Abstract
Background
Carbon dioxide (CO2) therapy refers to the transcutaneous administration of CO2 for therapeutic purposes. This effect has been explained by an increase in the pressure of O2 in tissues known as the Bohr effect. However, there have been no reports investigating the oxygen dissociation of haemoglobin (Hb) during transcutaneous application of CO2 in vivo. In this study, we investigate whether the Bohr effect is caused by transcutaneous application of CO2 in human living body.
Methods
We used a novel system for transcutaneous application of CO2 using pure CO2 gas, hydrogel, and a plastic adaptor. The validity of the CO2 hydrogel was confirmed in vitro using a measuring device for transcutaneous CO2 absorption using rat skin. Next, we measured the pH change in the human triceps surae muscle during transcutaneous application of CO2 using phosphorus-31 magnetic resonance spectroscopy (31P-MRS) in vivo. In addition, oxy- and deoxy-Hb concentrations were measured with near-infrared spectroscopy in the human arm with occulted blood flow to investigate O2 dissociation from Hb caused by transcutaneous application of CO2.
Results
The rat skin experiment showed that CO2 hydrogel enhanced CO2 gas permeation through the rat skin. The intracellular pH of the triceps surae muscle decreased significantly 10 min. after transcutaneous application of CO2. The NIRS data show the oxy-Hb concentration decreased significantly 4 min. after CO2 application, and deoxy-Hb concentration increased significantly 2 min. after CO2 application in the CO2-applied group compared to the control group. Oxy-Hb concentration significantly decreased while deoxy-Hb concentration significantly increased after transcutaneous CO2 application.
Conclusions
Our novel transcutaneous CO2 application facilitated an O2 dissociation from Hb in the human body, thus providing evidence of the Bohr effect in vivo.
Introduction
Carbon dioxide (CO2) therapy refers to the transcutaneous or subcutaneous administration of CO2 for therapeutic purposes especially in the treatment of peripheral vascular disorder [1]. One example of this is the use of spa therapy that emerged as an important treatment in Europe during the 1800 s and is still in use in many countries today [2]. Another example is the use of artificial CO2 enriched water for bathing, which has been clinically applied to improve ischemic limb symptom [3]–[5]. In plastic surgery, subcutaneous injection of CO2 is applied if skin irregularity and/or adiposity occurs [6], [7]. Recently, some reports showed that the transcutaneous administration of CO2 rich spa gas improves microcirculation and symptoms in patients who have intermittent claudication [8], [9] and Raynoud's phenomenon [10]. These therapeutic effects of CO2 are caused by an increase in blood flow and microcirculation assessed by Laser Doppler [10], and an increase of tcPO2 in ischemic tissues, which is explained by the Bohr effect [3], [5], [8], [9], [11].
The Bohr effect is represented by a rightward shift of the O2–Hb dissociation curve with an increase in pCO2 or decrease in pH [12]–[15]. It has frequently been studied in physiology. However, subjects' blood samples were used in these previous experiments, and the O2 affinity of Hb measured only in vitro or ex vivo. In addition, although the Bohr effect has often been referred to as an explanation for the therapeutic usefulness of CO2 therapies, no reports have actually provided evidence for the Bohr effect in CO2 therapies as well as evidence for the transcutaneous absorption of CO2. In addition, to the best of our knowledge, there have been no reports that have investigated the Bohr effect in vivo.
To produce the effect of CO2 therapy, an adequate amount of CO2 needs to be delivered to local tissues without difficulty and invasion. Previously, only three methods of CO2 therapy have been reported that have been able to accomplish this. The first method requires bathing in CO2-enriched water such as in a carbonated spa [2], or artificially carbonated water prepared by the chemical reaction of succinic acid and sodium bicarbonate [3], [4], or by blowing micro-bubbles into the water through a CO2 gas-permeable membrane [5], [16]–[19]. However, the CO2 concentration of saturated CO2-enriched water is only 0.1% [16]–[18] and there is no evidence of CO2 absorption into the human body. The second method is direct subcutaneous CO2 injection [6], [7]. Even though direct subcutaneous CO2 injection can deliver pure CO2 into local tissues, this method is invasive, involves an infection risk, and is difficult to use over a large area of the body.
The third method is the transctaneous administration of CO2 natural spa gas [8]–[10]. Previous reports have outlined this method of transctaneous administration of CO2 natural spa gas into the whole limb as follows: The subjects take a bath, humidify their skin and then CO2 gas is administered transcutaneously by covering the subject's body with a large bag. This method administrates an adequate CO2 concentration, however, it is difficult to obtain CO2 natural spa gas, and a large space is needed to set up the bath.
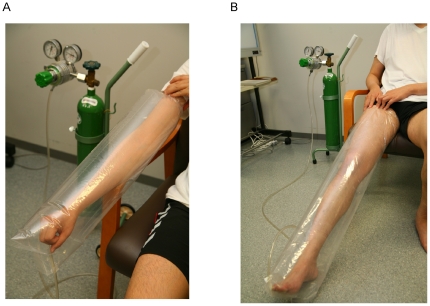
To solve these problems, we designed a novel transcutaneous CO2 application system using 100% CO2 gas, a transcutaneous CO2 absorption-enhancing hydrogel (CO2 hydrogel) and a CO2 adaptor that seals the body surface and traps the gas inside (Fig. 1, A and B). In this system, the CO2 hydrogel is applied to the skin to allow CO2 to dissolve and penetrate into the local tissue, which humidifies the skin without bathing, thereby forming a passage for CO2 to reach the local tissues. This system allows for the easy application of CO2 to any site of the body. In addition, the system provides for simple sterilization and is not invasive.
Figure 1. Rationale of the experimental outline.
(A) The application of our novel system for transcutaneous application of CO2 (For the upper limb). (B) The application of our novel system for transcutaneous application of CO2 (For the lower limb).
In this study, we investigated whether our transcutaneous CO2 application system caused CO2 absorption into local tissues and the Bohr effect in the human body, by real time and non-invasive measurement of changes in pH and oxygenated and deoxygenated-hemoglobin volume.
Methods
Novel system for transcutaneous CO2 application
A set of measuring devices for transcutaneous CO2 absorption, a plastic CO2 adaptor and transcutaneous CO2 absorption-accelerating hydrogel, (Formulation: carbomer (0.65%), glycerin (5.00%), sodium hydroxide (0.18%), sodium alginate (0.15%), sodium dihydrogen phosphate (0.15%), methylparaben (0.10%), and deionized water (balance)) were obtained from NeoChemir Inc., Kobe, Japan (International patent publication number: WO2004/002393). Pure CO2 gas was purchased from Kobe Sanso Inc., Kobe, Japan. The actual application to humans is shown in Figures 1A and 1B. When we applied CO2 transcutaneously using this system, sweating and redness of the skin were noted. In addition, the blood flow to the fingers increased, as shown by a Laser Doppler study (Data not shown).
Validation of transcutaneous CO2 absorption accelerating hydrogel using a measuring device for transcutaneous CO2 absorption
Subjects: Six Sprague–Dawley rats were purchased (CLEA Japan, Tokyo, Japan). The animal experiment plan was reviewed and approved by the Animal Research Committee of Kobe University Graduate School of Medicine. The approval ID is P00220.
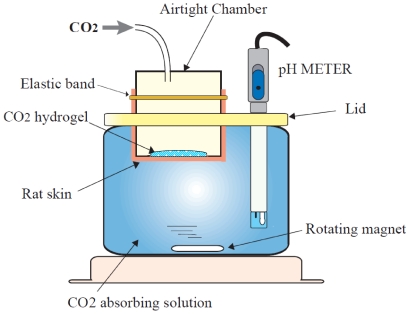
A set of measuring devices for transcutaneous CO2 absorption consisting of (1) a container filled with CO2 absorbing solution (600 mL of pure water) with a pH meter (D52: HORIBA, Kyoto, Japan) and a magnetic stirrer and (2) an airtight CO2 gas chamber, with a 5-cm diameter hole covered by a skin specimen positioned at the bottom of the chamber, was used to observe the permeability of CO2 gas thorough the rat skin specimen covered with, or without, the CO2 hydrogel. (Figure 2) The CO2-absorbing solution absorbs CO2 gas through the skin specimen, and the pH of the solution decreases depending on the volume of the absorbed CO2 (H2O + CO2 → H+ + HCO3 −). The skin from depilated Sprague–Dawley rats was harvested in 10×10-cm sections. Immediately after harvesting, a skin specimen was positioned over the hole of the chamber, in contact with the CO2-absorbing solution, the depilated surface facing upwards. CO2 hydrogel (0.5 g) was applied to the skin specimen in the Gel (+) groups but not in the Gel (−) groups, and the chamber was filled with pure CO2 gas. After filling, the pH of the solution was measured in 30-s intervals for 15 min. by a pH meter.
Figure 2. Measuring device to validate CO2 hydrogel in vitro using rat skin.
Measurement of intramuscular pH in vivo using phosphorus-31 magnetic resonance spectroscopy (31P-MRS) during transcutaneous application of CO2 in vivo
Subject: Five healthy male volunteers with no history of respiratory or vascular disease participated in this study. Subjects were 23–38 years old (average: 33.0±6.6). This study was approved and permitted by the Ethical Committee of Kobe University Graduate School of Medicine and informed consent was obtained by written from all subjects before the start of the study. The approval ID is 997.
31P-MRS: The intramuscular pH was measured in the triceps surae muscle. All MR studies were performed with a 1.5-T superconducting imaging system (Gyroscan NT-Intera; Philips Medical Systems, Best, The Netherlands) and a surface coil. CO2 hydrogel was applied to the subject's lower leg. A plastic CO2 adaptor was then attached to the subject's lower leg, and the surface coil was positioned over the adaptor. After measurement preparations, pure CO2 gas was flowed into the adaptor. Data were collected before infusion of CO2 and every 5 min. after infusion. Quantification of the 31P-MRS metabolite data was reported before [20], and pHi was determined from the chemical shift of Pi with respect to PCr.
Measurement of oxygenated and deoxygenated Hb concentration during transcutaneous application of CO2 in vivo
Subjects: Seven healthy male volunteers with no history of respiratory or vascular disease participated in this study. Subjects were 27–40 years of age (average: 32.0±4.6). This study was approved and permitted by the Ethical Committee of Kobe University Graduate School of Medicine and informed consent was obtained by written from all subjects before the start of the study. The approval ID is 619.
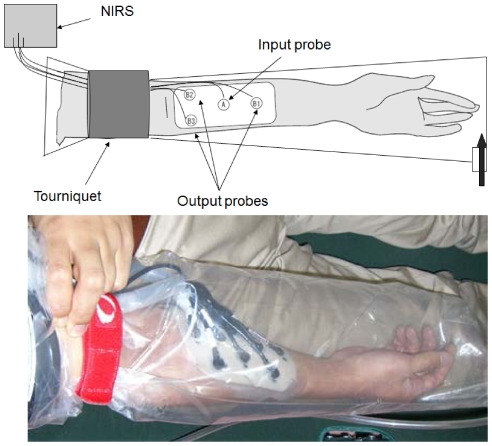
A near-infrared spectroscopy (NIRS), (NIRO-200 with multi-fiber adaptor: Hamamatsu Photonics. K. K. Hamamatsu, Japan), was used for Hb concentration measurement. Changes in oxygenated and deoxygenated Hb concentrations were measured using 3 channels by focusing on the differences in absorption of light at 775, 810, and 850 nm [21]–[23]. The recording probe was attached to the inner side of the subject's forearm. A pneumatic tourniquet system (ATS2000, Zimmer patient care division, Dover, OH) commonly used in orthopaedic surgery was used for avascularization of the arm.
Each subject entered the environmental chamber, which was maintained at an ambient temperature of 26°C and relative humidity of 45%. NIRS probes were attached to the subjects' forearms. A tourniquet was wound around the upper arms, and then Hb concentration was measured. After confirming that the oxy-/deoxy-Hb ratio had stabilized, the tourniquet was inflated to a pressure of 250 mmHg, a commonly used pressure level in surgeries to avoid bleeding from the forearm (Figure 3). Eight minutes after the inflation, CO2 hydrogel was applied to the subject's forearm, and the entire arm was enclosed by a CO2 adaptor. Ten minutes after the inflation, pure CO2 gas or air (control) was allowed to flow into the adaptor. The relative concentrations of the oxy- and deoxy-Hb were measured at 2-s intervals using NIRS. The duration of tourniquet inflation was limited to a maximum of 20 min to avoid ischemic damage to subjects' tissues.
Figure 3. Novel system for transcutaneous CO2 application in the forearm with NIRS probe.
Statistical analysis
Paired t-tests were used to compare all the variables in the control and CO2 groups. All values were analysed with measurement analysis of variance (ANOVA), followed by analysis of simple main effects. All data are presented as mean ± S.E.M. P<0.05 was considered statistically significant.
Results
Validation of transcutaneous CO2 absorption accelerating hydrogel using a measuring device for transcutaneous CO2 absorption
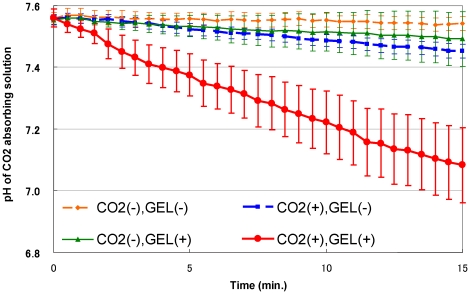
Before we used this system in humans, the validity of the CO2 hydrogel was confirmed in vitro using a measuring device for transcutaneous CO2 absorption (Figure 2). Four groups of rat skin specimens with or without CO2 hydrogel that had been filled with CO2 gas or air were used in the experiment. The CO2-absorbing solution receives CO2 gas through the skin specimen, and the pH of the solution decreases, depending on the volume of the absorbed CO2. The pH of the solution decreased time-dependently in the CO2 (+) groups, and the pH values were significantly lower in the CO2 (+) Gel (+) group compared to the CO2 (+) Gel (−) group after 3.5 min (Figure 4). These results showed that CO2 hydrogel actually enhanced CO2 gas permeation through the rat skin.
Figure 4. pH changes in CO2-absorbing solution during transcutaneous CO2 application through the rat skin with or without the CO2 hydrogel.
The graph shows pH changes during CO2 application with or without the CO2 hydrogel. (n = 6). Graph data are expressed as means ± S.E.M.
Measurement of intramuscular pH in vivo using 31P-MRS during transcutaneous application of CO2 in vivo
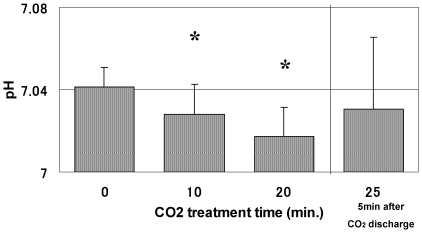
Next, to test this system in the human body, we measured the pH change in the muscle during transcutaneous application of CO2. We used phosphorus-31 magnetic resonance spectroscopy (31P-MRS) to measure the intracellular acid–base status in the subject's triceps surae muscle [20]. The room temperature was 25°C. CO2 hydrogel was first applied to the subject's lower leg, and the CO2 adaptor was attached to seal the lower leg; then the surface coil was applied over the adaptor adhering it to the calf. After preparing the subject for measurement, pure CO2 was infused into the adaptor. The measurements were performed before CO2 infusion and every 5 min. after infusion. The intracellular pH of the triceps surae muscle decreased significantly 10 min. after transcutaneous application of CO2 (Figure 5). The results showed that the intramuscular pH decreased by transcutaneous application of CO2 using this system in vivo. Although the pH change was expected to be buffered by body fluid and active blood flow, buffering was not enough to prevent the pH change. From these results, we confirmed that our novel system allowed transcutaneous penetration of CO2 in vivo.
Figure 5. Intramuscular pH changes in the triceps surae muscle during transcutaneous application of CO2 using 31P-MRS.
The graph shows that pH decreases during the accumulation of CO2. (n = 5) Graph data are expressed as means ± S.E.M.
Measurement of oxygenated and deoxygenated Hb concentration during transcutaneous application of CO2 in vivo
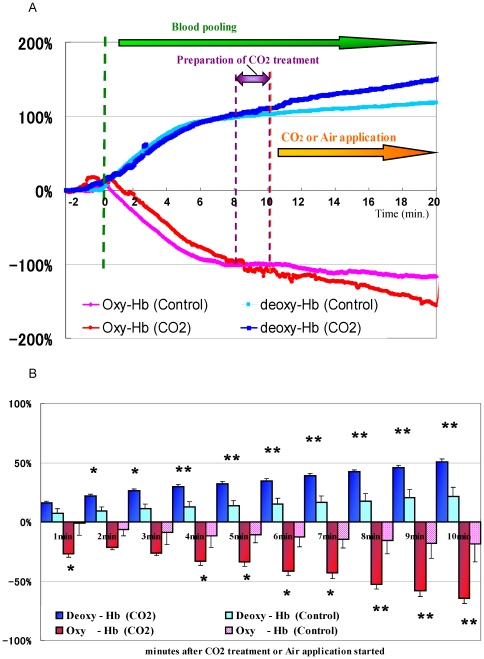
The oxy- and deoxy-Hb concentrations at all time points during the experiment are shown in Figure 6A. The oxy-Hb concentration decreased and the deoxy-Hb concentration increased after halting the blood flow (resting O2 consumption). The relative concentrations of oxy- and deoxy-Hb changed gradually, almost reaching a maximum after 8 min. Both the decrease in oxy-Hb and the increase in deoxy-Hb were greater in the CO2-applied arms than in the control arms.
Figure 6. Measurement of oxygenated and deoxygenated Hb concentration during transcutaneous application of CO2 using NIRS.
(A) Continuous measurement of oxy and deoxy-Hb concentrations using NIRS with pooling blood by a pneumatic tourniquet. The bold lines demonstrate the CO2 group data. All data show the changes in Hb concentrations from the starting point to the end point of measurement (n = 7). Data show the decrease in oxy-Hb and the increase in deoxy-Hb after pooling blood, followed by the greater decrease in oxy-Hb and greater increase in deoxy-Hb in CO2 group after transcutaneous CO2 application. (B) Relative changes in amounts of oxy/deoxy-Hb (the values 8 min. after blood pooling started were set as standards). Graph data are expressed as mean ± S.E.M. The averages and significance checks were calculated based on measurements of the 7 subjects. Statistical significance at P<0.05 is denoted by *, and P<0.01 is denoted by **. The graph shows a significant decrease in oxy-Hb and increase in deoxy-Hb in the CO2 group.
Oxy- and deoxy-Hb concentrations at 8 min. after inflation were assigned to be the control values. The mean values of 5 points around every second minute of measurement are shown in Figure 6B and Table. 1. The data show that the oxy-Hb concentration decreased significantly 4 min. after CO2 application (The relative changes of the oxy-Hb concentrations were −33.4±23.9% in the CO2 applied group and −11.8±8.0% in the air applied (control) group), and that deoxy-Hb concentration increased significantly 2 min. after CO2 application in the CO2-applied group compared to the control group (The relative changes of the deoxy-Hb concentrations were 21.9±3.6% in the CO2 applied group and 9.1±1.6% in the control group). Thus, it was confirmed that transcutaneous CO2 application facilitates a decrease in oxy-Hb and an increase in deoxy-Hb in the human body, providing evidence of O2 dissociation of Hb resulting from transcutaneous application of CO2 in vivo.
Table 1. Calculated data showing relative changes in relative amounts of oxy/deoxy-Hb (values 8 min. after blood pooling started were set as standards).
| Time | 1 min | 2 min | 3 min | 4 min | 5 min | 6 min | 7 min | 8 min | 9 min | 10 min | |
| Oxy - Hb | Control±S.E.M. | -0.9% | -6.3% | -8.8% | -11.8% | -10.7% | -12.5% | -14.8% | -15.8% | -18.3% | -18.5% |
| ±2.7% | ±2.2% | ±2.0% | ±3.3% | ±4.1% | ±3.9% | ±4.9% | ±4.2% | ±5.0% | ±4.7% | ||
| CO2±S.E.M. | -26.9% | -21.3% | -26.4% | -33.4% | -33.6% | -41.2% | -43.0% | -52.4% | -58.0% | -64.2% | |
| ±10.4% | ±5.2% | ±10.3% | ±9.7% | ±6.8% | ±8.6% | ±7.2% | ±10.9% | ±12.5% | ±15.0% | ||
| P Value | 0.0169 | 0.1678 | 0.104 | 0.0466 | 0.0351 | 0.0086 | 0.0099 | 0.0009 | 0.0003 | 0 | |
| Deoxy - Hb | Control±S.E.M. | 7.3% | 9.1% | 11.1% | 12.7% | 13.6% | 15.2% | 16.6% | 17.7% | 20.3% | 21.4% |
| ±1.5% | ±1.4% | ±1.5% | ±1.5% | ±1.7% | ±1.7% | ±1.8% | ±1.5% | ±2.0% | ±2.3% | ||
| CO2±S.E.M. | 16.3% | 21.9% | 26.3% | 30.0% | 32.4% | 34.7% | 39.1% | 42.2% | 46.0% | 50.6% | |
| ±3.8% | ±3.6% | ±4.1% | ±4.3% | ±4.6% | ±4.8% | ±5.4% | ±6.1% | ±6.8% | ±7.7% | ||
| P Value | 0.1088 | 0.0223 | 0.0072 | 0.0023 | 0.0009 | 0.0006 | 0.0001 | 0 | 0 | 0 | |
The data are expressed as mean ± S.E.M. The averages and significance levels were calculated based on the measurements of the 7 subjects. Statistical significance at P<0.05 is denoted by * and P<0.01 is denoted by **.
Discussion
In this study, we showed that our transcutaneous CO2 system could cause the absorption of CO2, and the Bohr effect in the human body. A number of studies into the physiological effects of CO2 therapy, especially CO2-enriched water bathing [3]–[5], [16]–[19] and CO2 natural spa gas therapy [8]–[10] have been published. The effect of CO2 therapy for peripheral vascular disorder has been explained by the vasodilation effect by CO2 1,11, and the Bohr effect [3], [5], [8]–[11]. For example, Hartmann et al demonstrated an increase in tissue oxygen pressure which was caused by CO2-enriched water bathing, and they concluded this increase was caused by the Bohr effect [3]. However, they showed no direct evidence for the Bohr effect in their study. To the best of our knowledge, there have been no reports that have investigated the Bohr effect in vivo.
The issue for successful investigation of the Bohr effect in vivo is that the O2 dissociation from Hb needs to be measured in an in vivo and real-time manner without taking blood samples. A study by Jöbsis demonstrated the feasibility of using near-infrared spectroscopy (NIRS) to assess the adequacy of O2 provision and utilization in living tissues [21]. NIRS can track changes in tissue oxy- and deoxy-Hb concentrations in a non-destructive, continuous, and real-time manner; thus, NIRS can be used to assess dynamic changes of the tissue oxy- and deoxy-Hb concentrations [22], [23]. In the present study, therefore, NIRS was used to confirm whether transcutaneous CO2 application actually causes O2 dissociation from the oxy-Hb, which is a characteristic phenomenon of the Bohr effect.
Another issue to be solved is that the blood flow flushes away CO2-absorbed erythrocytes in vivo. In our preliminary experiments, no changes were observed in oxy- and deoxy-Hb concentrations during transcutaneous CO2 application to the arm (data not shown). We hypothesized that this resulted from an active blood flow that caused an outflow of the deoxy-Hb enriched erythrocytes, which were then deoxygenated by the transcutaneously absorbed CO2, as well as by an inflow of the oxy-Hb enriched normal erythrocytes. Therefore, we employed a pneumatic tourniquet (commonly used in a number of surgeries) [24], [25] to halt the blood flow and keep the erythrocytes in the arm during the transcutaneous CO2 application. In addition, the pneumatic tourniquet reduced the problems inherent in measuring the O2 dissociation from the Hb, for example, by an increase in local tissue temperature caused by a hypercapnia-induced increase in blood flow. Thus, we believe this study can be regarded as the first to provide real evidence of the Bohr effect in the human body.
One potential flaw in this study is that NIRS measures not only Hb but also myoglobin [26], [27]. The O2 dissociation curve of myoglobin is a rectangular hyperbola, and myoglobin releases oxygen at a very low pO2 [27], as P50 of myoglobin is 2.03 mmHg at 35°C [28]. In a previous report, tissue pO2 was shown to be about 25–45 mmHg with blood pooling by applying a pneumatic tourniquet for 10–20 min [24], [25]. In addition, the relationship of myoglobin P50 with pH is linear [28]. In contrast, the relationship of Hb P50 with pH is exponential—the well-known Bohr coefficient [15], [29], [30]. Therefore, the contamination of myoglobin in NIRS measurement is expected to have only a minimal influence on the data.
CO2 therapy has a clinical effect in the treatment of ischemic legs and Raynoud's phenomenon. The effect is caused by the improvement of microcirculation, increasing in tcPO2, and causing the Bohr effect, which we report here. Our experimental results also show scientific evidence that our transcutaneous CO2 application can cause an “Artificial Bohr effect.” This artificial Bohr effect might be a potential new therapy for disorders in which a high quantity of O2 in local tissues is required for treatment as well as in the peripheral vascular disorder.
Acknowledgments
The authors would like to express their gratitude to Prof. Naruhito Kondo (Graduate School of Human Development and Environment, Kobe University), Prof. Shunsaku Koga (Kobe Design University) for providing the NIRS and room chamber and for their wealth of advice concerning Hb measurement, Dr. Hiroyuki Matsuda (Nano Medicine Merger Education Unit, Kyoto Graduate School of Medicine) for advising us about the simulation of O2 dissociation curve of Hb, and Dr. Hideaki Kawamitsu, Dr. Shoichi Fujii (Department of Radiology, Kobe University Hospital) for application of 31P-MRS.
Footnotes
Competing Interests: The hydro-gel was received as a gift from NeoChemir Inc. Takeshi Uhea is a full-time employee of NeoChemir Inc. Masaya Tanaka is Chief Executive Officer of NeoChemir Inc. and has patent licensing arrangements with the hydro-gel and CO2 application. The international patent publication number is WO2004/002393; the publication date is January 8, 2004. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Funding: This study was funded by NeoChemir Inc. through the employment of Masaya Tanaka and Takeshi Ueha who conceived, designed and performed the experiments, and analyzed the data for this study.
References
- 1.Blair DA, Glover WE, McArrdle L. The mechanism of the peripheral vasodilation following carbon dioxide inhalation in man. Clin Sci. 1960;19:407–423. [Google Scholar]
- 2.Matz H, Orion E, Wolf R. Balneotherapy in dermatology. Dermatol Ther. 2003;16:132–140. doi: 10.1046/j.1529-8019.2003.01622.x. [DOI] [PubMed] [Google Scholar]
- 3.Hartmann BR, Bassenge E, Pittler M. Effect of carbon dioxide-enriched water and fresh water on the cutaneous microcirculation and oxygen tension in the skin of the foot. Angiology. 1997;48:337–343. doi: 10.1177/000331979704800406. [DOI] [PubMed] [Google Scholar]
- 4.Hartmann BR, Bassenge E, Hartmann M. Effects of serial percutaneous application of carbon dioxide in intermittent claudication: results of a controlled trial. Angiology. 1997;48:957–963. doi: 10.1177/000331979704801104. [DOI] [PubMed] [Google Scholar]
- 5.Toriyama T, Kumada Y, Matsubara T, Murata A, Ogino A, et al. Effect of artificial carbon dioxide foot bathing on critical limb ischemia (Fontaine IV) in peripheral arterial disease patients. Int Angiol. 2002;21:367–73. [PubMed] [Google Scholar]
- 6.Brandi C, D'Aniello C, Grimaldi L, Bosi B, Dei I, et al. Carbon dioxide therapy in the treatment of localized adiposities: clinical study and histopathological correlations. Aesthetic Plast Surg. 2001;25:170–4. doi: 10.1007/s002660010116. [DOI] [PubMed] [Google Scholar]
- 7.Brandi C, D'Aniello C, Grimaldi L, Caiazzo E, Stanghellini E. Carbon dioxide therapy: effects on skin irregularity and its use as a complement to liposuction. Aesthetic Plast Surg. 2004;28:222–5. doi: 10.1007/s00266-004-2068-z. [DOI] [PubMed] [Google Scholar]
- 8.Savin E, Bailliart O, Bonnin P, Bedu M, Cheynel J, et al. Vasomotor effects of transcutaneous CO2 in stage II peripheral occlusive arterial disease. Angiology. 1995;46:785–91. doi: 10.1177/000331979504600904. [DOI] [PubMed] [Google Scholar]
- 9.Fabry R, Monnet P, Schmidt J, Lusson JR, Carpentier PH, et al. Clinical and microcirculatory effects of transcutaneous CO2 therapy in intermittent claudication. Randomized double-blind clinical trial with a parallel design. Vasa. 2009;38:213–24. doi: 10.1024/0301-1526.38.3.213. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt J, Monnet P, Normand B, Fabry R. Microcirculatory and clinical effects of serial percutaneous application of carbon dioxide in primary and secondary Raynaud's phenomenon. Vasa. 2005;34:93–100. doi: 10.1024/0301-1526.34.2.93. [DOI] [PubMed] [Google Scholar]
- 11.Duling BR. Changes in microvascular diameter and oxygen tension induced by carbon dioxide. Circ Res. 1973;32:370–6. doi: 10.1161/01.res.32.3.370. [DOI] [PubMed] [Google Scholar]
- 12.Bohr C, Hasselbach K, Krogh A. Ueber emen in biologischen Bezuehung wichtigen Einfluss, den die Kohlen saurespannung des Blutes anf dessen Samerstoffbinding ubt. Arch. Physiol. 1904;16:402–412. [Google Scholar]
- 13.Riggs A. The nature and significance of the Bohr effect in mammalian hemoglobins. J. Gen. Physiol. 1960;43:737–752. doi: 10.1085/jgp.43.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tyuma I. The Bohr effect and the Haldane effect in human hemoglobin. Jpn J Physiol. Jpn. J. Physiol. 1984;34:205–216. doi: 10.2170/jjphysiol.34.205. [DOI] [PubMed] [Google Scholar]
- 15.Jensen FB. Red blood cell pH, the Bohr effect, and other oxygenation-linked phenomena in blood O2 and CO2 transport. Acta. Physiol. Scand. 2004;182:215–227. doi: 10.1111/j.1365-201X.2004.01361.x. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto M, Yamamoto N. Decrease in heart rates by artificial CO2 hot spring bathing is inhibited by beta1-adrenoceptor blockade in anesthetized rats. J. Appl. Physiol. 2004;96:226–232. doi: 10.1152/japplphysiol.00812.2003. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto N, Hashimoto M. Spinal cord transection inhibits HR reduction in anesthetized rats immersed in an artificial CO2-hot spring bath. Int. J. Biometeorol. 2007;51:201–208. doi: 10.1007/s00484-006-0055-6. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto N, Hashimoto M. Immersion in CO2-rich water containing NaCl diminishes blood pressure fluctuation in anesthetized rats. Int. J. Biometeorol. 2007;52:109–116. doi: 10.1007/s00484-007-0102-y. [DOI] [PubMed] [Google Scholar]
- 19.Irie H, Tatsumi T, Takamiya M, Zen K, Takahashi T, et al. Carbon dioxide-rich water bathing enhances collateral blood flow in ischemic hindlimb via mobilization of endothelial progenitor cells and activation of NO-cGMP system. Circulation. 2005;111:1523–9. doi: 10.1161/01.CIR.0000159329.40098.66. [DOI] [PubMed] [Google Scholar]
- 20.Raymer GH, Green HJ, Ranney DA, Marsh GD, Thompson RT. Muscle metabolism and acid-base status during exercise in forearm work-related myalgia measured with 31P-MRS. J. Appl. Physiol. 2009;106:1198–1206. doi: 10.1152/japplphysiol.90925.2008. [DOI] [PubMed] [Google Scholar]
- 21.Jöbsis FF. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977;198:1264–1267. doi: 10.1126/science.929199. [DOI] [PubMed] [Google Scholar]
- 22.Boushel R, Piantadosi CA. Near-infrared spectroscopy for monitoring muscle oxygenation. Acta. Physiol. Scand. 2000;168:615–622. doi: 10.1046/j.1365-201x.2000.00713.x. [DOI] [PubMed] [Google Scholar]
- 23.Koga S, Poole DC, Ferreira LF, Whipp BJ, Kondo N, et al. Spatial heterogeneity of quadriceps muscle deoxygenation kinetics during cycle exercise. J. Appl. Physiol. 2007;103:2049–2056. doi: 10.1152/japplphysiol.00627.2007. [DOI] [PubMed] [Google Scholar]
- 24.Karalezli N, Ogun CO, Ogun TC, Yildirim S, Tuncay I. Wrist tourniquet: the most patient-friendly way of bloodless hand surgery. J. Trauma. 2007;62:750–754. doi: 10.1097/01.ta.0000249076.11239.96. [DOI] [PubMed] [Google Scholar]
- 25.Miller SH, Lung RJ, Graham WP, Davis TS, Rusenas I. The acute effects of tourniquet ischemia on tissue and blood gas tensions in the primate limb. J. Hand. Surg. Am. 1978;3:11–20. doi: 10.1016/s0363-5023(78)80113-0. [DOI] [PubMed] [Google Scholar]
- 26.Mancini DM, Bolinger L, Li H, Kendrick K, Chance B, Wilson JR. Validation of near-infrared spectroscopy in humans. J. Appl. Physiol. 1994;77:2740–2747. doi: 10.1152/jappl.1994.77.6.2740. [DOI] [PubMed] [Google Scholar]
- 27.Chance B, Dait MT, Zhang C, Hamaoka T, Hagerman F. Hagerman, Recovery from exercise-induced desaturation in the quadriceps muscles of elite competitive rowers. Am. J. Physiol. 1992;262:C766–775. doi: 10.1152/ajpcell.1992.262.3.C766. [DOI] [PubMed] [Google Scholar]
- 28.Ganong WF. New York: Lange medical Books; 2005. Review of medical physiology. pp. 666–670. [Google Scholar]
- 29.Schenkman KA, Marble DR, Burns DH, Feigl EO. Myoglobin oxygen dissociation by multiwavelength spectroscopy. J. Appl. Physiol. 1997;82:86–92. doi: 10.1152/jappl.1997.82.1.86. [DOI] [PubMed] [Google Scholar]
- 30.Hilpert P, Fleischmann RG, Kempe D, Bartels H. The Bohr effect related to blood and erythrocyte pH. Am. J. Physiol. 1963;205:337–340. doi: 10.1152/ajplegacy.1963.205.2.337. [DOI] [PubMed] [Google Scholar]