Abstract
The neural cell adhesion molecule L1 is critical for brain development and plays a role in learning and memory in the adult. Ethanol inhibits L1-mediated cell adhesion and neurite outgrowth in cerebellar granule neurons (CGNs), and these actions might underlie the cerebellar dysmorphology of fetal alcohol spectrum disorders. The peptide NAP potently blocks ethanol inhibition of L1 adhesion and prevents ethanol teratogenesis. We used quantitative RT-PCR and Western blotting of extracts of cerebellar slices, CGNs, and astrocytes from postnatal day 7 (PD7) rats to investigate whether ethanol and NAP act in part by regulating the expression of L1. Treatment of cerebellar slices with 20 mM ethanol, 10−12 M NAP, or both for 4 hours, 24 hours, and 10 days did not significantly affect L1 mRNA and protein levels. Similar treatment for 4 or 24 hours did not regulate L1 expression in primary cultures of CGNs and astrocytes, the predominant cerebellar cell types. Because ethanol also damages the adult cerebellum, we studied the effects of chronic ethanol exposure in adult rats. One year of binge drinking did not alter L1 gene and protein expression in extracts from whole cerebellum. Thus, ethanol does not alter L1 expression in the developing or adult cerebellum; more likely, ethanol disrupts L1 function by modifying its conformation and signaling. Likewise, NAP antagonizes the actions of ethanol without altering L1 expression.
Introduction
The L1 neural cell adhesion molecule is critical for brain development. L1 mediates cell-cell interactions, neuronal migration, neurite outgrowth, axon guidance and fasciculation, and neuronal survival in the developing nervous system [1]. L1 expression persists in the adult nervous system, where it is believed to play a role in learning, memory, and regeneration after injury [2]–[5]. L1 gene mutations cause a spectrum of dysmorphic lesions, including hydrocephalus, agenesis or hypoplasia of the corpus callosum, and cerebellar dysplasia, referred to as CRASH or L1 syndrome [6], [7]. The similarity of the lesions of L1 syndrome to those of fetal alcohol spectrum disorders (FASD) led to the hypothesis that ethanol causes FASD in part by disrupting L1-mediated processes [8], [9]. In support of this hypothesis, concentrations of ethanol attained after one drink inhibit L1-mediated cell-cell adhesion (L1 adhesion) in transfected fibroblasts, neural cell lines, and cerebellar granule neurons (CGNs) [8]–[10]. Furthermore, ethanol inhibits L1-mediated neurite outgrowth in CGNs at similarly low concentrations [11]. Finally, drugs that block ethanol inhibition of L1 adhesion also prevent ethanol teratogenesis in mouse embryos [12]–[15]. One such ethanol antagonist, the peptide NAPVSIPQ (NAP), blocks ethanol inhibition of L1 adhesion at femtomolar concentrations [16].
Several mechanisms might account for how ethanol disrupts L1 function. Recent data suggest that ethanol alters extracellular domain interactions that are critical for L1 homophilic binding [17], [18]. Ethanol also disrupts L1 activation of intracellular signaling events [19], [20]. It is unknown whether regulation of L1 expression also contributes to ethanol neurotoxicity. Reductions in L1 expression could not occur rapidly enough to account for acute ethanol inhibition of L1 adhesion; however, changes in L1 expression after longer periods of ethanol exposure would disrupt both L1 adhesion and L1-mediated neurite outgrowth. Furthermore, NAP-induced up-regulation of L1 expression could partly compensate for ethanol inhibition of L1 adhesion.
Ethanol damages the developing and adult cerebellum [21]–[23]. Because L1 is critical for cerebellar development and survival of cerebellar neurons [1], [24], ethanol could damage the cerebellum by altering the expression of L1. Indeed, another teratogen, polychlorinated biphenyls (PCBs), significantly reduced L1 expression in whole cerebellum [25]. The effects of ethanol on L1 expression have not been well studied. Chronic ethanol treatment did not reduce L1 protein expression in the NG108-15 neural cell line [9] and transiently increased L1 gene expression in B104 neuroblastoma cells [26]. However, it is unknown whether ethanol modulates the expression of L1 in cerebellum, nor whether NAP antagonizes ethanol inhibition of L1 function by increasing L1 expression.
We systematically investigated the effects of ethanol and NAP exposure on L1 mRNA and protein expression in cerebellar slices, CGNs, and astrocytes of postnatal day 7 (PD7) rats. Vulnerability to binge alcohol-induced cerebellum damage is greatest during PD4-PD9 in rats, the period that corresponds to gestational weeks 24–32 in humans [27], [28]. At this developmental stage, cerebellar neurons undergo neuritogenesis and express high levels of L1 [2], [29]. Because alcoholics frequently develop cerebellar degeneration [23], [30], we also examined the effects of long-term binge drinking on L1 expression in adult rat cerebellum. Here we present evidence that ethanol does not regulate L1 expression in the developing or adult cerebellum. Similarly, NAP or the combination of ethanol and NAP do not alter L1 mRNA or protein levels in the developing cerebellum.
Results
Quality control, assay reliability, and validation of endogenous controls
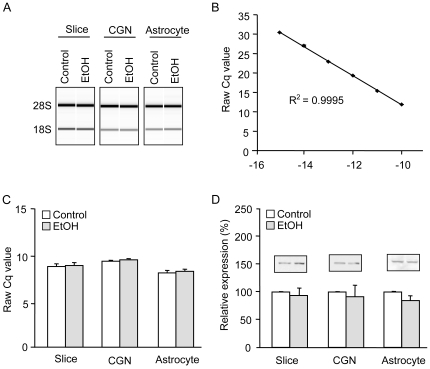
High-quality RNA preparations are necessary to insure that measured quantities of gene transcripts are representative of in vivo expression levels [31]. Therefore, the 28S:18S ribosomal RNA (rRNA) ratios, RNA integrity numbers (RINs), and yield were measured for every RNA sample prior to use in quantitative reverse transcription PCR (qRT-PCR). All samples had an RIN above 8.5 (data not shown), and treatment with ethanol did not degrade RNA quality (Fig. 1A). Average quantification cycle values (Cq) were linear (R2 = 0.9995) with log-transformed L1 transcript concentration over at least six log orders (Fig. 1B). Average quantification cycle (Cq) values from control and ethanol-treated samples were used to evaluate the stability of potential endogenous control genes, and 18S rRNA (18S) was found to be more stable than cyclophilin A (not shown) in all cell and tissue types (Fig. 1C). Due to its stability with ethanol treatment, 18S was used to normalize all L1 mRNA expression data. Similarly, we evaluated the effects of ethanol on levels of expression of candidate endogenous protein controls, including β-tubulin (Fig. 1D), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and actin (not shown). Among these, only β-tubulin protein expression was unaffected by ethanol treatment in all three culture preparations.
Figure 1. RNA quality, assay reliability, and validation of endogenous controls.
Total RNA quality was assessed using an Agilent Bioanalyzer 2100 and endogenous controls were validated for RT-qPCR and Western blot. A) Generated gel images show representative slice, CGN, and astrocyte samples with and without 24-hr ethanol treatment. B) A standard curve was performed using a dilution series of purified L1 template. Increases in template concentration result in decreasing Cq values, as expected for a well-functioning qPCR assay (n = 4). C) 18S Cq values are shown for all tissue/cell types with and without ethanol treatment. D) Representative Western blots for β-tubulin are shown for each sample type with and without 24-hr ethanol treatment. All bars (C, D) show the mean + SEM of 4 independent experiments.
Effects of ethanol and NAP on L1 expression in early postnatal cerebellum
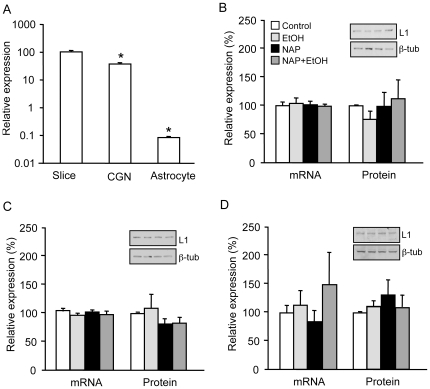
We first determined relative levels of L1 mRNA in the different culture preparations. L1 expression was 62.1±4.2% lower in CGNs than in slices and 99.9±0.01% lower in astrocytes than in slices (n = 4; p<0.0001 for each comparison)(Fig. 2A). To determine the effects of ethanol and NAP on L1 expression, cerebellar slices from PD7 rats were exposed to 20 mM ethanol, 10−12 M NAP, or both for 4 hours, 24 hours, or 10 days. Treatment with ethanol or NAP had no effect on levels of L1 mRNA or protein at any of the time points (Fig. 2B, Table 1). Treatment with ethanol and NAP for 10 days significantly reduced L1 mRNA, but had no significant effect on L1 total protein expression (Table 1).
Figure 2. L1 expression in cerebellar slices, CGNs, and astrocytes.
A) Comparison of L1 transcript levels in different culture preparations. * p<0.0001 compared to slices. B–D) Total RNA and cell lysates were collected from slice, CGN, and astrocyte cultures after 24-hr exposure to 20 mM ethanol, 10−12 M NAP, or the combination of NAP and ethanol. L1 mRNA expression was measured by qRT-PCR and normalized to 18S. L1 protein levels were measured by Western blot and normalized to β-tubulin (representative images shown above corresponding bars). L1 mRNA and protein levels are shown in cerebellar slices (B), CGNs (C), and astrocytes (D). Legend in B applies to B–D. All bars represent normalized mean + SEM of 4 independent experiments.
Table 1. Summary of ethanol and NAP effects on L1 mRNA and protein expression in three cerebellar culture systems.
| Sample type | L1 mRNA (% RQ±SEM) | n | p | L1 protein (% RQ±SEM) | n | p |
| Cerebellar slices | ||||||
| 4 hr | ||||||
| Control | 100±13 | 7 | - | 100 | 6 | - |
| EtOH | 102±9 | 7 | 0.92 | 82±16 | 6 | 0.30 |
| NAP | 91±4 | 4 | 0.59 | 121±59 | 6 | 0.73 |
| NAP+EtOH | 114±9 | 4 | 0.46 | 88±21 | 6 | 0.60 |
| 24 hr | ||||||
| Control | 100±6 | 4 | - | 100 | 4 | - |
| EtOH | 104±10 | 4 | 0.83 | 76±14 | 4 | 0.19 |
| NAP | 101±7 | 4 | 0.91 | 98±26 | 4 | 0.94 |
| NAP+EtOH | 99±4 | 4 | 0.85 | 112±33 | 4 | 0.75 |
| 10 day | ||||||
| Control | 100±10 | 4 | - | 100 | 3 | - |
| EtOH | 108±8 | 4 | 0.45 | 87±40 | 3 | 0.80 |
| NAP | 97±3 | 4 | 0.94 | 100±28 | 3 | 0.99 |
| NAP+EtOH | 65±7 | 4 | 0.03 | 113±42 | 3 | 0.78 |
| CGNs | ||||||
| 4 hr | ||||||
| Control | 100±12 | 5 | - | 100 | 4 | - |
| EtOH | 115±13 | 5 | 0.39 | 109±19 | 4 | 0.66 |
| NAP | 118±6 | 5 | 0.24 | 92±10 | 4 | 0.50 |
| NAP+EtOH | 131±7 | 5 | 0.07 | 99±32 | 4 | 0.98 |
| 24 hr | ||||||
| Control | 105±6 | 4 | - | 100 | 4 | - |
| EtOH | 96±4 | 4 | 0.25 | 109±25 | 4 | 0.76 |
| NAP | 103±4 | 4 | 0.77 | 81±10 | 4 | 0.16 |
| NAP+EtOH | 98±7 | 4 | 0.48 | 83±11 | 4 | 0.22 |
| Astrocytes | ||||||
| 4 hr | ||||||
| Control | 100±10 | 3 | - | |||
| EtOH | 85±13 | 3 | 0.47 | |||
| NAP | 77±11 | 3 | 0.20 | |||
| NAP+EtOH | 97±15 | 3 | 0.90 | |||
| 24 hr | ||||||
| Control | 100±15 | 8 | - | 100 | 4 | - |
| EtOH | 114±26 | 6 | 0.63 | 111±10 | 4 | 0.37 |
| NAP | 84±20 | 5 | 0.52 | 132±26 | 4 | 0.31 |
| NAP+EtOH | 150±57 | 3 | 0.23 | 109±22 | 4 | 0.72 |
Cerebellar slices comprise a mixture of cell types, so experiments on slices might obscure opposite effects on different cell types. Therefore, we conducted separate experiments on primary cultures of CGNs and astrocytes, the predominant cell types of the cerebellum. In CGNs, treatment with ethanol, NAP, or both for 4 or 24 hours had no significant effect on the expression of L1 mRNA or protein (Fig. 2C, Table 1). L1 expression was significantly lower in astrocytes compared with CGNs, as previously described [32], but also showed no significant changes with ethanol, NAP, or combined treatments (Fig. 2A,D). Higher concentrations of ethanol (100 mM) likewise had no effect on L1 expression in cerebellar slices, CGNs, and astrocytes (not shown).
Effects of ethanol and NAP on L1 expression in adult cerebellum
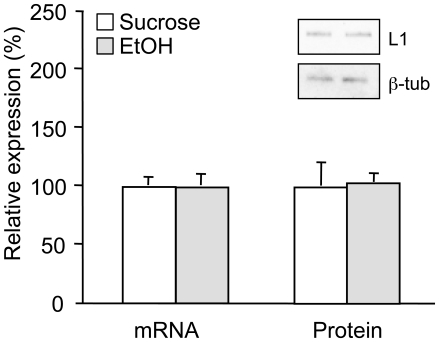
We used a chronic binge-drinking rat model [33] to evaluate the effects of ethanol on L1 expression in adult cerebellum. We measured L1 mRNA and protein expression in whole cerebellar homogenates from rats that had self-administered ethanol for more than 12 months, beginning at approximately 2 months of age. Subjects that self-administered ethanol attained mean blood ethanol concentrations of 100±14 mg/dl (n = 6). Cerebellar L1 mRNA and protein levels did not differ between ethanol-exposed rats (n = 6) and sucrose controls (n = 7)(Fig. 3).
Figure 3. L1 expression in adult cerebellum after chronic binge drinking.
Total RNA and tissue lysates were collected from cerebella of adult rats following one year of self-administration of ethanol (2% sucrose/10% ethanol) or sucrose (2% sucrose). L1 mRNA expression was measured by qRT-PCR and normalized to 18S. L1 protein levels were measured by Western blot and normalized to β-tubulin. The inset shows representative Western blot images above corresponding bars. Bars represent normalized mean + SEM (ethanol, n = 6; sucrose, n = 7).
Discussion
The major finding of this work is that ethanol does not regulate L1 gene or protein expression in the developing or adult cerebellum. Likewise, the alcohol antagonist NAP, either alone or in combination with ethanol, does not regulate L1 expression in three models of the developing cerebellum.
Validity of the experimental results
Accurate gene and protein expression analysis requires rigorous techniques and the appropriate selection of endogenous reference genes or proteins for the experimental conditions. We followed all of the recent recommendations for the reliable measurement of mRNA by qRT-PCR [34], [35]. In particular, we isolated high-quality RNA with an RIN that was consistently higher than 8.5 - well above the recommended threshold for qRT-PCR [31], [36]. Furthermore, we fully validated our primer pairs and performed standard curves to verify PCR efficiency and exclude the presence of PCR inhibitors. Finally, we also verified that our endogenous reference standards - 18S for RNA and β-tubulin for protein – were not influenced by ethanol treatment in any of the in vitro model systems.
Ethanol does not affect L1 expression in developing cerebellum
We studied PD7 rats, because the cerebellum is particularly vulnerable to ethanol exposure at this developmental time point [27], [28], when L1 plays a critical role in CGN differentiation, migration, and survival [2], [24], [37]–[40]. L1 regulates CGN migration and axon outgrowth and is a survival factor for CGNs [24], [38], [39]; therefore, ethanol-induced reductions in L1 expression could disrupt cerebellar development. We used three different model systems to evaluate the effects of ethanol exposure on L1 expression. Cerebellar slices preserve the integrity of cerebellar circuitry and neuronal-glial interactions in the developing cerebellum [41]. Cerebellar granule neurons and astrocytes are the most abundant neuronal and glial cell types, respectively, in the developing cerebellum and both show ethanol-induced cell death [42], [43]. Treatment with intoxicating (20 mM) or anesthetic (100 mM) concentrations of ethanol for 4 hours, 24 hours, or 10 days did not reduce L1 expression in cerebellar slices, CGNs, and astrocytes, with one exception. After 10 days of ethanol plus NAP treatment, there was a decrease in L1 mRNA in cerebellar slices, but this was of dubious functional significance, since there was no corresponding change in L1 protein expression.
Our failure to observe changes in L1 mRNA is not likely a consequence of the insensitivity of our assays or the unresponsiveness of our culture systems to ethanol. Our qRT-PCR assay was highly sensitive and linear in detecting differences in L1 transcript levels and allowed us to observe significant differences in L1 expression among cerebellar slices, CGNs, and astrocytes. Furthermore, the ethanol dose and duration of treatment in these experiments are sufficient to modify cerebellar physiology [44], [45], neuronal differentiation [11], [46], and gene expression [47]–[51]. Taken together, our findings suggest that ethanol does not disrupt cerebellar development by altering L1 expression.
Although previous work in CGNs demonstrated that ethanol inhibits L1 adhesion within 30 minutes [8] and L1-mediated neurite outgrowth within 12 hours [11], [19], our data indicate that neither of these effects can be attributed to ethanol-induced reductions in L1 expression. Indeed, recent studies suggest that ethanol inhibits L1 adhesion by disrupting the interactions of the Ig1 and Ig4 extracellular domains [17], [18]. Further work is required to learn whether ethanol modulates L1 expression in other cerebellar cell types, such as Purkinje cells, Golgi neurons, microglia, and oligodendrocytes. Likewise, it remains to be determined whether ethanol modulates the subcellular distribution of L1 in CGNs. Recent studies indicate that ethanol does not change the polarity of L1 sorting within dorsal root ganglion cells [19].
NAP does not regulate L1 expression in developing cerebellum
NAP is neuroprotective against a variety of insults, including fetal alcohol exposure, although the underlying mechanisms are unclear [12], [15], [52]–[54]. Our studies were designed to evaluate whether NAP up-regulates L1 expression, which could compensate for ethanol inhibition of L1 function. Treatment of cells with NAP, alone or in combination with ethanol, had no effect on L1 protein expression at any of the time points in any of the in vitro model systems. Similar concentrations of NAP induced axon outgrowth in CGNs and blocked ethanol-induced teratogenesis in mouse embryos [12], [46]. Therefore, it is unlikely that NAP prevents ethanol teratogenesis by regulating the expression of L1. In addition to blocking ethanol inhibition of L1 adhesion, NAP might prevent ethanol teratogenesis by blocking ethanol-induced decreases in levels of reduced glutathione and in GABAAβ3 receptor and BDNF gene expression [15], [55], [56].
Ethanol does not regulate L1 expression in adult cerebellum
Cerebellar atrophy is a common finding in alcoholics in both imaging and autopsy studies [57], [58]. Because L1 is a neuronal survival factor, ethanol effects on L1 expression could mediate alcoholic cerebellar degeneration. Our data demonstrate that one year of binge drinking to intoxicating blood ethanol concentrations did not alter L1 gene and protein expression in adult cerebellum. These findings make it less likely that ethanol causes cerebellar degeneration in part by down-regulating L1 expression. Further studies with other alcohol exposure paradigms and in other species would strengthen this conclusion.
Materials and Methods
Materials
Sprague Dawley rats from Charles River Laboratories (Wilmington, MA) were used for all cell and tissue culture experiments. Mothers and pups were allowed to acclimate for at least 24 hr prior to sacrifice. Male Long Evans rats (average age 7 weeks) were purchased from Harlan (Indianapolis, IN) and had an average body weight of 225 g at the start of training in binge-drinking experiments. Subjects were allowed to acclimate to the new environment for 5 days prior to any treatment. All animals were maintained on a light/dark cycle (0600 h to 1800 h) with access to food and water ad libitum. Animal care procedures were conducted in accordance with NIH guidelines and the approval of the Institutional Animal Care and Use Committees at the VA Boston Healthcare System and Boston University School of Medicine.
Neurobasal medium, Dulbecco's Modified Eagle Medium (DMEM), Hank's Balanced Salt Solution (HBSS), horse serum, bovine serum, Penicillin-Streptomycin-L-Glutamine (PSG), Penicillin-streptomycin (PS), HEPES buffer, and L-glutamine were acquired from Gibco (Carlsbad, CA). Glasgow Minimal Essential Medium (MEM), glucose, sodium bicarbonate, human apo-transferrin, L-thyroxine, selenium selenate, bovine insulin, bovine aprotinin, albumin from bovine serum (BSA), poly-L-lysine (pLL), and anti-GFAP antibody were acquired from Sigma Aldrich (St. Louis, MO). Hyclone bovine calf serum was obtained from Thermo Scientific (Waltham, MA), trypsin and DNase were obtained from Worthington (Lakewood, NJ), and 10× HBSS was obtained from Cellgro (Manassas, VA). Millipore (Billerica, MA) Millicell cell culture inserts were used in slice culture. Ethanol (anhydrous, 200-proof) from Sigma Aldrich was used for all treatments in cell and tissue culture. In the binge drinking experiments the ethanol solution was diluted from non-denatured 200-proof ethanol obtained from Pharmaco-AAPER, (Brookfield,CT), and the sucrose solution was made with Domino® sugar. The NAP peptide (NAPVSIPQ) was synthesized by New England Peptide (Gardner, MA).
All RNA preparation reagents were obtained from Qiagen (Valencia, CA), with the exception of ethanol from Sigma Aldrich and chloroform from Shelton Scientific (Shelton, CT). The RNA Nano Chip kit was acquired from Agilent (Santa Clara, CA). All reverse transcriptase PCR and quantitative PCR reagents were obtained from Promega (Madison, WI), with the exception of target-specific primers, which were synthesized by Invitrogen (Carlsbad, CA).
Radio-immunoprecipitation assay (RIPA) buffer, SDS-Tris-Glycine running buffer, transfer buffer, Tris-buffered saline (TBS), and Tris-buffered saline with Tween 20 (TBST) were from Boston BioProducts (Ashland, MA). Bovine serum albumin (BSA) fraction V was obtained from EMD (Gibbstown, NJ), instant non-fat dry milk was purchased at a local grocery store, and methanol was purchased from Sigma Aldrich. Complete Mini EDTA-free protease inhibitor cocktail was obtained from Roche (Basel, Switzerland). HALT phosphatase inhibitor, Pierce BCA protein concentration assay kit, and Pierce ECL Western blotting substrate were purchased from Thermo Scientific. Mini-PROTEAN TGX pre-cast gels (4–15%) and Trans-Blot nitrocellulose membranes were obtained from BioRad (Hercules, CA), and Re-blot Plus was acquired from Millipore. L1 goat polyclonal primary antibody (SC1508) and rabbit polyclonal ß-tubulin antibody (SC9104) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), and all secondary antibodies were acquired from Jackson ImmunoResearch (West Grove, PA).
Culture of cerebellar slices, CGNs, and astrocytes
All cultured cells and tissues were derived from PD7 rat cerebella. Pups were sacrificed with CO2, followed by cervical dislocation and decapitation. Cerebella were removed and meninges and blood vessels were dissected away in ice-cold HHGN (HBSS with 2.5 mM HEPES, 35 mM glucose, and 4 mM sodium bicarbonate).
Cerebellar slices were prepared as previously described [59]. Briefly, cerebella were cut into 350 µm slices using an 800 series McIlwain Tissue Chopper. Slices were manually separated and plated on Millicell cell culture inserts. Cultures were maintained in Glasgow MEM with 25% horse serum, 12.5 mM HEPES, 2.5% 10× HBSS, 1% PSG, and 22 mM glucose.
Primary CGN cultures were prepared as previously described [60]. Briefly, coarsely-chopped cerebella were incubated in 1% trypsin/0.05% DNase for 16 min and washed with HBSS. Cells were dissociated in 0.05% DNase solution by mechanical trituration. CGNs were separated by centrifugation (120 g) through a cushion of HBSS and Neurobasal medium with 15% bovine serum. Pelleted CGNs were washed with HBSS, followed by culture medium. Cells were plated on pLL-coated plates and maintained in Neurobasal medium supplemented with 1 mg/ml BSA, 10 µg/ml human apo-transferrin, 4 nM L-thyroxine, 30 nM selenium selenate, 1 µl/ml bovine aprotinin, 1 µg/ml insulin, and 2 µM L-glutamine.
Primary astrocytes were cultured as described [61], with modifications. Total cerebellar cells were dissociated as described for CGN culture. Cells were plated on pLL-coated plates in DMEM containing 10% Hyclone bovine calf serum and 1% PS. After approximately 10 days in culture, cells were shaken at 200 rpm for 6 hours to remove microglia and oligodendrocytes. Adherent astrocytes were maintained for further culture. Astrocyte purity was assessed using immunocytochemistry for GFAP and determined to be greater than 98% in representative cultures (data not shown).
Slices and CGNs were cultured for 24 to 48 hr before treatment. Astrocytes were maintained in culture for 3 to 8 passages (6–10 weeks) prior to treatment. All cultures were treated by refreshing medium and supplementing with 20 mM ethanol, 10−12 M NAP, or both.
Chronic binge-drinking animal model
Self-administration training was conducted in operant chambers (Med-Associates, St. Albans, VT) equipped with a light, retractable lever, and retractable double ball bearing sipper to prevent leakage. All subjects were initially trained by drinking 10% sucrose for several days and then randomly divided into ethanol (n = 6) and sucrose (n = 7) groups using a modification of the sucrose fading procedure [62], [63]. Training was started on a continuous reinforcement schedule with a fixed ratio (FR1-FR4) that transitioned to a response requirement (RR4 to RR20). Subjects had access to the sipper tube daily for 20 minutes, 5 days per week and attained an average ethanol daily intake of 1.15±0.003 g/kg. Two weeks prior to the end of the experiment, blood samples were collected from snipped tails following a 20-minute drinking session. Blood ethanol concentrations were determined using an Analox GM7 Analyzer (Analox Instruments, Lunenburg, MA). After one year of drinking and two hours after the last drink, animals were sacrificed, and the cerebella were removed for mRNA and Western blot analysis.
RNA isolation and cDNA synthesis
Total RNA was prepared using RNeasy Lipid Tissue Mini-kit, following the manufacturer's protocol. Briefly, Qiazol buffer was added to all samples. Afterwards, slices were disrupted by manual grinding and cell culture samples were lysed and suspended by scraping. All samples were sonicated for 1 min on ice to achieve complete homogenization. RNA was purified using spin columns. For chronic binge drinking animals, a portion of each cerebellum was preserved in RNAlater before processing for RNA preparation. RNA yield and quality were measured with an RNA Nano Chip kit using an Agilent Bioanalyzer 2100.
Total RNA samples were reverse-transcribed using GoScript Reverse Transcription System, following the manufacturer's protocol. Reactions were performed with 0.5 µg total RNA, 0.5 µg random hexamers, 3.75 mM MgCl2, 0.5 mM PCR nucleotides, 20 U RNasin, and 1 µL GoScript RT enzyme in 20 µL total volume. RNA and random hexamers were combined and incubated at 70°C for 5 min before combination with other components. Reactions were incubated at 25°C for 5 min to allow primer annealing, followed by 42°C for 1 hr for extension. The RT enzyme was inactivated by incubation at 70°C for 15 min. “No RT” reactions were performed for each sample by omitting the RT enzyme.
Quantitative real-time PCR
Primer pairs to amplify L1, 18S, and cyclophilin A (CypA) were used directly or with modifications from previously published sequences (Table 2) [64]–[66]. The sequences were analyzed using Primer-BLAST (NCBI) to assess amplicon specificity, size, and location. To confirm primer specificity, melting curves were performed and showed a single peak for each reaction, indicating a single amplicon and no primer dimerization. Additionally, gel electrophoresis confirmed amplicon size.
Table 2. Primers used for PCR amplification of target genes.
| Target (RefSeq) | Primer sequence | Location |
| L1 (NM_017345) | F-GCCTGACACCAAATATGAGATCCACC | 3346 |
| R-CTGACAAAGGCGATGAACCA | 3489 | |
| 18S (M11188.1) | F-GGACACGACAGGATTGACA | 1278 |
| R-ACCCACGGATCGAGAAAGA | 1327 | |
| CypA (NM_017101.1) | F-TGTGCCAGGGTGGTGACTT | 224 |
| R-TCAAATTTCTCTCCGTAGATGGACTT | 293 |
GoTaq Master Mix kits were used to amplify target genes for quantitative real time PCR (qPCR). This chemistry utilizes a SYBR-Green dye analog to bind double-stranded DNA. Reactions were performed in triplicate and each contained 1× GoTaq master mix (12.5 µL), 1× carboxy-X-rhodamine dye (0.25 µL), 100 nM forward and reverse primers, and 10 ng cDNA in 25 µL total volume. Amplification and data collection were performed in an ABI 7900 Signal Detector using a 96-well format. Cycling consisted of an initial denaturation step (95°C for 2 min), followed by 40 cycles at 95°C for 15 sec and 60°C for 1 min.
Standard curves were constructed by serially diluting purified PCR products for each gene target. Curves contained six template concentrations spanning 1 fg to 100 pg and the plots of log-transformed template concentrations against quantification cycle (Cq) values showed linear relationships with R2 values greater than 0.99 for each target. When calculated from these curves, the efficiencies of L1 and 18S amplification were 85% and 89%, respectively. All experimental Cq values fell within the range of the standard curves, insuring that they were above the limit of detection and within the linear dynamic range. A similar dilution series was prepared with pooled slice, CGN, and astrocyte cDNA to test for PCR inhibitors. A linear relationship was seen between log-transformed template concentration and Cq value, indicating no significant PCR inhibition.
Additionally, “no RT” reactions were performed for each sample. cDNA samples were considered free from genomic DNA contamination if Cq values of “no RT” samples were at least 10 cycles higher than matched sample, or no amplification was seen within 40 cycles. All samples met this criterion. No-template control reactions were run on each plate to confirm that no exogenous DNA contamination was present. A pooled sample in which all targets were detectable at known levels was run on each plate as a positive control and to monitor inter-assay variation.
Cell lysate preparation
For total protein preparations, cells and slices were washed once in ice-cold PBS and lysates were prepared in RIPA buffer with protease and phosphatase inhibitors. For chronic binge-drinking animals, cerebellar lysate was prepared in RIPA buffer with protease and phosphatase inhibitors from fresh tissue. All samples were sonicated for 1 min on ice and centrifuged at 14,000 rpm for 20 min. The protein concentration in the supernatant was measured using a BCA Protein Assay Kit.
Western blotting
Cell and tissue lysates (20–50 µg total protein) were separated by SDS-PAGE electrophoresis, and protein was transferred to nitrocellulose membranes for 1.5 hours in transfer buffer with 15% methanol. Membranes were blocked for 1 hr in TBST with 2% BSA and 3% milk, then incubated with L1 antibody (1∶1000) in blocking solution for 16–18 hrs at 4°C. Blots were then incubated with HRP-conjugated anti-goat secondary antibody (1∶4000) in blocking buffer for 1 hr at room temperature. For detection of β-tubulin, anti-β-tubulin antibody (1∶000) and HRP-conjugated anti-rabbit secondary antibody (1∶4000) were used following the same protocol. Blots were either cut at 75 kD so that L1 and β-tubulin could be processed simultaneously, or membranes were stripped with Re-Blot Plus following L1 blotting and then processed for β-tubulin as described. Signals were detected with ECL Western blot substrate, and blots were then exposed to x-ray film and developed.
Data analysis
All real time PCR data were managed and analyzed using the web-base JAVA application QPCR [67] (http://esus.genome.tugraz.at/rtpcr). The AnalyzerMiner algorithm was used to generate efficiency and Cq values for each reaction and to perform endogenous control normalization and efficiency corrections [68]. Permutation mean tests (performed in the QPCR application) were used to generate relative expression values and corresponding standard error values for each statistical class and to determine statistical significance.
For Western blotting, films were scanned and densitometry was performed using TINA 2.0 software. For each sample, L1 OD-background values were normalized to corresponding β-tubulin values and then, within each experiment, the control sample was set to 100% and all treatments were scaled accordingly. GraphPad Prism v4.0 was used to perform the one-sample t-test comparing the normalized means of treatment groups to 100%, the relative value assigned to control.
Acknowledgments
We thank Diego Arambula, Carrie E. Menkari, and Lauren E. Frank for excellent technical assistance.
Footnotes
Competing Interests: MC is a member of the scientific advisory board for Allon Therapeutics, which is developing clinical applications for NAPVSIPQ (NAP). He holds stock in Allon Therapeutics. MC has two patents related to the study to declare. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials, as detailed online in the guide for authors. 1. Charness ME and Wilkemeyer MF, co-inventors. “Antagonists of alcohol inhibition of cell adhesion.” US Patent 6,359,015, issued March 19, 2002. 2. Wilkemeyer MF and Charness ME, co-inventors “Method for antagonizing inhibition effects of alcohol on cell adhesion” US Patient 6,977,272 B2, issued December 20, 2005.
Funding: Funding for this work was provided by: The Foundation for Alcohol Research Award to SC (www.abmrf.org), Merit Review Award from Department of Veterans Affairs to MC (www.research.va.gov), National Institute on Alcohol Abuse and Alcoholism (NIAAA) R37-AA12974 to MC (www.niaaa.nih.gov), NIAAA U24-AA014811, Canadian Incidence Study|Foetal Alcohol Spectrum Disorder to MC (www.niaaa.nih.gov), NIAAA AA015194 9 to KL-M, Research service, Department of Veterans Affairs, and Department of Psychiatry, Boston University School of Medicine to KL-M. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Maness PF, Schachner M. Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat Neurosci. 2007;10:19–26. doi: 10.1038/nn1827. [DOI] [PubMed] [Google Scholar]
- 2.Liljelund P, Ghosh P, van den Pol AN. Expression of the neural axon adhesion molecule L1 in the developing and adult rat brain. J Biol Chem. 1994;269:32886–32895. [PubMed] [Google Scholar]
- 3.Lüthi A, Laurent J-P, Figurov A, Muller D, Schachner M. Hippocampal long-term potentiation and neural cell adhesion molecules L1 and NCAM. Nature. 1994;372:777–779. doi: 10.1038/372777a0. [DOI] [PubMed] [Google Scholar]
- 4.Chaisuksunt V, Zhang Y, Anderson PN, Campbell G, Vaudano E, et al. Axonal regeneration from CNS neurons in the cerebellum and brainstem of adult rats: correlation with the patterns of expression and distribution of messenger RNAs for L1, CHL1, c-jun and growth-associated protein-43. Neuroscience. 2000;100:87–108. doi: 10.1016/s0306-4522(00)00254-2. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Bo X, Schoepfer R, Holtmaat AJ, Verhaagen J, et al. Growth-associated protein GAP-43 and L1 act synergistically to promote regenerative growth of Purkinje cell axons in vivo. Proc Natl Acad Sci U S A. 2005;102:14883–14888. doi: 10.1073/pnas.0505164102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fransen E, Lemmon V, Vancamp G, Vits L, Coucke P, et al. CRASH syndrome - Clinical spectrum of corpus callosum hypoplasia, retardation, adducted thumbs, spastic paraparesis and hydrocephalus due to mutations in one single gene, L1 [Review]. Eur J Hum Genet. 1995;3:273–284. doi: 10.1159/000472311. [DOI] [PubMed] [Google Scholar]
- 7.Fransen E, Dhooge R, Vancamp G, Verhoye M, Sijbers J, et al. L1 knockout mice show dilated ventricles, vermis hypoplasia and impaired exploration patterns. Human Mol Genet. 1998;7:999–1009. doi: 10.1093/hmg/7.6.999. [DOI] [PubMed] [Google Scholar]
- 8.Ramanathan R, Wilkemeyer MF, Mittal B, Perides G, Charness ME. Ethanol inhibits cell-cell adhesion mediated by human L1. J Cell Biol. 1996;133:381–390. doi: 10.1083/jcb.133.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charness ME, Safran RM, Perides G. Ethanol inhibits neural cell-cell adhesion. J Biol Chem. 1994;269:9304–9309. [PubMed] [Google Scholar]
- 10.Wilkemeyer MF, Pajerski M, Charness ME. Alcohol inhibition of cell adhesion in BMP-treated NG108-15 cells. Alcohol Clin Exp Res. 1999;23:1711–1720. [PubMed] [Google Scholar]
- 11.Bearer CF, Swick AR, O'Riordan MA, Cheng G. Ethanol inhibits L1-mediated neurite outgrowth in postnatal rat cerebellar granule cells. J Biol Chem. 1999;274:13264–13270. doi: 10.1074/jbc.274.19.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilkemeyer MF, Chen SY, Menkari C, Brenneman D, Sulik KK, et al. Differential effects of ethanol antagonism and neuroprotection in peptide fragment NAPVSIPQ prevention of ethanol-induced developmental toxicity. Proc Natl Acad Sci (USA) 2003;100:8543–8548. doi: 10.1073/pnas.1331636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen S-Y, Wilkemeyer MF, Sulik KK, Charness ME. Octanol antagonism of ethanol teratogenesis. FASEB J. 2001;15:1649–1651. doi: 10.1096/fj.00-0862fje. [DOI] [PubMed] [Google Scholar]
- 14.Wilkemeyer MF, Sebastian AB, Smith SA, Charness ME. Antagonists of alcohol inhibition of cell adhesion. Proc Natl Acad Sci (USA) 2000;97:3690–3695. doi: 10.1073/pnas.050545697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spong CY, Abebe DT, Gozes I, Brenneman DE, Hill JM. Prevention of fetal demise and growth restriction in a mouse model of fetal alcohol syndrome. Journal of Pharmacology & Experimental Therapeutics. 2001;297:774–779. [PubMed] [Google Scholar]
- 16.Wilkemeyer MF, Menkari C, Spong CY, Charness ME. Peptide antagonists of ethanol inhibition of L1-mediated cell-cell adhesion. J Pharmacol Exp Ther. 2002;303:110–116. doi: 10.1124/jpet.102.036277. [DOI] [PubMed] [Google Scholar]
- 17.Dou X, Menkari CE, Shanmugasundararaj S, Miller KW, Charness ME. Two alcohol binding residues interact across a domain interface of the L1 neural cell adhesion molecule and regulate cell adhesion. J Biol Chem. 2011;286:16131–16139. doi: 10.1074/jbc.M110.209254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arevalo E, Shanmugasundararaj S, Wilkemeyer MF, Dou X, Chen S, et al. An alcohol binding site on the neural cell adhesion molecule L1. Proc Natl Acad Sci U S A. 2008;105:371–375. doi: 10.1073/pnas.0707815105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeaney NK, He M, Tang N, Malouf AT, O'Riordan MA, et al. Ethanol inhibits L1 cell adhesion molecule tyrosine phosphorylation and dephosphorylation and activation of pp60(src). J Neurochem. 2009;110:779–790. doi: 10.1111/j.1471-4159.2009.06143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang N, He M, O'Riordan MA, Farkas C, Buck K, et al. Ethanol inhibits L1 cell adhesion molecule activation of mitogen-activated protein kinases. J Neurochem. 2006;96:1480–1490. doi: 10.1111/j.1471-4159.2006.03649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarren SK, Alvord EJ, Sumi SM, Streissguth AP, Smith DW. Brain malformations related to prenatal exposure to ethanol. J Pediatr. 1978;92:64–67. doi: 10.1016/s0022-3476(78)80072-9. [DOI] [PubMed] [Google Scholar]
- 22.Sowell ER, Jernigan TL, Mattson SN, Riley EP, Sobel DF, et al. Abnormal development of the cerebellar vermis in children prenatally exposed to alcohol: size reduction in lobules I–V. Alcohol Clin Exp Res. 1996;20:31–34. doi: 10.1111/j.1530-0277.1996.tb01039.x. [DOI] [PubMed] [Google Scholar]
- 23.Jaatinen P, Rintala J. Mechanisms of ethanol-induced degeneration in the developing, mature, and aging cerebellum. Cerebellum. 2008;7:332–347. doi: 10.1007/s12311-008-0034-z. [DOI] [PubMed] [Google Scholar]
- 24.Chen S, Mantei N, Dong L, Schachner M. Prevention of neuronal cell death by neural adhesion molecules L1 and CHL1. Journal of Neurobiology. 1999;38:428–439. doi: 10.1002/(sici)1097-4695(19990215)38:3<428::aid-neu10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Nguon K, Baxter MG, Sajdel-Sulkowska EM. Perinatal exposure to polychlorinated biphenyls differentially affects cerebellar development and motor functions in male and female rat neonates. Cerebellum. 2005;4:112–122. doi: 10.1080/14734220510007860. [DOI] [PubMed] [Google Scholar]
- 26.Miller MW, Mooney SM, Middleton FA. Transforming growth factor beta1 and ethanol affect transcription and translation of genes and proteins for cell adhesion molecules in B104 neuroblastoma cells. J Neurochem. 2006;97:1182–1190. doi: 10.1111/j.1471-4159.2006.03858.x. [DOI] [PubMed] [Google Scholar]
- 27.Dikranian K, Qin YQ, Labruyere J, Nemmers B, Olney JW. Ethanol-induced neuroapoptosis in the developing rodent cerebellum and related brain stem structures. Brain Res Dev Brain Res. 2005;155:1–13. doi: 10.1016/j.devbrainres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- 29.Nybroe O, Dalseg AM, Bock E. A developmental study of soluble L1. Int J Dev Neurosci. 1990;8:273–281. doi: 10.1016/0736-5748(90)90033-x. [DOI] [PubMed] [Google Scholar]
- 30.Charness ME, Simon RP, Greenberg DA. Ethanol and the nervous system. N Engl J Med. 1989;321:442–454. doi: 10.1056/NEJM198908173210706. [DOI] [PubMed] [Google Scholar]
- 31.Fleige S, Walf V, Huch S, Prgomet C, Sehm J, et al. Comparison of relative mRNA quantification models and the impact of RNA integrity in quantitative real-time RT-PCR. Biotechnol Lett. 2006;28:1601–1613. doi: 10.1007/s10529-006-9127-2. [DOI] [PubMed] [Google Scholar]
- 32.Takeda Y, Asou H, Murakami Y, Miura M, Kobayashi M, et al. A nonneuronal isoform of cell adhesion molecule L1: tissue-specific expression and functional analysis. J Neurochem. 1996;66:2338–2349. doi: 10.1046/j.1471-4159.1996.66062338.x. [DOI] [PubMed] [Google Scholar]
- 33.Czachowski CL, Samson HH, Denning CE. Independent ethanol- and sucrose-maintained responding on a multiple schedule of reinforcement. Alcoholism-Clinical and Experimental Research. 1999;23:398–403. [PubMed] [Google Scholar]
- 34.Bustin SA, Beaulieu JF, Huggett J, Jaggi R, Kibenge FS, et al. MIQE precis: Practical implementation of minimum standard guidelines for fluorescence-based quantitative real-time PCR experiments. BMC Mol Biol. 2010;11:74. doi: 10.1186/1471-2199-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clinical Chemistry. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 36.Birdsill AC, Walker DG, Lue L, Sue LI, Beach TG. Postmortem interval effect on RNA and gene expression in human brain tissue. 2010. Cell Tissue Bank. August 12.[Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 37.Lindner J, Zinser G, Wetz W. Experimental modification of postnatal cerebellar granule cell migration in vitro. Brain Res. 1986;377:298–304. doi: 10.1016/0006-8993(86)90872-3. [DOI] [PubMed] [Google Scholar]
- 38.Lindner J, Rathjen FG, Schachner M. L1 monoclonal antibodies modify cell migration in early postnatal mouse cerebellum. Nature. 1983;305:427–430. doi: 10.1038/305427a0. [DOI] [PubMed] [Google Scholar]
- 39.Chang S, Rathjen FG, Raper JA. Extension of neurites on axons is impaired by antibodies against specific neural cell surface glycoproteins. J Cell Biol. 1987;104:355–362. doi: 10.1083/jcb.104.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yazaki T, Martuza RL, Rabkin SD. Expression of L1 in primary astrocytes via a defective herpes simplex virus vector promotes neurite outgrowth and neural cell migration. Brain Res Mol Brain Res. 1996;43:311–320. doi: 10.1016/s0169-328x(96)00186-6. [DOI] [PubMed] [Google Scholar]
- 41.Gaudilliere B, Konishi Y, de la Iglesia N, Yao G, Bonni A. A CaMKII-NeuroD signaling pathway specifies dendritic morphogenesis. Neuron. 2004;41:229–241. doi: 10.1016/s0896-6273(03)00841-9. [DOI] [PubMed] [Google Scholar]
- 42.Vaudry D, Rousselle C, Basille M, Falluel-Morel A, Pamantung TF, et al. Pituitary adenylate cyclase-activating polypeptide protects rat cerebellar granule neurons against ethanol-induced apoptotic cell death. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:6398–6403. doi: 10.1073/pnas.082112699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCaffery P, Koul O, Smith D, Napoli JL, Chen N, et al. Ethanol increases retinoic acid production in cerebellar astrocytes and in cerebellum. Brain Research Developmental Brain Research. 2004;153:233–241. doi: 10.1016/j.devbrainres.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 44.Howerton TC, Collins AC. Ethanol-induced inhibition of GABA release from LS and SS mouse brain slices. Alcohol. 1984;1:471–477. doi: 10.1016/0741-8329(84)90024-7. [DOI] [PubMed] [Google Scholar]
- 45.Su LD, Sun CL, Shen Y. Ethanol Acutely Modulates mGluR1-Dependent Long-Term Depression in Cerebellum. Alcohol Clin Exp Res. 2010;34:1140–1145. doi: 10.1111/j.1530-0277.2010.01190.x. [DOI] [PubMed] [Google Scholar]
- 46.Chen S, Charness ME. Ethanol inhibits neuronal differentiation by disrupting activity-dependent neuroprotective protein signaling. Proc Natl Acad Sci U S A. 2008;105:19962–19967. doi: 10.1073/pnas.0807758105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Acquaah-Mensah GK, Leslie SW, Kehrer JP. Acute exposure of cerebellar granule neurons to ethanol suppresses stress-activated protein kinase-1 and concomitantly induces AP-1. Toxicol Appl Pharmacol. 2001;175:10–18. doi: 10.1006/taap.2001.9229. [DOI] [PubMed] [Google Scholar]
- 48.Yagle K, Costa LG. Effects of alcohol on immediate-early gene expression in primary cultures of rat cortical astrocytes. Alcohol Clin Exp Res. 1999;23:446–455. [PubMed] [Google Scholar]
- 49.Pickering C, Wicher G, Rosendahl S, Schioth HB, Fex-Svenningsen A. A low ethanol dose affects all types of cells in mixed long-term embryonic cultures of the cerebellum. Basic Clin Pharmacol Toxicol. 2010;106:472–478. doi: 10.1111/j.1742-7843.2009.00528.x. [DOI] [PubMed] [Google Scholar]
- 50.Heaton MB, Madorsky I, Paiva M, Siler-Marsiglio KI. Vitamin E amelioration of ethanol neurotoxicity involves modulation of apoptotis-related protein levels in neonatal rat cerebellar granule cells. Brain Res Dev Brain Res. 2004;150:117–124. doi: 10.1016/j.devbrainres.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 51.Wu J, Lee MR, Kim T, Johng S, Rohrback S, et al. Regulation of ethanol-sensitive EAAT2 expression through adenosine A1 receptor in astrocytes. Biochem Biophys Res Commun. 2011;406:47–52. doi: 10.1016/j.bbrc.2011.01.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gozes I. Activity-dependent neuroprotective protein: From gene to drug candidate. Pharmacol Ther. 2007;114:146–154. doi: 10.1016/j.pharmthera.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 53.Zhou FC, Sari Y, Powrozek TA, Spong CY. A neuroprotective peptide antagonizes fetal alcohol exposure-compromised brain growth. J Mol Neurosci. 2004;24:189–199. doi: 10.1385/JMN:24:2:189. [DOI] [PubMed] [Google Scholar]
- 54.Chen SY, Charness ME, Wilkemeyer MF, Sulik KK. Peptide-mediated protection from ethanol-induced neural tube defects. Dev Neurosci. 2005;27:13–19. doi: 10.1159/000084528. [DOI] [PubMed] [Google Scholar]
- 55.Vink J, Incerti M, Toso L, Roberson R, Abebe D, et al. Prenatal NAP+SAL prevents developmental delay in a mouse model of Down syndrome through effects on N-methyl-D-aspartic acid and gamma-aminobutyric acid receptors. American Journal of Obstetrics and Gynecology. 2009;200:524 e521–524. doi: 10.1016/j.ajog.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Incerti M, Vink J, Roberson R, Benassou I, Abebe D, et al. Prevention of the alcohol-induced changes in brain-derived neurotrophic factor expression using neuroprotective peptides in a model of fetal alcohol syndrome. Am J Obstet Gynecol. 2010;202:457 e451–454. doi: 10.1016/j.ajog.2010.03.050. [DOI] [PubMed] [Google Scholar]
- 57.Nicolas JM, Fernandez-Sola J, Robert J, Antunez E, Cofan M, et al. High ethanol intake and malnutrition in alcoholic cerebellar shrinkage. Qjm. 2000;93:449–456. doi: 10.1093/qjmed/93.7.449. [DOI] [PubMed] [Google Scholar]
- 58.Torvik A, Torp S. The prevalence of alcoholic cerebellar atrophy. A morphometric and histological study of an autopsy material. Journal of the Neurological Sciences. 1986;75:43–51. doi: 10.1016/0022-510x(86)90049-3. [DOI] [PubMed] [Google Scholar]
- 59.Shalizi A, Bilimoria PM, Stegmuller J, Gaudilliere B, Yang Y, et al. PIASx is a MEF2 SUMO E3 ligase that promotes postsynaptic dendritic morphogenesis. J Neurosci. 2007;27:10037–10046. doi: 10.1523/JNEUROSCI.0361-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keilhauer G, Faissner A, Schachner M. Differential inhibition of neurone-neurone, neurone-astrocyte and astrocyte-astrocyte adhesion by L1, L2 and N-CAM antibodies. Nature. 1985;316:728–730. doi: 10.1038/316728a0. [DOI] [PubMed] [Google Scholar]
- 61.Wu J, Wrathall JR, Schachner M. Phosphatidylinositol 3-kinase/protein kinase Cdelta activation induces close homolog of adhesion molecule L1 (CHL1) expression in cultured astrocytes. Glia. 2010;58:315–328. doi: 10.1002/glia.20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Czachowski CL, Samson HH, Denning CE. Blood ethanol concentrations in rats drinking sucrose/ethanol solutions. Alcoholism, Clinical and Experimental Research. 1999;23:1331–1335. [PubMed] [Google Scholar]
- 63.Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcoholism, Clinical and Experimental Research. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- 64.Shtutman M, Levina E, Ohouo P, Baig M, Roninson IB. Cell adhesion molecule L1 disrupts E-cadherin-containing adherens junctions and increases scattering and motility of MCF7 breast carcinoma cells. Cancer Res. 2006;66:11370–11380. doi: 10.1158/0008-5472.CAN-06-2106. [DOI] [PubMed] [Google Scholar]
- 65.de la Monte SM, Xu XJ, Wands JR. Ethanol inhibits insulin expression and actions in the developing brain. Cellular and Molecular Life Sciences. 2005;62:1131–1145. doi: 10.1007/s00018-005-4571-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Farnell YZ, Allen GC, Nahm SS, Neuendorff N, West JR, et al. Neonatal alcohol exposure differentially alters clock gene oscillations within the suprachiasmatic nucleus, cerebellum, and liver of adult rats. Alcoholism, Clinical and Experimental Research. 2008;32:544–552. doi: 10.1111/j.1530-0277.2007.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pabinger S, Thallinger GG, Snajder R, Eichhorn H, Rader R, et al. QPCR: Application for real-time PCR data management and analysis. BMC Bioinformatics. 2009;10:268. doi: 10.1186/1471-2105-10-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao S, Fernald RD. Comprehensive algorithm for quantitative real-time polymerase chain reaction. Journal of Computational Biology. 2005;12:1047–1064. doi: 10.1089/cmb.2005.12.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]