Abstract
Mice mutant for the bHLH gene Hes1, which is known to keep cells in a proliferative state, mostly lack thymus. Transfer of Hes1-null fetal liver cells into RAG2-null host mice normally reconstitutes B cells but fails to generate mature T cells in the thymus. In the reconstituted thymus, T cell differentiation is arrested at the CD4−CD8− double negative (DN) stage. Both the initial T cell receptor (TCR)-independent and the subsequent TCR-dependent selective expansion during the DN stage are severely affected. Thus, Hes1 is essential for the earliest thymocyte expansion in a cell-autonomous manner.
Keywords: bHLH, Hes1, T cell, thymocyte, thymus
T-cell precursors in the thymus are initially negative for expression of the surface antigens CD4 and CD8 [double negative (DN); Anderson et al. 1996; Shortman and Wu 1996; von Boehmer and Fehling 1997]. These DN cells then differentiate into CD4+CD8+ [double positive (DP)] cells, which finally become CD4+CD8− or CD4−CD8+ mature single-positive (SP) cells. DN cells are subdivided further into four distinct differentiation stages based on changes in the surface expression of CD44 and CD25 in the following order: CD44+CD25− → CD44+CD25+ → CD44−CD25+ → CD44−CD25− (Godfrey and Zlotnik 1993; Godfrey et al. 1993, 1994; Pénit et al. 1995). At the first stage (CD44+CD25−) DN cells are resting and they start proliferation at the next stage (CD44+CD25+). At the third stage (CD44−CD25+), DN cells undergo rearrangement of T cell receptor (TCR) β locus and, only when the β locus is productively rearranged, these cells selectively proliferate and differentiate into DP cells. Thus, expansion of DN cells occurs at two distinct phases: the initial TCR-independent and the subsequent TCR-dependent selective proliferation (Pénit et al. 1995).
Although cell proliferation is very important for T-cell development because the majority of thymocytes eventually die after selection, the molecular mechanisms that control thymocyte proliferation are still largely unknown. The basic helix–loop–helix (bHLH) gene Hes1 (Sasai et al. 1992), which is expressed in the developing thymus from very early stages, is one of the candidate genes that regulate thymocyte proliferation, as persistent expression of Hes1 keeps cells in a proliferative state (Ishibashi et al. 1994; Tomita et al. 1996). Hes1 is a transcriptional repressor and functionally inhibits differentiation factors such as MyoD and Mash1 (Sasai et al. 1992; Ishibashi et al. 1995; Tomita et al. 1996; Chen et al. 1997). In the developing nervous system, Hes1 is expressed in dividing precursor cells, and only after Hes1 activity is lost do precursor cells stop cell division and start the differentiation program (Kageyama and Nakanishi 1997; Ström et al. 1997).
Here, we examined roles of Hes1 in T cell development by reconstituting the lymphoid system of RAG2-null mice with Hes1-null fetal liver cells. We found that T cell development is arrested at the DN stage in the absence of Hes1 and that Hes1 is essential for both the initial TCR-independent and the subsequent TCR-dependent selective expansion of thymocytes.
Results and Discussion
Hes1 is essential for T cell development
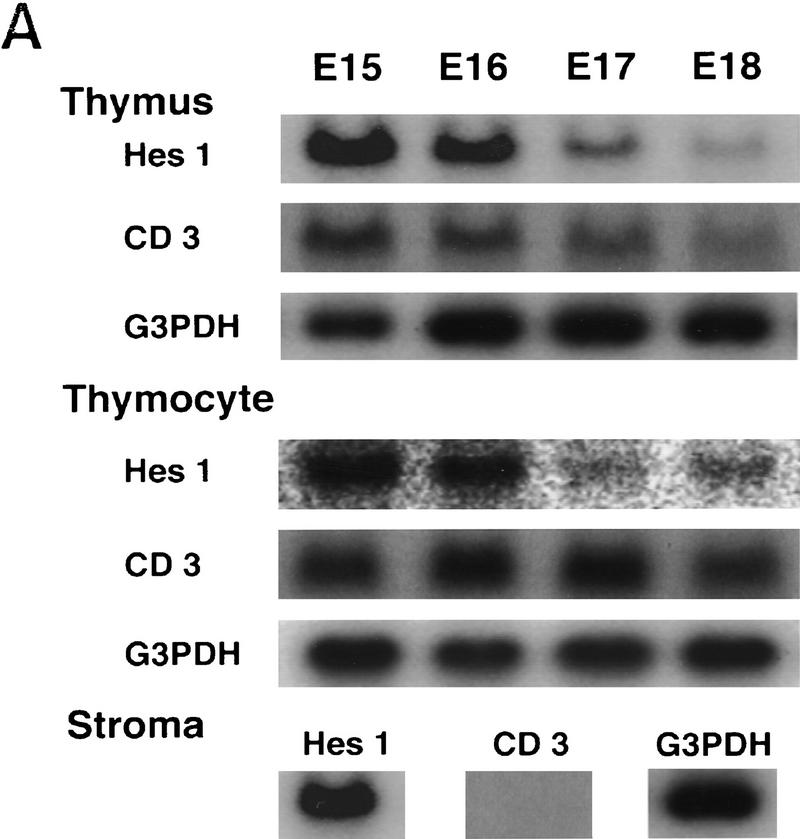
Hes1 expression during normal thymic development was first determined by Northern blot analysis. Hes1 was already expressed at a high level in the developing thymus at embryonic day 15 (E15), but the expression gradually decreased afterwards (Fig. 1A). To determine the cell types that expressed Hes1, thymocytes and thymic stroma were separately prepared. Thymic stroma was prepared by treating the thymus with deoxyguanosine (dGUO) and it was negative for the thymocyte-specific CD3 expression (Fig. 1A). Hes1 was expressed in both thymocytes and thymic stroma (Fig. 1A), suggesting that Hes1 functions in both cell types.
Figure 1.
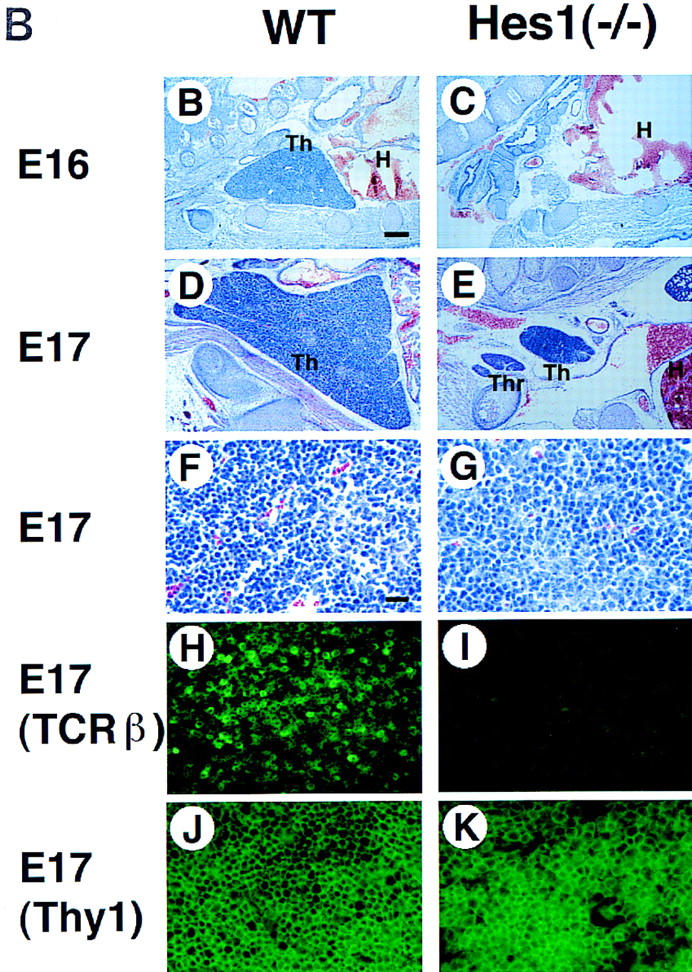
Hes1 expression in the developing thymus and the thymic defects of mice mutant for Hes1. (A) Hes1 expression in the thymus was determined by Northern blot analysis. Hes1 was already expressed at a high level at E15, but expression decreased gradually afterward. Hes1 was expressed by both thymocytes and stroma while CD3 was expressed only by thymocytes. G3PDH expression was examined as a control. (B–K) Thymic sections were prepared from wild-type (B,D,F,H,J) and Hes1-null (C,E,G,I,K) embryos and subjected to HE staining (B–G) and immunohistochemistry with anti-TCRβ (H,I) and anti-Thy1 antibodies (J,K). Hes1-null embryos lacked thymus (C) or had a very small thymus (E) compared to wild type (D). (F,G) A higher magnification of D and E. (H,I) Even when the thymus existed, αβ TCR+ thymocytes were absent in Hes1-null embryos (I), whereas they were present in wild-type thymus (H). (J,K) In the Hes1-null thymus, there were Thy1+ cells (K) as in the wild-type thymus (J). Scale bar, 150 μm (B–E); 25 μm (F–K). (H) Heart; (Th) thymus; (Thr) thyroid.
To determine the Hes1 function in T cell development, we analyzed the mice mutant for Hes1. Hes1-null mice exhibit severe defects of neural tube and eye morphogenesis and die during gestation or just after birth (Ishibashi et al. 1995; Tomita et al. 1996). Surprisingly, >90% of Hes1-null embryos completely lacked the thymus (Fig. 1C), and in the rest the thymus was much smaller in size than that of the wild type (Fig. 1D,E). In the thymus of Hes1-null mice, there were some thymocytes (Thy1+, Fig. 1G,K) but TCRβ (Fig. 1I) and TCRγδ expression (data not shown) was not detectable, suggesting that mature T cells were virtually absent. These results demonstrate that Hes1 is essential for T cell development.
Because Hes1 is also expressed in embryonal liver and spleen (Sasai et al. 1992), other lymphohematopoietic lineages in Hes1-null mice were examined by studying fetal liver cells. The number of the recovered Hes1-null fetal liver cells [(2.0 ± 0.4) × 107] was comparable to that of the wild type cells [(2.5 ± 0.5) × 107]. Flow cytometric analysis revealed that erythroids (TER-119+, Fig. 2A), myeloids (Gr-1+, Fig. 2B), and monocytes (Mac-1+, Fig. 2C) were normally generated in the absence of Hes1. In addition, Hes1-null fetal liver cells cultured on PA6 stromal cells in the presence of IL-7 (Sudo et al. 1989; Rolink et al. 1993) generated B-lineage cells comparably to wild-type cells (IgM+, B220+, Fig. 2D,E). Thus, cells of lymphohematopoietic lineages other than thymocytes appeared to develop normally in mice mutant for Hes1.
Figure 2.
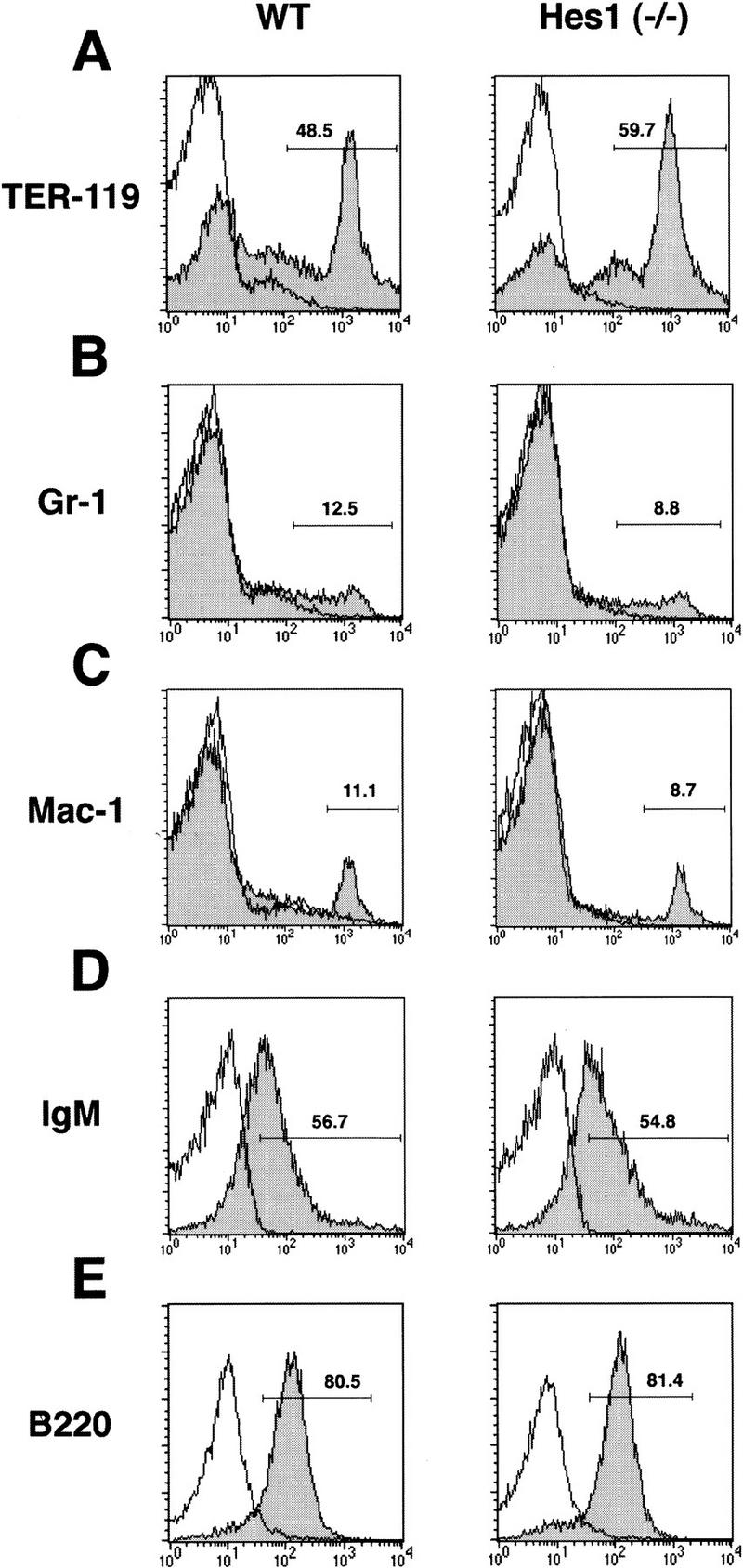
Flow cytometric analysis of fetal liver cells. Flow cytometric analysis was performed to determine the surface expression of TER-119 (A), Gr-1 (B), and Mac-1 (C). For B cell lineage analysis, fetal liver cells were cultured on PA6 stromal cells in the presence of IL-7 and examined for expression of IgM (D) and B220 (E). Cells were analyzed after incubation in the presence (shaded area) or absence (bold line only) of FITC-conjugated antibodies. No abnormality was detected in Hes1-null cells.
Hes1 is critical for thymocyte expansion in a cell-autonomous manner
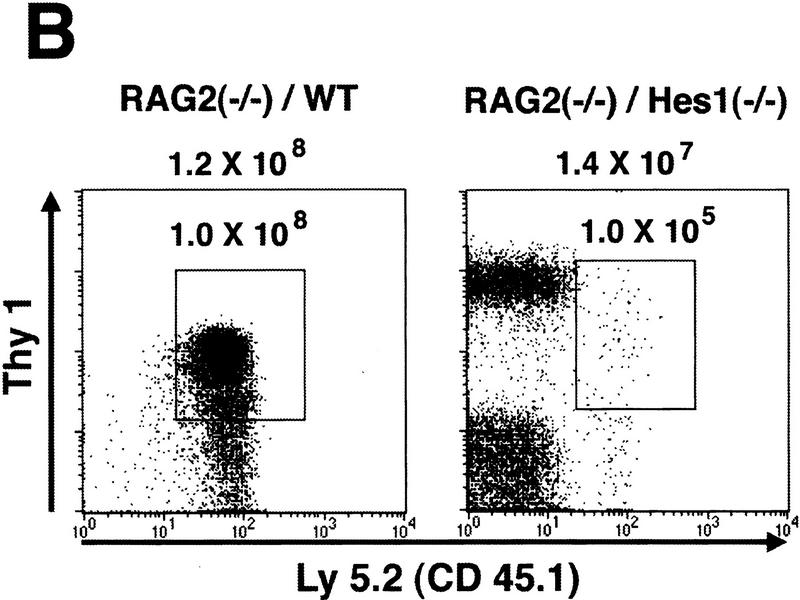
Because Hes1 is expressed in both stromal cells and thymocytes, the thymic phenotypes of Hes1-null mice could be due to abnormality of stromal cells and/or thymocytes. To determine whether Hes1-null thymocytes are intrinsically affected, we made bone marrow chimeras using RAG2-null mutant mice, which have no mature T or B lymphocytes (Shinkai et al. 1992). Fetal liver cells from Hes1-null or wild-type donor embryos were injected intravenously into irradiated RAG2-null mice of C57BL/6 background to allow differentiation of donor progenitors in the environment of normal thymic stromal cells. Lymphoid cells (Thy1+) of the donor origin were distinguished by Ly5.2 expression from those of possible host origin that are negative for Ly5.2 (Fig. 3B). This analysis could also help determine more precisely the stage at which T cell development is impaired in the absence of Hes1.
Figure 3.
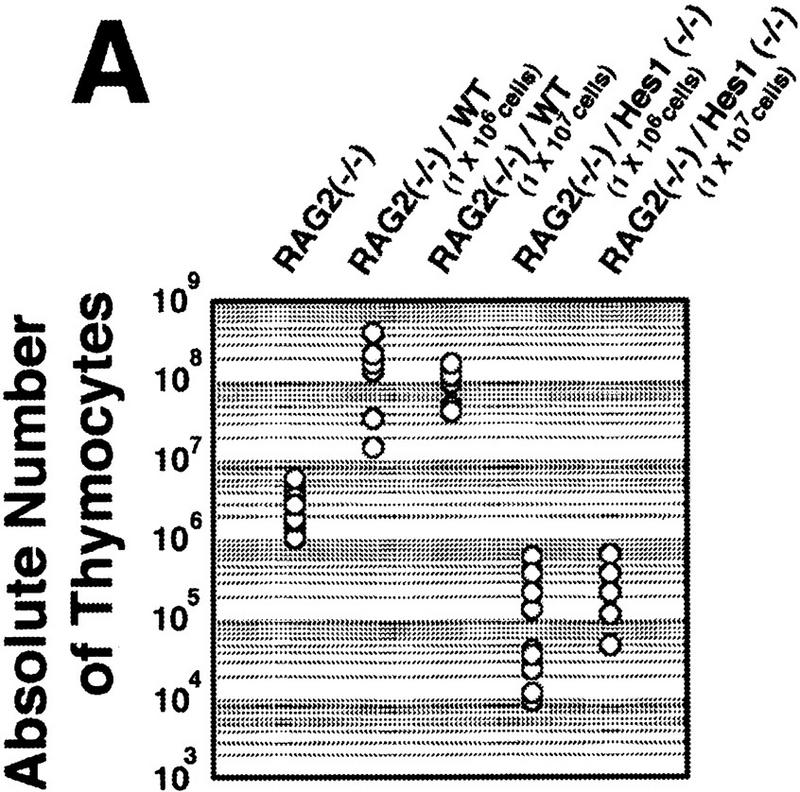
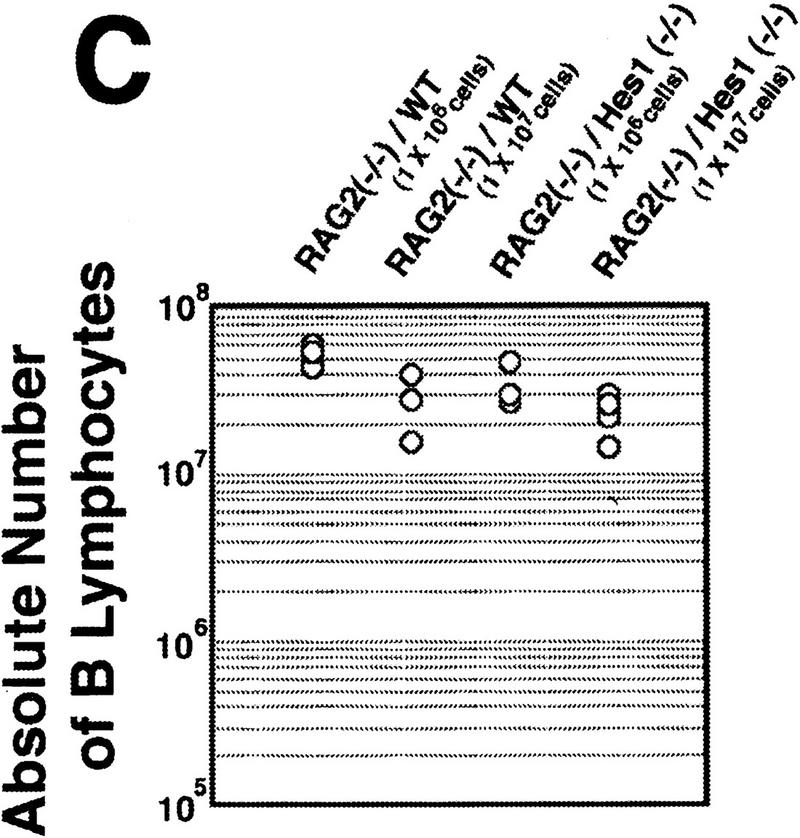
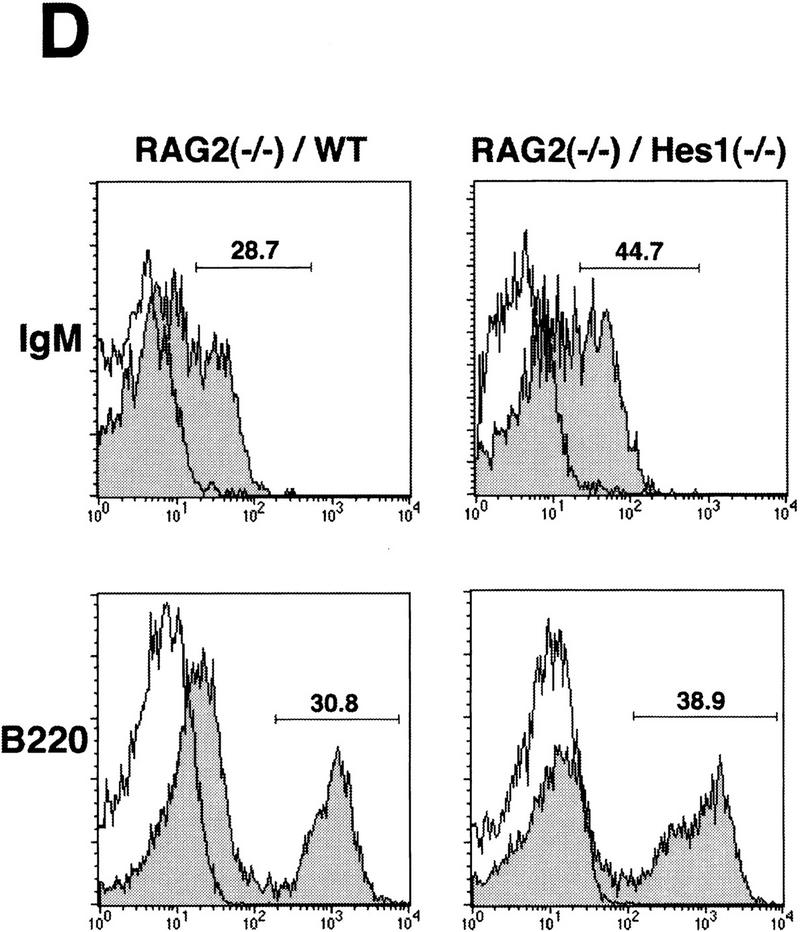
Reconstitution of the lymphoid system of RAG2 mutant mice by transfer of wild-type and Hes1-null fetal liver cells. Either wild-type or Hes1-null fetal liver cells (1 × 106 or 1 × 107, indicated in parentheses above the panel) were transferred intravenously into RAG2 mutant mice to reconstitute the lymphoid system, and after 4–6 weeks of transfer, the lymphoid cells were examined. (A) The absolute numbers of total thymocytes. From the reconstituted thymuses, only Ly5.2+ cells were counted. The average thymocyte number with standard error is RAG2−/−, (3.2 ± 0.6) × 106 (n = 10); RAG2−/−/WT(1 × 106 cells injected), (1.6 ± 0.3) × 108 (n = 13); RAG2−/−/WT(1 × 107 cells injected), (1.0 ± 0.2) × 108 (n = 6); RAG2−/−/Hes1−/−(1 × 106 cells injected), (1.7 ± 0.7) × 105 (n = 9); RAG2−/−/Hes1−/−(1 × 107 cells injected), (4.1 ± 1.0) × 105 (n = 8). The number of Hes1-null thymocytes averaged 200- to 1000-fold less than that of wild-type thymocytes and 10- to 20-fold less than that of irradiated and nonreconstituted RAG2-null thymocytes. (B) Flow cytometric analysis of the reconstituted thymus with α-Ly5.2 and α-Thy1. The absolute number of total cells is indicated above each panel; the number of Ly5.2+Thy1+ cells is shown above the gated region. (C) The absolute numbers of B cells in the spleen. The B cell number of Hes1-null origin was normal. (D) Flow cytometric analysis of the reconstituted spleen with α-IgM and α-B220. B cells developed normally in the absence of Hes1.
When reconstituted with wild-type or Hes1-null fetal liver cells, the RAG2-null hosts had a comparable number of mature B cells in the spleen (IgM+, B220+, Fig. 3C,D), indicating that reconstitution was successful and that B cells develop normally in the absence of Hes1. In contrast, the absolute number of thymocytes of the Hes1-null origin was by far less than that of the wild-type origin in the reconstituted RAG2-null thymus (Fig. 3A). When 1 × 106 and 1 × 107 fetal liver cells of wild-type origin were injected, an average of 1.6 × 108 (n = 13) and 1.0 × 108 (n = 6) thymocytes, respectively, was recovered. In contrast, when 1 × 106 Hes1-null cells were injected, only 1.7 × 105 thymocytes were recovered (n = 9); and even when 1 × 107 Hes1-null cells were injected, only 4.1 × 105 thymocytes were recovered (n = 8), indicating that the thymocyte number of the Hes1-null origin was 200- to 1000-fold less than that of wild-type origin. The thymocyte number of the Hes1-null origin was still 10- to 20-fold less than that of the nonreconstituted RAG2-null host mice (3.2 × 106 cells; n = 10) (Fig. 3A). These results demonstrate that thymocytes are intrinsically affected by Hes1 mutation and that Hes1 is critical for expansion of thymocytes.
Thymocyte expansion at the DN stage is impaired by Hes1 mutation
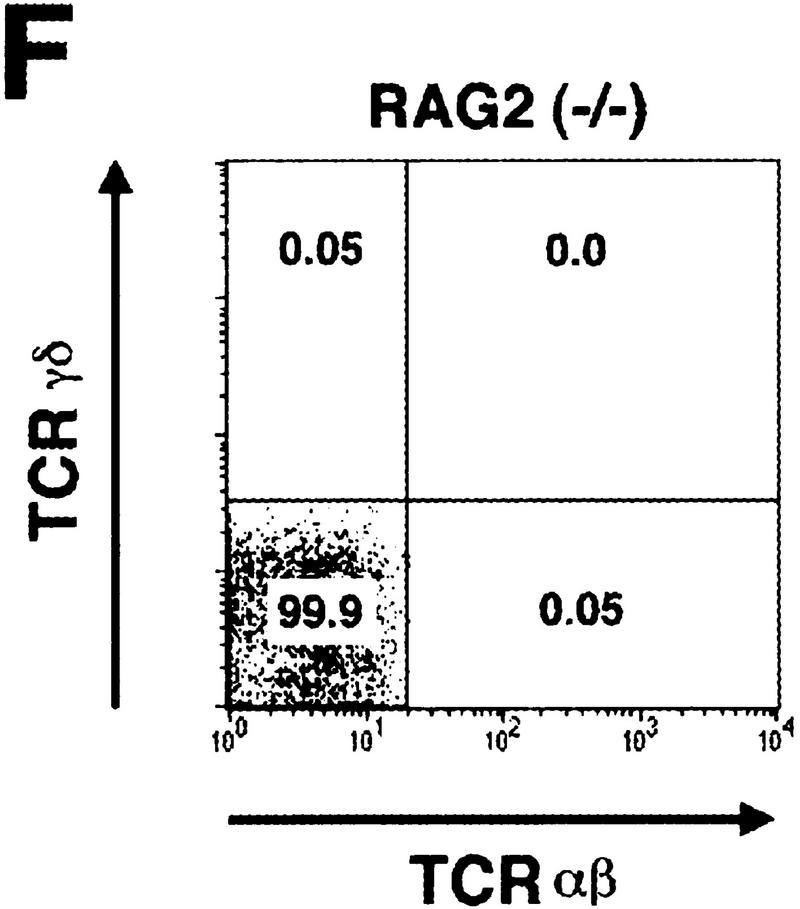
To determine the stage at which T cell development is impaired in the absence of Hes1, thymocytes of the donor origin were analyzed further with various surface markers. Whereas both αβ and γδ T cells were generated from wild-type donors, neither mature T cells were detected in the thymus reconstituted with Hes1-null donor cells (Fig. 4A). Furthermore, the vast majority of thymocytes of Hes1-null origin were negative for expression of the surface antigens CD4 and CD8 (Fig. 4B) but highly expressed the early marker heat-stable antigen (HSA) in the virtual absence of CD3 (Fig. 4C). Thus, in the absence of Hes1, T cell development is arrested at the CD3−DN stage. Essentially the same abnormality was observed irrespective of the number of the injected cells (1 × 106 or 1 × 107, Fig. 4A–C).
Figure 4.
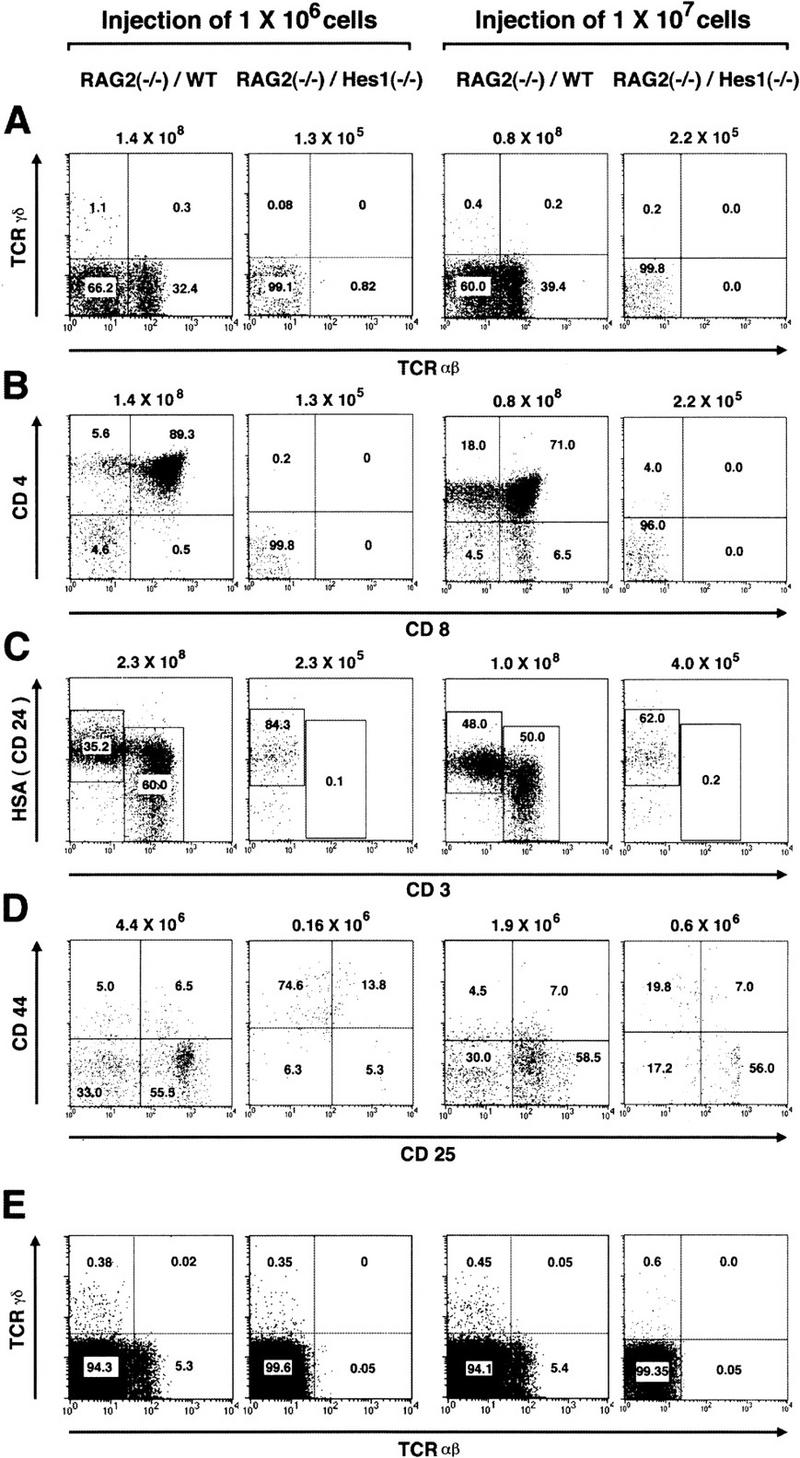
Flow cytometric analysis of reconstituted T-lineage cells. Flow cytometric analysis of reconstituted thymocytes (A–D) and spleen cells (E) and nonreconstituted host spleen cells (F) was performed to determine the surface expression of αβ and γδ TCR (A,E,F), CD4 and CD8 (B), HSA and CD3 (C), and CD44 and CD25 (D). The absolute number of Ly5.2+ thymocytes is indicated above each panel (A–C). For analysis of surface expression of CD44 and CD25, DN thymocytes from donor origin were analyzed (D), and the number is shown above each panel (D). In the absence of Hes1, αβ and γδ TCR+ cells, CD4+CD8+ DP cells, and CD3+ cells were virtually absent in the thymus (A–C). In the spleen reconstituted with Hes1-null cells, γδ T cells were present at a comparable level to the wild type, whereas αβ T cells were not detected (E).
Because DN cells are further divided into four distinct differentiation stages based on the profiles in the surface expression of CD44 and CD25 (Godfrey and Zlotnik 1993; Godfrey et al. 1993, 1994; Pénit et al. 1995), donor DN thymocytes were analyzed with these markers (Fig. 4D). At the first stage (CD44+CD25−) DN cells are normally resting and the absolute number of Hes1-null DN thymocytes was, on an average, 1.3 × 105 cells (n = 4) and 0.6 × 105 cells (n = 4) when 1 × 106 and 1 × 107 cells, respectively, were injected. These numbers were comparable to those of wild-type DN cells (1.2 × 105 cells; n = 9). However, when 1 × 106 Hes1-null cells were injected, at the subsequent stages the number of Hes1-null thymocytes was by far less than that of the wild-type cells, which are known to proliferate TCR independently first and then TCR dependently during the DN stages (Fig. 4D). When 1 × 107 Hes1-null cells were injected, DN thymocytes increased at the CD44−CD25+ stage (2.2 × 105 cells; n = 4), but the number was still much less than the wild type (1.5 × 106 cells; n = 9) (Fig. 4D). Thus, proper expansion of DN thymocytes was severely impaired in the absence of Hes1.
The spleen reconstituted with Hes1-null donors contained virtually no αβ T cells, but interestingly it had some γδ T cells (Fig. 4E). Because the RAG2-null host mice did not have such T cells (Fig. 4F), they were derived from Hes1-null fetal liver cells. Thus, some γδ T cells may develop normally in the absence of Hes1, although it remains to be determined whether they differentiate at the intrathymic or extrathymic environments.
Both TCR-independent and -dependent thymocyte expansion is affected by Hes1 mutation
DN cells normally undergo rearrangement of TCRβ locus at the CD44−CD25+ stage, and only when the β locus is productively rearranged are these cells allowed to selectively proliferate and mature into the last stage (CD44−CD25−) (Godfrey and Zlotnik 1993; Godfrey et al. 1993, 1994; Pénit et al. 1995). Because the thymocyte number at the CD44−CD25+ and CD44−CD25− stages was reduced significantly by Hes1 mutation, it is possible that TCR gene rearrangement is affected in Hes1-null thymocytes. We therefore examined rearrangement of TCR genes by amplifying the genomic DNA of the reconstituted thymi (Anderson et al. 1992; Itohara et al. 1993; Maki et al. 1996). Because in reconstituted mice Hes1-null thymocytes were, on an average, 200- to 1000-fold less in number than wild-type thymocytes, we used a 500-fold dilution of the wild-type DNA as a template to normalize the cell numbers. In the thymus and spleen of RAG2 mutant mice reconstituted with wild-type cells, TCR gene rearrangements clearly occurred (+/+ in Fig. 5A–H). In contrast, when RAG2 mutant mice were reconstituted with 1 × 106 Hes1-null cells, complete rearrangement was not detected at the TCRα or β locus (−/− in Fig. 5A,E), although only a low level of D–J recombination could be detected at the TCRβ locus (−/− in Fig. 5C). TCRγ gene rearrangement was not detected either in the thymus reconstituted with 1 × 106 Hes1-null cells (−/− in Fig. 5G). These results demonstrated that the Hes1 mutation severely affected expansion of immature thymocytes before the initiation of TCR gene rearrangement. However, when RAG2 mutant mice were reconstituted with 1 × 107 Hes1-null cells, TCR gene rearrangements became detectable (−/− in Fig. 5B,D,F,H), suggesting that Hes1 is not a prerequisite for the TCR gene rearrangements per se. Even when TCR gene rearrangements could take place, Hes1-null thymocytes did not expand properly at the CD44−CD25+ and CD44−CD25− stages (see above), suggesting that TCR-dependent selective proliferation seemed to be disturbed in the absence of Hes1. However, the amount of TCR gene rearrangements was still low in the absence of Hes1; therefore, future experiments with TCR transgenic in the Hes1−/− background will be required to determine the role of Hes1 in TCR-dependent expansion and differentiation.
Figure 5.
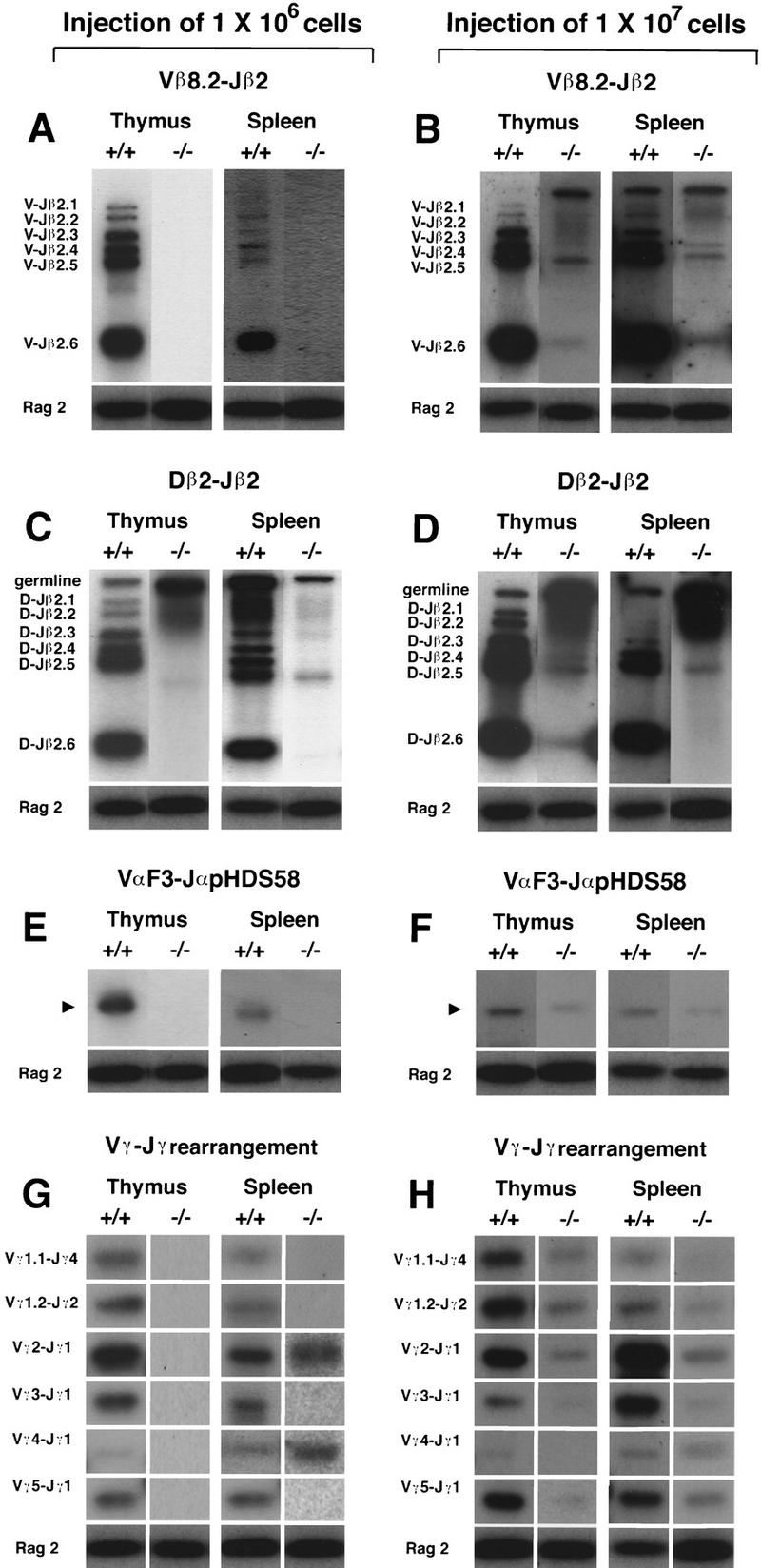
Rearrangement of TCR genes. Either 1 × 106 (A,C,E,G) or 1 × 107 (B,D,F,H) fetal liver cells of wild-type (+/+) and Hes1-null origin (−/−) were transferred intravenously into RAG2 mutant mice, and rearrangements of TCR genes were examined by PCR followed by Southern blot analysis with 32P-labeled oligonucleotide probes. In the thymus reconstituted with 1 × 106 Hes1-null cells, no apparent rearrangement was detected in the TCRα and TCRβ loci (A,E), although only a low level of D–J recombination could be detected (C). Whereas TCRγ gene rearrangement was not detected in the thymus reconstituted with 1 × 106 Hes1-null cells, TCRγ2 and TCRγ4 genes (Maki et al. 1996) were rearranged in the spleen in the absence of Hes1 (G). When 1 × 107 Hes1-null cells were injected, TCR gene rearrangement was detected (B,D,F,H). As an internal control for PCR, the RAG2 gene was amplified. The RAG2 signal was derived only from the donor cells.
Interestingly, TCRγ2 and TCRγ4 gene rearrangements were consistently and selectively detectable in the spleen but not in the thymus when RAG2 mutant mice were reconstituted with 1 × 106 Hes1-null fetal liver cells (−/− in Fig. 5G). Thus, γδ T cells with restricted repertoire could develop normally in the absence of Hes1.
Unique and essential functions of Hes1 in T cell development
In this study, we have demonstrated that early events of αβ and γδ T cell development in the thymus, such as cell expansion and TCR gene rearrangement, are severely impaired in Hes1-null mice. Particularly, Hes1 seems to be essential for cell expansion at the very early stages, as Hes1-null cells did not expand at the CD44+CD25+ stage in the reconstituted thymus when 1 × 106 fetal liver cells were injected. Homing of Hes1-null progenitors to the thymus appeared unaffected, as the number of Hes1-null thymocytes at the CD44+CD25− stage was comparable with that of wild-type cells. It is likely that Hes1-null thymocytes might remain as resting cells or be more susceptible to apoptosis. This defect of expansion might contribute to the lack of or a small-sized thymus in Hes1 mutant mice, although it is possible that certain functional defects of the Hes1-null stromal cells additionally contribute to the thymic aplasia.
It has been shown that the selective proliferation of DN cells that express functional TCRs involves various transcription factors such as HMG-box factors LEF-1 and TCF-1 and zinc finger transcription factor GATA-3 (Ting et al. 1996; Okamura et al. 1998). However, none of these factors appears to be involved in cell expansion before TCR gene rearrangement. Thus, Hes1 is unique in regulating TCR-independent expansion of thymocytes at the earliest stage. Interestingly, in the absence of IL-7 receptor, thymocyte expansion is severely disturbed before TCR gene rearrangement (Peschon et al. 1994; Candéias et al. 1997), suggesting that IL-7 is one of the major growth factors involved in initial TCR-independent thymocyte proliferation. It is therefore tempting to speculate that Hes1 functions downstream of the IL-7 signaling pathway. In addition to TCR-independent expansion, Hes1 seems to be involved in expansion at later stages, as—even though TCR gene rearrangement occurred in the thymus of RAG2-null mice reconstituted with as many as 1 × 107 fetal liver cells—Hes1-null thymocytes were still confined exclusively to the DN fraction with negligible increase in cell number. In the developing nervous system, Hes1 is known to keep cells in a proliferative state and down regulation of Hes1 expression leads to transition to a nonproliferative differentiation stage (Kageyama and Nakanishi 1997). Present results have indicated that similarly in the thymus Hes1 promotes the initial expansion of immigrant progenitor cells, which is essential for the extensive clonal diversification and selection to generate mature T cells.
Hes1 is also known as a target gene of the Notch signaling (Jarriault et al. 1995; Nishimura et al. 1998). It has been shown that both Notch and Hes1 regulate CD4 SP versus CD8 SP fate choice of T cell development (Robey et al. 1996; Kim and Siu 1998). Although this function could not be examined here, as T cell development was arrested much earlier by Hes1 mutation, collectively these data suggest that Hes1 might function at multiple steps of T cell development.
Materials and methods
Hes1 expression in the thymus
Northern blot analysis was done as described previously (Sasai et al. 1992). The thymic stroma devoid of thymocytes was prepared as reported (Ikuta et al. 1990), with some modifications: Fetal thymic lobes (E15) were cultured at 37°C for 6 days in 7% CO2 on filters in 12-well plates containing RPMI1640 supplemented with 10% fetal calf serum, 1.35 mm dGUO, and 50 μm β-mercaptoethanol.
Histological analysis of mouse embryos
Whole embryos were fixed with 4% paraformaldehyde in PBS at 4°C for 1 hr and incubated in 30% sucrose in PBS at 4°C overnight. Frozen sections were prepared at 16 μm thickness and subjected to hematoxylin–eosin (HE) staining and immunohistochemistry. For immunohistochemistry, sections were incubated with biotinylated anti-TCRβ (Pharmingen, H57-597) and anti-Thy1 antibody (Phamingen, 53-2.1) followed by visualization with avidin-labeled fluorescein (Vector).
Fetal liver cell analysis
Single cell suspensions from fetal livers of E17 wild-type or Hes1-null embryos were prepared and subjected to flow cytometric analysis (Becton-Dickinson). The antibodies used were as follows: anti-TER-119 (Pharmingen, TER-119), anti-Gr-1 (Pharmingen, RB6-8C5), and anti-Mac-1 (Pharmingen, M1/70). Cultured fetal liver cells on PA6 stromal cells with IL-7 were subjected to immunostaining with anti-IgM (Pharmingen, R6-60.2), and anti-B220 antibodies (Pharmingen, RA3-6B2). Detailed culture conditions were described previously (Sudo et al. 1989; Rolink et al. 1993).
Reconstitution of RAG2 mutant mice
Fetal liver cells (1 × 106 to 10 × 106) from E13–E16 wild-type and Hes1-null embryos (Ly5.2+) were injected intravenously into 4 Gy irradiated RAG2 mutant mice (Ly5.2−). After 4–6 weeks, single cell suspensions were prepared from thymuses and spleens of the reconstituted RAG2 mutant mice and subjected to flow cytometric analysis (Becton-Dickinson). Only Ly5.2+ cells (the donor origin) were analyzed. The antibodies used were as follows: anti-TCRβ (Pharmingen, H57-597), anti-γδ TCR (Pharmingen, GL-3), anti-CD4 (Pharmingen, RM4-5), anti-CD8 (Pharmingen, 53-6.7), anti-CD25 (Pharmingen, 7D4), anti-CD44 (Pharmingen, IM7), anti-CD24 (HSA) (Pharmingen, M1/69), anti-CD3 (Pharmingen, 145-2C11), anti-Thy1 (Phamingen, 53-2.1), and anti-CD45.1 (Ly5.2) (Pharmingen, A20).
PCR analysis for TCR gene rearrangement
The genomic DNA extracted from the reconstituted thymuses was amplified by PCR and subjected to Southern blot analysis with 32P-labeled oligonucleotide probes as described previously (Anderson et al. 1992; Itohara et al. 1993; Maki et al. 1996). Because the numbers of Hes1-null thymocytes were 200- to 1000-fold less than those of wild-type thymocytes, we used a 500-fold dilution of the wild-type samples to normalize the cell numbers. As a PCR control, we amplified the RAG2 gene, which was derived only from donor cells. In each experiment, a similar intensity of the RAG2 band was detected, indicating that the number of donor cells was normalized.
Acknowledgments
We thank Koichi Ikuta for RAG2 mutant mice and discussion, Kazuhiro Iwai, Shunichi Takeda, Yoshimoto Katsura and Kazushige Maki for discussion, and Tetsuo Sudo for IL-7. This work was supported by Special Coordination Funds for Promoting Science and Technology and research grants from the Ministry of Education, Science, Sports, and Culture of Japan and from Japan Society for the Promotion of Science.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked ‘advertisement’ in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL rkageyam@virus.kyoto-u.ac.jp; FAX 81-75-751-4807.
E-MAIL hattori@med.kyoto-u.ac.jp; FAX 81-75-753-4403.
References
- Anderson G, Moore NC, Owen JJT, Jenkinson EJ. Cellular interactions in thymocyte development. Annu Rev Immunol. 1996;14:73–99. doi: 10.1146/annurev.immunol.14.1.73. [DOI] [PubMed] [Google Scholar]
- Anderson SJ, Abraham KM, Nakayama T, Singer A, Perlmutter RM. Inhibition of T-cell receptor β-chain gene rearrangement by overexpression of the non-receptor protein tyrosine kinase p56lck. EMBO J. 1992;11:4877–4886. doi: 10.1002/j.1460-2075.1992.tb05594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candéias S, Muegge K, Durum SK. IL-7 receptor and VDJ recombination: Trophic versus mechanistic actions. Immunity. 1997;6:501–508. doi: 10.1016/s1074-7613(00)80338-6. [DOI] [PubMed] [Google Scholar]
- Chen H, Thiagalingam A, Chopra H, Borges MW, Feder JN, Nelkin BD, Baylin SB, Ball DW. Conservation of the Drosophila lateral inhibition pathway in human lung cancer: A hairy-related protein (HES-1) directly represses achaete-scute homolog-1 expression. Proc Natl Acad Sci. 1997;94:5355–5360. doi: 10.1073/pnas.94.10.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey DI, Zlotnik A. Control points in early T-cell development. Immunol Today. 1993;14:547–553. doi: 10.1016/0167-5699(93)90186-O. [DOI] [PubMed] [Google Scholar]
- Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3− CD4− CD8− triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993;150:4244–4252. [PubMed] [Google Scholar]
- Godfrey DI, Kennedy J, Mombaerts P, Tonegawa S, Zlotnik A. Onset of TCR-β gene rearrangement and role of TCR-β expression during CD3− CD4− CD8− thymocyte differentiation. J Immunol. 1994;152:4783–4792. [PubMed] [Google Scholar]
- Ikuta K, Kina T, MacNeil I, Uchida N, Peault B, Chien Y, Weissman IL. A developmental switch in thymic lymphocyte maturation potential occurs at the level of hematopoietic stem cells. Cell. 1990;62:863–874. doi: 10.1016/0092-8674(90)90262-d. [DOI] [PubMed] [Google Scholar]
- Ishibashi M, Moriyoshi K, Sasai Y, Shiota K, Nakanishi S, Kageyama R. Persistent expression of helix-loop-helix factor HES1 prevents mammalian neural differentiation in the central nervous system. EMBO J. 1994;13:1799–1805. doi: 10.1002/j.1460-2075.1994.tb06448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi M, Ang S-L, Shiota K, Nakanishi S, Kageyama R, Guillemot F. Targeted disruption of mammalian hairy and Enhancer of split homolog-1 (HES-1) leads to up-regulation of neural helix-loop-helix factors, premature neurogenesis, and severe neural tube defects. Genes & Dev. 1995;9:3136–3148. doi: 10.1101/gad.9.24.3136. [DOI] [PubMed] [Google Scholar]
- Itohara S, Mombaerts P, Lafaille J, Iacomini J, Nelson A, Clarke AR, Hopper ML, Farr A, Tonegawa S. T cell receptor δ gene mutant mice: Independent generation of αβ T cells and programmed rearrangement of γδ TCR genes. Cell. 1993;72:337–348. doi: 10.1016/0092-8674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A. Signalling downstream of activated mammalian Notch. Nature. 1995;377:355–358. doi: 10.1038/377355a0. [DOI] [PubMed] [Google Scholar]
- Kageyama R, Nakanishi S. Helix-loop-helix factors in growth and differentiation of the vertebrate nervous system. Curr Opin Genet Dev. 1997;7:659–665. doi: 10.1016/s0959-437x(97)80014-7. [DOI] [PubMed] [Google Scholar]
- Kim HK, Siu G. The Notch pathway intermediate HES-1 silences CD4 gene expression. Mol Cell Biol. 1998;18:7166–7175. doi: 10.1128/mcb.18.12.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki K, Sunaga S, Ikuta K. The V-J recombination of T cell receptor-γ genes is blocked in interleukin-7 receptor-deficient mice. J Exp Med. 1996;184:2423–2427. doi: 10.1084/jem.184.6.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M, Isaka F, Ishibashi M, Tomita K, Tsuda H, Nakanishi S, Kageyama R. Structure, chromosomal locus, and promoter of mouse Hes2 gene, a homologue of Drosophila hairy and Enhancer of split. Genomics. 1998;49:69–75. doi: 10.1006/geno.1998.5213. [DOI] [PubMed] [Google Scholar]
- Okamura RM, Sigvardsson M, Galceran J, Verbeek S, Clevers H, Grosschedl R. Redundant regulation of T cell differentiation and TCR α gene expression by the transcription factors LEF-1 and TCF-1. Immunity. 1998;8:11–20. doi: 10.1016/s1074-7613(00)80454-9. [DOI] [PubMed] [Google Scholar]
- Pénit C, Lucas B, Vasseur F. Cell expansion and growth arrest phases during the transition from precursor (CD4−8−) to immature (CD4+8+) thymocytes in normal and genetically modified mice. J Immunol. 1995;154:5103–5113. [PubMed] [Google Scholar]
- Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, Meyer JD, Davison BL. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey E, Chang D, Itano A, Cado D, Alexander H, Lans D, Weinmaster G, Salmon P. An activated form of Notch influences the choice between CD4 and CD8 T cell lineages. Cell. 1996;87:483–492. doi: 10.1016/s0092-8674(00)81368-9. [DOI] [PubMed] [Google Scholar]
- Rolink A, Haasner D, Nishikawa S-I, Melchers F. Changes in frequencies of clonable pre B cells during life in different lymphoid organs of mice. Blood. 1993;81:2290–2300. [PubMed] [Google Scholar]
- Sasai Y, Kageyama R, Tagawa Y, Shigemoto R, Nakanishi S. Two mammalian helix–loop–helix factors structurally related to Drosophila hairy and Enhancer of split. Genes & Dev. 1992;6:2620–2634. doi: 10.1101/gad.6.12b.2620. [DOI] [PubMed] [Google Scholar]
- Shinkai Y, Rathbun G, Lam K-P, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, Alt FW. Rag-2-deficient mice lack mature lymphocytes owning to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Shortman K, Wu L. Early T lymphocyte progenitors. Annu Rev Immunol. 1996;14:29–47. doi: 10.1146/annurev.immunol.14.1.29. [DOI] [PubMed] [Google Scholar]
- Ström A, Castella P, Rockwood J, Wagner J, Caudy M. Mediation of NGF signaling by post-translational inhibition of HES-1, a basic helix-loop-helix repressor of neuronal differentiation. Genes & Dev. 1997;11:3168–3181. doi: 10.1101/gad.11.23.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo T, Ito M, Ogawa Y, Iizuka M, Kodama H, Kunisada T, Hayashi S-I, Ogawa M, Sakai K, Nishikawa S, Nishikawa S-I. Interleukin 7 production and function in stromal cell-dependent B cell development. J Exp Med. 1989;170:333–338. doi: 10.1084/jem.170.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting C-N, Olson MC, Barton KP, Leiden JM. Transcription factor GATA-3 is required for development of the T-cell lineage. Nature. 1996;384:474–478. doi: 10.1038/384474a0. [DOI] [PubMed] [Google Scholar]
- Tomita K, Ishibashi M, Nakahara K, Ang S-L, Nakanishi S, Guillemot F, Kageyama R. Mammalian hairy and Enhancer of split homolog 1 regulates differentiation of retinal neurons and is essential for eye morphogenesis. Neuron. 1996;16:723–734. doi: 10.1016/s0896-6273(00)80093-8. [DOI] [PubMed] [Google Scholar]
- von Boehmer H, Fehling HJ. Structure and function of the pre-T cell receptor. Annu Rev Immunol. 1997;15:433–452. doi: 10.1146/annurev.immunol.15.1.433. [DOI] [PubMed] [Google Scholar]