Abstract
Markers of preparatory visual–spatial attention in sensory cortex have been described both as lateralized, slow-wave event-related potential (ERP) components and as lateralized changes in oscillatory-electroencephalography alpha power, but the roles of these markers and their functional relationship are still unclear. Here, 3 versions of a visual–spatial cueing paradigm, differing in perceptual task difficulty and/or response instructions, were used to investigate the functional relationships between posterior oscillatory-alpha changes and our previously reported posterior, slow-wave biasing-related negativity (swBRN) ERP activity. The results indicate that the swBRN reflects spatially specific, pretarget preparatory activity sensitive to the expected perceptual difficulty of the target detection task, correlating in both location and strength with the early sensory-processing N1 ERP to the target, consistent with reflecting a preparatory baseline-shift mechanism. In contrast, contralateral event-related decreases in alpha-band power were relatively insensitive to perceptual difficulty and differed topographically from both the swBRN and target N1. Moreover, when response instructions emphasized making immediate responses to targets, compared with prescribing delayed responses, contralateral alpha-event-related desynchronization activity was particularly strong and correlated with the longer latency target-P3b activity. Thus, in contrast to the apparent perceptual-biasing role of swBRN activity, contralateral posterior alpha activity may represent an attentionally maintained task set linking stimulus-specific information and task-specific response requirements.
Keywords: attention, biasing, control, EEG, ERP
Introduction
Covertly shifting attention to a location different from where the eyes are directed increases the likelihood of being able to rapidly and accurately process stimuli presented at that location (Posner et al. 1980). The prevailing view is that these behavioral improvements are brought about by preparatory activity induced by a frontal–parietal attentional control network (reviewed in Corbetta and Shulman 2002), which in turn is thought to initiate and maintain a state of goal-directed stimulus-specific readiness for expected target stimuli. This stimulus-selective perceptual-processing readiness is often referred to as sensory biasing (e.g., Hopfinger et al. 2000; Kastner and Ungerleider 2001; Foxe et al. 2005; Grent-'t-Jong and Woldorff 2007), and it is thought to be accomplished by a prestimulus baseline shift of activity in stimulus-selective sensory areas, which in turn is believed to lead to the increase in perceptual sensitivity (Desimone and Duncan 1995; Luck et al. 1997; Kastner et al. 1999).
Although there is substantial consensus on this general model of anticipatory, visual–spatial attentional control, certain aspects of this model are still unclear. One aspect that is still not very clear, for example, is the precise nature of the target location–specific biasing activity in sensory cortex that appears to be contingent upon frontal–parietal attentional control activity and that would be expected to be particularly clearly manifested during the later part of the cue-target delay interval in cueing paradigms. Electroencephalographic (EEG) recordings from human participants in such paradigms have revealed both oscillatory changes (particularly in the alpha frequency range) as well as a number of slower event-related potential (ERP) changes. In regards to ERP markers, sensory cortex pretarget ERP activity was initially reported as a contralateral positive polarity wave, termed the late directing attention positivity (LDAP; e.g., Harter et al. 1989; Hopf and Mangun 2000; Eimer et al. 2002; Green et al. 2005; Jongen et al. 2006; van der Lubbe et al. 2006). More recently, however, contralateral negative polarity preparatory ERP activity have been reported (Van der Stigchel et al. 2006; Grent-'t-Jong and Woldorff 2007; Dale et al. 2008), which have been referred to as the late directing attention negativity (LDAN) in one of these studies (Van der Stigchel et al. 2006) and as the biasing-related negativity (BRN) in another (Grent-'t-Jong and Woldorff 2007).
The functional interpretations of these markers have varied greatly between studies. For example, the positive polarity LDAP was initially postulated as reflecting an increase in the excitability of visual occipital cortical neurons enhancing the response to the target stimulus (Harter et al. 1989) and later as reflecting the buildup and maintenance in occipital–temporal areas of an attentional trace of the expected visual target (Hopf and Mangun 2000). Subsequently, this positive polarity posterior wave was interpreted as reflecting parietal cortex activity related to the deployment and maintenance of spatially specific attention at the cued location (Eimer et al. 2002, 2003), as encoding of the to-be-ignored location (rather than the to-be-attended) location (McDonald and Green 2008) or as a marker of covert manual response preparation (Praamstra et al. 2005; Gherri et al. 2009), with the last of these deviating substantially from a location-specific “perceptual” biasing interpretation. The posterior negative waves contralateral to the direction of attention (BRN/LDAN), on the other hand, have been interpreted by our group as reflecting baseline-shift activity that biases target-specific brain areas to enhance perceptual sensitivity, following the instantiation of attentional control activity in the frontoparietal network (BRN: Grent-'t-Jong and Woldorff 2007), and by others as reflecting a combination of “pretarget oculomotor programming” and “attentional orienting” (LDAN: Van der Stigchel et al. 2006).
In addition to ERP markers of preparatory activity, ample evidence exists for the involvement of induced preparatory “oscillatory” signals, especially in the form of late, sustained, spatially selective, occipital–parietal cortex modulations of ongoing activity in the alpha band (8–14 Hz). As with the ERP components, some variability has been reported in the directionality (polarity) of these spatial location–specific alpha frequency modulations. For example, some studies have reported observing predominantly sustained pretarget decreases (desynchronization) in oscillatory alpha power over occipital or parietal scalp sites “contralateral” to the direction of attention (Sauseng et al. 2005; Yamagishi et al. 2005; Thut et al. 2006; Trenner et al. 2008; Kelly et al. 2009), whereas others have reported observing predominantly sustained pre-target increases (synchronization) in alpha power over “ipsilateral” sites (Worden et al. 2000; Kelly et al. 2006; Rihs et al. 2007). In general, decreases in visual cortex alpha power have been interpreted as reflecting cortical activation or enhanced cortical excitability, whereas increases in alpha power have been linked to cortical deactivation (Pfurtscheller 2001) and/or to active decoupling of cortical processing or disengagement of visual attention (Vanni et al. 1997; Fu et al. 2001). More recently, the ipsilateral alpha power increases seen in visual–spatial cueing studies have also been interpreted as an active inhibition mechanism (Kelly et al. 2006; Rihs et al. 2007) for the purpose of suppressing task-irrelevant or distracting visual input, an interpretation along the lines of the inhibition-timing hypothesis put forward by Klimesch et al. (2007).
These observations of different EEG/ERP markers of preparatory visual cortex activity, along with their widely varying functional interpretations, lead to two important questions that comprise the focus of the current study. First, what is the relationship between our previously reported slow-wave BRN (swBRN) ERP marker and previously reported oscillatory alpha-band markers of pretarget sensory cortex activity, and second, what are the functional roles of the neural activations these markers reflect? (Note that, because the basic paradigm of the present studies, which manipulates attention within the lower visual field, has previously been shown to trigger a negative polarity contralateral BRN component over posterior scalp in the absence of any positive-polarity contralateral LDAP wave, the contralateral posterior ERP effect that we will be focusing on here will be the negative-polarity BRN.). Answering these questions is of considerable interest for at least two reasons. First, it is still unclear whether ERP and oscillatory markers reflect different aspects of a related underlying mechanism (see also studies by: Kelly et al. 2009; Green and McDonald 2010). And secondly, the fact that so many different functional roles have been presented for the different visual–spatial attentional control markers that have been observed in the literature (LDAP/BRN ERP effects, alpha power changes) points to the possibility that there might actually be more than one process or mechanism reflected by these visual-field-specific preparatory activations in visual cortex. Thus, gathering better understanding of the functional roles reflected by these markers has the potential to improve and/or extend our current understanding of the mechanisms of top-down attentional control.
In order to investigate these questions, the current study used a multiexperiment approach that entailed analyses of 3 variants of a visual–spatial attentional control ERP paradigm, one variant of which we have reported on previously (Grent-'t-Jong and Woldorff 2007). The basic paradigm in all 3 experiments consisted of a foveally presented instructional cue (attend right, attend left, or control cue) that could be followed shortly later by a faint target dot in a lower left or right visual-field location, with the target needing to be detected and reported by a button press when it occurred. The main variations of this general paradigm in the 3 experiments reported on here included a manipulation of perceptual task difficulty (harder or easier detection) and a manipulation of behavioral–response instructions (responding immediately following the target or being delayed until after the onset of a visual report signal). Manipulating perceptual task difficulty was used to study sensitivity of the preparatory BRN and alpha-band marker activity to perceptual degradation, as such sensitivity would favor the interpretation of a baseline-shift mechanism for enhancing the sensory processing of the expected target. Response instructions were manipulated in order to investigate the possible involvement of sensorimotor linkage activity during the delay period, which would favor an interpretation related to engendering the task-set or stimulus-response mapping. Such an interpretation would fit well with data from single-cell and multi-unit studies in nonhuman primates suggesting that parietal regions not only code for the spatial position of an expected target stimulus or saccade location but also for its current task set, including behavioral relevance or valence (see review Bisley and Goldberg 2010).
Materials and Methods
Participants
A grand total of 59 healthy (primarily University student) participants with normal or corrected-to-normal vision gave written informed consent to participate in this study as approved by the Duke University Institutional Review Board. The participants were divided across 3 independently run experiments (abbreviated in the remainder of the article as exp1, exp2, and exp3). Included in the final analysis were 16 participants for exp1 (6 females, mean age 21 years, standard deviation [SD] 5.8 years, 2 left-handed), 16 participants for exp2 (10 females, mean age 24 years, SD 8.0 years, 5 left-handed), and 16 participants for exp3 (10 females, mean age 24 years, SD 5.5 years, all right-handed). Data from the remaining 11 participants were excluded from the analyses because of excessive eye blinks, eye movements, muscle activity, or skin potential drift. Participants were either paid $10/h or received university class credits for their participation.
General Paradigm
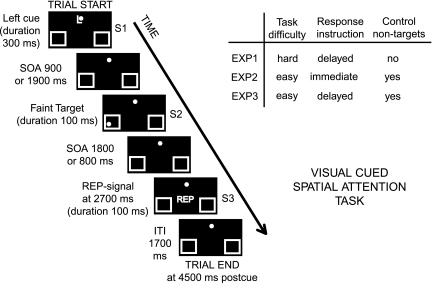
Participants were presented with a series of visually presented compound-event trials. Each of these lasted 4500 ms and began with an instructional upper case letter cue (“L,” “R,” or “P”) at fixation, which was sometimes followed by a target stimulus but which always ended with an End-of-Trial or report signal (for an example trial, see Fig. 1). The cues instructed the participants “to covertly shift attention” for that trial to a boxed location in the lower left (L) or lower right (R) visual field (3 deg lateral and 3 deg below the horizontal meridian) while maintaining central fixation (attention-directing cues) or “to not shift attention” on that trial and just continue to maintain central fixation (P, control or “interpret” cues). Targets consisted of a small, faint, unilateral, gray dot presented on a black background in the box on the cued side (100% validity). In exp1 (some of the data from which were included in our earlier published study, Grent-'t-Jong and Woldorff 2007), these faint gray dots occurred only after attend cues and were titrated for perceptual difficulty by changing the contrast level so that the detection rate averaged around 80%. In exp2 and exp3, all target dots had the same level of contrast that was clearly above threshold level, making the detection substantially easier. In addition, the faint dots could also be presented after the control cues in these 2 experiments, in which case they were task irrelevant and thus to be ignored.
Figure 1.
.Task paradigm. Left panel shows an example of an attend-left-cue-plus-target-trial. The box on the right contains information on different task/paradigm manipulations used in the 3 experiments in this study. In all experiments, a centrally presented cue (here, the letter “L”) instructed the participant to covertly attend to the lower left visual field box to detect whether a faint dot target stimulus was presented there in the period shortly following. On trials with a target, it could appear either early or late (50% probability) following the cue, at the cued location only. An End-of-Trial signal (the letters REP) presented at 2700 ms postcue signaled the participant in exp1 and exp3 to press a button to report if they had seen a target and signaled the end of the response window in exp2. Other trials included attend-right-cue-plus-target, attend-left-cue-only (no target), attend-right-cue-only (no target), control-cue-only (no target), control-cue-plus-non-target (only in exp2 and exp3), and no-stim trials (no cue, no target).
For all trial types (other than “nostims,” see below), an End-of-Trial or report signal (the letters REP, for “report”) was presented directly below fixation (see Fig. 1) 2700 ms after cue onset. Participants in exp1 and exp3 were instructed to press a response button with their right index finger after this REP signal, whereas participants in exp2 were told to respond immediately following the detection of a target dot, before the REP signal. In sum, the 3 experiments were identical in most aspects but differed in either perceptual task difficulty and/or response instruction, as well as on the inclusion of task-irrelevant dots following control cues (for a quick overview of the key differences between the experiments, see the Table in the upper right corner of Fig. 1). Task-irrelevant dots were included in 2 of the 3 experiments to provide a baseline for extracting expected attention effects (attended dots vs. control dots) on target occipital P1/N1 and/or parietal P3b component activity.
With respect to trial types, 25% of all trials were “attend-cue-plus-target” trials in which a target occurred either early (900 ms) or late (1900 ms) after cue onset (50% probability). A similar number of the trials (25%) were “attend-cue-only” trials in which only cues were presented, still requiring a covert shift of attention but no target was presented. Another 25% of trials consisted of “control cues” instructing to not shift attention. On these control trials in exp1, no faint target dot would occur (i.e., 25% “control-cue-only” trials). In exp2 and exp3, however, half of the control-cue trials were followed by faint dots (which were task irrelevant and to be ignored), randomly presented in the left or right lower visual field boxes, either early or late (50% probability), whereas the other half were control-cue-only trials. The remaining 25% of the trials consisted of nostim trials (periods of fixation only), which were randomized with the other trial types in order to provide a jittering of the intertrial intervals that would be necessary for effective functional magnetic resonance imaging (fMRI) versions of these experiments (e.g., Woldorff et al. 2004). In all experiments, participants received at least 2 practice runs of 64 trials each, followed by 12–14 experimental runs, each consisting of 64 trials and a run time of 4.8 min, during which EEG was recorded.
Recordings
The EEG was recorded from 64 electrodes mounted in a custom-designed extended-coverage electrocap (Duke64-cap layout, made by Electro-Cap International Inc.) and referenced to the right mastoid during recording. The 64 channels were equally spaced across the cap and covered the head from above the eyebrows to the lower occiput (slightly below the inion).
Vertical eye movements and eye blinks (vertical electrooculogram [VEOG]) were recorded from 2 electrodes placed below each eye, referenced to the scalp electrodes above the eye. Horizontal eye movements (HEOG) were recorded from 2 electrodes placed on the outer canthi of the eyes, referenced to each other. Eye movements were also monitored online with a video zoom lens camera. Participants were trained before starting the experiment on covertly orienting their attention without moving their eyes. Analyses of the horizontal EOG data indicated that the number of rejected trials due to eye movements was indeed very low in all conditions in all experiments (3% to 7%) and did not significantly differ between the different conditions or experiments.
Electrode impedances were maintained below 2 kΩ for the mastoids, below 10 kΩ for the EOG electrodes, and below 5 kΩ for all remaining electrodes. All EEG and EOG channels were continuously recorded with an online band-pass filter of 0.01–100 Hz (SynAmps amplifiers from Compumedics Neuroscan Inc.) and digitized with a 500-Hz sampling rate. Recordings took place in an electrically shielded, sound attenuated, dimly lit, experimental chamber. Stimuli were presented using the Presentation software package (Neurobehavioral Systems Inc.).
Behavioral Data Analyses
Behavioral performance estimates were extracted from the analyses of hit rates and false-alarm rates. Because the focus of this study was on the long-lasting preparatory activity induced by the cues, hit rates were determined based on the trials in which the targets occurred late in the cue-target interval, whereas false alarm rates were estimated from cue-only trials. Reaction time data were not analyzed because the delayed responses used in 2 of the 3 experiments rendered comparisons of those measures not meaningful. For comparisons between the experiments, the statistical analyses of hit rates included mixed-design repeated-measures analyses of variance (rANOVAs), including the between-subject factor EXPERIMENT (exp1, exp2, and exp3) and the within-subject factor LOCATION (right and left). Mixed-design rANOVAs for false alarm rates included the between-subject factor EXPERIMENT (exp1, exp2, and exp3) and the within-subject factor CONDITION (attend-left/right collapsed and control cue). Significance in all statistical tests was inferred for P values lower than 0.05.
EEG Data Analyses
EEG analyses were performed using EEGLAB 7.1.3.14b (Delorme and Makeig 2004), after downsampling of the data to 250 Hz. Cue-only and cue-plus-late-target trials were extracted from both the attend-cue and control-cue conditions (i.e., cue-plus-early-target trials were excluded from all analyses, although the inclusion of these early-target trials in the paradigm engendered more rapid attentional shifting across all the trials). The epochs analyzed for cue-related activity included data between 400 ms precue and 1900 ms postcue onset, whereas epochs for target-related activity included data between 200 ms pretarget and 800 ms posttarget onset. Cue and target ERPs were extracted from the same set of preprocessed trials.
Preprocessing started with the removal of trials that contained high-amplitude muscle artifacts or were clearly contaminated by eye blinks or eye movements. Trials with eye blinks were only rejected if the blink occurred around stimulus presentation times (−200 to +300 ms around cue and target onsets). Residual blink contamination was subsequently removed using independent components analysis (ICA) in the EEGLAB Matlab Toolbox. (Note that this approach differs from the one taken in the earlier published study containing data from exp1 participants (Grent-'t-Jong and Woldorff 2007) in that in the earlier study all trials with eyeblinks in the time windows of interest were excluded from the analyses, whereas in the present study ICA detection and removal was used. As a consequence, the data sets from more of the participants (16 vs. 13 before), as well as more trials from the individual participants, could be included, thereby increasing overall power.). After these preprocessing steps, the data were divided into 3 separate analysis pipelines, one for the cue ERPs, one for target-related ERP activity, and one for the cue-induced changes in alpha-band (8–12 Hz) power.
To extract the cue-induced, slow-wave, ERP activity, the data from the cue-only and cue-plus-late-target trials were collapsed together. Target ERPs were extracted only from the cue-plus-late-target trials. Both cue and target ERP averages were imported into ERPSS (ERP analysis software package; UCSD) for further analyses. These analyses included re-referencing of the data for all channels to the algebraic mean of the 2 mastoid electrodes, and the generation of contra-minus-ipsilateral activity (averaged across attend-right-cue and attend-left-cue trials to improve signal-to-noise ratio). In addition, to minimize overlap with cue-induced alpha-band activity, slow-wave cue-locked ERPs were computed by applying a low-pass filter to the data. This low-pass filter consisted of a 41-data-point running-average filter, which at our converted sampling rate of 250 Hz comprised a 164-ms boxcar filter kernel that strongly attenuates signal contributions from frequencies above 6 Hz. For target-locked ERPs, the data were filtered with a 7-data-point (28 ms) running-average low-pass filter that attenuates activity above 35 Hz.
For extracting the induced alpha-band responses, the analysis pipeline started with the computation of averaged event-related spectral perturbations (ERSPs) with the EEGLAB toolbox, separately for each condition and each channel, using Fast Fourier Transforms of single trials with a frequency resolution of 1.95 Hz. Subsequently, averaged event-related induced alpha-band (8–12 Hz) responses were extracted for all channels from these data. These data were then further analyzed and plotted in ERPSS similar to the cue ERPs (i.e., re-referenced to averaged mastoids and converted into contra-minus-ipsilateral data).
Statistical Analyses of EEG Data
Lateralized attend-cue-induced changes (contra-minus-ipsilateral activity) in slow-wave ERP and alpha-band activity were estimated across the cue-target interval in consecutive windows of 250 ms of averaged data from 400 to 1900 ms postcue onset, relative to a precue baseline window of 400 ms. Statistical tests included data from 2 occipital–parietal regions of interest (ROIs), consisting of sites approximately equivalent to O1, PO3, PO7, and PPO5 on the left (in the 10-5 system: Oostenveld and Praamstra 2001) and O2, PO4, PO8, and PPO6 on the right. Significant contralaterality of the slow-wave ERP and alpha-band changes were tested within these ROIs using rANOVAs.
For target-related activity, the amplitudes of the sensory ERP components P1 (90–110 ms) and N1 (exp1: 175–225 ms; exp2 and exp3: 150–200 ms) over the occipital–parietal ROI were tested for significant contralaterality with rANOVAs, using a 200-ms pretarget baseline. In addition, possible early attention effects (attended targets vs. nontargets) on these sensory P1/N1 components were tested for significance in exp2 and exp3 only, as exp1 lacked irrelevant nontargets as a baseline condition. In addition, longer-latency target attention effects in exp2 and exp3 were tested on the mean amplitude (350–500 ms) of the parietal P3b component, a component believed to represent stimulus evaluation and decision-making processes (e.g., review by Kok 2001). Statistical tests for early (P1/N1) attention effects on the targets included rANOVAs with the within-subjects factor ATTENTION (attended targets and ignored nontargets). Repeated-measures ANOVAs for the later latency, parietal, P3b component included an additional factor of ROI (left-parietal ROI: channels [PPO5, PO3, and PPO3h]; midline parietal ROI: channels [CPz, Pz, and POz]; and right parietal ROI: channels [PPO6, PO4, and PPO4h]).
Results
Behavioral Performance
Analyses of behavioral performance focused primarily on the accuracy measures of hit rates and false-alarm rates. Hit rates were at their expected level of accuracy, matching the titration goal of approximately 80% for the hard detection task in exp1 (75.9%, standard error [SE] 2.1%) and the goal of substantially easier detection tasks in exp2 and exp3 (exp2: 98.9%, SE 0.4%; exp3: 94.8%, SE 1.2%; for more details, see in Supplementary Table 1). Post hoc specific pairwise mixed-design rANOVAs indicated a significantly lower overall hit rate in exp1 compared with both exp2 (main effect of EXPERIMENT, F1,15 = 119.2, P < 0.001) and exp3 (main effect of EXPERIMENT, F1,15 = 68.8, P < 0.001). Hit rates were higher in exp2 relative to the delayed-response conditions of exp3 (main effect of EXPERIMENT, F1,15 = 9.4, P < 0.01). Finally, false-alarm rates were generally low (on average across all experiments between 0.1% and 2.0%; more details in Supplementary Table 1) and were found to not differ significantly between experiments.
In sum, the analyses of behavioral performance indicated proper overall attention and task compliance in all 3 experiments, while also showing the intended manipulation of perceptual task difficulty.
Target ERPs
The analyses of the target ERPs focused on early sensory components (P1/N1) at the selected occipital–parietal scalp ROIs and on the longer-latency parietal P3b component in the left, middle, and right parietal ROIs. In all 3 experiments, the occipital P1 amplitude to the faint target dots peaked between 90 and 110 ms. P1 amplitudes were generally very small, presumably because of the target being small and relatively faint, and were not significantly different in amplitude over the left and right ROIs as a function of target location. Attention effects on the P1 (i.e., larger for attended target dots vs. task-irrelevant nontarget dots) could be assessed only for exp2 and exp3 in that exp1 had no nontarget dot condition. These tests showed significant ATTENTION effects on the P1 amplitude in exp3 (F1,15 = 6.1, P < 0.03) but not in exp2 (P > 0.38).
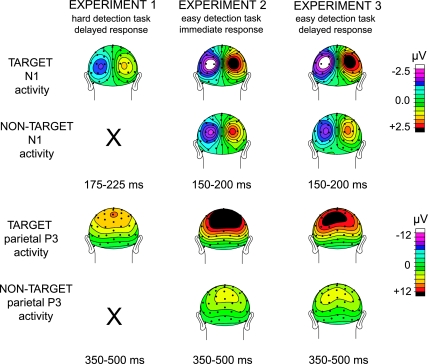
The first target ERP component that was significantly larger contralateral versus ipsilateral to the location of the target as well as to the direction of attention was the occipital–parietal N1 component (see Fig. 2), peaking between 175 and 225 ms in exp1 and between 150 and 200 ms posttarget onset in exp2 and exp3. The latency difference of the target N1s between experiments is presumably a result of the manipulation of perceptual contrast (being fainter in exp1 compared with exp2 and exp3), as perceptually degrading a stimulus is known to diminish the amplitude and to delay the peak activity of early sensory components (Johannes et al. 1995). Contralaterality of the N1 component was confirmed by a main effect of TARGET LOCATION in all 3 experiments (exp1: F1,15 = 52.6, P < 0.001; exp2: F1,15 = 46.6, P < 0.001; exp3: F1,15 = 52.8, P < 0.001). ATTENTION effects on these contralateral N1 components, again assessable only for exp2 and exp3, were again found for exp3 (F1,15 = 17.9, P < 0.001) only. In exp2, the overall ATTENTION effect for the N1 across all occipital ROI channels did not quite reach significance (P = 0.06), but there was a significant ATTENTION × ELECTRODE interaction effect (F1,15 = 3.9, P < 0.03). Post hoc tests revealed that only the more superior channels of the ROI (PO3/PO4 and PPO5/PPO6) showed a significant N1 amplitude enhancement (F1,15 = 5.4, P < 0.03).
Figure 2.
.Distributions and attention effects of target N1 and P3b ERP components. Top 2 rows: Grand-average (n = 16) distributions of early N1 activity (contralateral-minus-ipsilateral activity on the left; right side is opposite subtraction and thus contains redundant information) elicited by target (following attend cues) and nontarget faint dots (following control cues; only in exp2 and exp3). Bottom 2 rows: Grand-average (n = 16) distributions of P3b activity (nonlateralized), again separately for targets and nontargets. Columns separate the results from the 3 different experiments.
Finally, clear ATTENTION effects were found on the parietal P3b component (see Fig. 2: peaking between 350 and 500 ms posttarget onset) in both exp2 (F1,15 = 71.7, P < 0.001) and exp3 (F1,15 = 20.5 P = 0.004). In addition, in exp2 only, an ATTENTION × ROI interaction effect was found (F2,30 = 17.6, P < 0.0001) that resulted from attention effects being larger over the left parietal than over the middle and right parietal ROIs (attention effects were on average 9.63, 7.38, and 7.50 μV over left, middle, and right parietal ROIs, respectively).
In sum, the presence of clear early and late attention effects on the target ERPs in exp2 and exp3 indicates that subjects were attending as instructed even when target detection was relatively easy.
Cue-Induced Preparatory Occipital Slow-Wave BRN and Alpha-Band Activity
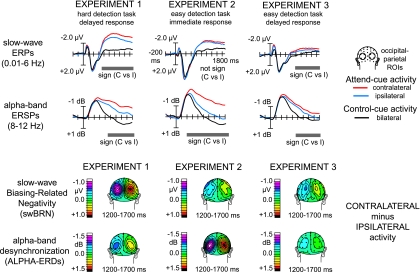
Sensory cortex preparatory activity was studied by investigating target-location-specific changes in cue-induced slow-wave ERP activity (0.01–6 Hz) and oscillatory event-related alpha-band (8–12 Hz) activity (Fig. 3).
Figure 3.
.Cue-locked pretarget preparatory activity in visual cortex. Top panel: Grand-average (n = 16) traces, shown separately for the 3 experiments, of cue-locked slow-wave (0.01–6 Hz) ERPs and alpha-band (8–12 Hz) ERD activity. These are shown over contralateral (red traces) and ipsilateral (blue traces) occipital ROIs for the attend condition, collapsed across attend-right and attend-left cues, and for the control condition (black traces), collapsed across right and left ROI channels, from 200 ms before to 1900 ms after cue onset, for cue-only and cue-plus-early-target trials only. Gray bars underneath the traces indicate significant differences between contralateral and ipsilateral responses to attend cues, revealing significant swBRN or alpha-ERD (ERD) preparatory activity. Bottom panel: Corresponding topographical distributions of the contralaterality of the swBRN and alpha-ERDs difference-wave activity between 1200 and 1700 ms following cue onset. Contralaterality of distribution is calculated as contralateral-minus-ipsilateral activity relative to the left hemisphere, so the activity over the right hemisphere reflects the inverse of the subtraction (i.e., ipsilateral-minus-contralateral).
Slow-Wave ERP Activity
All cue types (attend right, attend left, and control cues) triggered a late-onsetting, sustained, negative-polarity ERP wave over occipital–parietal scalp sites that was smallest and mostly bilateral for control-cue trials, somewhat larger for attend-cue trials over scalp sites ipsilateral to the direction of attention, and largest for attend-cue trials over scalp sites contralateral to the direction of attention. The difference between contralateral and ipsilateral activity reflects the ERP biasing marker previously termed the BRN (Grent-'t-Jong and Woldorff 2007), which we will refer to here as the “swBRN” because of the explicit 6 Hz low-pass filtering we applied to the ERP data in the current study. Exp1 triggered clear swBRN activity, whereas exp3, and particularly exp2, yielded much weaker versions of these responses. The rANOVAs revealed that the contralateral swBRN was significant between 650 and 1900 ms in exp1 (P values all < 0.01) and between 900 and 1900 ms in exp3 (P values all < 0.01) but did not reach significance (P values all > 0.1) in any test window in exp2 (for more details, see Supplementary Table 2). Additional rANOVAs, testing for differential late swBRN activity across experiments (1200–1700 ms data only), revealed a main effect of EXPERIMENT (F2,30 = 27.4, P < 0.0001). Post hoc specific pairwise comparisons showed that the late swBRN activity differed significantly between exp1 and exp2 (F1,15 = 50.5, P < 0.0001) and between exp1 and exp3 (F1,15 = 25.0, P < 0.0001) but not between exp2 and exp3 (P = 0.21), which both included easy-to-detect targets.
Alpha-Band (8–12 Hz) Fluctuations
In contrast to the swBRN activity, alpha-band responses showed a fairly consistent pattern across the 3 experiments. All cue types triggered an initial strong decrease in alpha-band power (compared with precue baseline power) until about 400–500 ms postcue onset. Following this initial alpha-band event-related desynchronization (ERD) elicited by all cue types, alpha-band power in control-cue trials moved quickly back to baseline level and even somewhat beyond (giving rise to a small degree of alpha event-related synchronization [ERS]). Alpha power in attend-cue trials, on the other hand, moved more slowly back to baseline level, particularly over scalp sites contralateral to the direction of attention. In contrast to the pattern for the swBRN, this ERD effect was particularly strong in exp2 relative to exp1 and exp3. Repeated-measures ANOVAs revealed that the alpha-ERDs were significantly lateralized (larger contralateral vs. ipsilateral to attention) between 900 and 1900 ms in exp1 (all P values < 0.03), between 400 and 1900 ms in exp2 (all P values < 0.01), and between 900 and 1900 ms in exp3 (all P values < 0.03). Additional rANOVAs, testing for differential late alpha-band power changes across experiments (1200–1700 ms data only), revealed a main effect of EXPERIMENT (F2,30 = 16.1, P < 0.0001). Follow-up post hoc pairwise comparisons indicated that the late alpha-ERDs differed significantly between exp1 and exp2 (F1,15 = 20.2, P < 0.0001) and between exp2 and exp3 (F1,15 = 25.8, P < 0.0001) but not between the 2 studies with delayed responses, exp1 and exp3 (P = 0.1).
In summary, very different patterns were found for the 2 markers of pretarget preparatory activity in visual cortex. The swBRN activity was strongest and earliest when the target stimulus was expected to be perceptually difficult to detect (exp1). Surprisingly, no significant swBRN was found in exp2, whereas a small but significant one was found in exp3, despite differing only in response instructions (immediate in exp2, delayed in exp3). In contrast, alpha-ERD responses were much more consistent across studies, thus showing much less sensitivity to perceptual task difficulty. The alpha-ERD marker appeared more sensitive to response instructions, however, starting earlier and becoming stronger when immediate response were required (exp2) compared with when they were delayed (exp1 and exp3).
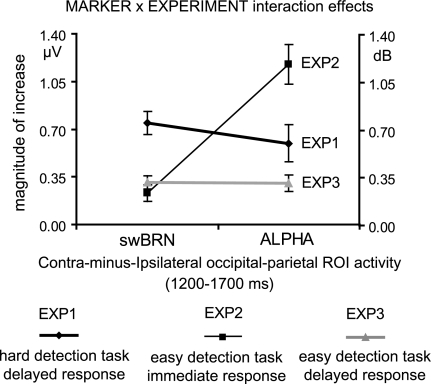
Direct Analyses of Differences between Marker Responses Across Experiments
The different response patterns of the 2 markers, summarized in Fig. 4, were investigated further by direct analyses across the 3 experiments, using a mixed-design rANOVA that included the within-subject factor MARKER (magnitude of change in swBRN or alpha-ERD activity) and the between-subject factor EXPERIMENT (exp1, exp2, or exp3). Both a significant main effect of MARKER (F1,2 = 13.6, P < 0.001) and a significant MARKER × EXPERIMENT interaction effect (F2,45 = 23.3, P < 0.0001) were found. In addition, pairwise post hoc comparisons revealed that the pattern of change in magnitude of the swBRN and alpha-ERD activity was significantly different between exp2 and exp1 (main effect of MARKER: F1,1 = 15.6, P < 0.001 and a MARKER × EXPERIMENT interaction effect: (F1,30 = 29.8, P < 0.001), and between exp2 and exp3 (main effect of MARKER: F1,1 = 29.2, P < 0.001 and a MARKER × EXPERIMENT interaction effect: (F1,30 = 30.1, P < 0.001), whereas no such differences were found between exp1 and exp3 (no MARKER effect: P = 0.29 and no MARKER × EXPERIMENT effect: P = 0.33). In conclusion, these results suggest that the marker responses in the immediate-response conditions of exp2, lacking a clear swBRN but showing the strongest alpha-ERD responses of all 3 experiments, were “qualitatively” different from those recorded in the delayed-response conditions of exp1 and exp3, which induced both swBRN and alpha-ERD activity, mainly differing in strength (i.e., quantitatively) between the 2 experiments.
Figure 4.
.swBRN and alpha-ERD marker activity across experiments. These show a qualitatively different pattern of preparatory activity for exp2, compared with exp1 and exp3, indicating an especially strong effect of the manipulation of response instructions on the alpha-ERDs.
Topographical Distributions
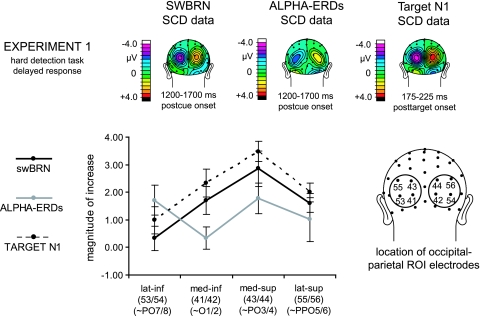
Another clear difference between the 2 preparatory activity markers was the scalp potential distributions (Topographic distributions or [TOPOs]) (see TOPOs in Fig. 3 and scalp current density [SCD] maps in Fig. 5). The distribution of the swBRN appeared to be more superior and medial, whereas the alpha-ERDs seemed to extend further inferiorly and laterally over occipital–parietal scalp sites. We explicitly tested for the presence of statistical distributional differences between the 2 markers using SCD transformed data (in order to focus on local distributions) extracted between 1200 and 1700 ms postcue onset from exp1, an experiment that induced clear activity for both markers. In addition, we compared the marker distributions from exp1 with the target-N1 distribution from that experiment. We reasoned that if one, or both, of these markers reflects a baseline-shift biasing mechanism to improve perceptual sensitivity for processing the targets, one would expect this baseline-shift activity to occur over scalp sites closely corresponding to those where the contralateral target-N1 sensory component peaked.
Figure 5.
.Comparison between swBRN, alpha-ERD, and target-N1 distributions. Top panel shows grand-average (n = 16) SCD distribution data from exp1, separately for swBRN, alpha-ERDs, and target-N1 activity, plotted over posterior scalp sites (left hemisphere displays contra-minus-ipsi activity, right hemisphere ipsi-minus-contra activity). Bottom panel shows the mean activity and standard error of the mean for all 3 components, separately for each of the 4 channels included in the occipital–parietal ROI (see for exact locations the lower right cartoon head). Note the pattern of activity across sites differed for the alpha-ERD relative to the swBRN and target-N1.
Figure 5 provides an overview of the results of this analysis. Repeated-measures ANOVAs were performed on the contra-minus-ipsilateral topographic distributions, using the factors of MARKER (swBRN vs. alpha-ERD, swBRN vs. target-N1, alpha-ERD vs. target-N1, in pairwise comparisons) and ELECTRODES (4 occipital–parietal ROI channels), revealed significant MARKER × ELECTRODE interaction effects for the swBRN versus alpha-ERD distributions (F2.2,32.4 = 4.8, P < 0.02, Greenhouse–Geisser [GG] corrected) as well as for the contrast of the alpha-ERDs and target-N1 distributions (F2.5,37.9 = 9.2, P < 0.0001, GG corrected), whereas no significant differences were found between the swBRN and the target-N1 distributions (P = 0.96). Inspection of the distributions indicated that the swBRN and the target-N1 distributions had the same focal peak distribution within the ROIs, whereas the alpha-ERDs had a broader and more diagonal swath of peak activity that reached from medial parietal scalp locations to more infero-lateral ones (Fig. 2). Thus, it appears that the alpha-ERD power changes were “hemifield” specific but not so target-location specific, differing in distribution from the target N1, whereas the swBRN was both hemifield specific and target-location specific. Accordingly, the swBRN would seem to be more likely than alpha-ERDs to reflect a baseline-shift mechanism.
Correlations between Preparatory Biasing Markers and Target ERP Activity
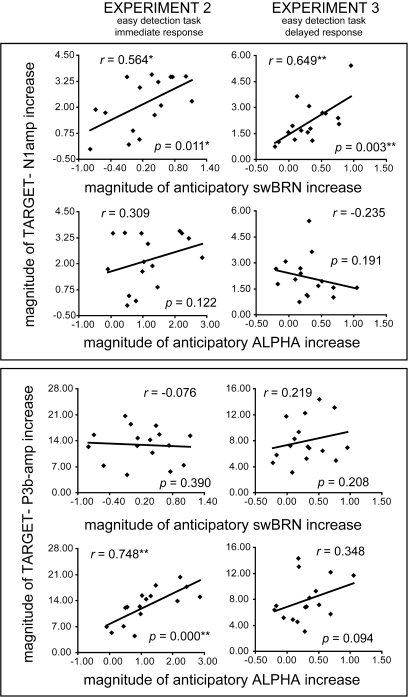
The functional roles of the swBRN and alpha-ERD were further investigated by correlating the preparatory activity of each of these with the subsequent brain responses to the targets (Fig. 6). In particular, using Pearson's r correlation statistics, activity levels of the cue-triggered contra-minus-ipsilateral swBRN and alpha-ERD activity between 1200 and 1700 ms were tested for their possible correlation across subjects with the amplitudes of the contra-minus-ipsilateral sensory target-N1 (150–200 ms) over the lateralized occipital–parietal ROIs and with the longer latency parietal target-P3b (350–500 ms) over the left, middle, and right parietal ROIs (i.e., where these ERP components were shown to be affected by attention). These pretarget swBRN and alpha-ERP activity marker were also assessed for their possible correlations across subjects with the mean target-detection reaction times (exp2 only) and hit rates (both exp2 and exp3). Data from exp1 were excluded from these analyses because the online titration of perceptual task difficulty level in that experiment would likely have confounded the correlations of interest. We predicted that if the swBRN indeed reflects a baseline-shift mechanism, as our main analyses seemed to suggest, then a correlation between the swBRN and target N1 amplitude should be observed, whereas such an effect would likely be absent for the alpha-ERDs because its topographic distribution did not match the N1 distribution well. Supporting this notion, target N1 amplitudes were found to correlate positively with the preceding cue-triggered swBRN activity (see Fig. 6; upper box, first row, right and left column) in both experiments (exp2: r = 0.564, P = 0.011; exp3: r = 0.649, P = 0.003) but not with the preceding alpha-ERDs (see Fig. 6; upper box, second row, right and left column). Thus, increased target N1 amplitudes were preceded by stronger swBRN preparatory activity, thereby further linking the swBRN marker to a functional role of increasing baseline activity for enhancing sensory and perceptual processing.
Figure 6.
.Anticipatory swBRN and alpha-ERD activity and their correlations across subjects with subsequent target N1 and P3 activity. Scatterplots showing the correlations (Pearsons r coefficients and corresponding, one-tailed, P values) between the contra-minus-ipsilateral magnitude of increase of swBRN and alpha-ERD activity between 1200 and 1700 ms postcue and subsequently elicited magnitude of increase of the contra-minus-ipsilateral N1 amplitudes (upper panels) and the longer latency P3b (lower panels) to the targets, separately for exp2 (left column) and exp3 (right column). N1 activity is averaged over the 4 channels of the occipital–parietal ROI, and the P3b activity is from the left-parietal ROI where maximum correlations were found in exp2. All activity is collapsed across attend-right and attend-left conditions.
The pattern of correlations between the swBRN and alpha-ERDs and the later parietal P3b in exp2 and exp3 were less straightforward but in part showed the converse pattern. (A complete overview of all the parietal ROI correlations is presented in Supplementary Table 3.). More specifically, preparatory swBRN activity did not correlate with the P3b amplitude in either of the 2 experiments in any of the parietal P3b ROIs or collapsed across all of them. (See Fig. 6, left parietal ROI correlations in lower target P3b box, first row, right and left column.). In contrast, alpha-ERD activity correlated significantly with P3b amplitude in exp2 with P3b amplitude, however (Fig. 6; lower box, second row, right column; r = 0.748, P < 0.001), although not in exp3 (Fig. 6; lower box, second row, left column; r = 0.348, P = 0.094). In addition, in exp2, the alpha-ERDs correlated more strongly with the P3b over the left parietal ROI (r = 0.748, P < 0.001) than over the middle (r = 0.652, P = 0.003) and right (r = 0.662, P = 0.003) parietal ROIs, mirroring the stronger left parietal effects found for attention effects on the target P3b amplitudes in that experiment. Thus, a stronger decrease in alpha-band power (larger alpha-ERDs) resulted in a subsequently higher target P3b amplitude (especially over left parietal sites), but only when response instructions favored immediate-response decisions and executions and not when responses were delayed.
Finally, correlational analyses between preparatory swBRN and/or alpha-ERD activity and subsequent behavioral performance (hit rates and mean reaction times) were performed for the 2 studies that could be tested (exp2 and exp3), but no significant correlations were found. Although all these correlational relationships were in the direction of a positive relationship with task performance, the P values were all larger than 0.3, except for the correlation between swBRN activity and hit rates in exp3 (easy detection/delayed response), which was 0.08 (r = −0.37). In other words, in exp3, participants with stronger preparatory swBRN activity (greater contralateral amplitude decrease) tended to show better target-detection performance (higher hit rates). Considering the rather small number of target trials in these experiment, however, we hypothesize that we may not have had sufficient power for these correlational analyses with behavior to reach significance.
Within-Subject Marker Correlations
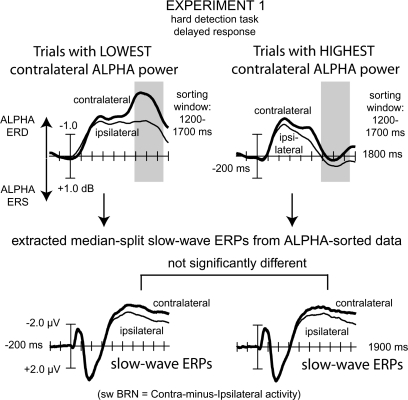
Finally, although clearly significant differences were found between the swBRN and alpha-ERDs markers of visual–spatial biasing activity, these significant differences could in theory be heavily driven by individual differences as all tests used between-subject variance. Thus, as a final test, we also explored the relationship between swBRN and alpha-ERD activity at a within-subject level by selecting data from exp1 (which included both markers), looking for different swBRN activity in a subject-based median split of the single-trial amplitudes of the occipital-parietal alpha activity between 1200 and 1700 ms.
For this final test (results summarized in Fig. 7), trials were sorted by the induced alpha-power desynchronization, separately for attend-right and attend-left cue trials on the channel of strongest swBRN activity found in the grand average (i.e., channel 43 [∼PO3] for attend-left cues and channel 44 [∼PO4] for attend-right cues) between 1200 and 1700 ms postcue onset. After sorting, the data were split for each subject individually into 2 data sets of high and low alpha-ERSPs (median split). Subsequently, averaged alpha-ERSP and slow-wave ERP data was extracted for further analyses comparable with the original analyses on the whole data set. Finally, the data from 6 windows of 250 ms of averaged data between 400 and 1900 ms were submitted to rANOVAs, separately for both markers, including the factor CONTRAvsIPSI (contralateral, ipsilateral) and MSDATA (median split: data1, data2). As would be expected, due to the sorting, these data showed large differences in the alpha-ERSPs, which was also reflected in the rANOVAs showing significant CONTRAvsIPSI × MSDATA interaction effects for the alpha-ERSPs (upper traces in Fig. 7) between 1150 and 1400 ms (F1,15 = 12.2, P < 0.01) and between 1400 and 1650 ms (F1,15 = 16.6, P = 0.001). In contrast, for the same trials, there was no evidence of any such interaction effects in any of the tested windows for the swBRN (lower traces in Fig. 7).
Figure 7.
.Within-subject marker correlations across trials in exp1. Top panel shows grand-average (n = 16) median-split alpha-ERSP activity, separately for the contralateral and ipsilateral occipital–parietal ROIs. These activitation traces were extracted from alpha-power-sorted data at the contralateral scalp sites that showed maximum overlapping lateralized alpha and swBRN responses (PO3/PO4) between 1200 and 1700 ms postcue onset. Bottom panel shows the corresponding swBRN ERP responses extracted from these alpha-sorted median-split data sets. Note that, despite the very large, contralateral alpha-power differences resulting from the median-split separation imposed, the swBRN responses (contralateral minus ipsilateral ERPs) did not show any difference in magnitude between these 2 sets of trials.
In conclusion, in the current multiexperiment study, changes in the magnitude of alpha-ERD activity did not appear to correlate with changes in the magnitude of swBRN activity in the same trials, supporting the view that these 2 markers indeed reflect different processes.
Discussion
In the current study, we used different variants of a cued visual-attention paradigm in 3 separate experiments to investigate the functional roles of 2 markers of pretarget preparatory neural activity in visual cortex, namely the swBRN ERP component and the simultaneously induced alpha-ERD activity. The results provide strong support for the view that these markers do not reflect the same underlying mechanism of preparatory sensory cortex activity. First, the swBRN was very sensitive to the manipulation of perceptual task difficulty and not to the manipulation of response instructions, whereas the alpha-ERDs showed the opposite pattern. Second, the activity of these 2 markers did not correlate in either their strength or timing of onset. Third, their location over occipital-parietal scalp sites also differed, showing a broader swath of hemifield-specific responses with a more inferior maximum for alpha-ERDs, and a more superior, hemifield- and target-location-specific response for the swBRN activity, with the latter corresponding more closely to the N1 sensory-ERP distributions of the targets that would follow. Fourth, a further dissociation was found between the across-subject correlation of the amplitudes of both markers with the activity elicited by the targets that followed. More specifically, the swBRN correlated strongly with early sensory-evoked target-N1 amplitude but not with that of the later parietal P3b activity, whereas alpha-ERDs did not correlate with the early N1 but correlated with the later P3b activity. Finally, the strengths of the swBRN and alpha-ERD activity also did not correlate across trials within subjects.
Functional Significance of the BRN
The sensitivity of the swBRN component to the perceptual difficulty level of the expected target stimulus and its correlation with subsequent early sensory N1 activity over identical brain areas is in line with the predictions from our previously proposed model (Grent-'t-Jong and Woldorff 2007). This model, based on the combination of ERP recordings with fMRI measures of brain activity from a closely matched neuroimaging study (Woldorff et al. 2004), proposed a temporal cascade of attentional control processes following an instructional cue to covertly shift visual–spatial attention. This cascade begins with activity in the frontal eye fields (FEF), followed shortly later by activity in medial parietal regions, which together lead to the induction and maintenance of pretarget preparatory activity in visual sensory cortex contralateral to the direction of attention. This preparatory contalateral activity was reflected by both the contralateral BRN ERP wave and corresponding increased fMRI signal in low-level visual sensory cortex. In this previous work, we proposed that the pretarget contralateral BRN may reflect preparatory biasing activity in visual sensory cortex in response to control signal activity from frontal and parietal cortex, and thus we termed this negative polarity electrophysiological activity a “biasing-related” negativity.
In the present study, we directly investigated this hypothesis concerning the role of this pretarget negative wave activity. In particular, as noted above, we manipulated perceptual difficulty between experiments and found that the magnitude of the swBRN was, as hypothesized, larger when targets were expected to be perceptually more difficult to detect and thus it would be particularly advantageous to invoke sensory biasing. Also, as our ERP-behavior correlational analyses of exp3 suggests, even when detection was relatively easy, participants with stronger swBRN responses tended to show better performance in terms of their percentage of correct detections (higher hit rates). We also tested whether increased pretarget swBRN amplitudes would correlate with the amplitude of posttarget sensory-evoked N1 activity and showed that this indeed was the case. In addition, we compared the topographical distributions of the pretarget swBRN and the target-elicited N1 sensory component and showed that the swBRN distribution was both hemisphere and target-location specific. The results of all these analyses support the view that the BRN is a sensitive marker of sensory biasing and support the hypothesis that a baseline shift in target-location-specific sensory cortical areas in advance of an expected target stimulus enhances the early sensory processing of such targets, thereby facilitating their detection.
The interpretation of the BRN as reflecting pretarget biasing activity that facilitates early sensory processing activity of the target fits with reports of baseline-shift activity in nonhuman primate single-cell recordings that is thought to result in subsequent modulation of activity in extrastriate cortex for attended stimuli (Luck et al. 1997; Reynolds et al. 1999). Moreover, it is consistent with the findings that microstimulation of sensory-ERP, at a level below the threshold needed to trigger a saccade, can produce modulation of firing rates in retinotopic visual areas such as V4 that resemble spatial attention effects (Moore and Armstrong 2003; Ekstrom et al. 2009). The increase in sensory biasing ERP activity shown here with higher levels of expected perceptual task difficulty is also consistent with such findings reported by Ress et al. (2000) with fMRI. And finally, the fact that the baseline shift interpretation fits the swBRN, rather than the higher frequency alpha-band power changes, is in line with earlier reports of slow cortical negative polarity potentials representing increased excitability of underlying cortical areas (Brunia and van Boxtel 2001), which have been shown to correlate with shifts in sensory thresholds (Devrim et al. 1999).
Functional Significance of Preparatory Desynchronization of Alpha-Band Activity
In the present study, there was no comparable correlation for the pretarget preparatory alpha-ERDs with the evoked sensory N1 activity of the target. In addition, alpha-ERD activity was not sensitive to the expected perceptual difficulty of the target stimulus. On the other hand, in contrast to the perceptual difficulty effects on the swBRN, the manipulation of response instruction in the present study clearly and robustly affected preparatory alpha-ERD activity. In particular, alpha-ERD activity was much stronger when instructions emphasized immediate responding (exp2) compared with when responses were delayed (exp1 and exp3). In addition, only when participants were preparing for possible immediate responses to potential target stimuli (exp2) did their alpha-ERD activity correlate strongly with the subsequently elicited, long-latency parietal P3b activity to the targets, a correlation that was strongest over the left parietal scalp sites, as compared with the middle or right parietal ones. This combination of results suggests that preparatory alpha-band desynchronization activity in posterior cortex (alpha-ERDs) reflects more than just stimulus-specific preparation. The parietal P3b component has been linked to stimulus evaluation and decision-making processes (e.g., Kok 2001) and thus it might well be that the contralateral alpha-ERD activity reflects the formation and maintenance of an attentional template or task set, containing both stimulus features as well as task-specific features (such as response instructions), which then is implemented for the processing of the target stimulus during the P3 latency window. Note that this interpretation is for contralateral alpha-ERDs only (i.e., alpha power decreases), as the present study did not produce ipsilateral alpha-ERS activity (i.e., alpha power increases) following attend cues, presumably because of a lack of to-be-ignored distractors. Accordingly, we cannot comment on the functional significance of ipsilateral alpha power increases.
Some earlier visual–spatial attention studies have reported correlations between preparatory lateralized alpha activity (greater ERDs or lower alpha power) and improved behavioral detection and/or discrimination performance (Thut et al. 2006; Trenner et al. 2008; Yamagishi et al. 2008; Kelly et al. 2009). In addition, other non-cueing, auditory (Jasiukaitis and Hakerem 1988; Price 1997) and visual nonspatial attention studies (Hanslmayr et al. 2007; Min and Herrmann 2007) have reported correlations between pretarget alpha activity (power) and behavioral performance. In addition, the Hanslmayr et al. (2007) study showed that prestimulus oscillatory alpha activity can correlate differentially with different aspects of cognitive processing and task performance (perception related vs. memory related in particular).
In the current study, however, no significant correlations between prestimulus alpha power changes and subsequent behavioral performance were found for either exp2 (hit rate and mean reaction time) or exp3 (hit rate only), the only 2 experiments here that could be used to investigate such correlations. It should be noted, however, that our tests were necessarily performed across participants because the low number of available target trials (on average maximally 56 per cue type) did not allow the use of within-subject single-trial correlational analyses. Importantly, however, the earlier reported significant correlations in the literature were of correlations with behavioral performance only, with no relationships with respect to target ERP activity being reported. Unfortunately, a correlation with later behavioral outcome does not delineate the underlying mechanisms that brought about these changes. Improved performance can be explained both by improved early perceptual processing as well as by improved late decision-making processes, or by a combination thereof, or by still other mechanisms. The current study revealed that pretarget alpha-ERD activity correlated with the later target P3b activity but not with the early target N1 activity (in contrast to the swBRN, which showed the opposite pattern), suggesting that the processes reflected by changes in prestimulus alpha power may bring about their behavioral effects by influencing later decision-making stages of information processing rather than by influencing earlier perceptual processes. Interestingly, the studies cited above all used the more typical instruction of immediate responses to target stimuli, and our current study showed that only under such instructions does prestimulus alpha-ERD activity correlate with the later P3b amplitudes of the targets (but still not with early sensory activity). Accordingly, the current pattern of results suggests that the behavioral improvement observed in those other studies resulted from improved task-set preparation, rather than improved perceptual performance due to a baseline sensory shift. Future studies that include both recordings of electrical brain activity and appropriate behavioral measures could further test this hypothesis.
Topographic Distribution of Markers of Visual Cortex Preparatory Activity
Although in the current study it was not feasible to effectively localize the sources of the preparatory electrophysiological marker activity in the brain, the distribution of the preparatory swBRN distribution did not differ from that of the target N1 sensory component, suggesting similar neural sources, presumably predominantly involved in perceptual processing activity. In contrast, the topography of the alpha-ERD activity differed from both the swBRN and N1 distributions, thus providing evidence for a different functional role than just the biasing of sensory regions to facilitate perceptual processing. In particular, as suggested above, it is possible that alpha-ERD activity reflects the coding and maintenance of an attentional trace (task-set representation) that includes linking of expected sensory and motor aspects of the upcoming task.
An increasing amount of data can be found in the literature supporting the presence of such preparatory sensorimotor activity in posterior brain areas. In the animal literature, for example, the lateral intraparietal lobule (LIP) and the parietal reach area in particular have shown increased firing rates during delay periods (e.g., Platt and Glimcher 1997; Andersen and Buneo 2002), coding both expected target location as well as action intentions (saccades, reaching). Recently, it has been proposed (Bisley and Goldberg 2010) that these parietal cortical areas code a priority map in which the amount of preparatory activity is proportional to the expected behavioral relevance and value, an idea that fits well with our observation of increased alpha-ERD activity with increasing motor readiness (immediate vs. delayed responses). In humans, brain areas in the intraparietal sulcus (Medendorp et al. 2005) have been shown to code the location for an upcoming saccade, both the direction of the expected target location for prosaccades as well as the opposite direction for antisaccades. In a follow-up study from the same research group (Medendorp et al. 2007), lateralized visual–spatial alpha-ERDs were found during delay periods that could be localized to areas in posterior parietal and occipital cortex, which the authors argued were close to areas V3A and LIP in the monkey that were found to be active during comparable tasks. In sum, our findings suggesting that the alpha-ERDs code both the hemifield of the expected target location as well as action intentions could well reflect activity from a human homologue of the monkey parietal cortex regions that have been proposed to code priority maps during delay periods.
Preparatory Top-Down Attentional Control Strategies
A perhaps somewhat surprising finding in the current study is the pattern of the effects on brain activity and behavior that the response instruction had. In particular, the data from exp2, in which participants were asked to immediately respond to target stimuli, were clearly qualitatively different from exp1 and exp3, in which responses were delayed. Participants in exp2 also detected more targets than in the other 2 experiments. Furthermore, their cue-related activity differed from the other 2 experiments in that it did not contain significant swBRN activity but, in contrast, contained a much stronger alpha-ERD response with a much earlier onset latency. In addition, in this experiment, preparatory attention affected target processing relatively late (at the level of the longer latency parietal P3b activity between 350 and 500 ms posttarget onset), not during, or very limited during, the earlier sensory-cortex level of analysis (lateral-occipital P1/N1 activity, before 200 ms). Such a pattern could be explained by differences in induced task strategies. For example, the participants in exp2 may have been using more of an “attention-for-action” strategy, with a stronger focus on the intention to respond as quickly as possible to detected target stimuli, whereas the participants in exp1 and exp3 may have used more of a pure attention-for-perception strategy. That is, in the latter case the data would appear to show a pretarget location–specific baseline shift for increasing perceptual sensitivity, leading to subsequently enhanced responses in early sensory cortex activity to the targets.
Conclusions
In conclusion, the present study adds more in-depth knowledge on the functional significance and relationships between 2 different electrophysiological markers of sensory cortex preparatory activity during the covert allocation of spatial attention, namely the contralateral swBRN and the contralateral alpha-band power decreases (alpha-ERDs). In particular, the swBRN correlates with early location-specific sensory-evoked (N1) responses to the targets, especially under situations of expected perceptually degraded task stimuli, consistent with reflecting a neural activity baseline shift for increasing the perceptual processing of these stimuli. In contrast, alpha-ERD activity correlates with the longer-latency target P3b activity, but only when immediate responses to targets are required. This could point to a role of preparatory alpha oscillations in establishing and maintaining an active task set (representing both stimulus and response requirements), which then can be used subsequently as an attentional template during final decision-making and task-performance output. Finally, this multiexperiment study clearly shows that key changes in the task paradigm, both in terms of expected perceptual difficulty and behavioral response requirements, can shift participants into different preparatory strategies. Such differential strategies, and the differential attention-related preparatory activation patterns they might lead to, are fundamental factors for understanding the neural processes underlying attentional control.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
National Institute of Mental Health (RO1-MH60415 to M.G.W.); National Institute of Neurological Disorders and Stroke (R01-NS051048to M.G.W.).
Supplementary Material
Acknowledgments
We would like to thank Greg Appelbaum for helpful comments on earlier versions of the manuscript. Conflict of Interest: None declared.
References
- Andersen RA, Buneo CA. Intentional maps in posterior parietal cortex. Annu Rev Neurosci. 2002;25:189–220. doi: 10.1146/annurev.neuro.25.112701.142922. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci. 2010;33:1–21. doi: 10.1146/annurev-neuro-060909-152823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunia CH, van Boxtel GJ. Wait and see. Int J Psychophysiol. 2001;43:59–75. doi: 10.1016/s0167-8760(01)00179-9. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Dale CL, Simpson GV, Foxe JJ, Luks TL, Worden MS. ERP correlates of anticipatory attention: spatial and non-spatial specificity and relation to subsequent selective attention. Exp Brain Res. 2008;188:45–62. doi: 10.1007/s00221-008-1338-4. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Devrim M, Demiralp T, Kurt A, Yucesir I. Slow cortical potential shifts modulate the sensory threshold in human visual system. Neurosci Lett. 1999;270:17–20. doi: 10.1016/s0304-3940(99)00456-5. [DOI] [PubMed] [Google Scholar]
- Eimer M, van Velzen J, Driver J. Cross-modal interactions between audition, touch, and vision in endogenous spatial attention: ERP evidence on preparatory states and sensory modulations. J Cogn Neurosci. 2002;14:254–271. doi: 10.1162/089892902317236885. [DOI] [PubMed] [Google Scholar]
- Eimer M, van Velzen J, Forster B, Driver J. Shifts of attention in light and in darkness: an ERP study of supramodal attentional control and crossmodal links in spatial attention. Brain Res Cogn Brain Res. 2003;15:308–323. doi: 10.1016/s0926-6410(02)00203-3. [DOI] [PubMed] [Google Scholar]
- Ekstrom LB, Roelfsema PR, Arsenault JT, Kolster H, Vanduffel W. Modulation of the contrast response function by electrical microstimulation of the macaque frontal eye field. J. Neurosci. 2009;29:10683–10694. doi: 10.1523/JNEUROSCI.0673-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe JJ, Simpson GV, Ahlfors SP, Saron CD. Biasing the brain's attentional set: I. cue driven deployments of intersensory selective attention. Exp Brain Res. 2005;166:370–392. doi: 10.1007/s00221-005-2378-7. [DOI] [PubMed] [Google Scholar]
- Fu KM, Foxe JJ, Murray MM, Higgins BA, Javitt DC, Schroeder CE. Attention-dependent suppression of distracter visual input can be cross-modally cued as indexed by anticipatory parieto-occipital alpha-band oscillations. Brain Res Cogn Brain Res. 2001;12:145–152. doi: 10.1016/s0926-6410(01)00034-9. [DOI] [PubMed] [Google Scholar]
- Gherri E, Van Velzen J, Eimer M. The instructed context of a motor task modulates covert response preparation and shifts of spatial attention. Psychophysiology. 2009;46:655–667. doi: 10.1111/j.1469-8986.2009.00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JJ, McDonald JJ. The role of temporal predictability in the anticipatory biasing of sensory cortex during visuospatial shifts of attention. Psychophysiology. 2010;47:1057–1065. doi: 10.1111/j.1469-8986.2010.01025.x. [DOI] [PubMed] [Google Scholar]
- Green JJ, Teder-Salejarvi WA, McDonald JJ. Control mechanisms mediating shifts of attention in auditory and visual space: a spatio-temporal ERP analysis. Exp Brain Res. 2005;166:358–369. doi: 10.1007/s00221-005-2377-8. [DOI] [PubMed] [Google Scholar]
- Grent-'t-Jong T, Woldorff MG. Timing and sequence of brain activity in top-down control of visual-spatial attention. PLoS Biol. 2007;5:0114–0126. doi: 10.1371/journal.pbio.0050012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S, Aslan A, Staudigl T, Klimesch W, Herrmann CS, Bauml KH. Prestimulus oscillations predict visual perception performance between and within subjects. Neuroimage. 2007;37:1465–1473. doi: 10.1016/j.neuroimage.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Harter MR, Anllo-Vento L, Wood FB. Event-related potentials, spatial orienting, and reading disabilities. Psychophysiology. 1989;26:404–421. doi: 10.1111/j.1469-8986.1989.tb01943.x. [DOI] [PubMed] [Google Scholar]
- Hopf JM, Mangun GR. Shifting visual attention in space: an electrophysiological analysis using high spatial resolution mapping. Clin Neurophysiol. 2000;111:1241–1257. doi: 10.1016/s1388-2457(00)00313-8. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Jasiukaitis P, Hakerem G. The effect of prestimulus alpha activity on the P300. Psychophysiology. 1988;25:157–165. doi: 10.1111/j.1469-8986.1988.tb00979.x. [DOI] [PubMed] [Google Scholar]
- Johannes S, Munte TF, Heinze HJ, Mangun GR. Luminance and spatial attention effects on early visual processing. Brain Res Cogn Brain Res. 1995;2:189–205. doi: 10.1016/0926-6410(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Jongen EM, Smulders FT, van Breukelen GJ. Varieties of attention in neutral trials: linking RT to ERPs and EEG frequencies. Psychophysiology. 2006;43:113–125. doi: 10.1111/j.1469-8986.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. The neural basis of biased competition in human visual cortex. Neuropsychologia. 2001;39:1263–1276. doi: 10.1016/s0028-3932(01)00116-6. [DOI] [PubMed] [Google Scholar]
- Kelly SP, Gomez-Ramirez M, Foxe JJ. The strength of anticipatory spatial biasing predicts target discrimination at attended locations: a high-density EEG study. Eur J Neurosci. 2009;30:2224–2234. doi: 10.1111/j.1460-9568.2009.06980.x. [DOI] [PubMed] [Google Scholar]
- Kelly SP, Lalor EC, Reilly RB, Foxe JJ. Increases in alpha oscillatory power reflect an active retinotopic mechanism for distracter suppression during sustained visuospatial attention. J Neurophysiol. 2006;95:3844–3851. doi: 10.1152/jn.01234.2005. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res Brain Res Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Kok A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology. 2001;38:557–577. doi: 10.1017/s0048577201990559. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- McDonald JJ, Green JJ. Isolating event-related potential components associated with voluntary control of visuo-spatial attention. Brain Res. 2008;1227:96–109. doi: 10.1016/j.brainres.2008.06.034. [DOI] [PubMed] [Google Scholar]
- Medendorp WP, Goltz HC, Vilis T. Remapping the remembered target location for anti-saccades in human posterior parietal cortex. J Neurophysiol. 2005;94:734–740. doi: 10.1152/jn.01331.2004. [DOI] [PubMed] [Google Scholar]
- Medendorp WP, Kramer GF, Jensen O, Oostenveld R, Schoffelen JM, Fries P. Oscillatory activity in human parietal and occipital cortex shows hemispheric lateralization and memory effects in a delayed double-step saccade task. Cereb Cortex. 2007;17:2364–2374. doi: 10.1093/cercor/bhl145. [DOI] [PubMed] [Google Scholar]
- Min BK, Herrmann CS. Prestimulus EEG alpha activity reflects prestimulus top-down processing. Neurosci Lett. 2007;422:131–135. doi: 10.1016/j.neulet.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Praamstra P. The five percent electrode system for high-resolution EEG and ERP measurements. Clin Neurophysiol. 2001;112:713–719. doi: 10.1016/s1388-2457(00)00527-7. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G. Functional brain imaging based on ERD/ERS. Vision Res. 2001;41:1257–1260. doi: 10.1016/s0042-6989(00)00235-2. [DOI] [PubMed] [Google Scholar]
- Platt ML, Glimcher PW. Responses of intraparietal neurons to saccadic targets and visual distractors. J Neurophysiol. 1997;78:1574–1589. doi: 10.1152/jn.1997.78.3.1574. [DOI] [PubMed] [Google Scholar]
- Posner MI, Snyder CR, Davidson BJ. Attention and the detection of signals. J Exp Psychol. 1980;109:160–174. [PubMed] [Google Scholar]
- Praamstra P, Boutsen L, Humphreys GW. Frontoparietal control of spatial attention and motor intention in human EEG.J. Neurophysiol. 2005;94:764–774. doi: 10.1152/jn.01052.2004. [DOI] [PubMed] [Google Scholar]
- Price GW. The effect of pre-stimulus alpha activity on the auditory P300 paradigm: a prospective study. Brain Topogr. 1997;9:169–176. doi: 10.1007/BF01190386. [DOI] [PubMed] [Google Scholar]
- Ress D, Backus BT, Heeger DJ. Activity in primary visual cortex predicts performance in a visual detection task. Nat Neurosci. 2000;3:940–945. doi: 10.1038/78856. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L, Desimone R. Competitive mechanisms subserve attention in macaque areas V2 and V4. J Neurosci. 1999;19:1736–1753. doi: 10.1523/JNEUROSCI.19-05-01736.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rihs TA, Michel CM, Thut G. Mechanisms of selective inhibition in visual spatial attention are indexed by alpha-band EEG synchronization. Eur J Neurosci. 2007;25:603–610. doi: 10.1111/j.1460-9568.2007.05278.x. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Stadler W, Schabus M, Doppelmayr M, Hanslmayr S, Gruber WR, Birbaumer N. A shift of visual spatial attention is selectively associated with human EEG alpha activity. Eur J Neurosci. 2005;22:2917–2926. doi: 10.1111/j.1460-9568.2005.04482.x. [DOI] [PubMed] [Google Scholar]
- Thut G, Nietzel A, Brandt SA, Pascual-Leone A. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci. 2006;26:9494–9502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenner MU, Heekeren HR, Bauer M, Rossner K, Wenzel R, Villringer A, Fahle M. What happens in between? Human oscillatory brain activity related to crossmodal spatial cueing. PLoS ONE. 2008;3:e1467. doi: 10.1371/journal.pone.0001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lubbe RH, Neggers SF, Verleger R, Kenemans JL. Spatiotemporal overlap between brain activation related to saccade preparation and attentional orienting. Brain Res. 2006;1072:133–152. doi: 10.1016/j.brainres.2005.11.087. [DOI] [PubMed] [Google Scholar]
- Van der Stigchel S, Heslenfeld DJ, Theeuwes J. An ERP study of preparatory and inhibitory mechanisms in a cued saccade task. Brain Res. 2006;1105:32–45. doi: 10.1016/j.brainres.2006.02.089. [DOI] [PubMed] [Google Scholar]
- Vanni S, Revonsuo A, Hari R. Modulation of the parieto-occipital alpha rhythm during object detection. J Neurosci. 1997;17:7141–7147. doi: 10.1523/JNEUROSCI.17-18-07141.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldorff MG, Hazlett CJ, Fichtenholtz HM, Weissman DH, Dale AM, Song AW. Functional parcellation of attentional control regions of the brain. J Cogn Neurosci. 2004;16:149–165. doi: 10.1162/089892904322755638. [DOI] [PubMed] [Google Scholar]
- Worden MS, Foxe JJ, Wang N, Simpson GV. Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha-band electroencephalography increases over occipital cortex. J Neurosci. 2000;20:1–6. doi: 10.1523/JNEUROSCI.20-06-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi N, Callan DE, Anderson SJ, Kawato M. Attentional changes in pre-stimulus oscillatory activity within early visual cortex are predictive of human visual performance. Brain Res. 2008;1197:115–122. doi: 10.1016/j.brainres.2007.12.063. [DOI] [PubMed] [Google Scholar]
- Yamagishi N, Goda N, Callan DE, Anderson SJ, Kawato M. Attentional shifts towards an expected visual target alter the level of alpha-band oscillatory activity in the human calcarine cortex. Brain Res Cogn Brain Res. 2005;25:799–809. doi: 10.1016/j.cogbrainres.2005.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.