothesis. Science. 1986;234:364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Sakata N, Patel HR, Terada N, Aruffo A, Johnson GL, Gelfand EW. Selective activation of c-Jun kinase mitogen-activated protein kinase by CD40 on human B cells. J Biol Chem. 1995;270:30823–30828. doi: 10.1074/jbc.270.51.30823. [DOI] [PubMed] [Google Scholar]
- Seyfert VL, Allman D, He Y, Staudt LM. Transcriptional repression by the proto-oncogene BCL-6. Oncogene. 1996;12:2331–2342. [PubMed] [Google Scholar]
- Sutherland CL, Heath AW, Pelech SL, Young PR, Gold MR. Differential activation of the ERK, JNK, and p38 mitogen-activated protein kinases by CD40 and the B cell antigen receptor. J Immunol. 1996;157:3381–3390. [PubMed] [Google Scholar]
- Tang AH, Neufeld TP, Kwan E, Rubin M. PHYL acts to down-regulate TTK88, a transcriptional repressor of neuronal cell fates, by a SINA-dependent mechanism. Cell. 1997;90:459–467. doi: 10.1016/s0092-8674(00)80506-1. [DOI] [PubMed] [Google Scholar]
- Tedder TF, Tuscano J, Sato S, Kehrl JH. CD22, a B lymphocyte-specific adhesion molecule that regulates antigen receptor signaling. Annu Rev Immunol. 1997;15:481–504. doi: 10.1146/annurev.immunol.15.1.481. [DOI] [PubMed] [Google Scholar]
- Vietor I, Schwenger P, Li W, Schlessinger J, Vilcek J. Tumor necrosis factor-induced activation and increased tyrosine phosphorylation of mitogen-activated protein (MAP) kinase in human fibroblasts. J Biol Chem. 1993;268:18994–18999. [PubMed] [Google Scholar]
- Xue F, Cooley L. Kelch encodes a component of intercellular bridges in Drosophila egg chamber. Cell. 1993;72:681–693. doi: 10.1016/0092-8674(93)90397-9. [DOI] [PubMed] [Google Scholar]
- Yan M, Templeton DJ. Identification of 2 serine residues of MEK-1 that are differentially phosphorylated during activation by raf and MEK kinase. J Biol Chem. 1994;269:19067–19073. [PubMed] [Google Scholar]
- Ye BH, Cattoretti G, Shen Q, Zhang J, Hawe N, Waard R, Leung C, Nouri-Shirazi M, Orazi A, Chaganti RSK, Rothman P, Stall AM, Pandolfi PP, Dalla-Favera R. The BCL-6 proto-oncogene controls germinal-centre formation and Th2-type inflammation. Nature Genet. 1997;16:161–170. doi: 10.1038/ng0697-161. [DOI] [PubMed] [Google Scholar]
- Ye BH, Chaganti S, Chang CC, Niu H, Corradini P, Chaganti RS, Dalla-Favera R. Chromosomal translocations cause deregulated BCL6 expression by promoter substitution in B cell lymphoma. EMBO J. 1995;14:6209–6217. doi: 10.1002/j.1460-2075.1995.tb00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye BH, Lista F, Lo Coco F, Knowles DM, Offit K, Chaganti RS, Dalla-Favera R. Alterations of a zinc finger-encoding gene, BCL-6, in diffuse large-cell lymphoma. Science. 1993;262:747–750. doi: 10.1126/science.8235596. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Carroll M, Papa FR, Hochstrasser M, D’Andrea AD. DUB-1, a deubiquitinating enzyme with growth-suppressing activity. Proc Natl Acad Sci. 1996;93:3275–3279. doi: 10.1073/pnas.93.8.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]