Abstract
The initiation of apoptosis often transpires in the presence of agents that regulate cell survival. This study evaluated the effects of stress-induced ceramide on the anti-apoptotic activity of the phosphoinositide-3 kinase [PI(3)K] pathway. PI(3)K activity is directly down-regulated by stress-induced ceramide in a dose-dependent manner with rapid kinetics and high specificity. Ceramide inhibition of PI(3)K is dependent on acid–sphingomyelinase. Down-regulation of PI(3)K by ceramide results in inhibition of the kinase Akt and decreased phosphorylation of the death effector Bad. Thus, ceramide levels could act as a general apoptotic rheostat controlling cell survival by regulating PI(3)K anti-apoptotic effector mechanisms.
Keywords: Apoptosis, ceramide, phosphatidylinositol-3 kinase
Many stimuli that induce apoptosis generate the lipid second messenger, ceramide (for review, see Hannun 1996; Spiegal et al. 1996). Cellular ceramide levels increase within 30–60 min in response to oxidative stress, UV and ionizing radiation, chemotherapeutic agents, and cytokines through the activation of acidic and neutral sphingomyelinases (for review, see Saba et al. 1996). Cells derived from Niemann–Pick (NP) patients, which lack acidic sphingomyelinase (Brady et al. 1966), are more refractory to dying by apoptosis when exposed to genotoxic stress (Santana et al. 1996). In addition, cells transformed by oncogenic Ras exhibit decreased sphingomyelinase activity (Laurenz et al. 1996), and resistance to radiation-induced apoptosis in Burkitt’s lymphoma cells is associated with defective ceramide signaling (Michael et al. 1997). These observations implicate ceramide generation as having a central and necessary, but as yet unidentified, role in apoptotic programs induced by stress, and deregulation of ceramide metabolism as a factor contributing to tumor progression and resistance to cancer therapy.
In fibroblasts and other cells of mesenchymal origin that ectopically express the oncogene c-myc, certain growth factors, including insulin-like growth factor-1 (IGF-1) and insulin, as well as matrix attachment, have been shown to have an anti-apoptotic effect (Harrington et al. 1994). Recently, a phosphatidylinositol-3 kinase [PI(3)K] modulated pathway responsible for this anti-apoptotic effect has been partially elucidated (Franke et al. 1997b). PI(3)K consists of a regulatory subunit (p85) that binds to an activated growth factor/cytokine receptor and undergoes phosphorylation, which results in the activation of its catalytic subunit (p110) (Rodriguez-Viciana et al. 1996). A product of PI(3)K phosphorylation, PIP2(3,4), facilitates the recruitment of the protein kinase Akt to the plasma membrane (Franke et al. 1997a). Akt is then thought to be activated by the recently identified protein kinases, PDKs (Alessi et al. 1997). Overexpression of constitutively activated forms of PI3(K) or Akt results in a decreased rate of apoptosis in response to serum/growth factor deprivation, UV-B irradiation, or loss of matrix attachment (Kauffmann-Zeh et al. 1997; Kennedy et al. 1997; Khwaja et al. 1997; Kulik et al. 1997). Because apoptosis often occurs in the presence of cytokines and growth factors that stimulate the anti-apoptotic pathway modulated by PI(3)K (Franke et al. 1997b), we hypothesized that cells undergoing apoptosis possess a mechanism that can inhibit PI(3)K. To test this hypothesis, we exposed an apoptotically sensitive Rat-1 fibroblast cell line, containing an estrogen-inducible form of c-myc, to various stresses and investigated the regulation of PI(3)K activity.
Results and Discussion
Stress-induced ceramide down-regulated PI(3)K activity in cultured fibroblasts
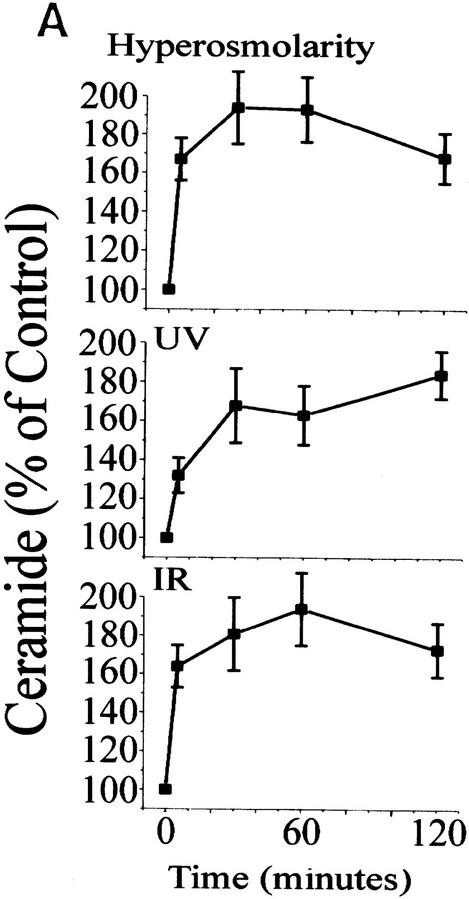
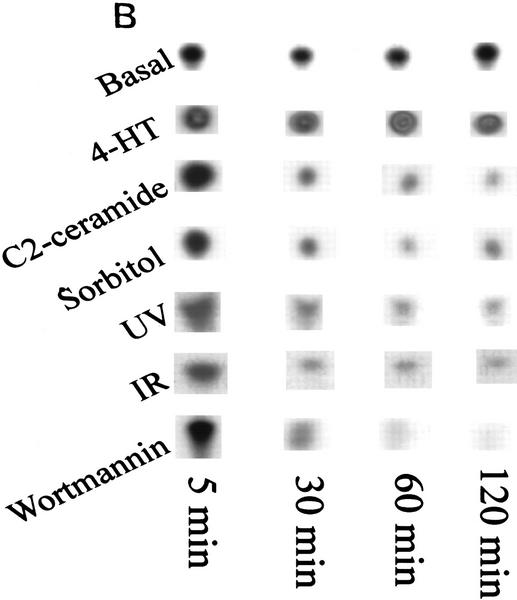
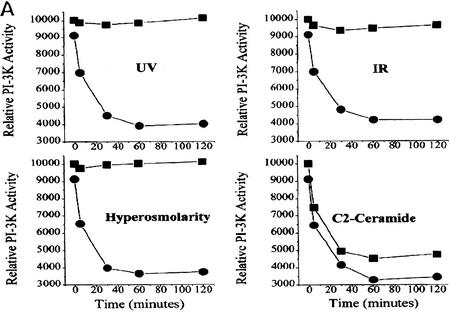
In response to sorbitol (hyperosmolar stress), γ-irradiation (IR) and UV radiation (UV-C), intracellular ceramide levels increased to twice the control levels within 2 hr after exposure (Fig. 1A). Expression of c-Myc alone did not alter the kinetics or intensity of ceramide generation in response to stress. To determine whether ceramide and agents that stimulate sphingomyelinase activity can modulate the activity of PI(3)K, we quantitated PI(3)K activity from serum-stimulated cells against a preferred PI(3)K substrate, phophatidylinositol-4-phosphate. Cells treated with hyperosmolar stress, UV-C, IR, or a cell-permeable form of ceramide, C2 ceramide, rapidly down-regulated PI(3)K activity to 10%–30% of the activity found in serum-stimulated control cells, which is comparable to the inhibition of PI(3)K activity by the p110-specific inhibitor wortmannin (Fig. 1B; Wymann et al. 1996). Expression of the inducible form of c-myc alone had no effect on PI(3)K activity or the response of stress-generated ceramide.
Figure 1.
Ceramide induction and PI(3)K activity by stress. (A) Time course of ceramide induction. Rat-1 Myc–ER cells were treated for 60 min prior to assay with 100 nm 4-HT and for indicated times with sorbitol (250 mm), IR (10 Gy), or UV-C (10 Jm−2). At the indicated times ceramide was extracted and quantified by the DAG kinase assay. Error bars indicate s.d. from three independent experiments. (B) PI(3)K activity. Cells were treated with 4-HT as in A and with wortmannin (100 nm), C2 ceramide (10 μm), sorbitol (250 mm), IR (10 Gy), or UV-C (10 Jm−2) for the indicated times. Cells were harvested, lysed, immunoprecipitated with anti-phosphotyrosine antibody, and assayed for PI(3)K activity. Equal amounts of PI(3)K were confirmed by immunoblotting of the PI(3)K regulatory subunit in cell lysates. Shown are representative phosphorimages.
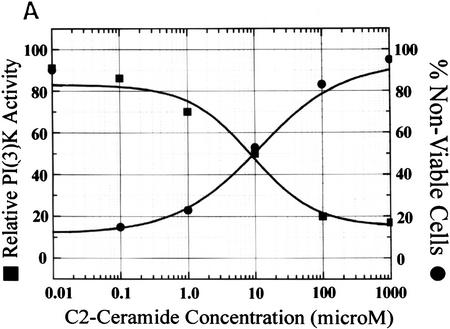
PI(3)K can act as an anti-apoptotic agent in cells exhibiting deregulated c-myc oncogene expression when exposed to stress (Kauffmann-Zeh et al. 1997; Kennedy et al. 1997). To relate the kinetics of PI(3)K inhibition with apoptotic cell death, we compared the dose response of PI(3)K inactivation with cell death induced by treatment of cells with cell-permeable C2 ceramide. We found an inverse relationship between ceramide-induced PI(3)K inhibition and ceramide-induced cell death, with the 50% lethal dose (LD50) of cell viability at 10 μm ceramide in c-myc-sensitized fibroblasts (Fig. 2A).
Figure 2.
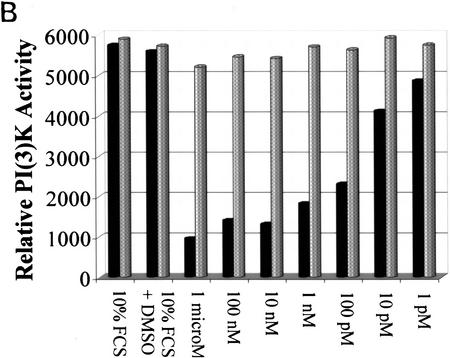
Specificity of ceramide activity. (A) Relationship between PI(3)K inhibition by ceramide and apoptosis. Rat-1 Myc–ER cells were pretreated with 100 nm 4-HT for 60 min prior to 2-hr incubation with the indicated concentrations of C2 ceramide, then assayed for PI(3)K activity as in Fig. 1B (▪). Cells were pretreated similarly prior to incubation for 24 hr, harvested, and cell viability determined by trypan blue exclusion (•). (B) Cells were treated with 4-HT as in A, lysed, pooled, and equally aliquoted, followed by immunoprecipitation with anti-PI(3)K antibody, and treatment with the indicated amounts of C2 ceramide (solid bars), or dihydro-C2 ceramide (shaded bars), and assayed for PI(3)K activity. (10% FCS) Basal levels. Shown are representative results from three independent experiments.
PI(3)K is regulated by ceramide specifically and at physiologic concentrations in vitro
To determine whether the observed kinase inhibition was a specific effect of ceramide on PI(3)K activity we added ceramide or an inactive analog, dihydroceramide, to PI(3)K immunoprecipitated from extracts of serum-stimulated cells (Fig. 2B). Ceramide inhibited PI(3)K activity at subnanomolar concentrations, whereas dihydroceramide exhibited no effect on PI(3)K activity, indicating that the effect of ceramide on PI(3)K inhibition is specific and occurs at low, biologically relevant concentrations. Ceramide analogs containing glucosyl moieties were the only other sphingolipids that affected PI(3)K activity, but only at high, nonphysiological concentrations (data not shown), further supporting a specific role for ceramide in modulating PI(3)K activity in response to stress.
PI(3)K down-regulation by stress is dependent on acid–sphingomyelinase
Generation of ceramide in response to stress is catalyzed by several distinct sphingomyelinases that are characterized by their activity at distinct pH ranges and their requirement for divalent cations (Hannun 1996). Human acid–sphingomyelinase (Schuchman et al. 1992) is found deleted or mutated in individuals afflicted with NP disease (Brady et al. 1966; Schneider and Kennedy 1967). Lymphoblasts from NP patients exhibit apoptotic resistance to ceramide-generating stresses but not to ceramide-induced cell death (Santana et al. 1996). Using lymphoblasts from a NP patient that exhibit <1% of normal acid–sphingomyelinase activity (Santana et al. 1996), we evaluated whether NP cells had altered PI(3)K activity in response to stress. IR, UV-C, and hyperosmotic stress induced a rapid decrease in PI(3)K activity in normal human lymphoblasts but had no effect on the activity of PI(3)K in NP lymphoblasts (Fig. 3A). However, the addition of cell-permeable C2 ceramide down-regulated PI(3)K activity in both cell types, genetically implicating ceramide in the inactivation of PI(3)K in NP lymphoblasts in response to stress.
Figure 3.
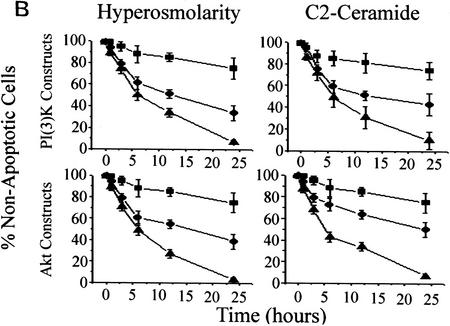
(A) Stress-induced inhibition of PI(3)K is mediated by acid–sphingomyelinase. MS-1418 NP acid–sphingomyelinase-deficient EBV-transformed (▪) and JY wild-type EBV-transformed human lymphoblasts (•) were treated with C2 ceramide (10 μm), sorbitol (250 mm), IR (10 Gy), or UV-C (10 Jm−2) for the indicated times, and assayed for PI(3)K activity as in Fig. 1B. (B) In cells treated with C2 ceramide or hyperosmolar stress, dominant negative PI(3)K or Akt increases the kinetics of apoptosis and constitutively active PI(3)K or Akt promotes survival. (Top panels) Rat-1 Myc–ER cells were transfected with either an empty vector (•), a constitutively active (p110–SH2) (▪) or a dominant-negative (p85Δ) (▪) form of PI(3)K in addition to a GFP plasmid for transfection identification. The cells were treated for 60 min with 100 nm 4-HT prior to C2 ceramide (10 μm) or sorbitol (250 mm) addition (•). At 24 hr, the cells expressing the GFP plasmid were assayed for apoptosis by Hoechst 33342 and propidium iodide staining. (Bottom panels) Rat-1 Myc–ER cells were transfected with either an empty vector (•), a constituatively active (Myr–Akt) (▪) or a dominant-negative (ΔAkt) (▴) form of c-Akt in addition to a GFP plasmid for transfectant identification. The cells were treated the same as above. Error bars represent the mean of triplicate cultures from a representative experiment.
Ceramide affects PI(3)K upstream of the p110 catalytic subunit
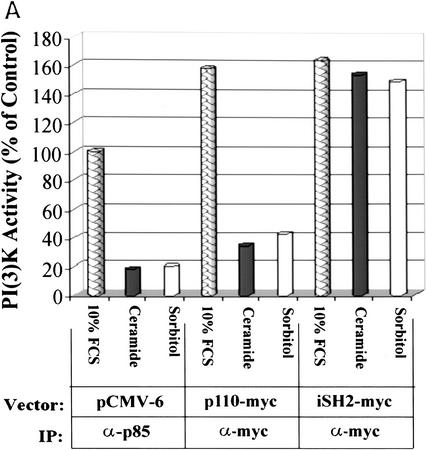
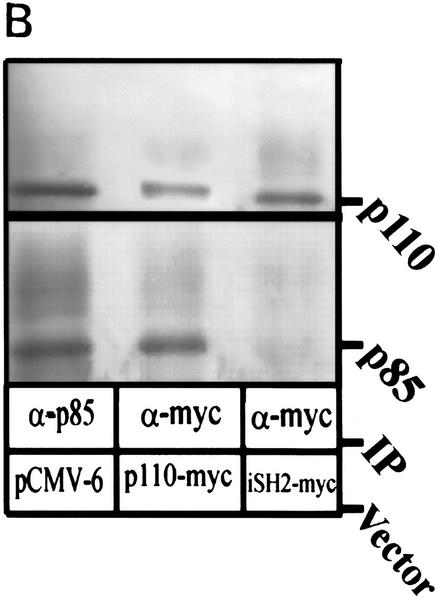
To verify PI(3)K as an effector of cell survival in Rat-1 Myc–ER cells, we used constitutively active or dominant inhibitory forms of PI(3)K or Akt in transient transfection studies to assess the ability of these genes to alter the apoptotic sensitivity of cells to ceramide or agents that generate ceramide (Fig. 3B). Apoptosis induced by 10 μm ceramide or 250 μm sorbitol, agents that both decrease PI(3)K activity to 50% of control values, was significantly reduced by overexpression of activated PI(3)K and Akt. In contrast, inhibition of PI(3)K or Akt by the dominant inhibitory form of the p85 subunit (Hara et al. 1995) or an inhibitory form of Akt (Franke et al. 1997b) increased the kinetics of cell death in response to hyperosmotic conditions or ceramide exposure. The constitutively activated PI(3)K (Franke et al. 1997b) is generated by cotransfection of the p85 regulatory subunit inner SH2 domain (iSH2) and the wild type–p110 catalytic subunit. Because this form of PI(3)K appears to retain activity (Franke et al. 1997b), it is likely that ceramide does not inhibit PI(3)K by modifying or interacting with the p110 catalytic subunit of PI(3)K. To investigate this hypothesis, we immunoprecipitated transiently transfected Myc-tagged p110 and iSH2 of p85 for use in PI(3)K assays (Fig. 4A). Ceramide and hyperosmolarity inhibited PI(3)K activity in both p110- and p85-immunoprecipitated PI(3)K complexes but had no effect on complexes from iSH2 immunoprecipitates. To determine what PI(3)K subunits were present in this experiment, the immunoprecipitates were probed with anti-p110 and anti-p85 antibodies (Fig. 4B). The anti-myc-p110 and the anti-p85 immunoprecipitates contained both p110 and p85 subunits, whereas the anti-myc–iSH2 immunoprecipitates contained only the p110 subunit. Ceramide was also unable to inhibit PI(3)K activity in iSH2 immunoprecipitates in vitro (data not shown). These results suggest that the interaction of ceramide on PI(3)K is independent of its p110 catalytic subunit.
Figure 4.
(A) Rat-1 Myc–ER cells were transiently transfected for 24 hr with either an empty pCMV-6 vector (mock), p110-myc, or iSH2–myc (Franke et al. 1997b) followed by treatment with 100 nm 4-HT for 30 min prior to C2 ceramide (10 μm) or sorbitol (250 μm) addition. Immunoprecipitation was performed as indicated and PI(3)K activity measured. (B) Aliquots from immunoprecipitates were probed for p110 and p85 by Western blot. Shown are representative results from independent experiments.
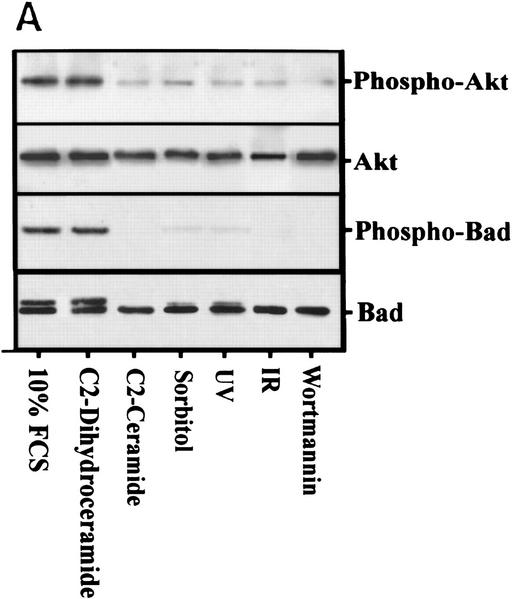
Akt and Bad phosphorylation decreases in response to ceramide
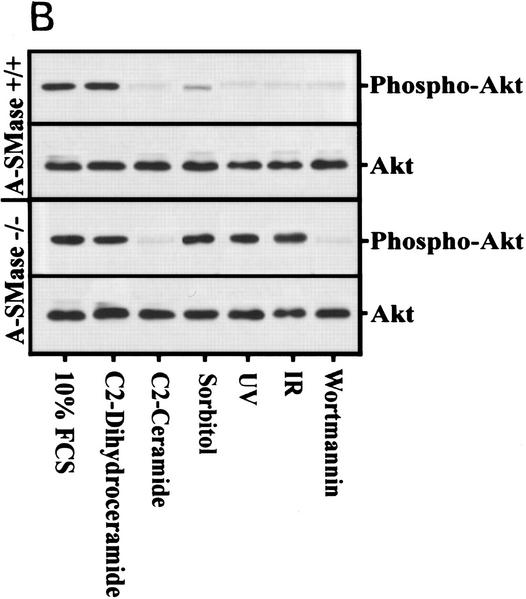
Because Akt is required for the anti-apoptotic effects generated by PI(3)K agonists (Marte and Downward 1997), we examined the effects of different ceramide-inducing stresses on Akt and its downstream effector Bad. Akt phosphorylation is inhibited in response to ceramide, osmotic stress, UV, and IR (Fig. 5A). The small amount of residual Akt activity seen can likely be accounted for by p85α-independent mechanisms thought to be mediated by G proteins (Moule et al. 1997). However, the inhibition of Akt phosphorylation by these stresses is strongly dependent on ceramide generated by acid–sphingomyelinase as NP cells exhibit no difference in Akt phosphorylation after these stresses (Fig. 5B). Treatment of NP cells with cell-permeable ceramide reconstituted PI(3)K inhibition and subsequent inhibition of Akt phosphorylation.
Figure 5.
(A) Rat-1 Myc–ER cells were treated for 60 min with 100 nm 4-HT (all) and with wortmannin (100 nm), C2 ceramide (10 μm), sorbitol (250 mm), IR (10 Gy), or UV-C (10 Jm−2) for 3 hr (Akt data) or 4 hr (Bad data). Akt and phospho–Akt were detected by immunoblot analysis. Shown are representative results from three independent experiments. For Bad phosphorylation, Myc–ER cells were labeled with 100 μCi/ml [32]Porthophosphate for 6 hr prior to treatment followed by immunoprecipitation with anti-Bad antibody, electrophoresis, and phosphorimaging. Cells treated identically except for the lack of 32P-labeling were immunoprecipitated with anti-Bad antibody and probed with anti-Bad antibody. (B) NP MS-1418 and JY wild-type EBV-transformed cells were treated as above, prior to blotting for Akt and phospho–Akt. Shown are representative results from three independent experiments.
The Bcl-2 family member, Bad, has been suggested to mediate the anti-apoptotic effects of the PI(3)K pathway (Datta et al. 1997; del Peso et al. 1997). In response to phosphorylation by Akt, Bad is sequestered by 14-3-3 proteins, thereby preventing its binding to Bcl-2 (Zha et al. 1996). If Bad is a downstream effector of Akt, then inhibition of Akt activity by ceramide should also result in inhibition of Bad phosphorylation. Ceramide also inhibited Bad phosphorylation, directly linking ceramide-mediated apoptosis to the cell death effector pathway (Fig. 5A). Taken together, these results suggest ceramide promotes the induction of apoptosis by inhibiting a constitutively active anti-apoptotic pathway regulated by PI(3)K, Akt, and Bad.
Recently, agents that activate PI(3)K and c-Akt have been shown to antagonize various forms of cell death (for review, see Franke et al. 1997b). Because ceramide has been shown to mediate other lipid-mediated signaling mechanisms, such as phospholipase D and protein kinase C (Venable et al. 1994; Jones and Murray 1995; Chmura et al. 1996; Lee et al. 1996; Spiegel and Merrill 1996; Sawai et al. 1997), and can also regulate receptor-mediated signaling (Kanety et al. 1996), it was a reasonable hypothesis that PI(3)K activity could also be regulated by ceramide. Inhibition of the anti-apoptotic effects of PI(3)K by stress-induced ceramide production provides new insight into how lipid second messengers control cell fate in response to both stress and oncogenic transformation. Ceramide contributes to apoptosis not only by regulating effector mechanisms such as caspases and c-Jun, but by deregulation of the anti-apoptotic PI(3)K/Akt/Bad pathway. In addition, p85 knockouts have recently been shown to be resistant to oxidative apoptosis in a PI(3)K-independent, p53-dependent fashion (Yuxin et al. 1998). Because ceramide modulates the PI(3)K response at extremely early times following stress, it is feasible that the effect of ceramide on p85 could modulate p85’s affinity for p110 and thus make it available for binding other potential targets that could be proapoptotic.
An important implication of this study is that during tumorigenesis, when ceramide levels become limiting (Laurenz et al. 1996), PI(3)K activity is unchecked, leading to increased survival and resistance to chemo- and radiotherapies. Tumors that possess activated signaling mechanisms that utilize the PI(3)K catalytic subunit or its downstream effectors, such as Akt, also could bypass the regulatory mechanism of ceramide generated during apoptotic stress. A second implication is that in degenerative diseases in which ceramide levels are elevated, survival mechanisms are constitutively down-regulated leading to increased cell death. Thus, modulators of ceramide metabolism represent an important class of anti-cancer and disease-modifying pharmaceuticals that have yet to be exploited.
Materials and methods
Cell culture and reagents
The Rat-1 clone YY8ME4 containing the 4-hydroxytomoxifin-inducible c-myc–estrogen receptor fusion construct (Graeber et al. 1996) was maintained in DMEM containing 10% (vol/vol) FBS (GIBCO-BRL) and 1.5 μg/ml puromycin (Sigma). All experiments were performed at 80%–100% confluence. The MS-1418 NP acid–sphingomyelinase-deficient and the JY wild-type Epstein–Barr virus-transformed human lymphoblasts were a generous gift from Dr. R. Kolesnick (Memorial Sloan-Kettering Cancer Center, New York, NY). These cell lines were maintained in a 1:1 mixture of DMEM and RPMI-1640 containing 15% (vol/vol) FBS. Lipid (Biomol and Matreya) stocks were solvated in DMSO or ddH2O as per the manufacturer’s recommendation. Sorbitol stocks were solvated in DMEM. IR was performed in a Shepherd Mark I137Cs Irradiator at a dose rate of 543 rads/min. UV irradiation was performed using a cathode lamp and were calibrated with a probe connected to a germicidal photometer at 0.63 J/m2 per sec.
Ceramide quantitation
Ceramide was quantified by the diacylglycerol (DAG) kinase assay as described previously (Dressler and Kolesnick 1990). Briefly, following treatment, total lipid extraction was performed. Lipids in the organic partition were dried under N2 and subjected to mild alkaline hydrolysis (500 μl of 0.1 n KOH/sample for 1 hr at 37°C), re-extracted with 500 μl of CHCl3, 270 μl of saline, and 30 μl of EDTA, and dried under N2. A reaction mix (per time point) containing 6 μl of 25 mg/ml cardiolipin (Avanti Polar Lipids), and 20 μl of 1.0 mm DETAPAC was bath sonicated and vortexed with 6.2 μl of 825 mm Octyl-β-d-glucopyranoside. To this mix (per time point) was added 50 μl of 2× Rx buffer (100 mm NaCl, 100 mm imidazole, 2 mm EDTA, 25 mm MgCl2) at pH 6.5, 8 μl of imidazole/DETAPAC (10 mm/1 mm), 2 μl of 100 mm DTT, 1 μl of 100 mm ATP, 8 μl of ddH2O, and 3.5 μl of 1 mg/ml DAG kinase (Calbiochem). The mix was vortexed and incubated at 37°C for 30 min to phosphorylate any contaminating DAG. The amount of 1.0 μl of [γ32P]ATP (3000 Ci/mmole) (NEN) was added to mix per point and vortexed. The amount of 100 μl of reaction mix was added to each tube of dried lipid and incubated at room temperature for 30 min. The reaction was stopped and ceramide-l–phosphate extracted by adding 1.0 ml of KILL, 170 μl of saline, and 30 μl of 100 mm EDTA. The organic phase was transferred to a new tube, dried under N2, and resuspended in 50 μl of CHCl3. Forty microliters per reaction was loaded onto TLC plates and run in a pre-equilibrated TLC chamber using a solvent system containing CHCl3/CH3OH/acetic acid (65:15:5). The plates were air-dried and autoradiographed. Bands corresponding to ceramide-1-phosphate were cut out and counted in a scintillation counter.
PI(3) kinase activity quantitation
PI(3)kinase assays were performed as described previously (Whitman et al. 1987). Briefly, cells were exposed to prolonged PI(3)K stimulation with 10% FCS followed by treatment with the indicated reagents. After an iced PBS wash containing 1 mm CaCl2, 1 m m MgCl2, and 100 μm sodium orthovanadate, cells were lysed in iced PBS containing 1 mm CaCl2, 1 mm MgCl2, 1% (vol/vol) NP-40, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 1 μm PMSF, and 100 μm sodium orthovanadate. Lysates were assayed for protein concentration using the bicinchoninic acid technique (Pierce Biochemicals). Equal amounts of protein from control or treated cells were incubated with 5 μl of anti-phosphotyrosine antibody (UBI) and immunoprecipitated with protein A–Sepharose beads (Sigma). Immunoprecipitates were washed 3× with lysis buffer, 1× with 100 mm Tris (pH 7.4) containing 5 mm LiCl and 100 μm sodium orthovanadate, and 1× with TNE (10 mm Tris at pH 7.4 containing 150 mm NaCl, 5 mm EDTA, and 100 μm sodium orthovanadate). The immunoprecipitates were resuspended in 25 μl of TNE containing 20 μg of l-α-phosphatidylinositol-4 monophosphate (Sigma), and 5 μl of 100 mm MgCl2. Kinase reactions were carried out by adding 30 μCi of [γ-32P]ATP in 2.5 μl of 0.88 mm ATP per reaction. TLC was performed with a CHCl3/MeOH/H2O/NH4OH (40:48:10:5) solvent system. Results were visualized by autoradiography and quantitated by ImagePC (Scion Corporation) or PhosphorImager (Molecular Dynamics). For in vitro reactions, the cells were grown in 10% FCS as a PI(3)K stimulus and PI(3)K activity determined as above with the following modifications. The cellular lysates were pooled, aliquoted equally, and immunoprecipitated with a polyclonal anti-PI(3)K antibody (UBI) Lipids were diluted with TBS containing 0.01% BSA. One microliter of lipid or solvent was added per 40 μl of kinase reaction mixture.
Cell viability and apoptosis quantitation
Cell viability was determined by trypan blue exclusion. Apoptosis was quantified on a morphological basis as described previously (Graeber et al. 1996). Briefly, following treatment, the cells were incubated with 2 μg/ml each of bis-benzamide and propidium iodide (P.I.) for 15 min. Viability ratios (no. of apoptotic cells/total no. of cells) were determined by scoring low-magnification fields of randomly selected fields for cells with condensed and fragmented nuclei and loss of membrane integrity. Low-magnification fields of cells expressing enhanced green flourescent protein (EGFP) and p85, p110–iSH2, AktΔ, or Myr–Akt were referenced to Hoechst/P.I. of the same field.
Plasmids and transfections
Ten micrograms of a mutant form of the p85 subunit of PI(3)K (dominant negative) that lacks amino acids 479–513 and contains 2 extra amino acids, Ser–Arg (p85Δ) (provided by Dr. Kasuga, University of Kobe School of Medicine, Japan) and was cotransfected with 2 μg of EGFP reporter plasmid (Clontech). Five micrograms of a plasmid containing the wild-type myc-tagged p110 coding sequence of PI(3)K and 5 μg of a plasmid containing the myc-tagged inner iSH2 domain of the p85 regulatory subunit of PI(3)K, acting as a constitutively active PI(3)K (p110-iSH2), was kindly provided by Dr. T. Franke (Harvard Medical School, Boston, MA) and was cotransfected with 2 μg of EGFP reporter plasmid. Ten micrograms of a plasmid containing an inhibitory form of Akt (AktΔ) was generously provided by Dr. T. Franke and was cotransfected with 2 μg of EGFP reporter. Ten micrograms of a constitutively active Akt (Myr–Akt) containing a myristolation sequence, generously provided by Dr. R. Roth (Kohn et al. 1996), was cotransfected with 2 μg of EGFP reporter plasmid. All transfections were performed using lipofectamine (GIBCO-BRL). Twelve hours following transfections the cells were washed with media three times, replated at 2 × 105, and allowed to equilibrate 24 hr prior to assay.
Immunoblot and ortho-32P labeling
Akt and phospho–Akt were immunoprecipitated and blotted using PhosphoPlus Akt (Ser-473) Antibody Kit (New England Biolabs), detected by Vistra Western ECF Blotting Kit (Amersham L.S.), and visualized by Fluorimager (Molecular Dynamics). For other immunoprecipitations and Western analyses, cell pellets were lysed in immunoprecipitation buffer [20 mm Tris (pH 7.5), 150 mm NaCl, 1 mm EDTA, 1% Triton X-100, 2.5 mm sodium pyrophosphate, 1 mm β-glycerol phosphate, 1 mm Na3VO4, 1 mg/ml leupeptin, 1 mmPMSF, 10 mm sodium fluoride, 10 μg/ml aprotinin, 0.7 μg/ml pepstatin), immunoprecipitated using anti-Bad sc-943-G, polyclonal anti-p85 06-195 (UBI), or anti-myc (kind gift from T. Wang, Stanford University School of Medicine, Stanford, CA), separated by electrophoresis, and transferred to PVDF blotting paper. Blots were probed with anti-Bad sc-943-G, anti-p85α sc-423, anti-p110α sc1331 (Santa Cruz), or anti-myc (T. Wang) and detected as above.
Acknowledgments
We thank T. Franke, R. Kolesnick, Y. Hannun, M. Kasuga, and R. Roth for cell lines, plasmid constructs, and advice, and N. Denko and N. Mazure for assistance with the manuscript. W.Z. thanks M. Heintzelman for his mentorship. W.Z. was supported by a Ford Foundation Doctoral Fellowship for Minorities and a Markey Trust Fellowship. This work is supported by a Howard Hughes Young Investigator Award, an American Cancer Society Junior Faculty Research Award, and National Institutes of Health grants CA 64489 and CA 67166 to A.J.G.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL giaccia@leland.stanford.edu; FAX (415) 723-7382.
References
- Alessi D, James S, Downes C, Holmes A, Gaffney P, Reese C, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase B alpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- Brady RO, Kanfer JN, Mock MB, Fredrickson DS. The metabolism of sphingomyelin. II. Evidence of an enzymatic deficiency in Niemann-Pick disease. Proc Natl Acad Sci. 1966;55:366–369. doi: 10.1073/pnas.55.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmura S, Nodzenski E, Crane M, Virudachalam S, Hallahan D, Weichselbaum R, Quintans J. Cross-talk between ceramide and PKC activity in the control of apoptosis in WEHI-231. Adv Exp Med Biol. 1996;406:39–55. doi: 10.1007/978-1-4899-0274-0_5. [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- Dressler K, Kolesnick R. Ceramide 1-phosphate, a novel phospholipid in human leukemia (HL-60) cells. Synthesis via ceramide from sphingomyelin. J Biol Chem. 1990;265:14917–14921. [PubMed] [Google Scholar]
- Franke T, Kaplan D, Cantley L, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997a;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- Franke T, Kaplan D, Cantley L. PI3K: Downstream AKTion blocks apoptosis. Cell. 1997b;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- Graeber T, Osmanian C, Jacks T, Housman D, Koch C, Lowe S, Giaccia A. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;679:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- Hannun Y. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- Hara K, Yonezawa K, Sakaue H, Kotani K, Kotani K, Kojima A, Waterfield M, Kasuga M. Normal activation of p70 S6 kinase by insulin in cells overexpressing dominant negative 85kD subunit of phosphoinositide 3-kinase. Biochem Biophys Res Commun. 1995;208:735–741. doi: 10.1006/bbrc.1995.1399. [DOI] [PubMed] [Google Scholar]
- Harrington E, Bennett M, Fanidi A, Evan G. c-Myc-induced apoptosis in fibroblasts is inhibited by specific cytokines. EMBO J. 1994;13:3286–3295. doi: 10.1002/j.1460-2075.1994.tb06630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M, Murray A. Evidence that ceramide selectively inhibits protein kinase C-alpha translocation and modulates bradykinin activation of phospholipase D. J Biol Chem. 1995;270:5007–5013. doi: 10.1074/jbc.270.10.5007. [DOI] [PubMed] [Google Scholar]
- Kanety H, Hemi R, Papa M, Karasik A. Sphingomyelinase and ceramide suppress insulin-induced tyrosine phosphorylation of the insulin receptor substrate-1. J Biol Chem. 1996;271:9895–9897. doi: 10.1074/jbc.271.17.9895. [DOI] [PubMed] [Google Scholar]
- Kauffmann-Zeh A, Rodriguez-Vicciana P, Urlich E, Gilbert C, Coffer P, Downward J, Evan G. Suppression of c-Myc-induced apoptosis by Ras signaling through PI(3)K and PKB. Nature. 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- Kennedy S, Wagner A, Conzen S, Jordan J. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes & Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne PH, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn A, Takeuchi F, Roth R. Akt, a pleckstrin homology domain containing kinase, is activated primarily by phosphorylation. J Biol Chem. 1996;271:21920–21926. doi: 10.1074/jbc.271.36.21920. [DOI] [PubMed] [Google Scholar]
- Kulik G, Klippel A, Weber M. Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-Kinase, and Akt. Mol Cell Biol. 1997;17:1595–1606. doi: 10.1128/mcb.17.3.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenz J, Gunn J, Jolly C, Chapkin R. Alteration of glycerolipid and sphingolipid-derived second messenger kinetics in ras transformed 3T3 cells. Biochim Biophys Acta. 1996;1299:146–154. doi: 10.1016/0005-2760(95)00202-2. [DOI] [PubMed] [Google Scholar]
- Lee J, Hannun Y, Obeid L. Ceramide inactivates cellular protein kinase C alpha. J Biol Chem. 1996;271:13169–13174. doi: 10.1074/jbc.271.22.13169. [DOI] [PubMed] [Google Scholar]
- Marte BM, Downward J. PKB/Akt: Connecting phosphoinositide 3-kinase to cell survival and beyond. Trends Biochem Sci. 1997;22:355–358. doi: 10.1016/s0968-0004(97)01097-9. [DOI] [PubMed] [Google Scholar]
- Michael JM, Lavin MF, Watters DJ. Resistance to radiation-induced apoptosis in Burkitt’s lymphoma cells is associated with defective ceramide signaling. Cancer Res. 1997;57:3600–3605. [PubMed] [Google Scholar]
- Moule SK, Welsh GI, Edgell NJ, Foulstone EJ, Proud CG, Denton RM. Regulation of protein kinase B and glycogen synthase kinase-3 by insulin and beta-adrenergic agonists in rat epididymal fat cells. Activation of protein kinase B by wortmannin-sensitive and -insensitive mechanisms. J Biol Chem. 1997;272:7713–7719. doi: 10.1074/jbc.272.12.7713. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Nakashima S, Ojio K, Banno Y, Miyata H, Nozawa Y. Ceramide inhibits IgE-mediated activation of phospholipase D, but not of phospholipase C, in rat basophilic leukemia (RBL–2H3) cells. J Immunol. 1996;156:256–262. [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Marte B, Warne P, Downward J. Phosphatidylinositol 3′ kinase: One of the effectors of Ras. Philos Trans R Soc Lond B Biol Sci. 1996;351:225–231. doi: 10.1098/rstb.1996.0020. [DOI] [PubMed] [Google Scholar]
- Saba J, Obeid L, Hannun Y. Ceramide: An intracellular mediator of apoptosis and growth suppression. Philos Trans R Soc Lond B Biol Sci. 1996;351:233–240. doi: 10.1098/rstb.1996.0021. [DOI] [PubMed] [Google Scholar]
- Santana P, Pena L, Haimovitz-Friedman A, Martin S, Green D, McLoughlin M, Cordon-Cardo C, Schuchman E, Fuks Z, Kolesnick R. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell. 1996;86:189–199. doi: 10.1016/s0092-8674(00)80091-4. [DOI] [PubMed] [Google Scholar]
- Sawai H, Okazaki T, Takeda Y, Tashima M, Sawada H, Okuma M, Kishi S, Umehara H, Domae N. Ceramide-induced translocation of protein kinase C-delta and -epsilon to the cytosol. Implications in apoptosis. J Biol Chem. 1997;272:2452–2458. doi: 10.1074/jbc.272.4.2452. [DOI] [PubMed] [Google Scholar]
- Schneider P, Kennedy E. Sphingomyelinase in normal human spleens and in spleens from subjects with Niemann-Pick disease. J Lipid Res. 1967;8:202–209. [PubMed] [Google Scholar]
- Schuchman E, Suchi M, Takahashi T, Sandhoff K, Desnick R. Human acid sphingomyelinase. Isolation, nucleotide sequence and expression of the full-length and alternatively spliced cDNAs. J Biol Chem. 1992;266:8531–8539. [PubMed] [Google Scholar]
- Spiegel S, Merrill AJ. Sphingolipid metabolism and cell growth regulation. FASEB J. 1996;10:1388–1397. doi: 10.1096/fasebj.10.12.8903509. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Foster D, Kolesnick R. Signal transduction through lipid second messengers. Curr Opin Cell Biol. 1996;8:159–167. doi: 10.1016/s0955-0674(96)80061-5. [DOI] [PubMed] [Google Scholar]
- Venable M, Blobe G, Obeid L. Identification of a defect in the phospholipase D/diacylglycerol pathway in cellular senescence. J Biol Chem. 1994;269:26040–26044. [PubMed] [Google Scholar]
- Whitman M, Kaplan D, Roberts T, Cantley L. Evidence for two distinct phosphatidylinositol kinases in fibroblasts. Implications for cellular regulation. Biochem J. 1987;247:165–174. doi: 10.1042/bj2470165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wymann M, Bulgarelli-Leva G, Zvelebil M, Pirola L, Vanhaesebroeck B, Waterfield M, Panayotou G. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol Cell Biol. 1996;16:1722–1733. doi: 10.1128/mcb.16.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuxin Y, Terauchi Y, Solomon G, Aizawa S, Rangarajan P, Yazaki Y, Kadowaki T, Barrett J. Involvement of p85 in p53-dependent apoptotic response to oxidative stress. Science. 1998;391:707–710. doi: 10.1038/35648. [DOI] [PubMed] [Google Scholar]
- Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X. Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]