Abstract
Three closely related parthenogenetic species of root-knot nematodes, collectively termed the Meloidogyne incognita-group, are economically significant pathogens of diverse crop species. Remarkably, these asexual root-knot nematodes are capable of acquiring heritable changes in virulence even though they lack sexual reproduction and meiotic recombination. Characterization of a near isogenic pair of M. javanica strains differing in response to tomato with the nematode resistance gene Mi-1 showed that the virulent strain carried a deletion spanning a gene called Cg-1. Herein, we present evidence that the Cg-1 gene lies within a member of a novel transposable element family (Tm1; Transposon in Meloidogyne-1). This element family is defined by composite terminal inverted repeats of variable lengths similar to those of Foldback (FB) transposable elements and by 9 bp target site duplications. In M. incognita, Tm1 elements can be classified into three general groups: 1) histone-hairpin motif elements; 2) MITE-like elements; 3) elements encoding a putative transposase. The predicted transposase shows highest similarity to gene products encoded by aphids and mosquitoes and resembles those of the Phantom subclass of the Mutator transposon superfamily. Interestingly, the meiotic, sexually-reproducing root-knot nematode species M. hapla has Tm1 elements with similar inverted repeat termini, but lacks elements with histone hairpin motifs and contains no elements encoding an intact transposase. These Tm1 elements may have impacts on root-knot nematode genomes and contribute to genetic diversity of the asexual species.
Introduction
Root-knot nematodes (RKNs), comprising the genus Meloidogyne, are obligate parasites causing damage to plant roots [1] and weakening plants by acting as carbon sinks [2], [3]. Worldwide, RKNs represent a serious economic problem, causing a 5–12% reduction in crop productivity [4]. Species within the genus Meloidogyne differ widely in their modes of reproduction. The three species generally regarded as causing the most damage globally are part of what has been called the M. incognita (Mi)-group: M. incognita, M. javanica, and M. arenaria. All three are parthenogenetic species in which the eggs are products of mitosis and develop without fertilization [5], [6], [7]. These asexual species are closely related and are thought to have originated from interspecific hybridization followed by the loss of meiosis [8], [9], [10]. Despite their lack of meiosis, this group of RKNs is evolutionarily successful, capable of parasitizing thousands of plant species and acquiring heritable virulent phenotypes [7]. The mechanisms by which these asexual RKN species acquire the ability to parasitize additional host taxa are unknown, but genomic change mediated by transposable elements and repetitive sequences has been proposed [11], [12].
Transposable elements are potent mutagens and can shape genomes in many ways, ranging from large-scale chromosomal rearrangements to subtle alterations in gene regulation [13], [14]. Two general mechanistic classes of transposable elements exist [15]: Class I—retrotransposons, which replicate and insert in new genomic locations through an RNA intermediate; and Class II—DNA transposons, which transpose through cut-and-paste mechanisms without an RNA intermediate. Typical autonomous Class II elements encode a transposase gene flanked by terminal inverted repeats (TIRs). The transposase protein recognizes the TIRs and facilitates mobilization of the element to a new genomic location. Non-autonomous elements do not encode a functional transposase, but can be mobilized in trans by transposase proteins produced by an autonomous element. Most transposable elements are also flanked by target site duplications (TSDs) produced as a byproduct of the transposon integration process [16].
A widely studied example of the genetic variability within the apomictic RKNs is the ability to acquire virulence by escaping recognition by tomato plants with the root-knot nematode resistance gene, Mi-1. Mi-1 confers resistance that is broadly effective against the related Mi-group asexual root-knot nematode species [17], [18] and, surprisingly, some isolates of potato aphid and whitefly [19], [20]. However, Mi-1 does not confer resistance to the RKN species M. hapla, a facultative parthenogen in which gametes are products of meiosis, but in the absence of males, sister nuclei re-fuse to produce parthenogenetic diploid progeny [21], [22]. Populations of Mi-1-resistance breaking asexual nematodes have been found both in the field and after greenhouse selection [23], [24], [25], [26], [27], [28]. Gleason et al. [24] sought to identify genetic differences between an avirulent isolate of M. javanica (VW4) and a greenhouse-derived virulent derivative, VW5. Results of both gDNA and cDNA AFLP analyses were consistent with these two nematode strains being isogenic with the exception of the deletion spanning a putative gene named Cg-1 in VW5. The known 727 bp of the Cg-1 locus was found to express 456 nt-long mature transcript without obvious coding potential, capable of producing peptides of only 3–32 amino acids, and containing a histone-hairpin motif near its 3′ end. Except for its discovery in Cg-1, this motif has been identified only in the 3′ UTR of metazoan, but not plant, histone genes where it is required for processing of histone mRNAs and export of these transcripts from the nucleus [29], [30]. Southern analyses and PCR demonstrated that Cg-1 is a member of a gene family with 8 copies in the M. javanica genome, and transcript analysis indicated that several of these sequences are expressed. Soaking preinfective juveniles of VW4 in dsRNA corresponding to parts of the Cg-1 sequence resulted in a virulent phenotype capable of parasitizing Mi-1 tomato, supporting a role for Cg-1 in Mi-1-mediated recognition by the resistant host. However, because of the apparent lack of coding potential, the molecular function of Cg-1 and its role in mediating response to tomato with Mi-1 has remained elusive.
Recently, the genome sequences of two root-knot nematodes species, M. incognita and M. hapla, became available and provide a resource for further investigation of the Cg-1 locus [10], [31]. Herein we present a detailed analysis of the Cg-1 locus, and demonstrate that the previously described Cg-1 gene is within a novel class II transposable element with structurally complex terminal-inverted repeats similar to those of Foldback (FB) transposable elements. We have named this element Tm1 (for Transposon in Meloidogyne-1). We analyze and contrast the Tm1 transposon families of M. incognita and M. hapla. While Tm1 elements display evidence of transposition activity during the radiation of RKN species, our analyses suggest Tm1 elements are currently inactive and a decaying transposon family in M. incognita, M. javanica, and M. hapla. We propose that despite the lack of obvious recent transposition activity, Tm1 elements may be part of the molecular toolkit asexual root-knot nematodes use to acquire genetic diversity.
Results
The Cg-1 locus contains a transposon-like structure
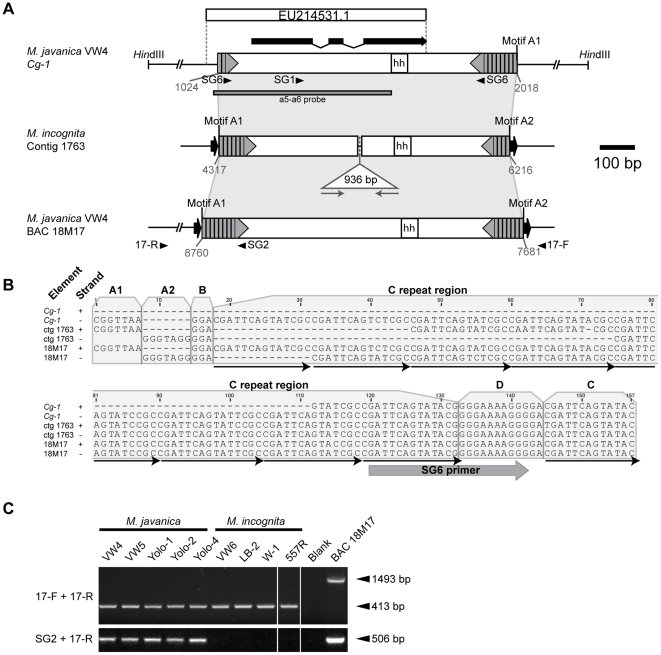
We used the available 727 bp of the Meloidogyne javanica VW4 Cg-1 locus (GenBank EU214531.1) [24] to query the published genome sequence of M. incognita, a RKN species closely related to M. javanica. The highest-scoring BLASTN match (E = 6.70e−48) was on M. incognita contig 1763 (GenBank CABB01001763.1). We noted that sequences 5′ of the M. javanica Cg-1 transcript are present on contig 1763 in an inverted-repeat orientation flanked by 9 bp direct repeats, suggestive of a transposable element (Figure 1A). The sequences between these inverted repeats are similar to Cg-1 with the exception of the presence of a second, unrelated 936 bp inverted-repeat element flanked by 7 bp target site duplications being inserted within the region of homology (Figure 1A). In other words, the sequence on M. incognita contig 1763 appears to contain two nested transposable elements: a 936 bp element residing within another novel transposable element sharing sequence identity to M. javanica Cg-1 (Figure 1A). We designed two PCR primers to test whether M. javanica Cg-1 was also flanked by inverted repeats. The 3′ end of primer SG1 binds a 5 bp sequence (CAATGA) present in Cg-1 but absent in other members of the gene family [24]. A second primer, SG6, binds within the presumptive inverted repeat. Using SG1 and SG6, we amplified a 629 bp fragment from genomic DNA of M. javanica strain VW4, but not VW5 (data not shown), confirming specific amplification of the Cg-1 locus and supporting the presence of inverted repeats flanking sequences coding for the Cg-1 transcript.
Figure 1. Inverted-repeat element carrying Cg-1 and related elements.
(A) Alignment between elements (open boxes) at the M. javanica VW4 Cg-1 locus (GenBank EU214531.2), M. incognita contig 1763 (CABB01001763.1), and M. javanica VW4 BAC 18M17 (HQ122410.1). Similar regions between elements are designated with light grey shading. Position of Cg-1 transcript [24] is shown with thick black lines representing exons and thin lines, introns. Dark grey arrows within open boxes represent the TIRs with vertical bars indicating internal repeats. Target site duplications are indicated black arrows; hh designates the position of the histone hairpin. Binding sites for primers SG2, SG6, 17-F and 17-R are indicated by arrowheads. The grey bar (a5–a6 probe) shows the position of the PCR amplicon used for Southern analysis. (B) Alignment of TIRs of the elements shown in (A). Strand designations+and−represent the 5′ and 3′ ends for the elements as drawn above. Motifs and binding site of primer SG6 are indicated. Thin black arrows below the alignment denote repeat units of Motif C. (C) Agarose gel showing amplification products from genomic DNA of M. incognita and M. javanica isolates using primers 17-F and 17-R for amplification of empty sites and primer set SG2 and 17-R to detect an insertion similar to that on BAC 18M17.
To investigate the M. javanica Cg-1 locus further, we screened a genomic DNA library containing ∼3 kb VW4 HindIII fragments, a size previously demonstrated to harbor Cg-1 [24], using primers SG1 and SG6. We obtained a 3154 bp clone containing Cg-1 (GenBank EU214531.2; Figure 1A), and sequence analysis confirmed that the Cg-1 sequence is flanked by inverted repeats. Sequences outside the inverted repeat element of M. javanica Cg-1 diverge completely from those outside the element on M. incognita contig 1763, further suggesting that these inverted-repeat sequences correspond to the boundaries of transposable elements. However, the inverted repeats flanking M. javanica Cg-1 do not have target site duplications and the 5′ TIR appears to be truncated (Figure 1A). The element at Cg-1 is 82.7% identical to that on M. incognita contig 1763 excluding the nested 936-bp insertion.
In an attempt to identify additional sequence flanking Cg-1, we screened a BAC library of M. javanica VW4 by PCR using primers SG1 and SG6. Despite several attempts, we were unable to identify a clone corresponding exactly to Cg-1 in the BAC library. However, by lowering the annealing temperature of our PCR reaction (Materials and Methods), we obtained a 26 kb BAC clone (clone 18M17, GenBank HQ122410.1). The sequence of this clone identified an element similar to that found at Cg-1 (Figure 1A). This element was 1080 bp with terminal inverted repeats flanked by 9 bp target site duplications. The sequence is 84.9% identical to Cg-1, but sequences outside the element on BAC 18M17 are different from those found flanking elements at Cg-1 and Mi contig 1763, again suggestive of a transposable element.
The three putative transposable elements shown in Figure 1 are similar in sequence and display no obvious protein-coding potential. Between their inverted repeats, each contains a histone hairpin motif (Figure 1A) [24], [29]. Analysis of the TIRs of each element reveals a composite structure (Figure 1B). The TIRs of the elements on contig 1763 and BAC 18M17 have asymmetric 7 bp motifs at their outer ends, internal to the 9 bp target site duplications, here called Motif A1 (5′-CGGTTAA-3′) and Motif A2 (5′-CCTACCC-3′). Internal to Motif A1 and A2 is a 3 bp sequence, Motif B (5′-GGA-3′). The largest portion of the TIR is composed of a variable number of 13 to 15 bp (most often 14 bp) tandem repeats with the consensus 5′- CGATTCAGTATCCGC-3′ (Motif C). Due to differing numbers of repeats of Motif C, the TIRs at each end of the same element, as well as between elements, differ in size. The internal ends of the TIRs (closer to the element center) contain a conserved 12 bp purine-rich sequence stretch (Motif D; 5′-GGGAAAAGGGGA-3′), followed by one additional unit of Motif C.
To determine whether the nematode genomes carried paralogous loci lacking an element and the associated target site duplication (e.g., empty sites) corresponding to the insertion site of the element on BAC 18M17, we surveyed several M. javanica and M. incognita isolates by amplifying genomic DNA with primers 17-F and 17-R, which flank the putative transposable element (Figure 1A). We obtained ∼0.4 kb amplicons from all tested RKN isolates (Figure 1C), approximately 1 kb shorter than the 1493 bp amplicon produced from BAC clone 18M17 and corresponding to the expected length for an empty site. Sequencing of the 0.4-kb amplicon from VW4 (GenBank HQ122411.2) confirmed that these fragments correspond to a paralogous locus without the inverted repeat element and target site duplications. A second PCR test using a primer within the inverted-repeat element (SG2) and the flanking 17-R primer (Figure 1A) demonstrates that the tested M. javanica isolates, but not the M. incognita isolates, contain a paralog with an inverted repeat element similar to that on BAC 18M17 (Figure 1C).
Taken together, the identification of 3 similar elements in distinct genomic contexts, two of which are flanked target site duplications, and the identification of paralogous loci with and without an element support the hypothesis these sequences are transposable elements. We have named this transposon family, members of which have novel TIRs with internally redundant motifs, Tm1 (Transposon in Meloidogyne-1). Elements closely resembling those found at Cg-1, Mi contig 1763, and on BAC 18M17 and containing a histone-hairpin motif will be referred to as Tm1-HH.
Identification of a Tm1 element encoding a 496 amino acid putative transposase
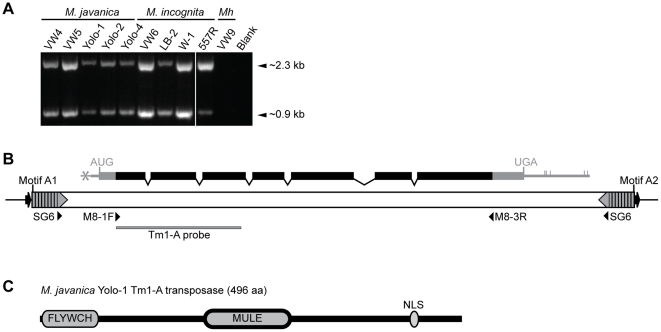
The three Tm1-HH elements discussed previously lack potential to code for a transposase protein and are thus non-autonomous elements. Reasoning that an autonomous Tm1 element is likely to share the same terminal inverted repeats, we amplified nematode DNA using only the SG6 primer, which binds within the TIRs (Figure 1B). We obtained predominant amplicons of approximately 0.9 kb and 2.3 kb in all tested M. javanica and M. incognita isolates, but no amplicons from the more distantly related M. hapla VW9 (Figure 2A). The 0.9 kb amplicons are the size expected from Tm1-HH elements such as those found at Cg-1 and on BAC 18M17 (Figure 1A). Three of the 2.3-kb amplicons (one each from M. javanica VW4, M. javanica Yolo-1, and M. incognita LB-2) were cloned and sequenced (GenBank accessions HM470231.1, HM470232.1, and HM470230.1, respectively). These three clones were very similar in sequence, with the two M. javanica amplicons sharing 99% identity and the M. javanica VW4 and M. incognita LB-2 amplicons sharing 97% identity. Because the binding site of SG6 was within the TIRs (Figure 1B), the cloned amplicons from VW4, Yolo-1, and LB-2 lack the full TIR. Therefore, we used BLASTN to query the M. incognita genome and identified a highly similar element on contig 274 (GenBank CABB01000274.1) (E value = 0). This element is 2593 bp long, and is flanked by 9 bp target site duplications (Figure 2B). The TIRs are structurally similar to those of the Tm1 elements described above and carry the same set of motifs (Figure 2B and Table S1).
Figure 2. Discovery and analysis of Tm1-A elements.
(A) Agarose gel of PCR amplification products with primer SG6 showing the presence of 0.9 kb and 2.3 kb products with M. javanica and M. incognita isolates. (B) Diagram of 2.5 kb Tm1-A element from M. incognita contig 274 (GenBank CABB01000274.1). Transcript, diagrammed above element, is predicted using experimental data acquired from M. javanica Yolo-1 (black regions) and genomic sequence (grey regions). Asterisk indicates the predicted 5′ SL1 splice site, and vertical marks, possible polyadenylation sites. All other annotations are as described in Figure 1. “Tm1-A probe” indicates the position of the subclone used for Southern analysis in Figure 3. (C) Predicted 496 aa protein domain protein encoded by the M. javanica Yolo-1 Tm1-A element with positions of FLYWCH-MULE domains and nuclear localization signal (NLS) identified by Pfam and PSORT II, respectively, are indicated.
A query of the Pfam protein database [32] with the DNA sequences of the 2.5 kb Tm1 element on contig 274 element revealed coding potential for a MULE domain transposase [33], [34]. Thus, we refer to the 2.5 kb Tm1 element as Tm1-A (Tm1-Autonomous). However, no long contiguous ORF was found suggesting the presence of introns. To identify transcripts encoded by Tm1-A elements, we used primers within the predicted coding region (M8-1F and M8-3R; Figure 2B) to amplify partial Tm1-A transcripts from cDNA of M. javanica Yolo-1 (GenBank HQ122409.1; Figure 2B) and M. incognita LB-2 (not shown). We were not successful in obtaining full-length cDNA, so the 5′ and 3′ ends of the transcript were deduced by comparing the Yolo-1 Tm1-A cDNA sequence to the genomic Yolo-1 Tm1-A sequence. The nearest in-frame start codon (AUG) and stop codon (UGA) flanking the cloned cDNA were found; a spliced leader 1 (SL1) acceptor site (UUUUCAG) [35] was found 5′ of the predicted start codon, and several potential polyadenylation signal sequences (composed of AUUAAA, AAUAAU, GAUAAA, and two repeats of AAAAAA) [35] were identified 3′ of the stop codon. Taken together, the Yolo-1 Tm1-A element is predicted to express a transcript comprised of 7 exons (Figure 2B) and encoding a protein of 496 amino acids (Figure 2C).
The highest BLASTP hits of the predicted full-length 496 amino acid protein encoded by the M. javanica Yolo-1 Tm1-A element (GenBank ADM16638.1) correspond to predicted proteins deduced from the genome sequences of aphids and mosquitoes (Table 1). Pfam v. 24.0 [32] revealed that these predicted proteins have a domain structure similar to transposases encoded by Phantom elements, a recently described subclass of Mutator Class II transposable elements that is widely distributed in eukaryotic taxa including many animals [36] (Figure 2C). Residues 5–66 of the putative Yolo-1 Tm1-A transposase have similarity (E = 1.3e−7) to a FLYWCH zinc-finger domain (Pfam PF04500), a protein interaction domain in some isoforms of Drosophila Mod(mdg4) proteins [37]. Pfam also identified Yolo-1 Tm1-A residues 197–294 as a MULE domain (E = 9e−13) (Pfam PF10551) [33], [34]. MULE domains are found in transposases of the Mutator family originally identified in plants [38] and the prokaryotic IS256 family [39].
Table 1. Top BLASTP hits of Tm1-A transposase in GenBank.a .
| Accession # | Organism | E Val. | Size (aa) | Description |
| XP_003243189.1 | Acyrthosiphon pisum | 2e-123 | 468 | hypothetical protein with FLYWCH and MULE domains |
| XP_003240826.1 | Acyrthosiphon pisum | 3e-87 | 482 | hypothetical protein with MULE domain |
| XP_003244058.1 | Acyrthosiphon pisum | 1e-85 | 409 | hypothetical protein with MULE domain |
| XP_003244156.1 | Acyrthosiphon pisum | 3e-40 | 481 | hypothetical protein with FLYWCH and MULE domains |
| XP_001657863.1 | Aedes aegypti | 1e-36 | 488 | hypothetical protein with FLYWCH and MULE domains |
| XP_003240985.1 | Acyrthosiphon pisum | 5e-36 | 483 | hypothetical protein with MULE domain |
| XP_001659669.1 | Aedes aegypti | 1e-35 | 488 | hypothetical protein with FLYWCH and MULE domains |
| XP_003241855.1 | Acyrthosiphon pisum | 4e-32 | 433 | hypothetical protein with MULE domain |
| XP_001866309.1 | Culex quinquefasciatus | 6e-32 | 485 | hypothetical protein with MULE domain |
| XP_001848871.1 | Culex quinquefasciatus | 2e-31 | 487 | hypothetical protein with MULE domain; recombinase-like |
| YP_001029420.1 | Glypta fumiferanae ichnovirus | 3e-31 | 404 | GfV-C17-ORF1; hypothetical protein with MULE domain; recombinase-like |
Analysis done 08 August 2011.
PSORT II [40] identified two putative overlapping nuclear localization signals (NLSs) near the carboxy end of the Yolo-1 Tm1-A transposase: PPQKARK (residues 438–444) and PQKARKY (439–445). Both are pattern-7 SV40-type NLSs [40], [41] (Figure 2C).
Tm1 elements are low copy and do not display evidence of recent transposition
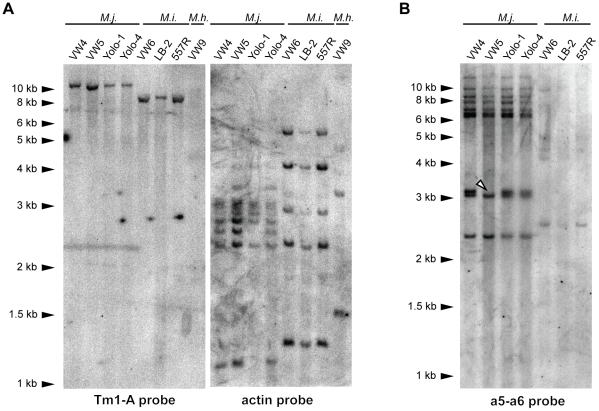
To determine the copy number of Tm1-A elements, a Southern blot of HindIII-digested genomic DNA from several isolates representing three RKN species was probed with an internal portion of the Tm1-A element (Figure 2B). All surveyed M. javanica isolates have a single ∼11 kb hybridizing HindIII fragment, while all surveyed M. incognita isolates have a single ∼8 kb hybridizing fragment (Figure 3A). No HindIII fragments hybridizing to the Tm1-A probe were detected in M. hapla VW9, consistent with PCR results (above) and bioinformatic analyses (discussed below). The same blot was stripped and re-probed with a fragment of beta actin (GenBank AF532605; Materials and Methods). The beta actin hybridization patterns were also similar between isolates of the same species, although M. javanica Yolo-1 lacked hybridizing fragments of approximately 2.5 and 1.25 kb when compared to other M. javanica isolates. A Southern blot of M. javanica VW4 genomic DNA cut with BamHI, XbaI, or XhoI and probed with Tm1-A also revealed only a single hybridizing fragment (13 kb, 12 kb, 11 kb, respectively; data not shown). These results are consistent with the presence of a single copy of Tm1-A in the genome of tested M. javanica and M. incognita isolates, and the absence of a conserved copy in M. hapla VW9. Furthermore, the similar size of the HindIII fragments suggests this element is likely in the same genomic location within the isolates of each species.
Figure 3. Tm1 elements are low copy and do not display recent transposition.
(A) A Southern blot of HindIII-digested genomic DNA from indicated root-knot nematode isolates probed with Tm1-A and actin. (B) Similar HindIII-digested blot probed with the a5–a6 probe from Cg-1. The position of the missing Tm1 element in VW5 is marked with an arrowhead). Probes are as diagrammed in Figures 1 (a5–a6) and 2 (Tm1-A).
To determine whether Tm1-HH elements like that at Cg-1 are in distinct genomic positions between isolates, a portion of the element at Cg-1 (Figure 1A) [24] was used to probe a second Southern blot of HindIII-digested genomic DNA (Figure 3B). With the exception of the previously noted absence of a 3.1 kb hybridizing fragment in M. javanica VW5 representing the deletion of Cg-1 [24], the same pattern of approximately nine strongly hybridizing bands was seen in all M. javanica isolates (VW4, Yolo-1, and Yolo-4; Figure 3B). This is in agreement with previous findings obtained by cloning and sequencing PCR products that M. javanica has 9 copies of sequences with 87 to 100% identity to Cg-1 [24]. In contrast, only weakly hybridizing fragments are observed in the M. incognita isolates suggesting that any copies present are sufficiently diverged and incapable of hybridizing with the probe at the stringent conditions used. This is consistent with our query of the M. incognita genome, which found the Tm1-HH element on contig 1763 to be the most similar sequence (72.4% identity over the length of the probe). The similar hybridization patterns present in the tested M. javanica isolates suggest that like Tm1-A, non-autonomous Tm1-HH elements have not been mobilized since these isolates diverged.
Bioinformatic comparison of Tm1 elements in M. incognita and M. hapla
We sought to identify additional Tm1 elements in the published genomes of M. incognita and M. hapla using a bioinformatics approach. In short, we used a BLASTN-based algorithm to search for two or more matches on the same contiguous sequence with similarity to a 38 bp region within the Tm1 TIRs in an inverted orientation (Materials and Methods). This method identified 39 elements in M. incognita and 22 elements in M. hapla, ranging in length from 0.17 to 2.5 kb (Tables S1 and S2; summarized in Table 2).
Table 2. Summary of Tm1 elements in M. incognita and M. hapla.
| Tm1 Class | Description | M. incognita | M. hapla | Average Length |
| Tm1-A | Putative autonomous element | 1 | 0 | 2593 |
| Tm1-D | Deletion derivatives of Tm1-A | 0 | 8 | 1307 |
| Tm1-HH | Contains histone haripin element | 8 | 0 | 1182 |
| Tm1-ML | MITE-Like | 30 | 12 | 431 |
| Other | No resemblance to other Tm1 elements | 0 | 2 | 788 |
| Total | 39 | 22 |
Tm1 elements of M. incognita could be divided into three categories based upon features of sequences between the TIRs (Table S1). Only 1 transposase-encoding element, on contig 274 as previously noted, was identified. Eight Tm1 elements, including the aforementioned element on contig 1763, included sequence that matched or resembled the histone hairpin of the Cg-1 element. The region with similarity to the Cg-1 element varied, but generally spanned the ∼24 nt histone hairpin and 300–400 nt 3′ of this sequence, in some cases extending to the TIR. We refer to these elements as the histone-hairpin class (Tm1-HH). Tm1-HH elements are about 900 bp except for two, including the previously described element on contig 1763, which contain insertions (Table S1). Upstream of the histone hairpin region, elements in this group vary in their similarity to Cg-1, with the element of contig 1763 being the most similar and some other elements showing no similarity. The third category of Tm1 elements is composed of elements typically less than 0.5 kb (with the exception of an element on Mi contig 3754, which contains a nested 878 bp inverted repeat sequence). Elements in this third class have inverted repeat sequences between their TIRs, thus resembling MITEs (miniature inverted-repeat transposable elements [42]). These MITE-like Tm1 (Tm1-ML) elements lack Motif D (Table S1) and thus do not have the SG6 primer-binding site within their TIRs, accounting for our failure to detect them by PCR. The Tm1-ML elements are the largest category with 30 of 39 copies identified in M. incognita and share 85.9% sequence identity as a group.
No elements capable of encoding an intact transposase were identified in M. hapla, but 8 elements with short patches of similarity mainly spanning approximately 200 to 600 bp 3′ to the MULE domain of the Tm1A transposase gene were noted (Table S2). These 8 M. hapla Tm1 elements appear to be derivatives of Tm1-A (hereafter called Tm1-D elements). None of these elements retained any homology to the MULE domain, and, aside from portions sharing similarity to the Tm1-A element, share only 52.3% pairwise sequence identity as a group. Queries of the M. hapla genome with both the nucleotide sequence and protein sequence of the Tm1-A element failed to detect full-length contiguous sequences, further suggesting M. hapla does not have an autonomous Tm1-A element.
No elements resembling the Tm1-HH class were found in M. hapla. Like M. incognita, the largest category of Tm1 elements in M. hapla (12 of 22) is Tm1-ML elements, sharing 90.3% sequence identity as a group. As with the ML elements of M. incognita, those of M. hapla lack Motif D in their TIRs (Table S2). The Tm1-ML element class is thus the only one clearly shared between M. incognita and M. hapla, though a neighbor-joining consensus tree generated from an alignment of Tm1-ML elements demonstrates the M. hapla elements are more closely related to each other than the majority of M. incognita elements (Figure S1). Two elements in M. hapla (on contigs 402 and 53) contain internal sequences that did not fit within the Tm1-A, -D, -HH, or -ML classes (noted as “other” in Table S2).
None of the non-autonomous Tm1 elements have significant coding potential, and BLASTX fails to detect similarity to known or predicted proteins in the GenBank nr (non-redundant) database (all E values >1). Similarly, conceptual translations of predicted genes and ORFs residing within Tm1 elements produce only short peptides not significantly similar to known or predicted proteins (all E values >1; data not shown).
Tm1 TIRs have both conserved and variable motifs
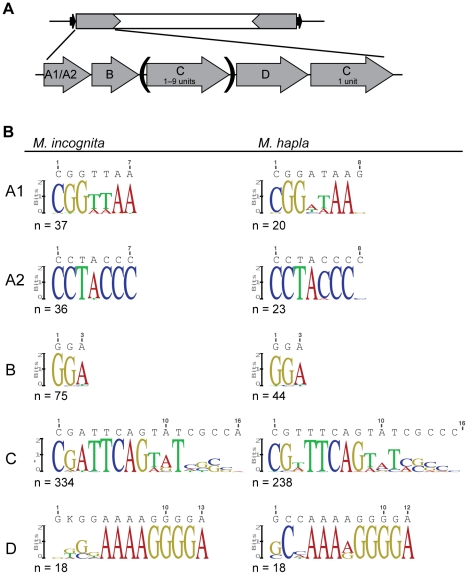
The TIRs of Tm1 elements annotated in the M. incognita and M. hapla genomes resemble the TIRs of Tm1 elements described previously (Figure 1A, 2B), but have some differences in the composition and sequence of motifs (Figure 4A). Most Tm1 elements identified in M. incognita (24/39) and M. hapla (20/22) have the 7 bp A1 Motif exterior to the TIR at one end and A2 at the other end of the element (Tables S3 and S4). However, for 6 elements, only one terminal motif was detected, and in one case (Tm1-ML on M. hapla contig 1532) Motif A2 was found on both ends of the element. Motif A2 (5′-CCTACCC-3′) is highly conserved in both M. incognita and M. hapla Tm1 elements (Figure 4B). However, Motif A1 displays some variability (Figure 4B), with a consensus of 5′-CGGTTAA-3′ in M. incognita, and 5′-CGGATAA-3′ in M. hapla. Most Tm1 elements have a 3 bp Motif B internal to Motifs A1 and A2 with a clear 5′-GGA-3′ consensus in both M. incognita and M. hapla.
Figure 4. Motifs in TIRs of Tm1 elements.
(A) Diagram showing the TIR motif structure of a generic Tm1 element. A1 is generally present at one terminus and A2 at the other. (B) Sequence logos demonstrating variability of Tm1 TIR motifs in M. incognita and M. hapla. Sequence logos and the associated consensus sequence are only shown for positions where the bit score is greater than 0.
Tm1 TIRs differ in length due to the variable number of Motif C units (Figure 4A). Tm1 TIRs contain 1–9 consecutive units of Motif C, and differences in the number of Motif C repeats occur both between elements and for opposite ends of the same element (Tables S1 and S2). In total, the 39 M. incognita elements contain 334 units of Motif C within their TIRs, ranging in length from 8 to 17 bp with the most common length being 14 bp (191 of 334). Similarly, within the 22 M. hapla elements, there are 238 Motif C units with lengths of 12 to 21 bp, but most commonly 14 bp (144 of 238). Sequence logos demonstrate a core consensus sequence of Motif C (5′-CGATTCAGTATCGC-3′ for M. incognita; 5′-CGTTTCAGTATCGC-3′ for M. hapla) with repeats more highly conserved at their 5′ ends than the 3′ ends (Figure 4B).
While Tm1-ML elements lack the GA-rich Motif D, this motif is generally present and highly conserved in other Tm1 elements (Tables S1 and S2). The sequence consensus of Motif D differs between M. incognita (5′-GKGGAAAAGGGGA -3′; where K = G or T) and M. hapla (5′-GCCAAAAGGGGA -3′). In both species, the 5′ end of Motif D is less conserved than is the 3′ end (Figure 4B). The SG6 primer binding site spans part of the variable portions of Motif D (Figure 1B), accounting for our inability to detect all Tm1 elements in M. javanica, M. incognita, and M. hapla via PCR.
In addition to differences in motif composition, the TIRs of each element differ in sequence. BLAST2 was used to identify local regions of similarity between the two TIRs of each element, allowing automatic exclusion of asymmetric regions such as Motif A1, A2, and asymmetric numbers of Motif C units. Subsequently, the BLAST2 identified regions were aligned for comparison. Of the 61 elements analyzed, only 5 elements (Tables S1 and S2) display 100% sequence identity between the BLAST2-defined portions of their TIRs. On average, the two TIRs of a single element are 93.15% identical due to SNPs and small indels.
Tm1 elements are commonly flanked by 9 bp target site duplications
Analysis of complete Tm1 elements having both terminal motifs A1 and A2 revealed that in most cases (45 of 53), elements are flanked by 8–10 bp (though most commonly 9 bp) target site duplications (Tables S3 and S4). Comparison of TSD sequences did not reveal a clear consensus sequence, suggesting Tm1 elements do not have target sequence preferences at their integration site.
Discussion
A deletion in the M. javanica genome encompassing a candidate effector gene led us to the discovery of a novel transposable element family that we have named Tm1. These elements are defined by the shared novel structure and sequence of their TIRs, which contain 4 sequence domains and varying numbers of an internally redundant ∼14 bp sequence motif and, thus, resemble Foldback (FB) elements, a heterogeneous group of transposons with composite TIRs of multiple domains and tandem repeats [43], [44], [45], [46]. Tm1 elements contain at their boundaries asymmetric 7 bp terminal motifs, generally Motif A1 at one end of the element and Motif A2 at the other, that are outside of the inverted repeat. The presence of short, non-inverted sequences at the ends of elements is unusual, but has been observed in other Mutator-like elements, which are thus sometimes said to have sub-terminal inverted repeats (sub-TIRs) [36], [47]. Additional conserved motifs characterizing Tm1 elements include a 3 bp Motif B and a GA-rich motif D, which is generally followed by a unit of Motif C.
Based on bioinformatic analysis of available genomic sequence, M. incognita Tm1 elements can be placed in three general categories: Tm1-A, Tm1-HH, and Tm1-ML. The isolates of M. incognita and M. javanica that we examined carry a single Tm1-A element encoding a protein resembling a Mutator-like (MULE) transposase. The presence of 9 bp TSDs flanking most Tm1 transposons is consistent with the size commonly generated by MULE domain transposases [47], [48], [49] and other members of the IS256/MULE transposon family [50], [51], [52], [53]. The domain structure of the putative transposase places Tm1-A and the Tm1 family within the Phantom subclass of Mutator elements with highest similarity to sequences from insects [36]. The predicted transposases of Phantom elements are characterized by an N-terminal, FLYWCH zinc-finger DNA binding domain and a MULE transposase domain. It is unclear whether the Tm1-A transposase can bind DNA as it lacks the final histidine residue to form a complete C2H2 zinc-finger motif within the FLYWCH domain. However, it is possible Tm1-A can localize to DNA in a manner similar to the Drosophila FLYWCH domain isoforms of Mod(mdg4) proteins, which do not bind DNA directly but modulate gene expression through interactions with DNA binding proteins [37], [54], [55]. Further biochemical analysis of the Tm1-A-encoded protein is needed to clarify any function.
Recently some Foldback elements have been grouped with Mutator transposons into a superfamily because of similarity between their encoded transposases [56]. Our finding of a Phantom-like transposase within FB-like TIRs supports this grouping. However, it is clear that despite their structural similarity, FB-like elements are not necessarily phylogenetically related as different classes of transposases have been shown to reside within their TIRs and mediate their transposition. For example, the FB transposon Galileo in Drosophila harbors a P element transposase [57] whereas the Arabidopsis FB element FARE contains a MULE domain transposase [46]. In addition, while some Phantom transposases are flanked by FB-like TIRs or sub-TIRs, others are flanked by structurally simpler TIRs more common in the Mutator superfamily [36]. Such observations have led others [36], [57] to propose that FB-like transposon families evolved independently from structurally simple transposons through the gradual expansion of TIR length. A similar case of TIR expansion toward a FB-like structure appears to have occurred during the evolution of the RKN Meloidogyne chitwoodi Tc1/Mariner-like element Mcmar, which has 355 bp TIRs composed of direct repeats and palindromes unlike most Tc1/Mariner elements, which have simple 26–30 bp TIRs [58].
We hypothesize the non-autonomous Tm1 elements are derived from an ancestral autonomous Tm1-A element, in a manner consistent with models of transposon family evolution [59]. For example, Pack-MULES are derivatives of plant Mutator transposons that have acquired fragments of host genes [60], [61]. Tm-1-HH elements, characterized by the presence of a highly conserved histone hairpin motif, may represent a similar acquisition of host sequences by a Tm1 element. Prior to the discovery of Cg-1 [24], histone-hairpin motif had been found only in the 3′ UTR of transcripts of replication-dependent histone genes in metazoans. The highly-conserved 24-nt hairpin and sequences 3′ serve as binding sites for an elaborate processing machinery that produces a specific endonucleolytic cleavage of the mRNA just 3′ of the hairpin. As a result of this cleavage, these histone mRNAs are not polyadenylated [29]. Interestingly, the region of the Tm-1-HH element that is most conserved among this group is 3′ of the histone hairpin structure, that is, the 3′ end of the transcript that is predicted to be cleaved off by hairpin processing machinery. Further studies will be required to determine if the histone hairpin region is under selection and if RNAs produced by Tm1-HH elements have a function.
Transposable elements can be acquired through horizontal gene transfer (HGT; for reviews see [62], [63], [64]). However, despite the similarity of the Tm1-A transposase to insect Phantom transposases (Table 1), Tm1-A lacks sufficient DNA sequence conservation with these elements to demonstrate HGT as has been done for other transposons [65], [66], [67], [68]. A more parsimonious assumption is that the Tm1 elements described here evolved from a functional Tm1-A-like element present in the ancestral Meloidogyne species rather than a recent horizontal gene transfer event from insects. Though firm conclusions cannot be drawn from current data, our observations are consistent with a model in which the Tm1-HH and Tm1-D classes arose as distinct deletion/insertion derivatives of Tm1-A in the M. incognita group and M. hapla lineages, respectively. Additionally, though the Tm1-ML elements are structurally similar between M. incognita and M. hapla, most show evidence of having evolved independently as deletion derivatives of larger Tm1 elements in each lineage: the majority of (20 of 30) M. incognita Tm1-ML elements have a M. incognita-type domain A1 (CGGTTAA; Figure 4) and the majority of (9 of 12) M. hapla Tm1-ML elements have a M. hapla-type domain A1 (CGGATAA; Figure 4). Additionally, the M. hapla Tm1-ML elements form a well-supported clade distinct from most M. incognita Tm1-ML elements (Figure S1).
Our data is consistent with Tm1 elements having been mobile during the evolution of root-knot nematode species. The presence of an additional 8 Tm1-HH elements similar in sequence to that at Cg-1 in M. javanica but not in M. incognita (Figure 3B) suggests that this particular element has expanded in the M. javanica lineage or lost in the M. incognita lineage. The presence of an insertion in the site flanked by primers 17-R and 17-F in M. javanica, but an empty site only in M. incognita suggests that this may be a relatively recent transposon insertion site (Fig. 1C). However, our Southern analyses also suggest neither Tm1-A or Tm1-HH elements have experienced recent transposition activity. Each isolate of M. javanica and M. incognita has a species-specific, rather than isolate-specific, hybridization pattern with the Tm1 probes (Figure 3). In fact, our Southern analysis indicates that the beta actin genes of Meloidogyne are more polymorphic than Tm1 elements (Figure 3A). It is important to note that most nematode isolates tested in this study (with the exception of M. incognita 557R, which was derived from a field collection in North Carolina) were collected from different locations in the California Central Valley and a wider sampling may be necessary to reveal rare Tm1 transposition events.
Consistent with the molecular observations suggesting recent Tm1 element inactivity, bioinformatically-detected Tm1 elements in the M. incognita and M. hapla genomes display sequence features consistent with senescence and deterioration. As Class II transposon families age, the number of autonomous elements decreases while the number of non-autonomous members increase and diverge in sequence [59], [69]. Moreover, as transposable elements are commonly deleterious, asexual lineages are predicted to contain largely inactive and decaying transposable elements [70]. Consistent with these hypotheses, tested M. javanica and M. incognita isolates have retained only a single putatively autonomous Tm1-A element while M. hapla encodes no elements capable of encoding a transposase, and the majority of the Tm1 family in both M. incognita and M. hapla is composed of MITE-like elements. Furthermore, no two elements are identical in sequence, suggesting they have accumulated mutations, including insertions, since the expansion of the Tm1 family. Additionally, some Tm1 elements are missing TIR motifs, differ in copy number of Motif C repeats, or lack TSDs. One Tm1 element in M. hapla (contig 1532) has Motif A2 at the end of both TIRs and lacks a TSD, and may have arisen as a crossover event between two Tm1 elements. Moreover, the Tm1-HH element at Cg-1 appears to be degenerate and lacks terminal Motif A2 (Figure 1A), possibly due to an intra or inter-element recombination event (Figure 1A). Together these observations support that Tm1 elements represent a currently inactive and senescent transposon family in root-knot nematodes.
The role of the loss of the Tm1-HH element at the Cg-1 locus in the acquisition of ability to bypass resistance in tomato mediated by the Mi-1 gene remains unclear. Current models based on similar plant resistance genes predict that the Mi-1 protein either directly or indirectly recognizes the presence of a nematode product (elicitor) to trigger defense responses [71]. Previous results [24] suggest the virulent M. javanica VW5 strain is missing the elicitor that is present in its progenitor strain VW4 and support the importance of the Cg-1 transcript for Mi-1-mediated resistance. However, several Tm1-HH elements similar to that at Cg-1 are present in both strains VW4 and VW5, and DNA blots showed that Cg-1 is present in the virulent M. javanica field isolates Yolo-1 and Yolo-4 (Figure 3 and [24]). It may be that the Tm1-HH element carrying Cg-1 regulates expression of a cis-linked nematode gene that is responsible for triggering resistance. Transposable elements contain enhancers and promoters, often in their TIRs, and thus can act as regulators of cis-linked genes (reviewed in [13]). RNAi silencing of Cg-1 may reduce or eliminate expression of the cis-linked gene by, for example, modifying chromatin structure in the region. In the case of VW5, the deletion of Cg-1 extends at least 2 kb 5′ of the Tm1-HH element (S. Gross and V. Williamson, unpublished observations) and, thus, the cis-linked elicitor gene may be deleted. However, these ideas remain untested as the extent of the deletion has not been determined and a cis-linked effector has not yet been identified.
Given the presence of multiple Tm1-HH elements in our virulent and avirulent isolates, the truncated nature of the Tm1-HH element at Cg-1, data suggesting the deletion extends beyond the Tm1 element at Cg-1, and the apparent inactivity of extant Tm1 elements, it is unlikely that canonical cut-and-paste transposition of Tm1 caused deletion of Cg-1 in VW5. However, other rearrangements involving the Tm1-HH element at Cg-1 may have occurred, such as ectopic recombination and chromosome breakage [72], [73]. In fact, internally repetitive TIRs of Foldback elements appear to be hotspots for ectopic recombination, as has been shown in studies of chromosome rearrangements in natural populations of Drosophila [74], [75]. In asexual lineages, which cannot acquire beneficial alleles that may arise in other members of the population, recombination between repetitive sequences leading to genome alterations may prove advantageous. Similar to Barbara McClintock's “Genome Shock” theory [76], asexual RKN parasites may use genomic change to their advantage when faced with the need to surmount plant resistance genes or perish. Such a mechanism has been proposed for some pathogenic bacteria, where effector molecules are clustered in “pathogenicity islands” flanked by transposable and repetitive elements and readily altered to avoid host defense responses [77], [78]. Perhaps because its asexual reproduction mode benefits from use of repetitive sequences for adaptation, the genome of M. incognita has much more repetitive DNA than that of the sexual species M. hapla [10], [11]. Whether Tm1-mediated genomic alterations produced the deletion of an effector molecule that allowed this nematode strain to bypass recognition by the tomato resistance gene product remains to be determined. However, such activity is consistent with hypotheses that transposons and repetitive elements contribute to creating genetic diversity in asexual organisms such as the root-knot nematodes M. javanica and M. incognita [11], [12], and may represent an example of transposable element exaptation [13], [79].
Materials and Methods
Collection and culture of nematodes
M. javanica nematode strains VW4 and VW5 [24]; M. incognita strain VW6 [80] and M. hapla strain VW9 [21], [31], [81] have been described previously. Additional isolates (M. javanica isolates Yolo-1, Yolo-2, and Yolo-4 from Yolo County, CA; M. incognita isolates Los Banos-2 from Merced County, CA and W-1 from Yolo County, CA) were obtained from processing tomato fields. Meloidogyne incognita strain 557R was provided by A.C. Triantaphyllou, North Carolina State University. Nematodes were identified to species by PCR using species-specific and mitochondrial primers [82], [83]. Nematodes were maintained on tomato cultivars VFNT (Mi/Mi) or UC82 (mi/mi). Eggs and second-stage juveniles (J2s) were collected as described previously [84] then frozen in liquid nitrogen and stored at −80°C.
Molecular analyses
To prepare DNA, 200–300 µl of packed frozen eggs were pulverized in a mortar and pestle with liquid nitrogen. An equal volume of homogenization buffer (200 mM NaCl, 200 mM Tris pH 7.5, 20 mM EDTA, 2% SDS, 0.04 M 2-mercaptoethanol) was added to the mortar and grinding continued until eggs were completely homogenized. The homogenate was incubated at 40°C for 30 minutes with 0.2 mg/ml proteinase K. Homogenate was extracted using an equal volume of a 1∶1 mixture of phenol∶chloroform, then repeated with chloroform only. DNA was precipitated with 2 volumes ethanol and 0.1 volumes 3 M sodium acetate, washed in 70% ethanol, and resuspended in TE (10 mM Tris pH 8.0, 1 mM EDTA). Phenol∶chloroform extraction and ethanol precipitation were repeated, and the DNA was resuspended in TE, treated with RNAse A, and quantified using a fluorometer. Prior to all PCR reactions, DNA was diluted to 5 ng/µl in TE.
Primer sequences used in this work are listed in Table 3. Amplification conditions for primer sets: SG1+SG6: 35 cycles of 95°C—30 s, 58°C—30 s, 72°—60 s; 17-F+17-R: of 95°C—30 s, 60°C—30 s, 72°C—75 sec; 17-R+SG2: 95°C—30 s, 58°C—30 s, 72°C—30 sec. Reactions were performed according to the manufacturer's guidelines with Taq polymerase (New England Biolabs, Ipswich, MA) in 1× Thermo Pol buffer supplemented with MgCl2 to a final concentration of 1 mM. PCR reactions with the single primer SG6 were performed using LongAmp Polymerase (New England Biolabs, Ipswich, MA) according to the manufacturer's guidelines in buffer supplemented with KCl to a final concentration of 30 mM. Cycling conditions were 35 cycles of 95°C—30 s, 58°C—30 s, 65°C—8 min. All separations of PCR amplicons were performed in 1% LE agarose, 1× TBE gels. PCR amplicons were cloned into pGEM-T Easy Vector (Promega, Madison, WI) and transformed into E. coli XL1-Blue (Stratagene, La Jolla, CA) according to the manufacturer's instructions. The SG1+SG6 and 17-F+17-R PCR amplicons were sequenced directly; SG6 amplicons were sequenced from plasmid clones. The UC Davis College of Biological Science DNA Sequencing Facility performed all sequencing services.
Table 3. Primers used in this study.
| Name | Sequence (5′ to 3′) | GenBank Accession(s) | Reference |
| a5 | GAGCCGTCCATTTTAAACCA | EU214531.2 | [24] |
| a6 | GGGTTAAGGTTGTTGTTGCC | EU214531.2 | [24] |
| SG1 | CGAGAATTCTACACTGACAATG | EU214531.2 | This work |
| SG2 | ACTGAATCGTCCCCTTTTCC | HQ122410.1 | This work |
| SG6 | GATTCAGTATACGGGGAAAAGG | EU214531.2; HM470230.1–HM470232.1 | This work |
| M8-1F | TGGCTTTCTATATGTTTTTCATGC | HM470230.1–HM470232.1 | This work |
| M8-3R | GTAAGTTGCTGTCAGTGCAAGG | HM470230.1–HM470232.1 | This work |
| M8-6R | TCAGAATCTGCCAAAAGAAACC | HM470230.1–HM470232.1 | This work |
| MjActin RT-F | AAGCCGTTCTTTCTTTGTATGC | AF532604.1 | [24] |
| MjActin RT-R | AAGAATAACCACGTTCAGTGAGG | AF532604.1 | [24] |
| Mj bActin-F | TAGGTATGTTGCCATCCAAGC | AF532604.1 | This work |
| Mj bActin-R | CAAAGCAGTAATTTCCTTCTGC | AF532604.1 | This work |
| 17-F | AGAGCTCGGGACTGAAACGTCC | HQ122410.1 | This work |
| 17-R | TCTCCCTCGCCTCATCTCCACG | HQ122410.1 | This work |
RNA was isolated from approximately 200–300 µl of packed nematode eggs using Trizol reagent (Invitrogen, Carlsbad, CA). Reverse transcription was performed with 5 µg DNAse I-treated RNA using SuperScript III (Invitrogen) and oligo(dT)12–18 (Invitrogen) according to the manufacturer's directions in a total volume of 20 µl. One µl of resulting cDNA was used per 25 µl PCR reaction with NEB Long Amp polymerase (as above) for the following conditions the primer pair M8-1F+M8-3R, 50 cycles of 95°C—30 s, 58°C—30 s, 65°C—80 s. As a positive control, beta actin was amplified from cDNA using Taq polymerase and primers Mj-Actin-RT-F and Mj-Actin-RT-R for M. javanica samples (35 cycles of 95°C—30 sec., 58°C—30 sec., 72°C—30 sec.). Amplicons were sequenced directly as described above.
Restriction digests of either 5 or 10 µg of genomic DNA were performed according to the restriction enzyme manufacturer's directions (New England Biolabs). DNA was separated in 1.0% LE agarose and 1× TBE and blotted to Hybond-N nylon membranes (GE Healthcare Life Sciences, Piscataway, NJ) following standard protocols [85]. Tm1-A probe templates were synthesized from a M8-1F and M8-6R PCR amplicon generated from a plasmid containing a 2.3 kb SG6 amplicon from VW4. Template for Tm1-HH probe was generated by amplification with primers a5 and a6 from a plasmid containing a cloned VW4 a5-a6 amplicon [24]. Beta actin probe was amplified from M. javanica VW4 genomic DNA template using primers Mj bActin-F and Mj bActin-R (95°C—30 s; 58°C—30 sec, 72°C—1 min). Probe templates were isolated from 1% TBE gels using a QIAquick Gel Extraction Kit (Qiagen, Valencia, CA). Randomly-primed probes were labeled with [α32P]-dATP (Perkin Elmer, Waltham, MA) according to published protocols [86]. Blots were hybridized in aqueous buffer at 60°C following standard protocols [85].
A plasmid library of ∼3 kb HindIII fragments of M. javanica VW4 genomic DNA was constructed as follows: 50 µg genomic DNA was digested with HindIII and separated on a 0.8% LE agarose gel in 1× TBE. Four size fractions spanning the range of 2–4 kb were isolated from the gel, and DNA was extracted from these fractions using a QiaQuick Gel Extraction Kit (Qiagen, Valencia CA). The four fractions of gel-extracted DNA were amplified using primers SG1 and SG6 to identify the fraction containing the highest amount of Cg-1 PCR template. The HindIII fragments were cloned into Litmus38i (New England Biolabs) that had been previously treated with alkaline phosphatase. Approximately 10,000 independent clones were screened via PCR using pooling methods described previously [87].
A BAC library of M. javanica VW4 DNA was prepared with high molecular weight DNA isolated from J2s by Lucigen Corporation (Middleton, WI). A total of 12,672 BAC clones with an average insert size of 50 kb was screened via PCR with SG1 and SG6 primers using PCR conditions as above but with the annealing temperature lowered from 58°C to 54°C. The identified BAC clone (BAC 18M17) was isolated and purified according to the instructions provided by Lucigen. Sequencing and sequence assembly of BAC 18M17 was completed by the UC Davis College of Agricultural and Environmental Science Genomics Facility.
Genbank deposits
The following sequences were deposited at GenBank: M. incognita Los Banos-2 Tm1-A, HM470230.1; M. javanica VW4 Tm1-A, HM470231.1; M. javanica Yolo-1 Tm1-A, HM470232.1; M. javanica VW4 BAC 18M17, HQ122410.1; M. javanica VW4 0.4 kb 17-F+17-R amplicon, HQ122411.2. The original sequence of the M. javanica Cg-1 locus (EU214531.1) was updated and corrected with the sequence of the 3.1 kb HindIII clone, EU214531.2.
Bioinformatic analyses
The genome of Meloidogyne incognita (GenBank accessions CABB01000001–CABB01009538) [10] was searched using BLASTN [88] for sequences homologous to Cg-1 (GenBank EU214531). Additional elements flanked by similar inverted repeats were identified using BLASTN to search the genomes of M. incognita and M. hapla [31] (GenBank accessions ABLG01000001–ABLG01003450) for sequences similar to a 38 bp sequence in the inverted repeat flanking Cg-1 (5′–GATTCAGTAT ACGGGGAAAA GGGGACGATT CAGTATAC–3′) with the following parameters (word size = 7; low-complexity filter = on; max E value = 10; gap costs: open = 4, extend = 2; scoring: match = 1, mismatch = −3). Using this method, we obtained a total of 142 BLASTN hits in M. incognita, and 147 BLASTN hits in M. hapla. Two or more BLASTN hits residing on the same contig in opposite orientation relative to each other suggested presence of an intact Tm1 element. A BLAST2 analysis [89] with the following parameters (word size = 11, complexity filter = on, match = 1, mismatch = −2, open gap penalty = 5, gap extension penalty = 2, gap×dropoff = 50, E-value cutoff = 10) was performed to clarify the full extent of the terminal inverted repeats. Tandem repeats of Motif C were annotated manually. Sequence alignments were generated using Geneious v4.6 [90]. Tandem repeats were aligned using ClustalW v1.82 [91] in conjunction with Geneious v4.6 with the following parameters: free end gaps allowed, gap open cost = 15; gap extension cost = 6.66. Sequence logos [92] were generated using Geneious v4.6. Global alignments with free end gaps of Tm1 elements were done using Geneious Aligner, part of Geneious v4.6, with the following parameters: Cost matrix: 65%, match = 5, mismatch = −4, open gap penalty = 12, gap extension penalty = 3.
Predicted protein sequences were analyzed using Pfam v. 24.0 [32]. Nuclear localization signals were identified using PSORT II [40]. GeneMark-ES [93], [94] was used to predict putative genes, using the C. elegans ES-3.0 model for gene prediction parameters.
Supporting Information
Unrooted neighbor-joining consensus tree demonstrating the relationship between M. hapla (red) and M. incognita (blue) Tm1-ML elements. Branch bootstrap support is noted on branches. Tree was generated using Geneious Aligner and Geneious Tree Builder (Drummond et al. 2008). Branch lengths were transformed to uniform length for clarity.
(TIF)
Tm1 elements of Meloidogyne incognita . Target site duplication (TSD) length is indicated if both terminal motifs A1 and A2 are present; n.a. (not analyzed) indicates one or both of these motifs is missing; n.f. (not found) indicates that neither motif A1 or A2 was found. GenBank accession numbers, location on contig, TSD sequences, and sequences of 7 bp terminal motifs are provided in Table S3. When possible, elements are oriented with the Left TIR beginning with Motif A1 and the Right TIR terminating with Motif A2. Letters in parenthesis denote Tm1 elements on the same contig. Notes: a Identity between TIRs excluding motif A1 or A2. Tm1 elements with nested, uncharacterized transposable elements are noted as follows: b Contains 937 bp insertion flanked by 9 bp TSD, c Contains 923 bp insertion flanked by 7 bp TSD, d contains 877 bp insertion lacking TSD.
(DOCX)
Tm1 elements of Meloidogyne hapla . TSD length is indicated if both terminal motifs A1 and A2 are present; n.a. (not analyzed) indicates one or both of these motifs is missing; n.f. (not found) indicates that neither motif A1 or A2 was found. GenBank accession numbers, location of Tm1 elements, TSD sequences, and 7 bp terminal motif sequences are provided in Table S4. When possible, elements are oriented with the Left TIR beginning with Motif A1 and the Right TIR terminating with Motif A2. Letters in parenthesis denote Tm1 elements on the same contig. Note: a Identity between TIRs excluding 7 bp terminal motifs.
(DOCX)
Accessory information of Tm1 elements in Meloidogyne incognita . The 7 bp terminal motifs A1 and A2 are shown in boldface. TSDs are underlined. No flanking sequence is shown if TSDs or terminal motifs are not present. Mismatches within the presumed TSDs are shown as lowercase letters. Letters in parenthesis denote Tm1 elements on the same contig.
(DOCX)
Accessory information of Tm1 elements in Meloidogyne hapla . The 7 bp terminal motifs A1 and A2 are shown in boldface. TSDs are underlined. No flanking sequence is shown if TSDs or terminal motifs are not present. Mismatches within the presumed TSDs are shown as lowercase letters. Letters in parenthesis denote Tm1 elements on the same contig.
(DOCX)
Acknowledgments
The authors acknowledge Rushi Shah, Raquel Cordero, Herschel Espiritu, and Bay Ho for greenhouse and lab assistance; Steven Silva and staff of the UC Davis College of Agriculture and Environmental Science Greenhouses; Chengcang Wu (Lucigen Corp.) for BAC library advice and construction; and Jongmin Baek (UC Davis) for BAC sequencing.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was funded by the USDA, Cooperative State Research, Education, and Extension Service (CSREES), grant no. 2008-35302-18802. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Williamson VM, Gleason CA. Plant-nematode interactions. Curr Opin Plant Biol. 2003;6:327–333. doi: 10.1016/s1369-5266(03)00059-1. [DOI] [PubMed] [Google Scholar]
- 2.Bird AF, Loveys BR. The incorporation of photosynthates by Meloidogyne javanica. J Nematol. 1975;7:111–113. [PMC free article] [PubMed] [Google Scholar]
- 3.McClure MA. Meloidogyne incognita: a metabolic sink. J Nematol. 1977;9:88–90. [PMC free article] [PubMed] [Google Scholar]
- 4.Sasser JN, Freckman DW. A world perspective of nematology: the role of the society. In: Veech JA, Dickson DW, editors. Vistas on Nematol. Hyattsville, MD: Society of Nematologists; 1987. pp. 7–14. [Google Scholar]
- 5.Triantaphyllou AC. Oogenesis in the root-knot nematode Meloidogyne javanica. Nematologica. 1962;7:105–113. [Google Scholar]
- 6.Triantaphyllou AC. Oogenesis and the chromosomes of the parthenogenetic root-knot nematode Meloidogyne incognita. J Nematol. 1981;13:95–104. [PMC free article] [PubMed] [Google Scholar]
- 7.Trudgill DL, Blok VC. Apomictic, polyphagous root-knot nematodes: exceptionally successful and damaging biotrophic root pathogens. Annu Rev Phytopathol. 2001;39:53–77. doi: 10.1146/annurev.phyto.39.1.53. [DOI] [PubMed] [Google Scholar]
- 8.Hugall A, Stanton J, Moritz C. Reticulate evolution and the origins of ribosomal internal transcribed spacer diversity in apomictic Meloidogyne. Mol Biol Evol. 1999;16:157–164. doi: 10.1093/oxfordjournals.molbev.a026098. [DOI] [PubMed] [Google Scholar]
- 9.Lunt DH. Genetic tests of ancient asexuality in root knot nematodes reveal recent hybrid origins. BMC Evol Biol. 2008;8:194. doi: 10.1186/1471-2148-8-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abad P, Gouzy J, Aury JM, Castagnone-Sereno P, Danchin EG, et al. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat Biotechnol. 2008;26:909–915. doi: 10.1038/nbt.1482. [DOI] [PubMed] [Google Scholar]
- 11.Bird DM, Williamson VM, Abad P, McCarter J, Danchin EG, et al. The genomes of root-knot nematodes. Annu Rev Phytopathol. 2009;47:333–351. doi: 10.1146/annurev-phyto-080508-081839. [DOI] [PubMed] [Google Scholar]
- 12.Castagnone-Sereno P. Genetic variability and adaptive evolution in parthenogenetic root-knot nematodes. Heredity. 2006;96:282–289. doi: 10.1038/sj.hdy.6800794. [DOI] [PubMed] [Google Scholar]
- 13.Feschotte C. Transposable elements and the evolution of regulatory networks. Nat Rev Genet. 2008;9:397–405. doi: 10.1038/nrg2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kidwell MG, Lisch D. Transposable elements as sources of variation in animals and plants. Proc Natl Acad Sci U S A. 1997;94:7704–7711. doi: 10.1073/pnas.94.15.7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finnegan DJ. Eukaryotic transposable elements and genome evolution. Trends Genet. 1989;5:103–107. doi: 10.1016/0168-9525(89)90039-5. [DOI] [PubMed] [Google Scholar]
- 16.Haren L, Ton-Hoang B, Chandler M. Integrating DNA: transposases and retroviral integrases. Annu Rev Microbiol. 1999;53:245–281. doi: 10.1146/annurev.micro.53.1.245. [DOI] [PubMed] [Google Scholar]
- 17.Barham WS, Winstead NN. Inheritance of resistance to root-knot nematodes in tomatoes. Proc Am Soc Hortic Sci. 1957;69:372–377. [Google Scholar]
- 18.Gilbert JC, McGuire DC. Inheritance of resistace to severe root knot from Meloidogyne incognita in commericial type tomatoes. Proc Am Soc Hortic Sci. 1956;63:437–442. [Google Scholar]
- 19.Nombela G, Williamson VM, Muniz M. The root-knot nematode resistance gene Mi-1.2 of tomato is responsible for resistance against the whitefly Bemisia tabaci. Mol Plant Microbe Interact. 2003;16:645–649. doi: 10.1094/MPMI.2003.16.7.645. [DOI] [PubMed] [Google Scholar]
- 20.Rossi M, Goggin FL, Milligan SB, Kaloshian I, Ullman DE, et al. The nematode resistance gene Mi of tomato confers resistance against the potato aphid. Proc Natl Acad Sci U S A. 1998;95:9750–9754. doi: 10.1073/pnas.95.17.9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu QL, Thomas VP, Williamson VM. Meiotic parthenogenesis in a root-knot nematode results in rapid genomic homozygosity. Genetics. 2007;176:1483–1490. doi: 10.1534/genetics.107.071134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Triantaphyllou AC. Polyploidy and reproductive patterns in the root-knot nematode Meloidogyne hapla. J Morphol. 1966;118:403–413. doi: 10.1002/jmor.1051180308. [DOI] [PubMed] [Google Scholar]
- 23.Bost SC, Triantaphyllou AC. Genetic basis of the epidemiological effects of resistance to Meloidogyne incognita in the tomato cultivar Small Fry. J Nematol. 1982;14:540–544. [PMC free article] [PubMed] [Google Scholar]
- 24.Gleason CA, Liu QL, Williamson VM. Silencing a candidate nematode effector gene corresponding to the tomato resistance gene Mi-1 leads to acquisition of virulence. Mol Plant Microbe Interact. 2008;21:576–585. doi: 10.1094/MPMI-21-5-0576. [DOI] [PubMed] [Google Scholar]
- 25.Semblat JP, Rosso MN, Hussey RS, Abad P, Castagnone-Sereno P. Molecular cloning of a cDNA encoding an amphid-secreted putative avirulence protein from the root-knot nematode Meloidogyne incognita. Mol Plant Microbe Interact. 2001;14:72–79. doi: 10.1094/MPMI.2001.14.1.72. [DOI] [PubMed] [Google Scholar]
- 26.Xu J, Narabu T, Mizukubo T, Hibi T. A molecular marker correlated with selected virulence against the tomato resistance gene Mi in Meloidogyne incognita, M. javanica, and M. arenaria. Phytopathology. 2001;91:377–382. doi: 10.1094/PHYTO.2001.91.4.377. [DOI] [PubMed] [Google Scholar]
- 27.Xu JH, Narabu T, Li HM, Fu P. Preparation of Meloidogyne javanica near-isogenic lines virulent and avirulent against the tomato resistance gene Mi and preliminary analyses of the genetic variation between the two lines. Yi Chuan Xue Bao. 2002;29:212–216. [PubMed] [Google Scholar]
- 28.Castagnone-Sereno P, Wajnberg E, Bongiovanni M, Leroy F, Dalmasso A. Genetic variation in Meloidogyne incognita virulence against the tomato Mi resistance gene: evidence from isofemale line selection studies. Theor Appl Genet. 1994;88:749–753. doi: 10.1007/BF01253980. [DOI] [PubMed] [Google Scholar]
- 29.Marzluff WF. Metazoan replication-dependent histone mRNAs: a distinct set of RNA polymerase II transcripts. Curr Opin Cell Biol. 2005;17:274–280. doi: 10.1016/j.ceb.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Keall R, Whitelaw S, Pettitt J, Muller B. Histone gene expression and histone mRNA 3′ end structure in Caenorhabditis elegans. BMC Mol Biol. 2007;8:51. doi: 10.1186/1471-2199-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Opperman C, Bird D, Williamson V, Rokshar D, Burke M, et al. Sequence and genetic map of Meloidogyne hapla: A compact nematode genome for plant parasitism. Proc Natl Acad Sci U S A. 2008;105:14802–14807. doi: 10.1073/pnas.0805946105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finn RD, Mistry J, Tate J, Coggill P, Heger A, et al. The Pfam protein families database. Nucleic Acids Res. 2010;38:D211–222. doi: 10.1093/nar/gkp985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Babu MM, Iyer LM, Balaji S, Aravind L. The natural history of the WRKY-GCM1 zinc fingers and the relationship between transcription factors and transposons. Nucleic Acids Res. 2006;34:6505–6520. doi: 10.1093/nar/gkl888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hua-Van A, Capy P. Analysis of the DDE motif in the Mutator superfamily. J Mol Evol. 2008 doi: 10.1007/s00239-008-9178-1. [DOI] [PubMed] [Google Scholar]
- 35.Blumenthal T, Steward K. RNA processing and gene structure. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C elegans II. Plainview, N.Y.: Cold Spring Harbor Laboratory Press; 1997. pp. 117–146. [PubMed] [Google Scholar]
- 36.Marquez CP, Pritham EJ. Phantom, a new subclass of Mutator DNA transposons found in insect viruses and widely distributed in animals. Genetics. 2010;185:1507–1517. doi: 10.1534/genetics.110.116673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dorn R, Krauss V. The modifier of mdg4 locus in Drosophila: functional complexity is resolved by trans splicing. Genetica. 2003;117:165–177. doi: 10.1023/a:1022983810016. [DOI] [PubMed] [Google Scholar]
- 38.Lisch D. Mutator transposons. Trends Plant Sci. 2002;7:498–504. doi: 10.1016/s1360-1385(02)02347-6. [DOI] [PubMed] [Google Scholar]
- 39.Eisen JA, Benito MI, Walbot V. Sequence similarity of putative transposases links the maize Mutator autonomous element and a group of bacterial insertion sequences. Nucleic Acids Res. 1994;22:2634–2636. doi: 10.1093/nar/22.13.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakai K, Horton P. PSORT: a program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem Sci. 1999;24:34–36. doi: 10.1016/s0968-0004(98)01336-x. [DOI] [PubMed] [Google Scholar]
- 41.Hicks GR, Raikhel NV. Protein import into the nucleus: an integrated view. Annu Rev Cell Dev Biol. 1995;11:155–188. doi: 10.1146/annurev.cb.11.110195.001103. [DOI] [PubMed] [Google Scholar]
- 42.Wessler SR, Bureau TE, White SE. LTR-retrotransposons and MITEs: important players in the evolution of plant genomes. Curr Opin Genet Dev. 1995;5:814–821. doi: 10.1016/0959-437x(95)80016-x. [DOI] [PubMed] [Google Scholar]
- 43.Cheng C, Tsuchimoto S, Ohtsubo H, Ohtsubo E. Tnr8, a foldback transposable element from rice. Genes Genet Syst. 2000;75:327–333. doi: 10.1266/ggs.75.327. [DOI] [PubMed] [Google Scholar]
- 44.Potter SS. DNA sequence of a foldback transposable element in Drosophila. Nature. 1982;297:201–204. doi: 10.1038/297201a0. [DOI] [PubMed] [Google Scholar]
- 45.Rebatchouk D, Narita JO. Foldback transposable elements in plants. Plant Mol Biol. 1997;34:831–835. doi: 10.1023/a:1005855008823. [DOI] [PubMed] [Google Scholar]
- 46.Windsor AJ, Waddell CS. FARE, a new family of foldback transposons in Arabidopsis. Genetics. 2000;156:1983–1995. doi: 10.1093/genetics/156.4.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu Z, Yan X, Maurais S, Fu H, O'Brien DG, et al. Jittery, a Mutator distant relative with a paradoxical mobile behavior: excision without reinsertion. Plant Cell. 2004;16:1105–1114. doi: 10.1105/tpc.019802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bennetzen JL, Swanson J, Taylor WC, Freeling M. DNA insertion in the first intron of maize adh1 affects message levels: cloning of progenitor and mutant adh1 alleles. Proc Natl Acad Sci U S A. 1984;81:4125–4128. doi: 10.1073/pnas.81.13.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chalvet F, Grimaldi C, Kaper F, Langin T, Daboussi MJ. Hop, an active Mutator-like element in the genome of the fungus Fusarium oxysporum. Mol Biol Evol. 2003;20:1362–1375. doi: 10.1093/molbev/msg155. [DOI] [PubMed] [Google Scholar]
- 50.Chandler M, Mahillon J. Insertion sequences revisited. In: Craig NL, Craigie R, Gellert M, Lambowitz A, editors. Mobile DNA II. Washington D.C.: ASM Press; 2002. pp. 306–366. [Google Scholar]
- 51.Quintiliani R, Jr, Courvalin P. Characterization of Tn1547, a composite transposon flanked by the IS16 and IS256-like elements, that confers vancomycin resistance in Enterococcus faecalis BM4281. Gene. 1996;172:1–8. doi: 10.1016/0378-1119(96)00110-2. [DOI] [PubMed] [Google Scholar]
- 52.Tailliez P, Ehrlich SD, Chopin MC. Characterization of IS1201, an insertion sequence isolated from Lactobacillus helveticus. Gene. 1994;145:75–79. doi: 10.1016/0378-1119(94)90325-5. [DOI] [PubMed] [Google Scholar]
- 53.Takami H, Han CG, Takaki Y, Ohtsubo E. Identification and distribution of new insertion sequences in the genome of alkaliphilic Bacillus halodurans C-125. J Bacteriol. 2001;183:4345–4356. doi: 10.1128/JB.183.14.4345-4356.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gause M, Morcillo P, Dorsett D. Insulation of enhancer-promoter communication by a gypsy transposon insert in the Drosophila cut gene: cooperation between suppressor of hairy-wing and modifier of mdg4 proteins. Mol Cell Biol. 2001;21:4807–4817. doi: 10.1128/MCB.21.14.4807-4817.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghosh D, Gerasimova TI, Corces VG. Interactions between the Su(Hw) and Mod(mdg4) proteins required for gypsy insulator function. EMBO J. 2001;20:2518–2527. doi: 10.1093/emboj/20.10.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feschotte C, Pritham EJ. DNA transposons and the evolution of eukaryotic genomes. Annu Rev Genet. 2007;41:331–368. doi: 10.1146/annurev.genet.40.110405.090448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marzo M, Puig M, Ruiz A. The Foldback-like element Galileo belongs to the P superfamily of DNA transposons and is widespread within the Drosophila genus. Proc Natl Acad Sci U S A. 2008;105:2957–2962. doi: 10.1073/pnas.0712110105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leroy H, Castagnone-Sereno P, Renault S, Auge-Gouillou C, Bigot Y, et al. Characterization of Mcmar1, a mariner-like element with large inverted terminal repeats (ITRs) from the phytoparasitic nematode Meloidogyne chitwoodi. Gene. 2003;304:35–41. doi: 10.1016/s0378-1119(02)01144-7. [DOI] [PubMed] [Google Scholar]
- 59.Kidwell MG, Lisch DR. Perspective: transposable elements, parasitic DNA, and genome evolution. Evolution. 2001;55:1–24. doi: 10.1111/j.0014-3820.2001.tb01268.x. [DOI] [PubMed] [Google Scholar]
- 60.Jiang N, Bao Z, Zhang X, Eddy SR, Wessler SR. Pack-MULE transposable elements mediate gene evolution in plants. Nature. 2004;431:569–573. doi: 10.1038/nature02953. [DOI] [PubMed] [Google Scholar]
- 61.Lisch D. Pack-MULEs: theft on a massive scale. Bioessays. 2005;27:353–355. doi: 10.1002/bies.20219. [DOI] [PubMed] [Google Scholar]
- 62.Kidwell MG. Horizontal transfer. Curr Opin Genet Dev. 1992;2:868–873. doi: 10.1016/s0959-437x(05)80109-1. [DOI] [PubMed] [Google Scholar]
- 63.Lisch D. A new SPIN on horizontal transfer. Proc Natl Acad Sci U S A. 2008;105:16827–16828. doi: 10.1073/pnas.0809525105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Silva JC, Loreto EL, Clark JB. Factors that affect the horizontal transfer of transposable elements. Curr Issues Mol Biol. 2004;6:57–71. [PubMed] [Google Scholar]
- 65.Diao X, Freeling M, Lisch D. Horizontal transfer of a plant transposon. PLoS Biol. 2006;4:e5. doi: 10.1371/journal.pbio.0040005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lampe DJ, Witherspoon DJ, Soto-Adames FN, Robertson HM. Recent horizontal transfer of Mellifera subfamily mariner transposons into insect lineages representing four different orders shows that selection acts only during horizontal transfer. Mol Biol Evol. 2003;20:554–562. doi: 10.1093/molbev/msg069. [DOI] [PubMed] [Google Scholar]
- 67.Pace JK, 2nd, Gilbert C, Clark MS, Feschotte C. Repeated horizontal transfer of a DNA transposon in mammals and other tetrapods. Proc Natl Acad Sci U S A. 2008;105:17023–17028. doi: 10.1073/pnas.0806548105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Silva JC, Kidwell MG. Horizontal transfer and selection in the evolution of P elements. Mol Biol Evol. 2000;17:1542–1557. doi: 10.1093/oxfordjournals.molbev.a026253. [DOI] [PubMed] [Google Scholar]
- 69.Kaplan N, Darden T, Langley CH. Evolution and extinction of transposable elements in Mendelian populations. Genetics. 1985;109:459–480. doi: 10.1093/genetics/109.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wright S, Finnegan D. Genome evolution: sex and the transposable element. Curr Biol. 2001;11:R296–299. doi: 10.1016/s0960-9822(01)00168-3. [DOI] [PubMed] [Google Scholar]
- 71.Kaloshian I. Gene-for-gene disease resistance: bridging insect pest and pathogen defense. J Chem Ecol. 2004;30:2419–2438. doi: 10.1007/s10886-004-7943-1. [DOI] [PubMed] [Google Scholar]
- 72.Lim JK, Simmons MJ. Gross chromosome rearrangements mediated by transposable elements in Drosophila melanogaster. Bioessays. 1994;16:269–275. doi: 10.1002/bies.950160410. [DOI] [PubMed] [Google Scholar]
- 73.Mieczkowski PA, Lemoine FJ, Petes TD. Recombination between retrotransposons as a source of chromosome rearrangements in the yeast Saccharomyces cerevisiae. DNA Repair (Amst) 2006;5:1010–1020. doi: 10.1016/j.dnarep.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 74.Cáceres M, Ranz JM, Barbadilla A, Long M, Ruiz A. Generation of a widespread Drosophila inversion by a transposable element. Science. 1999;285:415–418. doi: 10.1126/science.285.5426.415. [DOI] [PubMed] [Google Scholar]
- 75.Casals F, Cáceres M, Ruiz A. The foldback-like transposon Galileo is involved in the generation of two different natural chromosomal inversions of Drosophila buzzatii. Mol Biol Evol. 2003;20:674–685. doi: 10.1093/molbev/msg070. [DOI] [PubMed] [Google Scholar]
- 76.McClintock B. The significance of responses of the genome to challenge. Science. 1984;226:792–801. doi: 10.1126/science.15739260. [DOI] [PubMed] [Google Scholar]
- 77.Arnold DL, Jackson RW, Waterfield NR, Mansfield JW. Evolution of microbial virulence: the benefits of stress. Trends Genet. 2007;23:293–300. doi: 10.1016/j.tig.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 78.Arnold DL, Pitman A, Jackson RW. Pathogenicity and other genomic islands in plant pathogenic bacteria. Mol Plant Pathol. 2003;4:407–420. doi: 10.1046/j.1364-3703.2003.00187.x. [DOI] [PubMed] [Google Scholar]
- 79.Sinzelle L, Izsvak Z, Ivics Z. Molecular domestication of transposable elements: from detrimental parasites to useful host genes. Cell Mol Life Sci. 2009;66:1073–1093. doi: 10.1007/s00018-009-8376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang C, Bruening G, Williamson VM. Determination of preferred pH for root-knot nematode aggregation using pluronic F-127 gel. J Chem Ecol. 2009;35:1242–1251. doi: 10.1007/s10886-009-9703-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu QL, Williamson VM. Genetic linkage map of root-knot nematode, Meloidogyne hapla. J Nematol. 2006;38:279–279. [Google Scholar]
- 82.Powers TO, Harris TS. A polymerase chain reaction method for identification of five major Meloidogyne species. J Nematol. 1993;25:1–6. [PMC free article] [PubMed] [Google Scholar]
- 83.Zijlstra C, Donkers-Venne DTHM, Fargette M. Identification of Meloidogyne incognita, M. javanica, and M. arenaria using sequenced characterized amplifed region (SCAR) based PCR assays. Nematology. 2000;2:847–853. [Google Scholar]
- 84.Branch C, Hwang CF, Navarre DA, Williamson VM. Salicylic acid is part of the Mi-1-mediated defense response to root-knot nematode in tomato. Mol Plant Microbe Interact. 2004;17:351–356. doi: 10.1094/MPMI.2004.17.4.351. [DOI] [PubMed] [Google Scholar]
- 85.Sambrook J, Russell DW. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 86.Feinberg AP, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 87.Takumi T. Rapid cDNA cloning by PCR screening (RC-PCR). Methods Mol Biol. 2002;192:385–389. doi: 10.1385/1-59259-177-9:385. [DOI] [PubMed] [Google Scholar]
- 88.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 89.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Drummond AJ, Ashton B, Cheung M, Heled J, Kearse M, et al. 2008. Geneious v4.0, Available from http://www.geneious.com/
- 91.Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, et al. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003;31:3497–3500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schneider TD, Stephens RM. Sequence logos: a new way to display consensus sequences. Nucleic Acids Res. 1990;18:6097–6100. doi: 10.1093/nar/18.20.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Besemer J, Borodovsky M. GeneMark: web software for gene finding in prokaryotes, eukaryotes and viruses. Nucleic Acids Res. 2005;33:W451–454. doi: 10.1093/nar/gki487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lomsadze A, Ter-Hovhannisyan V, Chernoff YO, Borodovsky M. Gene identification in novel eukaryotic genomes by self-training algorithm. Nucleic Acids Res. 2005;33:6494–6506. doi: 10.1093/nar/gki937. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Unrooted neighbor-joining consensus tree demonstrating the relationship between M. hapla (red) and M. incognita (blue) Tm1-ML elements. Branch bootstrap support is noted on branches. Tree was generated using Geneious Aligner and Geneious Tree Builder (Drummond et al. 2008). Branch lengths were transformed to uniform length for clarity.
(TIF)
Tm1 elements of Meloidogyne incognita . Target site duplication (TSD) length is indicated if both terminal motifs A1 and A2 are present; n.a. (not analyzed) indicates one or both of these motifs is missing; n.f. (not found) indicates that neither motif A1 or A2 was found. GenBank accession numbers, location on contig, TSD sequences, and sequences of 7 bp terminal motifs are provided in Table S3. When possible, elements are oriented with the Left TIR beginning with Motif A1 and the Right TIR terminating with Motif A2. Letters in parenthesis denote Tm1 elements on the same contig. Notes: a Identity between TIRs excluding motif A1 or A2. Tm1 elements with nested, uncharacterized transposable elements are noted as follows: b Contains 937 bp insertion flanked by 9 bp TSD, c Contains 923 bp insertion flanked by 7 bp TSD, d contains 877 bp insertion lacking TSD.
(DOCX)
Tm1 elements of Meloidogyne hapla . TSD length is indicated if both terminal motifs A1 and A2 are present; n.a. (not analyzed) indicates one or both of these motifs is missing; n.f. (not found) indicates that neither motif A1 or A2 was found. GenBank accession numbers, location of Tm1 elements, TSD sequences, and 7 bp terminal motif sequences are provided in Table S4. When possible, elements are oriented with the Left TIR beginning with Motif A1 and the Right TIR terminating with Motif A2. Letters in parenthesis denote Tm1 elements on the same contig. Note: a Identity between TIRs excluding 7 bp terminal motifs.
(DOCX)
Accessory information of Tm1 elements in Meloidogyne incognita . The 7 bp terminal motifs A1 and A2 are shown in boldface. TSDs are underlined. No flanking sequence is shown if TSDs or terminal motifs are not present. Mismatches within the presumed TSDs are shown as lowercase letters. Letters in parenthesis denote Tm1 elements on the same contig.
(DOCX)
Accessory information of Tm1 elements in Meloidogyne hapla . The 7 bp terminal motifs A1 and A2 are shown in boldface. TSDs are underlined. No flanking sequence is shown if TSDs or terminal motifs are not present. Mismatches within the presumed TSDs are shown as lowercase letters. Letters in parenthesis denote Tm1 elements on the same contig.
(DOCX)