Abstract
The activation of maturation-promoting factor (MPF) is required for G2/M progression in eukaryotic cells. Xenopus oocytes are arrested in G2 and are induced to enter M phase of meiosis by progesterone stimulation. This process is known as meiotic maturation and requires the translation of specific maternal mRNAs stored in the oocytes. We have used an expression cloning strategy to functionally identify proteins involved in G2/M progression in Xenopus oocytes. Here we report the cloning of two novel cDNAs that when expressed in oocytes induce meiotic maturation efficiently. The two cDNAs encode proteins of 33 kD that are 88% identical and have no significant homologies to other sequences in databases. These proteins, which we refer to as p33ringo (rapid inducer of G2/M progression in oocytes), induce very rapid MPF activation in cycloheximide-treated oocytes. Conversely, ablation of endogenous p33ringo mRNAs using antisense oligonucleotides inhibits progesterone-induced maturation, suggesting that synthesis of p33ringo is required for this process. We also show that p33ringo binds to and activates the kinase activity of p34cdc2 but does not associate with p34cdc2/cyclin B complexes. Our results identify a novel p34cdc2 binding and activating protein that regulates the G2/M transition during oocyte maturation.
Keywords: Cell cycle, meiotic maturation, MPF, M phase, oocyte, Cdc2
Entry of eukaryotic cells into M phase of the cell cycle is regulated by maturation-promoting factor (MPF), an activity composed of a B-type cyclin and the protein kinase p34cdc2. Cyclin B is usually synthesized and associates with p34cdc2 throughout late S phase and early G2, but the p34cdc2/cyclin B complex is maintained inactive by the phosphorylation of p34cdc2 on Thr-14 and Tyr-15. Dephosphorylation of p34cdc2 leads to activation of the MPF kinase activity, which can phosphorylate many proteins responsible for both the G2/M transition and progression through M phase (for review, see Nurse 1990; Coleman and Dunphy 1994; Morgan 1997).
Xenopus oocytes are naturally arrested in late G2 and are induced to enter into M phase of meiosis by progesterone. This process is known as meiotic maturation and is associated with the activation of MPF (Masui and Clarke 1979). In G2-arrested Xenopus oocytes there is a preformed stock of p34cdc2/cyclin B complexes (pre-MPF) that is maintained inactive by the phosphorylation of p34cdc2 on Thr-14 and Tyr-15 (Cyert and Kirschner 1988; Gautier and Maller 1991; Kobayashi et al. 1991b). The inhibitory phosphorylation of p34cdc2 in immature oocytes is likely to be due to the membrane-bound protein kinase Myt1 (Atherton-Fessler et al. 1994; Kornbluth et al. 1994; Mueller et al. 1995; Palmer et al. 1998), whereas the activating dephosphorylation probably involves the phosphatase Cdc25 (Dunphy and Kumagai 1991; Gautier et al. 1991; Kumagai and Dunphy 1991; Strausfeld et al. 1991). Thus, either an increased activity of Cdc25 or the inhibition of Myt1 may bring about the activation of pre-MPF during oocyte maturation.
An essential requirement for progesterone-induced maturation is the translation of maternal mRNAs stored in the oocyte. Although several mRNAs are known to be translated de novo during oocyte maturation (Sagata et al. 1988; Kobayashi et al. 1991b; Gabrielli et al. 1992; Rempel et al. 1995; Murakami and Vande Woude 1998), only the mRNA encoding the protein kinase p39mos has been found so far to be necessary for oocyte maturation (Sagata et al. 1988, 1989; Freeman et al. 1990; Sheets et al. 1995). Injection of either p39mos mRNA or recombinant p39mos protein into Xenopus oocytes induces maturation in the absence of progesterone (Sagata et al. 1988, 1989; Yew et al. 1992). Conversely, injection of p39mos antisense oligonucleotides blocks progesterone-induced MPF activation indicating that p39mos is necessary for the initiation of maturation (Sagata et al. 1988).
The function of p39mos in oocyte maturation is most likely related to its ability to activate the p42mpk1 MAP kinase pathway (Nebreda et al. 1993; Nebreda and Hunt 1993; Posada et al. 1993; Shibuya and Ruderman 1993). This is supported by the injection of neutralizing anti-MAP kinase kinase antibodies that inhibit p39mos-induced oocyte maturation (Kosako et al. 1994, 1996) and is also consistent with the observation that upon ectopic p39mos expression, activation of p42mpk1 always precedes the activation of pre-MPF (Nebreda and Hunt 1993; Posada et al. 1993; Shibuya and Ruderman 1993). The idea that activation of p42mpk1 plays an important role in meiotic maturation is further supported by the delay in progesterone-induced maturation observed upon injection of either anti-MAP kinase kinase antibodies (Kosako et al. 1994, 1996) or a specific MAP kinase phosphatase (Gotoh et al. 1995). Conversely, microinjection of constitutively active MAP kinase kinase (Huang et al. 1995) or active thiophosphorylated p42mpk1 (Haccard et al. 1995) can induce oocyte maturation in the absence of progesterone. Taken together, the results suggest that activation of the p42mpk1 cascade may be both necessary and sufficient to release the oocyte from the G2-phase arrest. A possible connection between the activation of p42mpk1 and MPF during oocyte maturation may be provided by the p42mpk1-activated protein kinase p90rsk, which can phosphorylate and down-regulate Myt1 (Palmer et al. 1998).
In spite of the central role of p39mos and the p42mpk1 pathway in oocyte maturation, there is evidence that synthesis of other proteins in addition to p39mos is also required for progesterone to initiate oocyte maturation (Nebreda et al. 1995; Barkoff et al. 1998). Thus, in many batches of oocytes, the ability of p39mos or a constitutively active MAP kinase kinase mutant to activate pre-MPF is significantly reduced when protein synthesis is inhibited, whereas p42mpk1 is normally activated under these conditions (Yew et al. 1992; Daar et al. 1993; Nebreda and Hunt 1993; Shibuya and Ruderman 1993; Huang et al. 1995; Murakami and Vande Woude 1997). These data indicate that p42mpk1 activation alone is not always sufficient to activate pre-MPF. In contrast, the ability of recombinant cyclin A to activate pre-MPF is the same when injected into either untreated or cycloheximide-treated oocytes (Nebreda et al. 1995). Furthermore, overexpression of a dominant-negative p34cdc2 mutant or injection of a neutralizing anti-p34cdc2 antibody blocks progesterone-induced p39mos accumulation and the activation of both p42mpk1 and MPF (Nebreda et al. 1995). These results were taken to suggest that activation of the free p34cdc2 present in the oocyte (which is in a notable excess over the p34cdc2 complexed with cyclin B) is normally required for progesterone-induced maturation. However, there is no evidence that newly synthesized A- and B-type cyclins, which would be the obvious candidates to activate free p34cdc2, are required for progesterone-induced MPF activation (Minshull et al. 1991).
In this paper we have used an expression cloning strategy to identify novel proteins that can trigger oocyte maturation. We have cloned two cDNAs that upon expression in oocytes (either as synthetic mRNAs or recombinant proteins), can potently induce MPF activation and germinal vesicle breakdown (GVBD) in the absence of progesterone. Moreover, antisense-directed ablation of the endogenous mRNAs in the oocyte inhibits progesterone-induced maturation. These cDNAs code for closely related proteins that can bind to and activate the protein kinase p34cdc2. Our results indicate that this novel p34cdc2 binding and activating protein plays an important role in oocyte maturation.
Results
Cloning of two novel cDNAs that potently induce G2/M progression in Xenopus oocytes
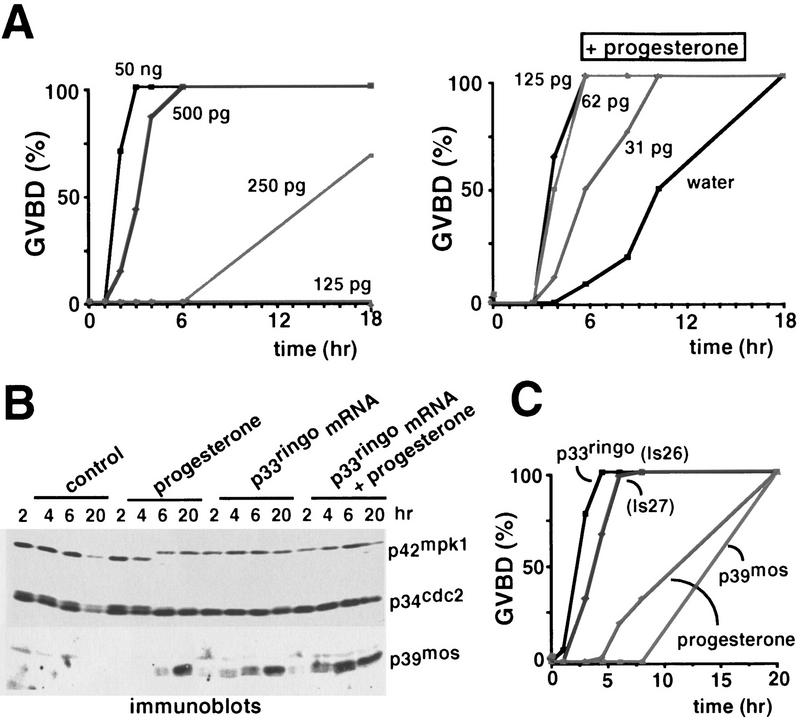
To identify novel proteins involved in G2/M progression during the meiotic maturation of Xenopus oocytes, we used an expression cloning strategy in which we constructed a Xenopus oocyte cDNA library in the FTX5 expression vector. The primary library was subdivided into pools of 150–200 colonies and plasmid DNA was purified from the pools and in vitro transcribed to obtain mRNAs. The mRNA pools that upon microinjection into oocytes were able either to induce oocyte maturation on their own or to accelerate progesterone-induced maturation were subdivided into smaller pools and reinjected until single positive clones were isolated. Using this approach, we isolated two clones named ls26 and ls27, which by DNA hybridization experiments did not correspond to proteins that are known to induce oocyte maturation including p39mos, Cdc25, and several A- and B-type cyclins (data not shown). The mRNAs prepared from the ls26 and ls27 clones were able to induce oocyte maturation in the absence of progesterone stimulation, even at concentrations as low as 250 pg per oocyte (Fig. 1A, left), as well as to significantly accelerate progesterone-induced maturation at a 10-fold lower concentration (Fig. 1A, right). Oocyte maturation induced by expression of ls26 or ls27 was accompanied by the appearance of a white spot at the animal pole of the oocyte as in progesterone-matured oocytes (Fig. 2), although a few hours later ls26/ls27-injected oocytes usually appeared ‘overmatured’. This overmaturation is sometimes similarly observed upon injection of high concentrations of cyclin A or p39mos (not shown). As in progesterone-treated oocytes, the maturation induced by ls26 or ls27 correlated with the activation of both p42mpk1 MAP kinase (p42mpk1 is phosphorylated resulting in an upward shift in immunoblots) and p34cdc2/cyclin B (pre-MPF, p34cdc2 is dephosphorylated resulting in a downward shift in immunoblots) as well as with the appearance of p39mos (Fig. 1B). We also found that ls26 and ls27 were able to induce oocyte maturation faster than p39mos, based on experiments where all mRNAs were prepared from the same expression vector and injected into oocytes at equivalent concentrations (Fig. 1C).
Figure 1.
Induction of Xenopus oocyte maturation by a novel protein. (A) The indicated concentrations (total amount per oocyte) of mRNA transcribed in vitro from the ls26 cDNA were injected into oocytes and GVBD was scored (left). Progesterone was added to the groups of injected oocytes that showed no white spot after 18 hr of incubation (right). Similar results were observed with mRNA prepared from the ls27 cDNA (not shown). (B) Immunoblot analysis using anti-p42mpk1, anti-p34cdc2, and anti-p39mos antibodies of lysates prepared from oocytes (four oocytes per time point) either untreated (control), treated with progesterone (5 μg/ml), injected with in vitro-transcribed mRNA encoding for p33ringo (ls26, 50 ng/oocyte) or both injected with mRNA encoding for p33ringo and treated with progesterone. In this experiment 50% GVBD was observed at 10, 3.5, and 1.5 hr with progesterone, p33ringo, and p33ringo + progesterone, respectively. (C) Oocytes were either injected with in vitro-transcribed mRNAs (500 pg/oocyte) encoding Myc-tagged forms of the two isoforms of p33ringo (ls26 and ls27) and p39mos or treated with progesterone (5 μg/ml) and then GVBD was scored.
Figure 2.
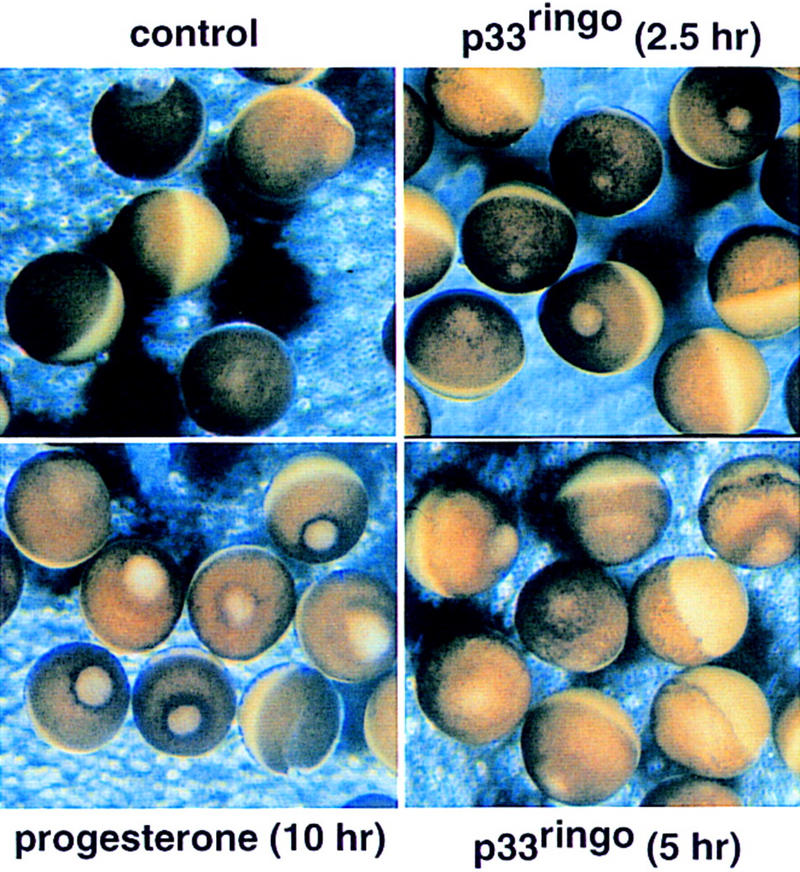
Morphological appearance of p33ringo mRNA-injected oocytes (50 ng/oocyte) after 2.5 and 5 hr of incubation. Oocytes treated with progesterone (5 μg/ml) for 10 hr and control (water-injected) oocytes are shown.
DNA sequencing of the clones isolated from the expression library showed that the ls26 and ls27 open reading frames (ORFs) encoded related proteins that were fused in-frame to the carboxyl terminus of the myc tag in the FTX5 vector. Using the partial cDNAs as probes, we cloned full-length cDNAs from a λ ZAP Xenopus oocyte cDNA library. The ls26 cDNA was 1574 bp long and encoded a protein of 300 amino acids, whereas ls27 was 1357 bp in length and encoded a protein of 298 amino acids. Both clones contained stop codons upstream of the first ATG and in the same frame. The predicted ls26 and ls27 proteins were 88% identical (Fig. 3). The cDNAs isolated from the oocyte expression screening encoded Myc-tagged proteins that either lacked the first 11 amino acids (ls26) or had an amino-terminal extension of 24 amino acids corresponding to the 5′-untranslated region (ls27). In a search of the ls26 and ls27 sequences using BLAST against DNA and protein sequence databases, we could detect no significant homologies, suggesting that ls26/ls27 belonged to a novel protein family. We were also unable to identify conserved protein motifs using PROSITE and GENEQUIZ programs. The only potentially relevant homologies were obtained from expressed sequence-tag (EST) databases. The best score in these searches was for the human EST clone 757814, which was 53% identical over a 49-amino-acid stretch. In contrast, no homologs were detected in the budding yeast genome. The close similarity between the sequences of ls26 and ls27 suggests that they might correspond to pseudoalloploid alleles; because they induced oocyte maturation with the same efficiency, we concentrated on ls26 for further characterization. We named this protein p33ringo for rapid inducer of G2/M progression in oocytes.
Figure 3.
Amino acid sequence comparison of the ls26 and ls27 p33ringo clones. Identity is indicated by a vertical bar.
Recombinant p33ringo can trigger GVBD and MPF activation in cycloheximide-treated oocytes
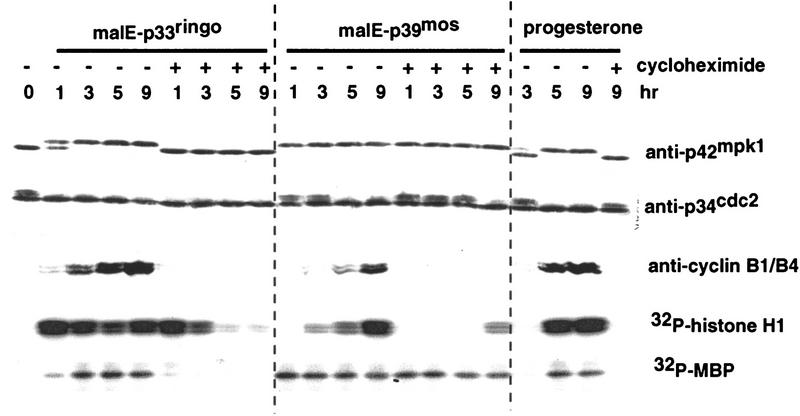
To further characterize the ability of p33ringo to induce oocyte maturation, we prepared a maltose-binding protein (malE)–p33ringo fusion protein. We found that the injection of 40 ng of bacterially produced malE–p33ringo was able to induce oocyte maturation considerably faster than progesterone treatment; 50% GVBD usually took ∼2.5 hr with malE–p33ringo versus 5–11 hr with progesterone. Moreover, injection of only 10 ng of malE–p33ringo per oocyte was still able to induce maturation in 100% of the injected oocytes (see Fig. 6a, below). We also tested whether malE–p33ringo was able to induce oocyte maturation in the presence of protein synthesis inhibitors. Preincubation of the oocytes with cycloheximide (10 μg/ml) is known to block progesterone-induced maturation, consistent with the known essential requirement for de novo translation of maternal mRNAs. However, we found that cycloheximide had no effect on the ability of malE–p33ringo to induce MPF activation and GVBD (Fig. 4).
Figure 6.
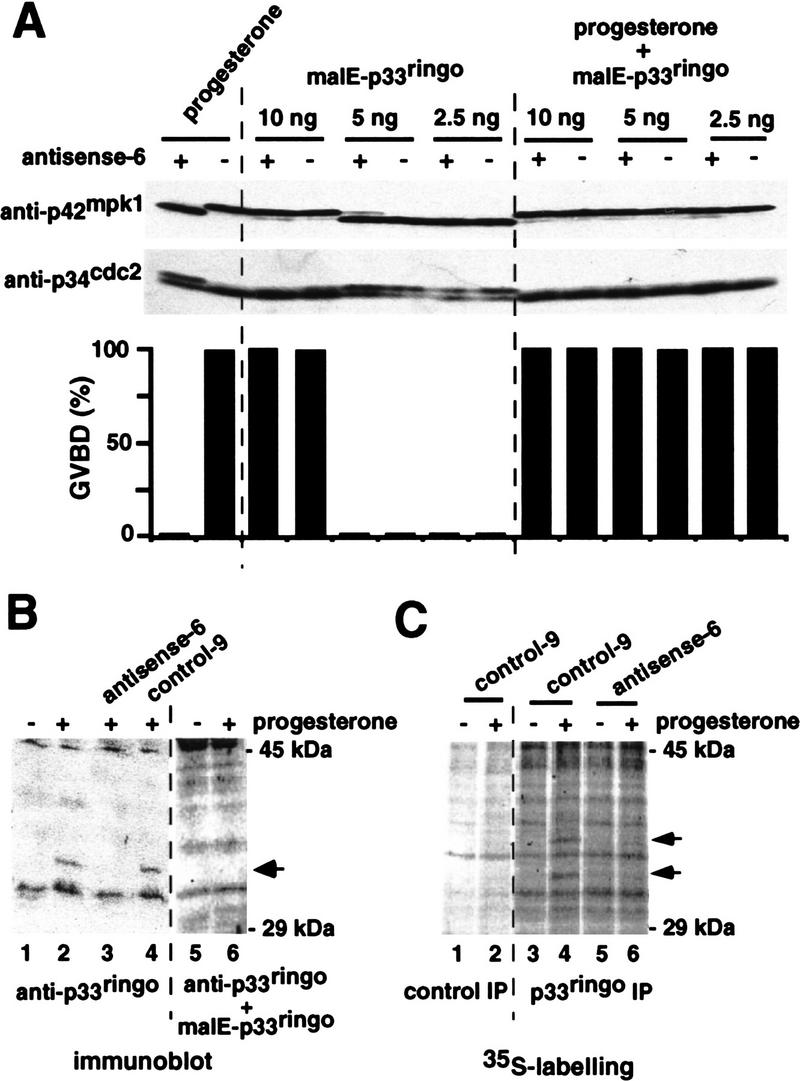
Requirement for endogenous p33ringo during progesterone-induced oocyte maturation. (A) Oocytes were injected with 100 ng of either antisense-6 or control-9 oligonucleotides (indicated by + and −, respectively) and incubated for 6 hr prior to the injection of the indicated amounts of malE–p33ringo and/or progesterone stimulation. After 8 hr, GVBD was scored and groups of five oocytes were taken, lysed, and analyzed by immunoblotting with anti-p34cdc2 and anti-p42mpk1 antibodies. (B) Oocytes were either noninjected or injected with 100 ng of the indicated oligonucleotides and incubated for 6 hr prior to progesterone stimulation. After 12 hr, lysates were prepared from groups of five oocytes and analyzed by immunoblotting using affinity-purified anti-p33ringo antibodies. In lanes 5 and 6, the antibodies were preincubated with malE–p33ringo protein before immunoblotting. The arrow indicates one band that cross-reacts with anti-p33ringo antibodies in mature oocytes. (C) Groups of 25 oocytes were injected with the indicated oligonucleotides and incubated with [35S]methionine in the presence or absence of progesterone. After 14 hr, oocytes were lysed and immunoprecipitated with either preimmune (lanes 1,2) or anti-p33ringo antiserum (lanes 3–6). The arrows indicate two proteins that are immunoprecipitated by anti-p33ringo antibodies in mature oocytes.
Figure 4.
MPF activation by recombinant malE–p33ringo in cycloheximide-treated oocytes. Oocytes were injected with malE–p33ringo (40 ng) or malE–p39mos (40 ng), or treated with progesterone (5 μg/ml) in the presence or absence of 10 μg/ml cycloheximide, as indicated. Cycloheximide was added 30 min prior to the injection or progesterone treatment and maintained during the subsequent incubation. At the indicated times, groups of five oocytes were collected, lysed, and analyzed both by in vitro kinase assay using as a substrate either histone H1 or MBP and by immunoblotting with anti-p42mpk1, anti-p34cdc2, and anti-cyclins B1 and B4 antibodies.
We investigated the kinetics of activation of p42mpk1 and p34cdc2/cyclin B in oocytes induced to mature by malE–p33ringo. Consistent with previous work, progesterone treatment activated both p42mpk1 and pre-MPF at the same time, whereas malE–p39mos injection activated p42mpk1 before pre-MPF (Fig. 4). In contrast, injection of malE–p33ringo rapidly activated histone H1 kinase (which correlated with Tyr dephosphorylation of p34cdc2) somewhat before myelin basic protein (MBP) kinase activity and p42mpk1 phosphorylation, indicating that p34cdc2/cyclin B preceded p42mpk1 activation. Interestingly, the ability of malE–p33ringo to activate p34cdc2/cyclin B, as determined by both increased histone H1 kinase activity and disappearance of the band with reduced electrophoretic mobility in p34cdc2 immunoblots, was unaffected in the presence of cycloheximide (Fig. 4). In these oocytes, however, the kinase activity on histone H1 was not stable and decreased after GVBD (Fig. 4) suggesting that p34cdc2/cyclin B might be transiently activated by p33ringo in cycloheximide-treated oocytes. On the other hand, oocytes injected with malE–p33ringo in the presence of cycloheximide had no detectable MBP kinase activity and most of their p42mpk1 was unphosphorylated, suggesting that activation of p42mpk1 was very much reduced (Fig. 4). This observation suggests that the activation of p42mpk1 by p33ringo may be the consequence of positive feedback loops that are blocked by cycloheximide. Thus, the function of p33ringo is more likely to be related to the activation of p34cdc2 rather than of p42mpk1. We also found that as in the case of progesterone and p39mos, oocyte maturation induced by p33ringo involves de novo synthesis of cyclins B1 and B4 (Fig. 4).
p33ringo is required for progesterone-induced oocyte maturation
To evaluate the importance of p33ringo for oocyte maturation we used antisense oligonucleotides. We first tested the oligonucleotides in an in vitro system and found that some antisense but not the control oligonucleotides could efficiently target the two isolated p33ringo clones (Fig. 5A, ls26 and ls27). The p33ringo antisense oligonucleotides, however, did not trigger degradation of the synthetic p39mos mRNA under the same conditions (Fig. 5A, p39mos). Next, we injected the p33ringo antisense oligonucleotides into oocytes and found that they were able to strongly delay progesterone-induced oocyte maturation, whereas injection of control oligonucleotides had no inhibitory effect (Fig. 5B). By Northern blot we found that the p33ringo antisense oligonucleotides were able to ablate the endogenous p33ringo mRNA(s) but not the endogenous p39mos mRNA (Fig. 5C).
Figure 5.
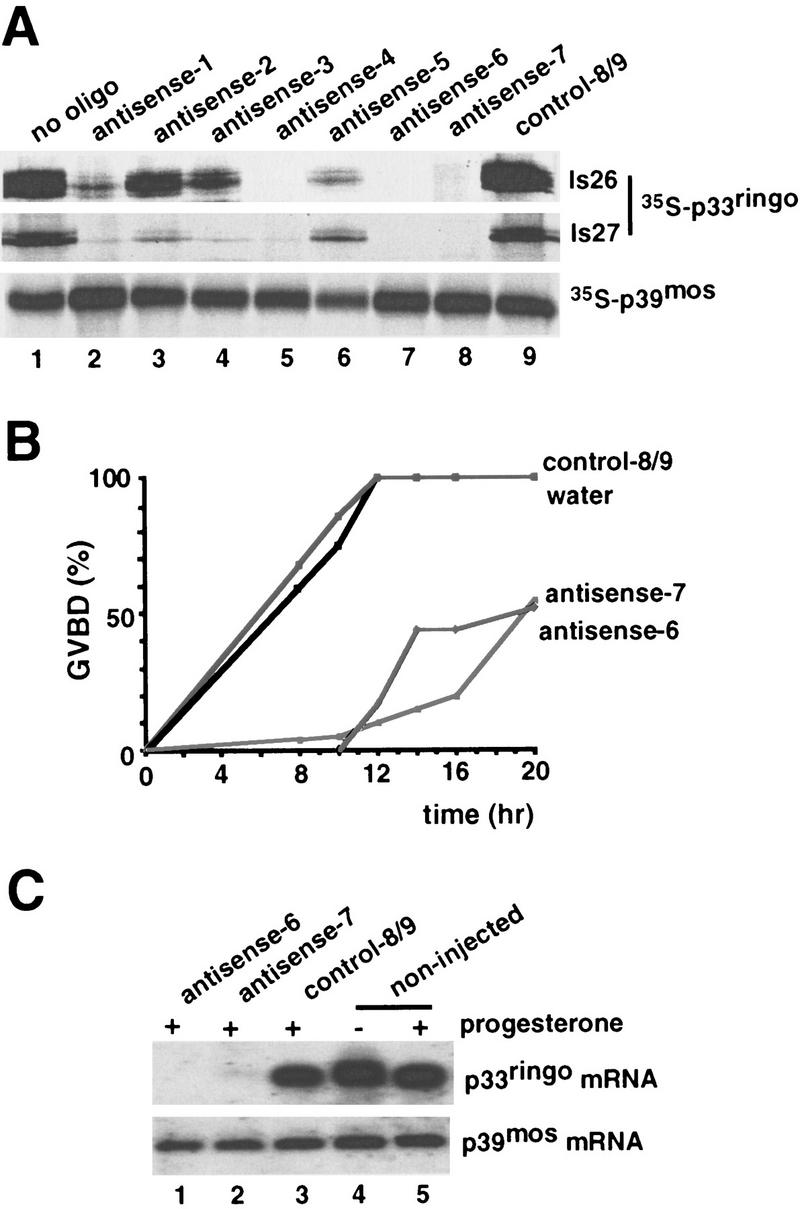
Inhibition of progesterone-induced oocyte maturation by p33ringo antisense oligonucleotides. (A) In vitro-transcribed mRNAs encoding the two isoforms of p33ringo (ls26 and ls27) and p39mos were translated in rabbit reticulocyte lysates with [35S]methionine either alone (lane 1) or in the presence of p33ringo antisense oligonucleotides (lanes 2–8) or with control oligonucleotides (lane 9) and analyzed by SDS-PAGE and autoradiography. (B) Oocytes were injected with 100 ng of the indicated oligonucleotides and incubated for 6 hr prior to progesterone stimulation. GVBD was scored at the indicated times. We observed essentially the same results in four experiments using two different oligonucleotide preparations. (C) RNA was extracted from groups of 20 oocytes taken after 16 hr of incubation as in B and analyzed by Northern blot using as a probe the p33ringo cDNA (top). The same RNA blot was reprobed with the p39mos cDNA (bottom).
To test the specificity of the antisense oligonucleotides, we tried to rescue the inhibition of progesterone-induced GVBD by adding back small amounts of purified p33ringo protein. For this experiment, oocytes that had been injected with control or antisense oligonucleotides were injected a second time with different amounts of purified malE–p33ringo prior to progesterone stimulation. As shown in Figure 6A, the inhibitory effect of the p33ringo antisense oligonucleotide on progesterone-induced maturation, could be readily reversed by coinjection of only 2.5 ng of malE–p33ringo, an amount that was not enough to trigger oocyte maturation in the absence of progesterone. These results indicate that the p33ringo antisense oligonucleotides are likely to inhibit progesterone-induced oocyte maturation via the specific targeting of the endogenous p33ringo mRNA(s).
We also investigated the endogenous p33ringo protein in oocytes. By immunoblotting with affinity-purified anti-p33ringo antibodies, we could detect one band of ∼34 kD in lysates prepared from progesterone-matured oocytes (Fig. 6B, lanes 2,4). This band was not present when the anti-p33ringo antibodies were preincubated with recombinant malE–p33ringo protein (Fig. 6B, lane 6) and was neither detected when the oocytes were injected with p33ringo antisense oligonucleotides prior to progesterone stimulation (Fig. 6B, lane 3). This indicates that the 34-kD protein was recognized specifically by the anti-p33ringo antibodies and that its appearance correlated with oocyte maturation.
Further evidence that p33ringo was synthesized during oocyte maturation was obtained by immunoprecipitation with anti-p33ringo antibodies of metabolically labeled oocytes. In these experiments we could detect two [35S]methionine-labeled proteins of ∼34 and 37 kD in lysates prepared from progesterone-matured oocytes (Fig. 6C, lane 4). These two proteins were immunoprecipitated neither from lysates of G2-arrested oocytes (Fig. 6C, lane 3) nor from lysates of oocytes injected with p33ringo antisense oligonucleotides prior to progesterone stimulation (Fig. 6C, lane 6), in agreement with the inhibitory effect of the oligonucleotides. These results indicate that the synthesis of p33ringo is up-regulated during progesterone-induced oocyte maturation.
p33ringo can associate independently with both p34cdc2 and cyclin B but not with p34cdc2/cyclin B complexes
The observation that p33ringo induced GVBD and the activation of MPF independently of new protein synthesis indicated that it was likely to act rather late in the signaling pathways that lead to the activation of pre-MPF. We investigated the possibility that p33ringo could directly associate with and/or modify the activity of p34cdc2/cyclin B complexes. Initially, we used extracts prepared from insect cells infected with p34cdc2-expressing baculovirus for pull-down experiments with malE–p33ringo bound to amylose beads. In these experiments we could detect that p34cdc2 bound to malE–p33ringo but not to malE alone (Fig. 7A, lanes 3–6). Moreover, when soluble cyclin B was added to the p34cdc2-containing extract prior to the amylose pull-down, we could still detect about the same amount of p34cdc2 bound to p33ringo but no cyclin B (Fig. 7A, lanes 7–10). This suggests that p33ringo did not bind efficiently to the p34cdc2/cyclin B complex and might preferentially bind to free p34cdc2. We also detected some binding of cyclin B to malE–p33ringo in the absence of p34cdc2 (Fig. 7A, lanes 11–14). In parallel experiments, we used Ni beads to directly recover the His-tagged cyclin B and found that when the cyclin B pull-down was done in the presence of a two-fold molar excess of soluble malE–p33ringo, there was a reduction in the amount of p34cdc2 bound to cyclin B (data not shown), suggesting that p33ringo might compete with cyclin B for binding to p34cdc2.
Figure 7.
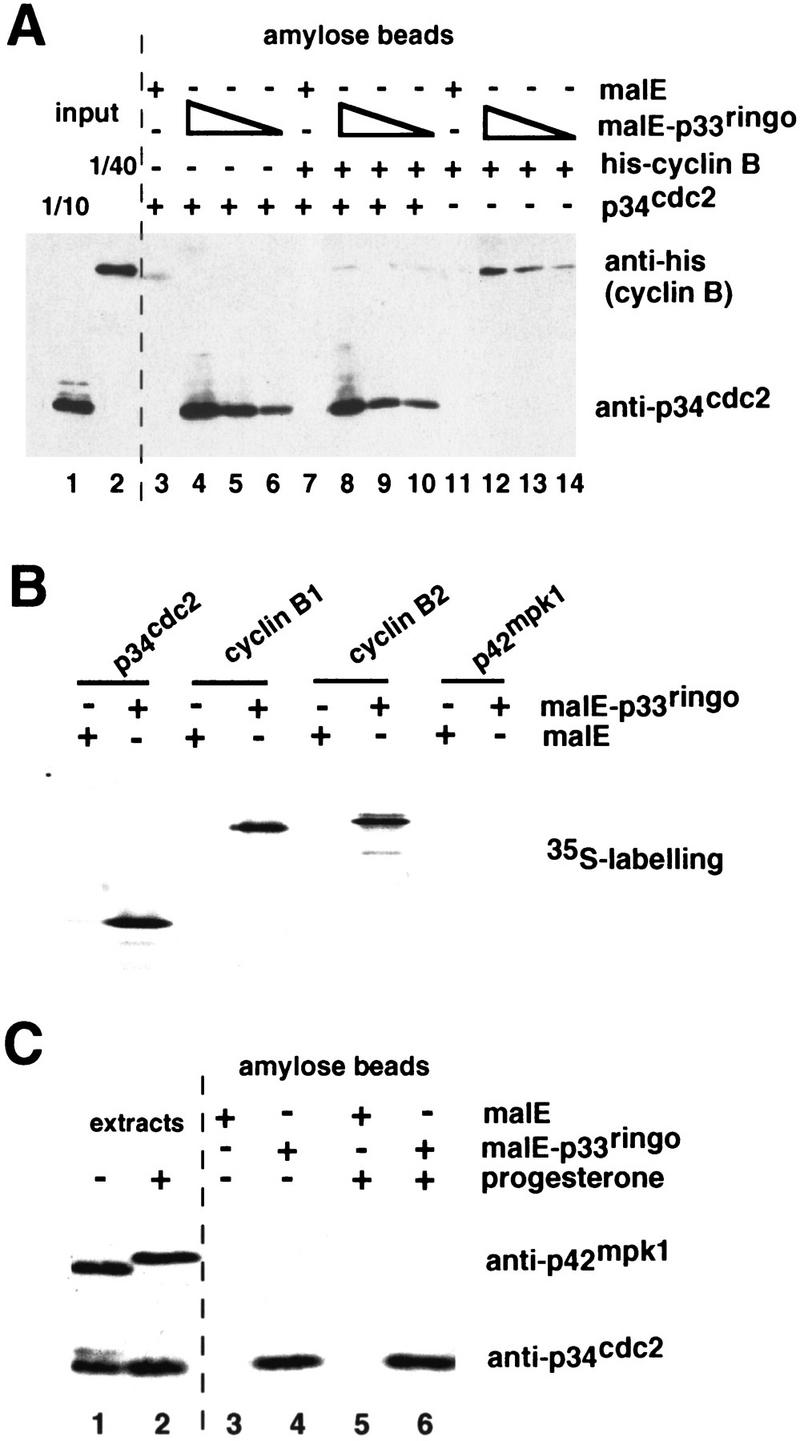
Interaction of p33ringo with free p34cdc2 and cyclin B but not with p42mpk1. (A) Purified malE–p33ringo (2, 0.2, and 0.02 μg) or malE (2 μg) was prebound to amylose beads and incubated with p34cdc2-expressing insect cell extracts (containing ∼10 μg of p34cdc2, lanes 3–6), purified His–cyclin B (8 μg, lanes 11–14) or p34cdc2-expressing extracts plus His–cyclin B (lanes 7–10). After extensive washing, the bead-associated proteins were analyzed by immunoblot with anti-p34cdc2 and anti-penta-His antibodies. Aliquots of the p34cdc2-expressing extract (lane 1) and purified His–cyclin B (lane 2) before the pull-down were also analyzed in the same immunoblot. (B) [35S]-Labeled p34cdc2, cyclin B1, cyclin B2, or p42mpk1 prepared in rabbit reticulocyte lysates was incubated with 1 μg of malE or malE–p33ringo bound to amylose beads. After extensive washing, the beads were analyzed by SDS-PAGE followed by autoradiography. (C) Lysates prepared from 20 control or progesterone-treated oocytes were incubated with malE or malE–p33ringo prebound to amylose beads, extensively washed, and analyzed by SDS-PAGE and immunoblotting with anti-p34cdc2 and anti-p42mpk1 antibodies. Aliquots of the lysates before the pull-downs (corresponding to one oocyte) were analyzed in the same gel (extracts).
The interaction between p33ringo and p34cdc2 was confirmed using [35S]methionine-labeled proteins prepared in rabbit reticulocyte lysates for malE–p33ringo pull-down experiments (Fig. 7B). We also observed in these experiments that p33ringo bound to cyclins B1 and B2 but did not bind to p42mpk1 significantly (Fig. 7B).
To extend these observations, we investigated whether p33ringo could also bind to the endogenous p34cdc2 in Xenopus oocytes. We found that p34cdc2 was present in malE–p33ringo pull-downs prepared from oocyte extracts (Fig. 7C, lanes 4, 6) but not in the malE pull-downs prepared in parallel from the same extracts (Fig. 7C, lanes 3, 5). Moreover, it appeared that the majority of the p34cdc2 bound to p33ringo might be free p34cdc2 rather than p34cdc2 complexed with cyclin B, based on the electrophoretic motility of the p34cdc2 isolated from immature oocytes (Fig. 7C). The binding of p33ringo to free p34cdc2 was also supported by the lack of detection of cyclin B1 in the p33ringo pull-downs prepared from progesterone-matured oocytes (data not shown). Because endogenous B-type cyclins in the oocytes are likely to be mostly bound to p34cdc2 (Kobayashi et al. 1991b), this is consistent with p33ringo not being able to associate efficiently with p34cdc2/cyclin B complexes. In these experiments we confirmed that p33ringo did not bind to the endogenous p42mpk1 in oocyte extracts (Fig. 7C).
Activation of p34cdc2 by p33ringo in cell-free extracts and in vitro
To gain further information on the possible function of p33ringo in oocyte maturation, we used cell-free extracts prepared by high speed centrifugation of oocyte lysates (Karaiskou et al. 1998). Addition of recombinant Cdc25 to these extracts can trigger the activation of p34cdc2/cyclin B complexes as indicated by the detection of H1 kinase activity and the disappearance of the slowly migrating p34cdc2 band in immunoblots (Fig. 8A, lanes 5, 6). As expected, the activity of Cdc25 was blocked by vanadate (Fig. 8A, lanes 11, 12). In contrast, p33ringo could induce the rapid appearance (5 min) of histone H1 kinase activity, which did not correlate with any change in the electrophoretic mobility of p34cdc2 in immunoblots (Fig. 8A, lanes 3, 4) and was not affected by the presence of vanadate (Fig. 8A, lanes 9, 10). Interestingly, Cdc25 and p33ringo appeared to have an additive effect on the level of H1 kinase activity (Fig. 8A, lanes 7, 8). Taken together, the results suggest that p33ringo can stimulate the H1 kinase activity of free p34cdc2.
Figure 8.
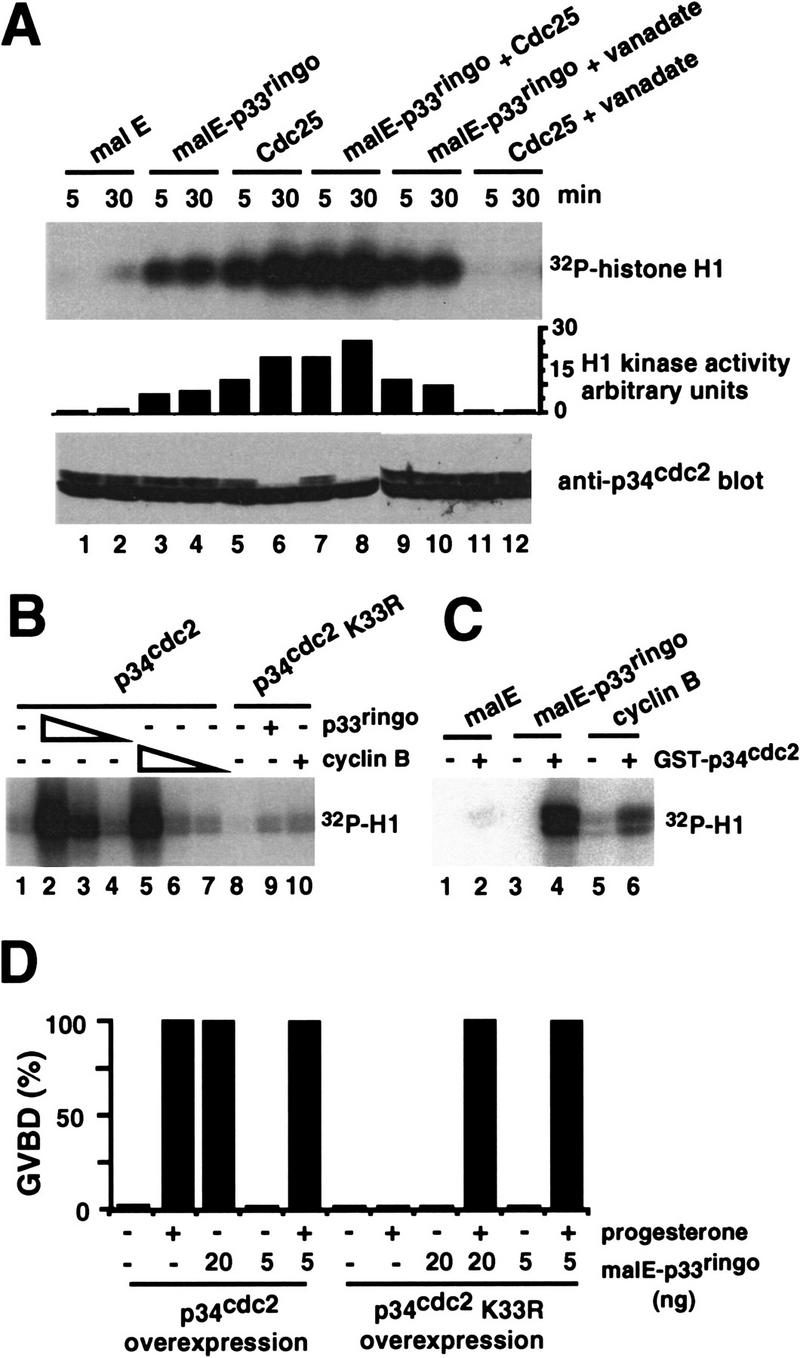
Activation of free p34cdc2 by p33ringo and inhibition of p33ringo-induced GVBD by kinase-inactive p34cdc2 K33R. (A) High-speed oocyte extracts (25 μl) were incubated with 1 μg of malE (lanes 1,2), 0.6 μg of malE–p33ringo (lanes 3,4,9,10), 0.2 μg GST–Cdc25 (lanes 5,6,11,12), or both malE–p33ringo and GST–Cdc25 (lanes 7,8). Where indicated (lanes 9–12), 5 mm vanadate was added 5 min prior to the addition of the recombinant proteins. At the indicated times, aliquots (5 μl) were withdrawn and analyzed both by in vitro kinase assay on histone H1 and by immunoblotting with anti-p34cdc2 antibodies. The phosphorylation of histone H1 was quantified in a PhosphorImager. (B) Insect cell extracts containing either wild-type p34cdc2 (lanes 1–7) or p34cdc2 K33R (lanes 8–10) were incubated with malE (75 μg/ml, lanes 1,8), malE–p33ringo (75 μg/ml, lanes 2,9; 7.5 μg/ml, lane 3; 0.75 μg/ml, lane 4) or His–cyclin B (50 μg/ml, lanes 5,10; 5 μg/ml, lane 6; 0.5 μg/ml, lane 7). After 20 min, histone H1 kinase activity in the total extracts was assayed. (C) Interphase Xenopus extracts either alone (lanes 1,3,5) or containing bacterially produced GST–p34cdc2 (1.5 μg, lanes 2,4,6) were incubated with glutathione–Sepharose beads. After centrifugation, the beads were washed extensively and incubated with 2 μg of either malE (lanes 1,2), malE–p33ringo (lanes 3,4), or His–cyclin B (lanes 5,6) for 20 min at 22°C and then used for histone H1 kinase assays. (D) Oocytes were injected with in vitro-transcribed mRNAs encoding either wild-type p34cdc2 or p34cdc2 K33R (Nebreda et al. 1995) and incubated for 16 hr prior to the injection of the indicated amounts of malE–p33ringo and/or progesterone stimulation. GVBD was scored after 14 hr.
To confirm the activation of free p34cdc2 by p33ringo, we investigated the ability of malE–p33ringo to stimulate the kinase activity of p34cdc2 in cell-free extracts prepared from baculovirus-infected insect cells. For this experiment, we added either cyclin B, malE–p33ringo, or malE alone to insect cell extracts containing p34cdc2 and then assayed their histone H1 kinase activity in vitro (Fig. 8B). We found that both cyclin B and malE–p33ringo, but not malE alone, up-regulated the histone H1 kinase activity of the p34cdc2-expressing extracts (Fig. 8B, lanes 1–7). In contrast, the increase in histone H1 kinase activity was very much reduced when we used extracts containing a kinase-inactive p34cdc2 mutant (p34cdc2 K33R) instead of wild-type p34cdc2 (Fig. 8B, lanes 8–10). Moreover, the histone H1 kinase activity triggered by either cyclin B or malE–p33ringo was recovered on p13suc1 beads (data not shown), suggesting further that it was due to the activation of p34cdc2. We also observed increased histone H1 kinase activity upon addition of malE–p33ringo to interphase Xenopus extracts prepared from activated eggs, where cyclins have been degraded and there are no preformed p34cdc2/cyclin complexes but only free p34cdc2 (not shown). On the other hand, p33ringo did not modify the histone H1 kinase activity of purified p34cdc2/cyclin B complexes (data not shown).
We also investigated whether malE–p33ringo was able to directly activate bacterially produced GST–p34cdc2. Incubation of the two recombinant proteins together in a buffer containing Mg/ATP did not result in any measurable kinase activity on histone H1 (data not shown). However, addition of malE–p33ringo to GST–p34cdc2, which had been preincubated in interphase Xenopus extracts and then recovered on glutathione beads, resulted in a significant increase in histone H1 kinase activity (Fig. 8C). These results suggest that p33ringo may be able to directly activate p34cdc2, although it is also possible that additional factors might have been recruited from the cell-free extract by the GST–p34cdc2.
A kinase-inactive p34cdc2 K33R mutant inhibits p33ringo-induced oocyte maturation
The above results suggested that p33ringo may trigger oocyte maturation by binding to and activating free p34cdc2. This possibility was supported by the observation that malE–p33ringo-induced maturation was inhibited by overexpression in oocytes of the kinase-inactive p34cdc2 K33R mutant (injection of synthetic mRNA results in about six times more p34cdc2 K33R than the endogenous p34cdc2 concentration) (Nebreda et al. 1995). In contrast, overexpression of wild-type p34cdc2 did not affect oocyte maturation induced by injection of malE–p33ringo (Fig. 8D). We also found that coinjection of a small amount of malE–p33ringo was able to rescue the inhibitory effect of p34cdc2 K33R on progesterone-induced maturation (Fig. 8D). This suggests that titration of endogenous p33ringo may account for the previously reported inhibition of progesterone-induced oocyte maturation by p34cdc2 K33R (Nebreda et al. 1995).
Discussion
In this report we describe the cloning and characterization of p33ringo, a novel protein that can efficiently induce entry into M phase of meiosis in Xenopus oocytes. At low concentrations p33ringo does not trigger maturation alone but strongly accelerates progesterone-induced maturation, suggesting that the pathway stimulated by p33ringo is normally activated during oocyte maturation. The induction of bona fide oocyte maturation by p33ringo is supported by the appearance of the characteristic white spot at the animal pole of oocytes and the detection of the same biochemical markers as in progesterone-matured oocytes, including the appearance of p39mos, cyclins B1 and B4, and the activation of both p42mpk1 and pre-MPF. We also observed that the oocytes injected with p33ringo tend to overmature to various extents after longer incubations, depending on the oocyte batch. This has been observed similarly upon overexpression in oocytes of other proteins that can trigger maturation such as p39mos or cyclin A (A.R. Nebreda, unpubl.) and is probably due to some side effects generated by the high levels of ectopic protein.
The ability of recombinant p33ringo to induce MPF activation and GVBD independently of protein synthesis indicates that it does not function by stimulating the translation of maternal mRNAs encoding p39mos or other triggering proteins (Barkoff et al. 1998). Moreover, the observation that p42mpk1 activation by p33ringo is strongly reduced by the presence of cycloheximide suggests that p33ringo is likely to function closer to the activation of p34cdc2 than of p42mpk1. Consistent with this idea, recombinant cyclin A or Cdc25 can also induce MPF activation and GVBD very potently, independently of protein synthesis but only activate p42mpk1 when protein synthesis is allowed (Nebreda et al. 1995). In contrast, the ability of activators of the p42mpk1 pathway to induce MPF activation in cycloheximide-treated oocytes versus nontreated oocytes is often reduced (see introductory section). In spite of the potent activity of p33ringo to induce MPF activation and GVBD in the presence of cycloheximide, the histone H1 kinase activity in these oocytes appears to be activated only transiently. This suggests that the oocytes enter into meiosis I but are unable to proceed through meiosis II and arrest at the meiotic interphase with low levels of MPF (and, hence, histone H1 kinase) activity. It therefore appears that synthesis of additional proteins is necessary for entry into meiosis II. Similar observations have been reported when p39mos protein is injected in the presence of cycloheximide (Murakami and Vande Woude 1997).
The experiments using antisense oligonucleotides suggest that the endogenous p33ringo mRNA is required for progesterone to induce oocyte maturation. Moreover, injection of small doses of recombinant malE–p33ringo, which cannot trigger oocyte maturation in the absence of progesterone, can rescue the inhibitory effect of the antisense oligonucleotides indicating that they are unlikely to target other important mRNAs. These results, together with the observation that synthesis of p33ringo is up-regulated upon progesterone stimulation, indicate that de novo synthesis of p33ringo is likely to be required for progesterone-induced maturation. We have also observed that antisense-directed interference with either p33ringo or p39mos mRNAs alone significantly delays progesterone-induced maturation but usually does not totally block the process (Fig. 5; data not shown). Thus, although synthesis of both p33ringo and p39mos may be normally required for progesterone-induced maturation, it appears that synthesis of only one of them can still trigger oocyte maturation albeit with reduced efficiency.
How can p33ringo trigger oocyte maturation? The amino acid sequence of p33ringo is not very informative as it shows no significant homologies to other proteins in databases. An interesting clue, however, is the ability of p33ringo to bind p34cdc2 (most likely free p34cdc2 rather than p34cdc2/cyclinB complexes) and up-regulate p34cdc2 kinase activity. This is likely to be a significant property of p33ringo, as malE–p33ringo can activate GST–p34cdc2 in vitro and the overexpression of kinase-inactive p34cdc2 K33R inhibits p33ringo-induced oocyte maturation. Moreover, both p33ringo and cyclin B1 activate the histone H1 kinase activity of p34cdc2 to a similar extent in extracts prepared from baculovirus-infected insect cells. In interphase Xenopus extracts, however, cyclin B1 induces higher levels of histone H1 kinase activity than p33ringo (data not shown). The reason for this difference in behavior, depending on the source of the extract, remains to be investigated. Proteins that are known to associate with p34cdc2 include A- and B-type cyclins, which are regulatory and activating subunits of p34cdc2 (for review, see Kobayashi et al. 1991a; Jackman and Pines 1997), and Suc1/Cks proteins, which may regulate substrate recognition by the p34cdc2/cyclin complex (Patra and Dunphy 1998). We have found no significant sequence homology between these proteins and p33ringo. Interestingly, there is at least one other example of a protein, p35, that has no sequence homology to cyclins but can bind to and activate p34cdc2-like cyclin-dependent kinase 5 (cdk5; for review, see Lew and Wang 1995). The only homology that we have detected between p35 and p33ringo is a region of 44 amino acids that is 24% identical. Although the similarity is not very high, it is intriguing that this region of p35 (amino acids 159–203) is included in the p35 fragment that is as effective as full-length p35 to induce a high level of histone H1 kinase activity in recombinant cdk5 (Lew and Wang 1995). Moreover, it appears that phosphorylation is not required for activation of cdk5 by p35 (Lew and Wang 1995), whereas Thr-161 phosphorylation is an essential requirement for the activity of p34cdc2/cyclin B complexes (for review, see Morgan 1997). It will be interesting to investigate whether or not activation of p34cdc2 by p33ringo involves Thr-161 phosphorylation.
While this paper was under revision, Lenormand et al. (1999) reported the cloning of a Xenopus cDNA that complements a rad1 mutant of Schizosaccharomyces pombe and encodes a protein 98% identical to p33ringo (ls27). They showed that this protein induces Xenopus oocyte maturation and suggested that this might depend on the activation of p42mpk1, but the mechanism was not elucidated (Lenormand et al. 1999). As discussed above, our results indicate that p33ringo probably induces oocyte maturation via the activation of p34cdc2. This is consistent with the ability of p33ringo to activate MPF in cycloheximide-treated oocytes independently of p42mpk1 activation.
In conclusion, we have identified a novel p34cdc2-binding and activating protein that can potently trigger Xenopus oocyte maturation. De novo synthesis of this protein appears to be required for progesterone-induced maturation. This may explain the requirement for the activation of free p34cdc2 during progesterone-induced maturation, which was proposed based on the inhibitory effects of dominant-negative p34cdc2 and a neutralizing anti-p34cdc2 antibody (Nebreda et al. 1995). Because newly synthesized cyclins are apparently not required for the activation of MPF induced by progesterone (Minshull et al. 1991; H. Hoccheger and T. Hunt, unpubl.), our results suggest that p33ringo may be a crucial protein that needs to be synthesized de novo upon progesterone stimulation to initiate the meiotic maturation of oocytes. Further work will be needed to determine the precise mechanism by which p33ringo-activated p34cdc2 can induce pre-MPF activation and what the possible connection between this pathway and the p39mos/p42mpk1 pathway might be during oocyte maturation.
Materials and methods
Preparation of the Xenopus oocyte cDNA expression library
Stage VI Xenopus oocytes were stimulated with progesterone (5 μg/ml) and collected when ∼50% of the oocytes had reached GVBD. RNA was prepared using the Oligotex Direct mRNA kit (Qiagen). Briefly, ∼900 oocytes were incubated with collagenase B (1 mg/ml, Sigma) for 1 hr in modified Barth’s medium (mBarth) at room temperature followed by extensive washing in PBS. The oocytes were lysed in 15 ml of OL1 lysis, diluted with 30 ml of ODB buffer, and poly(A)+ RNA was extracted by incubation with 150 μl of Oligotex beads for 15 min at room temperature. The beads were aliquoted (1.5 ml/tube), transferred to spin columns, and washed twice with 0.5 ml of OW2 buffer (10 mmTris-HCl at pH 7.5, 150 mm NaCl, 1 mm EDTA. The RNA was finally eluted with OEB buffer (preheated to 70°C), ethanol precipitated, and resuspended in 5 mm Tris (pH 7.5). Double-stranded cDNA was synthesized from 5 μg of poly(A)+ RNA using a commercial cDNA synthesis kit (catalog no. 200400, Stratagene) as recommended by the manufacturer. The cDNAs longer than 500 bp were gel purified and directionally cloned into the FTX5 expression vector (provided by C. Hill, ICRF, London, UK) digested previously with EcoRI and XhoI. The ligation mixture was transformed into XL-1 Blue MRF′ electroporation-competent cells (Stratagene). We estimated that the primary cDNA library consisted of ∼120,000 transformants.
Screening of small pools of the cDNA expression library by oocyte injection
The procedure to prepare small pools from the primary cDNA library was essentially as described by Lustig et al. (1997). The library was plated at ∼150–200 transformants per plate [10 cm, Luria–Bertani (LB) medium–ampicillin] and grown at 37°C overnight. Colonies from each plate were recovered as a pool in 2 ml of Terrific Broth from which 90 μl was used to prepare 20% glycerol stocks while the remaining was used to isolate plasmid DNA. The DNA from each pool was linearized with XbaI and in vitro transcribed using the Megascript T7 transcription kit (Ambion) according to the manufacturer’s instructions. The mRNA was purified by phenol–chloroform extraction followed by ethanol precipitation and finally dissolved in 40 μl of DEPC-treated water. For the screening, ∼80–100 ng of mRNA was injected into stage VI oocytes that were incubated for ∼30–36 hr to allow translation of the injected mRNAs and scored for GVBD. The groups of mRNA-injected oocytes that did not show GVBD were stimulated further with progesterone (5 μg/ml) and scored for acceleration of maturation. We analyzed 105 pools and found 4 that were able to trigger GVBD on their own, whereas 22 pools accelerated progesterone-induced maturation. All of the positive pools were analyzed by Southern blotting for the presence of known inducers of oocyte maturation including p39mos (Sagata et al. 1988), Cdc25 (Izumi et al. 1992; Kumagai and Dunphy 1992) and cyclins A1, A2, B1, B2, B3, B4, and B5 (provided by T. Hunt and collaborators, ICRF, South Mimms, UK). Two positive pools that did not contain any of the above-mentioned cDNAs were subdivided into smaller pools in two rounds, first of 20 transformants and then single clones, from which plasmid DNA and in vitro-transcribed mRNAs were prepared and injected into oocytes as described above. Using this protocol we isolated two clones that encoded Myc-tagged proteins and were able to potently induce GVBD.
Isolation of full-length p33ringo cDNAs
Full-length cDNAs of the two clones isolated in the oocyte expression screening were obtained from a λ ZAP Xenopus oocyte cDNA library (provided by J. Shuttleworth, University of Birmingham, UK). The phage library was transferred onto nylon membranes (Hybond N, Amersham) and probed independently with the two 32P-labeled cDNAs (Megaprime, Amersham) in Church buffer (0.5 m NaHPO4 at pH 7.2, 7% SDS, 1 mm EDTA, 10 grams/liter BSA) at 65°C for 16 hr. Several pBluescript phagemids were isolated containing ORFs that overlapped with the cDNAs isolated form the expression library. We selected as full-length cDNAs those clones that extended farther at the 5′ end and contained upstream stop codons followed by an ATG in the same frame as the ORF. The accession numbers for the full-length cDNAs are AJ133499 (ls26) and AJ133500 (ls27). The cDNAs isolated in the oocyte expression screening started at positions 246 (ls26) and 88 (ls27) of the corresponding full-length clones.
Oocyte maturation
Stage VI oocytes were sorted after collagenase B treatment (Boehringer Mannheim, 0.5 mg/ml, 30–60 min) and left at 18°C in mBarth for 2–16 hr before injection. For the experiment in Figure 4, frogs were injected with pregnant mare’s serum gonadotropin (100 I.U., Intervet) 3 days prior to operation and the oocytes were manually sorted. Meiotic maturation was induced by incubation with 5 μg/ml progesterone (Sigma) or by injection with 50 nl of either synthetic mRNAs or purified malE–p33ringo (see below) or malE–p39mos (Nebreda and Hunt 1993) proteins. In vitro-transcribed p39mos mRNA was obtained with the MEGAscript kit (Ambion) from a construct prepared by subcloning the full-length Xenopus c-mos proto-oncogene from murine leukemia virus (MLV)–mos (Nebreda et al. 1993) into FTX5. Maturation was scored by the appearance of a white spot at the animal pole of the oocyte, and GVBD was confirmed after fixation in 5% TCA.
Bacterial expression and purification of malE–p33ringo fusion proteins
The p33ringo cDNAs from FTX5 were subcloned into the pMalc2 expression vector (New England Biolabs) digested with BamHI and XbaI. For protein expression, the pMalc2–p33ringo constructs were transformed into Escherichia coli BL21(DE3). Fresh overnight cultures in LB–ampicillin (100 μg/ml) were diluted 1000-fold and incubated further at 37°C until OD600 was 0.6–0.8. The cultures were diluted 1:1 and induced with 100 μm isopropyl-β-d-thiogalactopyranoside (IPTG) for 6 hr at 23°C. Cells were collected, washed with cold PBS, and lysed in 50 mm Tris (pH 7.5), 50 mm NaCl, 5 mm EDTA, 1 mm phenylmethylsulfonyl fluoride (PMSF), and 1 mg/ml lysozyme for 30 min at 4°C. After sonication and centrifugation at 10,000g for 10 min, the supernatant was supplemented with 1 mm dithiothreitol (DTT) and incubated with amylose beads (New England Biolabs) for 30 min at 4°C. Beads were washed twice in PBS and once in 50 mm Tris (pH 8), 50 mm NaCl, 1 mm DTT, followed by elution with the same buffer supplemented with 0.1 mm EDTA and 12 mm maltose. The protein was finally dialyzed against 20 mm Tris (pH 8), 50 mm NaCl, 0.1 mm EDTA, 0.5 mm DTT, and 5% glycerol, stored at −70°C, and never subjected to more than two rounds of freeze–thawing.
Histone H1 and MBP kinase assays
The oocytes were lysed in 10 μl per oocyte of ice-cold H1K buffer [80 mm β-glycerophosphate (pH 7.5), 20 mm EGTA, 15 mm MgCl2, 1 mm DTT, 1 mm AEBSF) and centrifuged at 14,000 rpm (Eppendorf centrifuge) for 10 min. The supernatant (oocyte lysate), equivalent to about half an oocyte, was incubated for 15 min at 22°C with 3 μg of either histone H1 or MBP in 12 μl of H1K buffer supplemented with 2 μCi of [γ-32P]ATP (3000 Ci/mmole) and 50 μm cold ATP. The kinase reactions were analyzed by SDS-PAGE and autoradiography.
Immunoblotting and immunoprecipitation
Oocyte lysates were usually prepared in H1K buffer, as described above, except for the detection of endogenous p33ringo, where oocytes were lysed in 20 μl per oocyte of an ice-cold buffer containing 80 mm β-glycerophosphate (pH 7.6), 50 mm NaCl, 10 mm EDTA, 0.2 m sucrose, 2 mm PMSF, 1 μm microcystin, 50 nm calyculin A, 10 μg/ml pepstatin A, and a protease inhibitor cocktail (Complete, Boehringer Mannheim). The lysate equivalent of about one oocyte per lane was separated by SDS-PAGE and transferred to nitrocellulose membranes (0.22 μm Protran, Schleicher & Schuell) using a semidry blotting apparatus (Hoefer). The membrane was blocked for 30–60 min at room temperature in TTBS buffer (25 mm Tris-HCl at pH 8.0, 150 mm NaCl, 0.05% Tween 20) supplemented with 4% nonfat dry milk and probed with the corresponding antibody in TTBS supplemented with 1% milk, usually overnight at 4°C. The monoclonal antibody 3E1 (provided by J. Gannon and T. Hunt, ICRF, South Mimms, UK) was used to detect p34cdc2 (Nebreda et al. 1995), and the rabbit antiserum 3297.1 was used for the detection of p42mpk1 (Palmer et al. 1998). For p39mos we used a purified rabbit antibody (C-237, Santa Cruz Biotechnology) and for His-tagged cyclin B a monoclonal anti-penta-His antibody (Qiagen). Xenopus cyclins B1 and B4 were detected with affinity-purified antibodies provided by H. Hoccheger and T. Hunt (ICRF, South Mimms, UK). The anti-p33ringo antibodies were prepared in rabbits against bacterially produced malE–p33ringo protein and were affinity purified on a GST–p33ringo-containing nitrocellulose strip as described (Harlow and Lane 1988). In all cases, horseradish peroxidase-coupled secondary antibodies (Dakko) were used, and the binding was detected using the enhanced chemiluminescence detection system (Amersham).
For immunoprecipitation, groups of 25 oocytes were labeled in mBarth containing 1 mCi/ml of [35S]methionine for 14 hr. The oocytes were washed three times in mBarth and lysed in H1K buffer (10 μl/oocyte) supplemented with 2 μm microcystin, 2.5 mm benzamidine–HCl, 10 μg/ml each of aprotinin, leupeptin, and pepstatin A, and the Complete protease inhibitor cocktail (Boehringer Mannheim). The oocyte lysates were precleared with 25 μl of protein A–Sepharose beads for 2 hr at 4°C and then incubated with 3 μl of either preimmune serum or a rabbit antiserum prepared against the KLH-coupled peptides THDLHILQE and DGTALEWHHL corresponding to residues near the carboxyl terminus of p33ringo (ls26). After 90 min at 4°C, the lysates were mixed with 100 μl of a 10% solution of protein A–Sepharose beads in IP buffer (50 mm Tris-HCl at pH 7.5, 150 mm NaCl, 20 mm NaF, 5 mm EDTA, 5 mm EGTA, 100 μm sodium–vanadate, 1% NP-40), supplemented with microcystin and protease inhibitors as indicated above, and incubated further overnight at 4°C. The beads were washed four times in IP buffer, transferred to a new tube, and boiled in SDS sample buffer for elution of the immunocomplexes, which were analyzed by SDS-PAGE and autoradiography.
Antisense experiments
We designed four antisense oligonucleotides based on sequences around the first ATG of either ls27 (1-TGCATATGCCTCATTGTAGAAAGG and 2-AACTAAGGTGGCCCGGGTTGCACT) or ls26 (4-ACTCTGCATGTGCCTCATTGTAGA and 5-AATGGAGCTGGCCCGGGTTACACT) and three antisense oligonucleotides based on sequences common to the 5′ region of both the ls26 and ls27 p33ringo cDNAs (3-TTTTCGCCTTGCGCGCTCCAA; 6-ATATGCTAGAACCATTGCTATGAGA and 7-TCATTTTCTAGGAGCCTGTA). The two control oligonucleotides were a randomized sequence (8-TAGAGAAGATAATCGTCATCTTA) and the same sequence as antisense 4 but with four point mutations (9-ACTCTTCATCTGCCTCTTTGTCGA).
Oligonucleotides were prepared on an automatic DNA synthesizer using a special solid support carrying dimethoxytrityl (DMT)-protected 1,3-propanediol linked to amino-controlled pore glass through a standard succinil linkage (Van Aerschot et al. 1995) and the appropriate protected nucleoside phosphoramidites. The 200 nmole scale synthesis cycle was used. Oligonucleotide supports were treated with 1 ml of a concentrated ammonia solution (32%) at 55°C for 16 hr. The solid support was removed by filtration and the solution was concentrated to dryness. The residue was passed through a Dowex 50Wx4 Na+ (Fluka) to exchange the ammonium to sodium counterions. Finally, oligonucleotides were desalted on Sephadex G-25 (NAP-10 columns, Pharmacia-Upjohn), eluted with water, and concentrated using a Speed-vac rotatory evaporator until ∼2 mg/ml.
The ability of the antisense oligonucleotides to target the ls26 and ls27 p33ringo mRNAs was tested in vitro using rabbit reticulocyte lysates as described (Minshull and Hunt 1992). In vitro-transcribed mRNAs were added at a concentration of 10 μg/ml to rabbit reticulocyte lysates (10 μl, Promega) and incubated for 30 min at 30°C in the presence of the oligonucleotides (10 μg/ml), RNase H (0.2 U/μl, GIBCO–BRL) and [35S]methionine (0.5 mCi/ml, >1000 Ci/mmole). The 35S-labeled proteins were analyzed by SDS-PAGE and autoradiography. To test the effect of the oligonucleotides in oocyte maturation, oocytes were injected with ∼100 ng of each oligonucleotide and incubated for 5–6 hr prior to stimulation with progesterone (5 μg/ml) and/or injection of malE–p33ringo (2.5–10 ng per oocyte).
Northern blots
Total RNA was extracted from oocytes using the Triazol one-step extraction method (GIBCO) following the manufacturer’s protocol. About 20 μg of RNA was separated on a 1.2% agarose gel (20 mm MOPS, 5 mm sodium acetate, 1 mm EDTA, 2% formaldehyde), transferred to a nylon membrane (GeneScreen Plus, Nitran), and UV cross-linked. The probes were an 0.8-kb BamHI–BamHI DNA fragment that includes most of the ls26 p33ringo ORF and hybridizes to both the ls26 and ls27 cDNAs, and a 1-kb NcoI–XhoI DNA fragment including the p39mos ORF. For the hybridization, the DNA probes were labeled with [32P]dCTP (3000 Ci/mmole) and incubated with the RNA blot in Church buffer at 55°C for 14 hr. After four washes for 10 min at 55°C (two in 2× SSC, 0.1% SDS, and two in 0.5× SSC, 0.1% SDS), the blot was subjected to autoradiography.
Amylose pull-downs
Extracts from Sf9 insect cells expressing p34cdc2 were prepared as described (Kumagai and Dunphy 1997). The extracts (usually 20 μl) were diluted 1:5 in HBS buffer (10 mm HEPES–KOH at pH 7.4, 150 mm NaCl, 1% BSA, 1 mm PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin) and incubated for 20 min at room temperature with different concentrations of either malE or malE–p33ringo prebound to 10 μl of amylose beads. In some experiments, amylose pull-downs were performed in the presence of purified His-tagged cyclin B1 (Kumagai and Dunphy 1997) either alone or in combination with the p34cdc2-containing extracts. After centrifugation, the beads were washed four times in IP buffer and once in HBS buffer, and analyzed by immunoblotting.
For rabbit reticulocyte pulldowns, pET8c–p34cdc2 (Nebreda et al. 1995), pGEM1–cyclin B1 and pGEM1–cyclin B2 (provided by H. Hoccheger and T. Hunt) and FTX5–p42mpk1 (Gavin and Nebreda 1999) were in vitro-transcribed and -translated in a coupled reticulocyte lysate system (TNT-T7, Promega) in the presence of [35S]methionine. Aliquots of the reticulocyte lysates (20 μl) were diluted 1:10 in IP buffer supplemented with 1% BSA, 1 mm PMSF, and 10 μg/ml of both aprotinin and leupeptin and incubated for 40 min at 20°C with 1 μg of malE or malE–p33ringo prebound to 5 μl of amylose beads. The beads were washed six times in ice-cold IP buffer and analyzed by SDS-PAGE and autoradiography.
For pull-downs with oocyte extracts, 200 μl of extract prepared using 2 μl of H1K buffer per oocyte was diluted 1:1 in IP buffer supplemented with 1% BSA and incubated with 3 μg of malE or malE–p33ringo prebound to 15 μl of amylose beads. After 1 hr at 20°C, the beads were washed six times in IP buffer, resuspended in 20 μl of HBS buffer, and 4 μl was analyzed by immunoblotting with anti-p34cdc2 and anti-p42mpk1 antibodies.
Activation of p34cdc2 by p33ringo in cell-free extracts and in vitro
High speed oocyte extracts were obtained from immature oocytes using 2 μl per oocyte of H1K buffer supplemented with 10 mm DTT, an ATP-regenerating mixture, and 1 μg/ml cycloheximide as described (Karaiskou et al. 1998). The extract (25 μl) was preincubated with water or 5 mm vanadate for 5 min prior to the addition of malE (1 μg), malE–p33ringo (0.6 μg), and/or GST–XCdc25 (0.2 μg). Aliquots of 5 μl were used for histone H1 kinase assay and immunoblotting with anti-p34cdc2 antibodies.
Extracts (5 μl) prepared from baculovirus-infected Sf9 cells expressing either wild-type p34cdc2 or kinase-inactive p34cdc2 K33R were diluted 1:5 in HBS buffer supplemented with 250 μm ATP and 10 mm MgCl2, incubated for 20 min at room temperature with various concentrations of malE, malE–p33ringo, or His–cyclin B and analyzed by histone H1 kinase assay. For p13suc1 pull-downs, 100 μl of the extracts incubated with malE, malE–p33ringo, or His–cyclin B as above were mixed with 10 μl of p13suc1 linked to agarose beads (Calbiochem) for 30 min at 4°C. The beads were washed four times in IP buffer and once in HBS buffer, and then assayed for histone H1 kinase activity.
For the in vitro activation, 1.5 μg of bacterially expressed GST–p34cdc2 (Nebreda et al. 1995) was incubated in 15 μl of interphase Xenopus cell-free extracts prepared from activated eggs. After 40 min at 22°C, the extracts were diluted 10-fold in HIK buffer and incubated further with 5 μl of glutathione–Sepharose beads for 90 min at 4°C. The beads were washed four times in IP buffer and once in H1K buffer, resuspended in 5 μl of H1K buffer and incubated with 2 μg of purified malE, malE–p33ringo, or His–cyclin B for 20 min at 22°C. Half of the sample was then assayed for histone H1 kinase activity.
Acknowledgments
We are grateful to Akiko Kumagai and Bill Dunphy for the baculoviruses expressing p34cdc2, p34cdc2 K33R, and cyclin B1; Tim Hunt for sharing his collection of Xenopus A- and B-type cyclins; Toby Gibson and Gert Vriend for help with sequence analysis and database searching; Margaret Jones for excellent technical assistance; and Tewis Bouwmeester, Anne-Claude Gavin, and Giulio Superti-Furga for critically reading the manuscript. M.B was supported by the Schering Foundation.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked ‘advertisement’ in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL nebreda@EMBL-heidelberg.de; FAX 49 6221 387166.
References
- Atherton-Fessler S, Liu F, Gabrielli B, Lee MS, Peng CY, Piwnica-Worms H. Cell cycle regulation of the p34cdc2 inhibitory kinases. Mol Biol Cell. 1994;5:989–1001. doi: 10.1091/mbc.5.9.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkoff A, Ballantyne S, Wickens M. Meiotic maturation in Xenopus requires polyadenylation of multiple mRNAs. EMBO J. 1998;17:3168–3175. doi: 10.1093/emboj/17.11.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman TR, Dunphy WG. Cdc2 regulatory factors. Curr Opin Cell Biol. 1994;6:877–882. doi: 10.1016/0955-0674(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Cyert MS, Kirschner MW. Regulation of MPF activity in vitro. Cell. 1988;53:185–195. doi: 10.1016/0092-8674(88)90380-7. [DOI] [PubMed] [Google Scholar]
- Daar I, Yew N, Vande Woude GF. Inhibition of Mos-induced oocyte maturation by protein kinase A. J Cell Biol. 1993;120:1197–1202. doi: 10.1083/jcb.120.5.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy WG, Kumagai A. The cdc25 protein contains an intrinsic phosphatase activity. Cell. 1991;67:189–196. doi: 10.1016/0092-8674(91)90582-j. [DOI] [PubMed] [Google Scholar]
- Freeman RS, Kanki JP, Ballantyne SM, Pickham KM, Donoghue DJ. Effects of the v-mos oncogene on Xenopus development: Meiotic induction in oocytes and mitotic arrest in cleaving embryos. J Cell Biol. 1990;111:533–541. doi: 10.1083/jcb.111.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielli BG, Roy LM, Gautier J, Philippe M, Maller JL. A cdc2-related kinase oscillates in the cell cycle independently of cyclins G2/M and cdc2. J Biol Chem. 1992;267:1969–1975. [PubMed] [Google Scholar]
- Gautier J, Maller JL. Cyclin B in Xenopus oocytes: Implications for the mechanism of pre-MPF activation. EMBO J. 1991;10:177–182. doi: 10.1002/j.1460-2075.1991.tb07934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier J, Solomon MJ, Booher RN, Bazan JF, Kirschner MW. cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991;67:197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- Gavin AC, Nebreda AR. A MAP kinase docking site is required for phosphorylation and activation of p90rsk/MAPKAP kinase-1. Curr Biol. 1999;9:281–284. doi: 10.1016/s0960-9822(99)80120-1. [DOI] [PubMed] [Google Scholar]
- Gotoh Y, Masuyama N, Dell K, Shirakabe K, Nishida E. Initiation of Xenopus oocyte maturation by activation of the mitogen-activated protein kinase cascade. J Biol Chem. 1995;270:25898–25904. doi: 10.1074/jbc.270.43.25898. [DOI] [PubMed] [Google Scholar]
- Haccard O, Lewellyn A, Hartley RS, Erikson E, Maller JL. Induction of Xenopus oocyte meiotic maturation by MAP kinase. Dev Biol. 1995;168:677–682. doi: 10.1006/dbio.1995.1112. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies. A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- Huang W, Kessler DS, Erikson RL. Biochemical and biological analysis of Mek1 phosphorylation site mutants. Mol Biol Cell. 1995;6:237–245. doi: 10.1091/mbc.6.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T, Walker DH, Maller JL. Periodic changes in phosphorylation of the Xenopus cdc25 phosphatase regulate its activity. Mol Biol Cell. 1992;3:927–939. doi: 10.1091/mbc.3.8.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman MR, Pines JN. Cyclins and the G2/M transition. Cancer Surv. 1997;29:47–73. [PubMed] [Google Scholar]
- Karaiskou A, Cayla X, Haccard O, Jessus C, Ozon R. MPF amplification in Xenopus oocyte extracts depends on a two-step activation of cdc25 phosphatase. Exp Cell Res. 1998;244:491–500. doi: 10.1006/excr.1998.4220. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Golsteyn R, Poon R, Stewart E, Gannon J, Minshull J, Smith R, Hunt T. Cyclins and their partners during Xenopus oocyte maturation. Cold Spring Harbor Symp Quant Biol. 1991a;56:437–447. doi: 10.1101/sqb.1991.056.01.051. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Minshull J, Ford C, Golsteyn R, Poon R, Hunt T. On the synthesis and destruction of A- and B-type cyclins during oogenesis and meiotic maturation in Xenopus laevis. J Cell Biol. 1991b;114:755–765. doi: 10.1083/jcb.114.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornbluth S, Sebastian B, Hunter T, Newport J. Membrane localization of the kinase which phosphorylates p34cdc2 on threonine 14. Mol Biol Cell. 1994;5:273–282. doi: 10.1091/mbc.5.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosako H, Gotoh Y, Nishida E. Requirement for the MAP kinase kinase/MAP kinase cascade in Xenopus oocyte maturation. EMBO J. 1994;13:2131–2138. doi: 10.1002/j.1460-2075.1994.tb06489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosako H, Akamatsu Y, Tsurushita N, Lee KK, Gotoh Y, Nishida E. Isolation and characterization of neutralizing single-chain antibodies against Xenopus mitogen-activated protein kinase kinase from phage display libraries. Biochemistry. 1996;35:13212–13221. doi: 10.1021/bi960956f. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. The cdc25 protein controls tyrosine dephosphorylation of the cdc2 protein in a cell-free system. Cell. 1991;64:903–914. doi: 10.1016/0092-8674(91)90315-p. [DOI] [PubMed] [Google Scholar]
- ————— Regulation of the cdc25 protein during the cell cycle in Xenopus extracts. Cell. 1992;70:139–151. doi: 10.1016/0092-8674(92)90540-s. [DOI] [PubMed] [Google Scholar]
- ————— Regulation of Xenopus Cdc25 protein. Methods Enzymol. 1997;283:564–571. doi: 10.1016/s0076-6879(97)83044-3. [DOI] [PubMed] [Google Scholar]
- Lenormand JL, Dellinger RW, Knudsen KE, Subramani S, Donoghue DJ. Speedy: A novel cell cycle regulator of the G2/M transition. EMBO J. 1999;18:1869–1877. doi: 10.1093/emboj/18.7.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew J, Wang JH. Neuronal cdc2-like kinase. Trends Biochem Sci. 1995;20:33–37. doi: 10.1016/s0968-0004(00)88948-3. [DOI] [PubMed] [Google Scholar]
- Lustig K, Stukenberg P, McGarry T, King R, Cryns V, Mead P, Zon L, Yuan J, Kirschner M. Small pool expression screening: Identification of genes involved in cell cycle control, apoptosis, and early development. Methods Enzymol. 1997;283:83–99. doi: 10.1016/s0076-6879(97)83009-1. [DOI] [PubMed] [Google Scholar]
- Masui Y, Clarke HJ. Oocyte maturation. Int Rev Cytol. 1979;57:185–282. doi: 10.1016/s0074-7696(08)61464-3. [DOI] [PubMed] [Google Scholar]
- Minshull J, Hunt T. Antisense ablation of mRNA in frog and rabbit cell-free systems. In: Murray JAH, editor. Antisense RNA and DNA. New York, NY: Wiley–Liss; 1992. pp. 195–212. [Google Scholar]
- Minshull J, Murray A, Colman A, Hunt T. Xenopus oocyte maturation does not require new cyclin synthesis. J Cell Biol. 1991;114:767–772. doi: 10.1083/jcb.114.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DO. Cyclin-dependent kinases: Engines, clocks and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- Mueller PR, Coleman TR, Kumagai A, Dunphy WG. Myt1: A membrane-associated inhibitory kinase that phosphorylates Ccd2 on both threonine-14 and tyrosine-15. Science. 1995;270:86–90. doi: 10.1126/science.270.5233.86. [DOI] [PubMed] [Google Scholar]
- Murakami MS, Vande Woude GF. Mechanisms of Xenopus oocyte maturation. Methods Enzymol. 1997;283:584–600. doi: 10.1016/s0076-6879(97)83046-7. [DOI] [PubMed] [Google Scholar]
- ————— Analysis of the early embryonic cell cycles of Xenopus; regulation of cell cycle length by Xe-wee1 and mos. Development. 1998;125:237–248. doi: 10.1242/dev.125.2.237. [DOI] [PubMed] [Google Scholar]
- Nebreda AR, Hunt T. The c-mos protooncogene protein kinase turns on and maintains the activity of MAP kinase but not MPF, in cell-free extracts of Xenopus oocytes and eggs. EMBO J. 1993;12:1979–1986. doi: 10.1002/j.1460-2075.1993.tb05847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebreda AR, Hill C, Gomez N, Cohen P, Hunt T. The protein kinase Mos activates MAP kinase kinase in vitro and stimulates the MAP kinase pathway in mammalian somatic cells in vivo. FEBS Lett. 1993;333:183–187. doi: 10.1016/0014-5793(93)80401-f. [DOI] [PubMed] [Google Scholar]
- Nebreda AR, Gannon JV, Hunt T. Newly synthesized protein(s) must associate with p34cdc2 to activate MAP kinase and MPF during progesterone-induced maturation of Xenopus oocytes. EMBO J. 1995;14:5597–5607. doi: 10.1002/j.1460-2075.1995.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating cell cycle timing of M-phase. Nature. 1990;344:503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Palmer A, Gavin A-C, Nebreda AR. A link between MAP kinase and p34cdc2/cyclin B during oocyte maturation: p90rsk phosphorylates and inactivates the p34cdc2 inhibitory kinase Myt 1. EMBO J. 1998;17:5037–5047. doi: 10.1093/emboj/17.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra D, Dunphy WG. Xe-p9, a Xenopus Suc1/Cks protein, is essential for the Cdc2-dependent phosphorylation of the anaphase-promoting complex at mitosis. Genes & Dev. 1998;12:2549–2559. doi: 10.1101/gad.12.16.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada J, Yew N, Ahn N, VandeWoude GF, Cooper JA. Mos stimulates MAP kinase in Xenopus oocytes and activates a MAP kinase kinase in vitro. Mol Cell Biol. 1993;13:2546–2553. doi: 10.1128/mcb.13.4.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel RE, Sleight SB, Maller JL. Maternal Xenopus Cdk2-cyclin E complexes function during meiotic and early embryonic cell cycles that lack a G1 phase. J Biol Chem. 1995;270:6843–6855. doi: 10.1074/jbc.270.12.6843. [DOI] [PubMed] [Google Scholar]
- Sagata N, Oskarsson M, Copeland T, Brumbaugh J, Vande Woude GF. Function of c-mos proto-oncogene product in meiotic maturation in Xenopus oocytes. Nature. 1988;335:519–526. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- Sagata N, Daar I, Oskarsson M, Showalter SD, Vande Woude GF. The product of the mos proto-oncogene as a candidate ‘initiator’ for oocyte maturation. Science. 1989;245:643–646. doi: 10.1126/science.2474853. [DOI] [PubMed] [Google Scholar]
- Sheets MD, Wu M, Wickens M. Polyadenylation of c-mos mRNA as a control point in Xenopus meiotic maturation. Nature. 1995;274:511–516. doi: 10.1038/374511a0. [DOI] [PubMed] [Google Scholar]
- Shibuya EK, Ruderman JV. Mos induces the in vitro activation of mitogen-activated protein kinases in lysates of frog oocytes and mammalian somatic cells. Mol Biol Cell. 1993;4:781–790. doi: 10.1091/mbc.4.8.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausfeld U, Labbé JC, Fesquet D, Cavadore JC, Picard A, Sadhu K, Russell P, Dorée M. Dephosphorylation and activation of a p34cdc2/cyclin B complex in vitro by human CDC25 protein. Nature. 1991;351:242–245. doi: 10.1038/351242a0. [DOI] [PubMed] [Google Scholar]
- Van Aerschot A, Saison-Behmoaras T, Rozenski J, Hendrix C, Schepers G, Verhoeven G, Herdewijn P. Conjugation of oligonucleotides to 3′-polar moieties. Bull Soc Chim Belg. 1995;104:717–720. [Google Scholar]
- Yew N, Mellini ML, Vande Woude GF. Meiotic initiation by the Mos protein in Xenopus. Nature. 1992;355:649–652. doi: 10.1038/355649a0. [DOI] [PubMed] [Google Scholar]