Abstract
Growing consensus suggests that autism spectrum disorders (ASD) are associated with atypical brain networks, thus shifting the focus to the study of connectivity. Many functional connectivity studies have reported underconnectivity in ASD, but results in others have been divergent. We conducted a survey of 32 functional connectivity magnetic resonance imaging studies of ASD for numerous methodological variables to distinguish studies supporting general underconnectivity (GU) from those not consistent with this hypothesis (NGU). Distinguishing patterns were apparent for several data analysis choices. The study types differed significantly with respect to low-pass filtering, task regression, and whole-brain field of view. GU studies were more likely to examine task-driven time series in regions of interest, without the use of low-pass filtering. Conversely, NGU studies mostly applied task regression (for removal of activation effects) and low-pass filtering, testing for correlations across the whole brain. Results thus suggest that underconnectivity findings may be contingent on specific methodological choices. Whereas underconnectivity reflects reduced efficiency of within-network communication in ASD, diffusely increased functional connectivity can be attributed to impaired experience-driven mechanisms (e.g., synaptic pruning). Both GU and NGU findings reflect important aspects of network dysfunction associated with sociocommunicative, cognitive, and sensorimotor impairments in ASD.
Keywords: autism spectrum disorder, functional connectivity MRI, functional networks, underconnectivity, BOLD synchronization
Introduction
Neuroscientific studies of autism spectrum disorders (ASD) have accumulated an almost infinite wealth of empirical data in the past few decades. Despite many complexities and inconsistencies in this literature, it has become abundantly clear that ASD is not a localized brain disorder, but a disorder involving multiple functional networks (Geschwind and Levitt 2007; Müller 2007; Rippon et al. 2007). Neuroimaging studies of ASD have therefore increasingly focused on connectivity analysis. While the number of conventional functional magnetic resonance imaging (fMRI) studies aiming to localize task-evoked blood oxygen level–dependent (BOLD) effects has continued to grow, complementary implementations of the fMRI technique to examine functional cooperation between brain regions have become more common. These approaches are loosely held together by the term “functional connectivity MRI” (fcMRI), despite many methodological divergences that will be discussed below.
Several reviews on the neurobiology of ASD have focused on functional connectivity. Belmonte et al. (2004) suggested a combination of reduced long distance but increased local connectivity in ASD. This idea was further developed in a more extensive review by Rippon et al. (2007), who speculated that disordered long-distance connectivity may be accompanied by “noisy” processing at the local level. This may, in turn, relate to reports of increased density of cortical minicolumns with reduced lateral inhibition (Casanova and Trippe 2009) and other biological and genetic findings that suggest an increased cortical excitation/inhibition ratio in ASD (Rubenstein and Merzenich 2003). With respect to impaired long-distance connectivity, the hypothesis is supported by anatomical MRI findings of aberrant white matter growth patterns in the first few years in ASD (Courchesne et al. 2001; Sparks et al. 2002; Hazlett et al. 2005) and reduced white matter integrity later in life, as reported in several diffusion tensor imaging (DTI) studies (Alexander et al. 2007; Cheung et al. 2009; Fletcher et al. 2010; Shukla et al. 2011). Reviewing anatomical and functional imaging findings, Hughes (2007) considered underconnectivity as a potential “first firm finding” on ASD. However, with the growing number of fcMRI studies published in the past few years, it appears timely to reconsider the question of how firm the finding truly is.
At first glance, underconnectivity appears to be supported by a large set of empirical results. Based on their findings of reduced synchronization of the BOLD signal associated with sentence comprehension between a number of regions of interest (ROIs), Just et al. (2004) first formulated an “underconnectivity theory” and proposed that “autism is a cognitive and neurobiological disorder caused by underfunctioning integrative circuitry” (p. 1817). The proposal actually contains two separate claims, relating to 1) the empirical validity of the ‘underconnectivity’ theory and 2) the causal role of such underconnectivity in the development of the disorder. The present survey will focus on a thorough evaluation of the first claim, although we will return to the question of causality in the Discussion.
Subsequent to this original study and the formal underconnectivity proposal, the theory has found broad support in many fcMRI studies (Just et al. 2004, 2007; Villalobos et al. 2005; Bird et al. 2006; Cherkassky et al. 2006; Kana et al., 2006, 2007, 2009; Kennedy and Courchesne 2008; Kleinhans et al. 2008; Koshino et al. 2008; Mason et al., 2008; Lee et al. 2009; Mostofsky et al. 2009; Solomon et al. 2009; Damarla et al. 2010; Lombardo et al., 2010; Weng et al., 2010). Given the number of these studies and placement in high-impact journals, it may be easily overlooked that a significant number of other studies have reported mixed or increased fcMRI effects in ASD (Koshino et al. 2005; Welchew et al. 2005; Mizuno et al. 2006; Turner et al. 2006; Wicker et al. 2008; Monk et al. 2009; Noonan et al. 2009; Shih et al. 2010).
Empirical inconsistencies are not new in neuroscientific research on ASD. In many cases, this has been attributed to heterogeneity of the disorder, often coupled with small sample sizes, or with a lack of stringent diagnostic criteria. While such clinical issues may play a role, methodological factors cannot be ruled out. Thai et al. (2009), although not explicitly contradicting the underconnectivity view, raised several methodological concerns, including differences in response to tasks applied during fMRI scanning between ASD and control groups, details of ROI selection, as well as limits in spatial and temporal resolution. Jones et al. (2010) systematically examined effects of task regression in a data set acquired during different overt word generation conditions. They found underconnectivity between numerous ROI pairs for task-driven effects in their ASD group, which disappeared almost entirely when effects of task were removed, especially when each of their task conditions were modeled by separate regressors (presumably leaving only minimal residual task effects). Jones et al. (2010) also suggested that inverse findings of “overconnectivity” in ASD may relate to global signal regression, that is, removal of whole-brain signal fluctuations across time points.
The present survey attempts to elucidate the reasons for inconsistencies in the fcMRI literature in ASD, based on a comprehensive tabulation of methodological differences between studies. However, as will become clear below, the implications of these inconsistencies reach well beyond the methodological realm and relate to known or suspected patterns of neurodevelopmental disturbances in ASD. Rather than simply being a nuisance, the fact that not all fcMRI studies have been able to replicate underconnectivity is therefore an opportunity for an improved understanding of the disturbances in emerging functional networks, which ultimately determine the profile of socio-communicative and other impairments commonly seen in ASD.
Methods
We identified 32 fcMRI studies in ASD through PubMed searches (as listed in Table 1). The cutoff date for inclusion was 4 November 2010. Each publication was examined for a large number of methodological variables and for results, with each study being reviewed at least twice by two co-authors who were blind to each other's reviews. Any inconsistencies between reviewers were resolved through repeated close reading of respective journal articles. For practical purposes, we refrained from any attempts to obtain additional methodological information not stated in published articles from authors. The variables included in this survey are described in detail below.
Table 1.
Selected methodological variables by study type
| Study | Low-pass filter | Global signal removalbased on | Task regression selection | Seed selection based on | Whole-brain field of view |
| GU: Studies supporting general underconnectivity | |||||
| Anderson et al. (2010) | Yes | No | Noa | (anat)b | No |
| Assaf et al. (2010) | ns | No | Noa | aCOMB | No |
| Bird et al. (2006) | No | ns | No | aCOMB | No |
| Cherkassky et al. (2006) | No | No | No | aCOMB | No |
| Damarla et al. (2010) | ns | ns | No | aCOMB | No |
| Jones et al. (2010): M1 | Yes | No | No | aCOMB+aTD | No |
| Jones et al. (2010): M2/3 | Yes | No | Yes | aCOMB+aTD | No |
| Just et al. (2004) | ns | ns | No | aTD (litTD) | No |
| Just et al. (2007) | Yes | ns | No | aCOMB | No |
| Kana et al. (2006) | Yes | ns | No | aCOMB | No |
| Kana et al. (2007) | Yes | ns | No | aCOMB | No |
| Kana et al. (2009) | No | Yes | No | aCOMB | No |
| Kennedy and Courchesne (2008) | Yes | Yes | Noa | aTD | No |
| Kleinhans et al. (2008) | No | ns | No | aCOMB | Yes |
| Koshino et al. (2008) | Yes | ns | No | aCOMB | No |
| Lee et al. (2009) | No | No | No | aCOMB | No |
| Lombardo et al. (2010) | No | Yes | No | aTD (litTD) | No |
| Mason et al. (2008) | No | ns | No | aCOMB | No |
| Mostofsky et al. (2009) | No | ns | No | aCOMB | No |
| Solomon et al. (2009) | No | ns | No | aTD | No |
| Villalobos et al. (2005) | No | ns | Yes | anat | Yes |
| Weng et al. (2010) | Yes | ns | Noa | anat (litTD) | No |
| NGU: Studies inconsistent with general underconnectivity | |||||
| Agam et al. (2010)c | Yes | ns | No | anat+aCOMB | Yes |
| Ebisch et al. (2010)c | Yes | Yes | Noa | litTD+aTD | Yes |
| Jones et al. (2010): M3 + GSR | Yes | Yes | Yes | aCOMB+aTD | No |
| Koshino et al. (2005) | ns | ns | No | anat | No |
| Mizuno et al. (2006) | Yes | No | Yes | anat | Yes |
| Monk et al. (2009) | Yes | ns | Noa | aTD (litTD) | No |
| Noonan et al. (2009) | Yes | No | Yes | aCOMB | Yes |
| Shih et al. (2010) | Yes | No | Yes | litTD+anat | Yes |
| Turner et al. (2006) | Yes | No | Yes | anat | Yes |
| Welchew et al. (2005) | ns | ns | No | anat | Yes |
| Wicker et al. (2008) | ns | No | No | litTD+aTD | No |
Note: aCOMB, activation in TD and ASD groups combined; anat, anatomical landmarks. aTD, activation in TD group; litTD, expected activation site based on TD literature; ns, not stated.
Study used resting-state data (see Results for explanation why these were coded as non–task regressed).
Study tested for fcMRI effects for each anatomical brain voxel and its contralateral homolog.
Study graphically reports mixed fcMRI effects (both TD > ASD and ASD > TD).
Samples
For each group (ASD, typically developing [TD]), sample size, age, and IQ scores were entered and group-matching criteria were noted. For the ASD group, diagnostic tools were stated, including Diagnostic Statistical Manual of Mental Disorders (American Psychiatric Association 2000), Autism Diagnostic Observation Schedule (Lord et al. 1999), Autism Diagnostic Interview – Revised (Rutter et al., 2003), Childhood Autism Rating Scale (Schopler et al. 1980), and International Classification of Diseases (ICD; ICD-10, 1994), as well as sample composition with regard to differential diagnoses (autistic disorder, Asperger's disorder, Pervasive Developmental Disorder–Not Otherwise Specified).
Conditions
All experimental and control conditions were entered based on description provided by authors (i.e., no critical review of the adequacy of cognitive terminology was attempted). If all data were acquired during rest, this was considered the experimental condition. Design features, such as blocked versus event-related fMRI, were also entered. For blocked data sets, it was noted whether all or only selected types of blocks were included in fcMRI analyses.
Data Acquisition
Basic functional image acquisition parameters, such as repetition time (TR), voxel size, and number of time points, were entered. If data were acquired across different runs and concatenated for analysis, this was also noted.
Seed Identification and Field of View
We determined whether fcMRI seeds were identified based on activation results for the given data set (or results imported from other studies), or whether they were based on anatomical criteria. For activation-derived seeds, we further noted whether these were based on activation observed in the TD group, in the ASD group, in both groups pooled together, or on effects of significant activation differences between groups (TD>ASD or ASD>TD).
The field of view (FOV) specifies the search space for connectivity effects with a given seed. We entered whether the FOV was limited to activation-derived ROIs (using the specific distinctions described above) or to anatomically determined ROIs; or whether fcMRI statistics were performed for all other brain voxels (i.e., the maximal search space).
Preprocessing and Statistics
Two questions concerning head motion were considered. First, was head motion computed for each group and was a statistical test of potential group differences performed? Second, were motion time series used as orthogonal regressors (nuisance variables), and if so, in what way (e.g., a covariate at the group level or regressors at the single-subject level for each of the six translational and rotational axes; use of temporal derivatives)? These motion-related steps are relevant in fcMRI analyses, in at least two ways: 1) Residual unaccounted motion may result in artifactual correlations (and anticorrelations) of time series throughout the brain; and 2) motion (even when fully corrected) may result in noisy or washed-out time series in seeds or ROIs from signal interpolation during spatial realignment, which may reduce detection of true correlations.
We further entered whether any temporal filtering (high, low, or band-pass) was performed prior to fcMRI analyses. Whereas high-pass filtering serves the removal of low-frequency drift and other noise, low-pass filtering may be considered crucial for the detection of low-frequency fluctuations that have been shown to reflect network-specific intrinsic (i.e., non task-driven) connectivity most robustly (Biswal et al. 1995; Cordes et al. 2001; Fox and Raichle 2007). It was also noted whether global signal regression (or global intensity normalization) was performed, that is, the removal of effects associated with whole-brain signal fluctuations across time points within a time series. Global signal regression has been shown to result in the detection of negative correlations, for example, in studies of task-negative (or “default mode”) networks (Fox et al. 2009; Van Dijk et al. 2010), which may in turn affect comparisons between ASD and TD groups (Jones et al. 2010). A recent study by Schölvinck et al. (2010) suggests that global low-frequency fluctuations of the BOLD signal are partly accounted for by correlated fluctuations in local field potentials. Aside from further supporting the neural basis of low-frequency BOLD fluctuations, this finding suggests that global signal regression may remove true fluctuations in neuronal activity, resulting in potentially artifactual detection of interregional anticorrelations (cf. debate in Murphy et al. 2009 vs. Fox et al. 2009).
Furthermore, it was determined whether task effects (in studies using task paradigms) were removed and what the precise procedure for the removal of task effects was. We distinguished between gross removal of task effects (using only a single regressor for several conditions) as opposed to fine removal of task effects (using separate regressors modeling each task condition). This latter distinction corresponds to the one made by Jones et al. (2010) between their methods “M2” and “M3.” Inclusion or removal of task effects in fcMRI analyses is important because it determines whether detected correlations are primarily driven by activation (response to stimuli or task trials) or intrinsically generated (in the absence of task effects). Any additional preprocessing feature, for example, the use of physiological regressors, was also entered.
The type of single-subject analysis (e.g., regression) and the type of within- and between-group statistic (e.g., t-test) was entered. Some fundamental differences in statistical approaches, such as regression, structural equation modeling, or dynamic causal modeling, relate to other variables considered separately. For example, dynamic causal modeling typically implies the detection of task-related effects (Lee et al., 2006), as discussed above. Further statistical details, such as Fisher's r-to-z′ conversion (for comparing results from first level single-subject analyses in group level statistical tests) and the correction for multiple comparisons, were entered separately. Finally, we also noted whether negative correlations were observed in single-subject analyses and whether such potential negative correlations were included or discarded in analyses at the group level.
Findings and Definition of Study Types
Our goal was to elucidate potential methodological reasons for diverse findings in overall patterns of connectivity. Therefore, we did not attempt to enter findings comprehensively by listing all regions with significant between group differences as this was beyond the scope of our survey. We restricted our tabulation to an abridged characterization of findings of significantly greater fcMRI effects in TD compared with ASD groups (TD>ASD) and inverse findings (ASD>TD). Based on the overall pattern of findings from direct group comparisons, we assigned each study to one of the two types. Studies that exclusively reported effects of greater functional connectivity in TD compared with ASD groups were coded as “GU studies,” that is, studies consistent with a model of general underconnectivity (GU) in ASD (see Table 1, top). Note that this does not imply significant underconnectivity findings for every single pair of ROIs in these studies. For example, Just et al. (2004) found significantly reduced functional connectivity for only 10 of 186 ROI pairs but reported no single ROI pair with inverse findings. Conversely, studies with mixed or predominantly inverse effects (ASD > TD) were coded as “NGU studies,” that is, studies that were not consistent with GU (Table 1, bottom). One study (Brieber et al. 2010) was excluded from the listing in Table 1 and all quantitative analyses because it did not report any significant fcMRI group differences and could thus not be assigned to a study type. Two studies (Agam et al. 2010; Ebisch et al. 2010) were classified as NGU studies, although they presented their findings with exclusive focus on underconnectivity effects in ASD. However, both of these graphically presented inverse effects of greater connectivity in ASD, without discussing them (see figure 5A in Agam et al., 2010 and figures. 2–5 in Ebisch et al., 2010). The study by Jones et al. (2010), which used several methodological approaches (with different results) applied to the identical data set, was counted as three separate studies (i.e., method 1 without task regression and methods 2 and 3 with task regression as two GU studies, and method 3 with task and global signal regression as NGU study; cf. Supplementary Table 1). Table 1 thus lists a total of 22 GU studies and 11 NGU studies.
Results
A comprehensive matrix of methodological details for each study is presented in Supplementary Table 1. A first pass examination of the data showed that for many methodological variables, systematic differences between the two types of studies were unlikely. Among the demographic variables, both types included predominantly adults and adolescents, and IQs for ASD cohorts were in the normal range in both types. Task conditions varied greatly, but both types included sensorimotor as well as complex cognitive tasks. The only condition for which multiple studies from different groups were available was rest. Four of these had GU results (consistent with GU), two reported NGU results (not consistent with GU). Data acquisition parameters also varied somewhat across studies, but type-specific differences were not apparent. For example, most studies of both types used block designs. TR, which determines temporal resolution and thus the frequency range at which correlated oscillations can be detected, was between 1000 and 3000 ms in both types of studies.
Given the obvious impact of head motion on functional connectivity effects (Auer 2008; Weissenbacher et al. 2009), as described above, it was surprising to find that 19 of the 32 studies made no statement about this potential confound at all. Only four studies clearly stated the absence of significant group differences in motion.
While differences in participant characteristics, study conditions, and data acquisition were unlikely to be major contributors to overall differences in fcMRI results, more distinct patterns of differences between the two types were seen for several parameters related to preprocessing and statistical analysis. These are summarized in Table 1. Global signal removal is included here solely based on the findings from Jones et al. (2010). Information in Table 1 has been simplified for greater clarity (for details, see Supplementary Table 1). Results for each of these parameters are described below. Information was not always available from each study and numbers of included studies are stated for each parameter. As explained above, the study by Jones and colleagues was counted three times, based on its different methods applied.
Low-pass Filtering
This parameter was coded solely with respect to the question of whether low-pass or band-pass filtering was applied to specifically focus on low-frequency fluctuations < .1 Hz based on studies showing that network-specific intrinsic functional connectivity is predominantly detected in BOLD time series in the range of 0.01 < f < 0.1 Hz (Biswal et al. 1995; Cordes et al. 2001). The use of high-pass filters in many studies serves a different purpose (removal of drift and other noise at even lower frequencies, typically <0.01 Hz) and lends itself to conflation of low-frequency intrinsic fluctuations and task activation effects occurring predominantly at slightly higher frequencies.
Information was available for 27 out of 33 studies. Among 22 GU studies, 9 used low-pass filters, whereas 10 studies applied high-pass filters. All of the eight NGU studies, for which temporal filtering information was explicitly stated, used low-pass filtering.
Global Signal Removal
Only 16 studies overall provided explicit information regarding global signal removal. Three of nine GU studies and two of seven NGU studies performed global signal regression or some other procedure to remove effects of global signal changes.
Task Regression
Among the 18 GU studies that applied tasks, 16 took no measures to remove activation effects driven by a task paradigm. Two studies used orthogonal regressors to remove modeled task effects. Four further studies used resting-state data. Task regression is logically unfeasible in studies that lack controlled experimental conditions. However, it is understood that the resting state is associated with distinct mental activity (Mason et al. 2007), which is in principle uncontrolled and hard to monitor. In TD individuals, such mental activity is considered to correspond to the “default mode” (Raichle et al., 2001; Greicius et al., 2003; Fransson, 2005; Greicius et al., 2009), but this assumption cannot necessarily be made for participants with ASD (Kennedy et al., 2006). Since regression of activity changes related to cognitive processing is impracticable in resting-state studies, we coded these as non task-regressed. Among the nine NGU studies that applied tasks, five performed task regression. Two further studies used resting-state data.
Seed Selection
Of the 22 GU studies, 15 selected fcMRI seeds based on activation effects detected in both TD and ASD groups, coded as “aCOMB” in Table 1. Four studies, used seeds solely identified from activation analyses in the TD group (aTD), and three studies identified seeds based on anatomical criteria (including the study by Anderson et al., 2010, which tested BOLD correlations for every single brain voxel and its homolog in the contralateral hemisphere). In one of these (Weng et al. 2010), anatomy-based seed location was informed by the TD literature on expected domain-specific activation effects (coded “litTD” in Table 1). Of the 11 NGU studies, three used activation effects from TD and ASD groups combined, four used seeds derived from TD activation, and four applied purely anatomical criteria.
For data reduction, seeds were further coded with respect to possible bias toward TD or ASD groups. No distinct bias in favor of ASD participants was found in any study. Five of the 22 GU studies and 4 of 11 NGU studies used seeds that were distinctly biased in favor of TD groups (either aTD or litTD). A less conservative threshold for potential TD bias was used in a secondary comparison, including studies coded as aCOMB. As discussed in detail below, the reasoning was that activation effects detected for both TD and ASD groups may be predominantly driven by effects in TD groups that are less affected by interindividual variability. Results for the stringent and the less conservative definition of TD bias are presented separately in Figure 1.
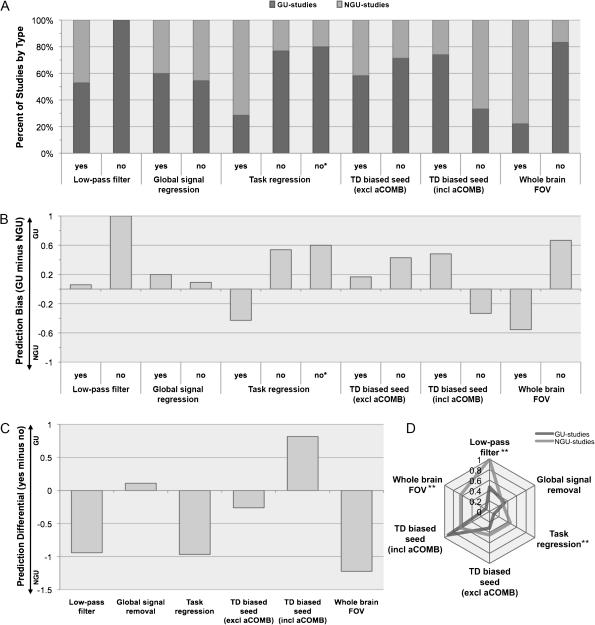
Figure 1.
(A) Fraction of studies by type for each selected methodological choice. (B) Prediction bias, calculated as the difference between study types (fraction GU − fraction NGU). (C) Prediction differential, calculated as the difference between Yes and No options for each methodological choice. (D) Radar plots for two types of studies showing differences in methodological fingerprints. *Excluding resting studies. **Significant difference between study types. For further details, see text.
Field of View
Among the 22 GU studies, only two reported fcMRI effects for the whole brain. The remaining 20 studies reported effects only for a limited number of ROIs. This pattern was different for the 11 NGU studies, 7 of which tested for fcMRI effects in the whole brain. The study by Welchew et al. (2005) is included among these, because the 90 ROIs used in their analyses covered the entire cerebrum and several subcortical structures. Four of the NGU studies did not report whole-brain findings.
Data Reduction and Visualization
Further data reduction was attempted to highlight the main patterns of differences between studies of the two types. In Figure 1A, the fraction of studies assigned to each type are shown, calculated separately for each of the main methodological parameters described above. For example, of the 17 studies using low-pass filtering, 9 were GU studies and 8 were NGU studies. Note that most fractions are higher for GU than for NGU studies, for the simple reason that overall more GU than NGU studies have been published. However, three exceptions become apparent, with greater fractions for NGU studies. These are the use of task regressors, the strict exclusion of seeds that could be biased in favor of TD groups (including those coded as aCOMB), and the use of a whole brain FOV.
In Figure 1B, these fractions are collapsed into difference scores (fraction GU minus fraction NGU) for each methodological choice. For example, whereas the choice of low-pass filtering implies little prediction bias, the choice of not using low-pass filtering is strongly predictive in favor of GU results. The latter reflects the finding that all studies that explicitly opted against low-pass filtering reported GU findings (cf. Fig. 1A). Aside from lack of low-pass filtering, the methods choices most strongly predictive of GU findings were lack of whole-brain FOV and lack of task regression. Conversely, removal of task activation effects and detection of fcMRI effects in the whole brain (rather than only in ROIs) were predictive of NGU findings.
We further calculated a prediction differential for each methodological parameter by subtracting the prediction bias scores from Figure 1B for each “No” option from those for the corresponding “Yes” option. For example, the prediction score of 1.0 for “low-pass filter no” was subtracted from the prediction score of 0.06 for “low-pass filter yes,” for a prediction differential of −0.94. As seen in Figure 1C, the use of seeds that were predominantly based on activation effects in TD groups (when studies coded as aCOMB were included, see Discussion) showed potential bias in favor of GU findings, whereas low-pass filtering, task regression, and whole-brain FOV favored NGU findings.
In Figure 1D, differences in methodological fingerprints between GU and NGU studies are depicted in a radar plot, representing the fraction of studies applying the six types of methodological choices of interest, calculated separately for each study type. Note that fractions are thus calculated differently from those shown in Figure 1A. For example, 7 of 11 NGU studies (64%) used a whole-brain FOV, whereas this was the case in only 2 of 22 GU studies (9%). The radar plot provides a different perspective on the data, highlighting that the two types of findings (GU vs. NGU) are indeed associated with substantially different methodological profiles.
Statistical Analysis
A Fisher's Exact test was used to examine the frequency of each methodological decision for each study outcome (as shown in Fig. 1D). NGU studies differed significantly (P < 0.05) from GU studies for low-pass filtering, task regression (with and without resting-state studies included), and whole-brain FOV. There was no difference between the study types for global signal regression (P = 1) or TD-biased seed selection (P = .47).
Discussion
We tabulated a large number of methodological variables for 32 fcMRI studies in ASD published between 2004 and November 2010. We first grossly separated studies based on overall patterns of findings, distinguishing those consistent with the hypothesis of general underconnectivity (GU) (Just et al. 2004), and those that were not (NGU). GU studies reported exclusively greater fcMRI effects in TD than in ASD groups, while those with mixed or predominantly inverse findings (ASD > TD) were labeled NGU studies. First inspection of the complete data summarized in Supplementary Table 1 showed that many demographic and methodological variables were unlikely to provide clues as to the inconsistencies in findings between the two study types. In an attempt to highlight the most likely factors, we focused on four parameters for which distinguishing patterns were apparent. These were low-pass filtering (isolating BOLD fluctuations <0.1 Hz), regression of task-driven activation effects, use of seeds explicitly or potentially biased toward activation in TD groups, and inclusion of the whole brain (rather than ROIs) in tests of fcMRI effects.
Global signal removal was further included as a variable of interest in Table 1 based on a recent study by Jones et al. (2010), which suggested that global signal regression might be an explanatory factor in findings of partially greater connectivity in ASD compared with TD groups. However, while only 16 studies provided explicit information in this respect, even these limited results suggest that global signal regression or similar procedures may have little consistent effect on the patterns of results. The findings by Jones et al. (2010) could be related to the use of a data set acquired during overt speech with unusually short 10-s blocks, which differs substantially from other fcMRI studies and may therefore limit the general interpretation of results. In addition, despite their thorough and systematic approach Jones et al. did not manipulate many of the methodological variables considered in the present survey. For example, most of their results were derived from analyses limited to seeds and ROIs based on detected or expected activation effects.
Treatment of head motion was not included as a variable of interest in Table 1 simply because of a lack of disclosure in the literature. In 28 of 32 studies, no statement regarding statistical tests to ascertain absence of group differences in head motion was found. This was surprising, given the obvious potential for head movement to affect fcMRI results (Auer 2008; Weissenbacher et al. 2009). Note that an appearance of reduced connectivity could in principle be exclusively explained by greater motion in one group compared with another. Since participants with ASD may tend to move more during fMRI scanning than their TD peers, the fact that this obvious confound was not addressed in most studies is troubling. However, one may hope that in many cases this reflects a failure of disclosure rather than a true methodological flaw.
Several steps of further data reduction allowed us to identify which methodological choices most strongly biased findings one way or the other. Absence of low-pass filtering and task regression as well as failure to test for fcMRI effects everywhere in the brain were the three choices that tended to be associated with findings consistent with GU (Fig. 1B). More specifically, all studies that explicitly opted against the use of low-pass filters presented findings of the GU type (only TD > ASD effects). Conversely, every single study combining low-pass filtering, task regression, and whole-brain FOV reported NGU findings, with mixed effects or even predominantly greater functional connectivity in ASD than TD groups. While this suggests a clear pattern of differences between study types (Fig. 1D), it also shows that no single methodological choice uniquely determines the type of fcMRI results (GU vs. NGU). Instead, it is most likely the confluence of several methodological choices that makes the difference. The three variables highlighted here (low-pass filtering, task regression, and FOV) are the most probable factors, based on the available literature. However, it is conceivable that other factors may come to light once a sufficient number of relevant studies become available. For example, the focus on task-driven effects in BOLD time series (in studies that opt against low-pass filtering and task regression) may be most strongly associated with GU findings when task paradigms are used that tap into domains of impairment in ASD.
While study sample size is an issue, especially given that only 11 NGU studies were available, the pattern of results generates, as a working hypothesis, the expectation that future studies opting not to focus on low-frequency fluctuations in the range 0.01 < f < 0.1 Hz through low-pass or band-pass filtering may tend to generate results supporting the GU hypothesis. On the other hand, future studies that implement low-pass filtering, task regression, and whole-brain FOV are more likely to generate findings inconsistent with this hypothesis. A facetious interpretation would imply that each group of researchers may generate the types of results that best fit their preconceived ideas about connectivity in ASD, simply by making a few crucial methodological choices. However, such considerations—while interesting from a methodological point of view—fail to capture the true significance of our findings for the study of connectivity in ASD. We will therefore first briefly discuss the implications of these pivotal methodological variables and then turn to the neurodevelopmental conclusions that can be drawn with respect to functional and anatomical connectivity in ASD.
Task-driven Versus Intrinsic Fluctuations in the BOLD Signal
The preponderance of GU findings in ASD is likely related to the focus on activation-driven correlations in many studies. These are studies that leave intact BOLD changes prompted by task and control conditions. The approach is undoubtedly of interest, but it is important to consider its implications. As suggested by Jones et al. (2010, p.408), fcMRI analyses that do not regress out task effects to isolate intrinsic BOLD fluctuations “simply reflect the differences in task-related response … and whether this should really be called a measure of ‘connectivity’ is debatable.” This comment may be considered radical, implying that 15 of 16 GU studies (not counting the study by Jones et al. themselves as well as four resting studies) that failed to remove task effects may have been mislabeled as “functional connectivity” studies in the strictest sense. From this perspective, almost the entire literature supporting general functional underconnectivity in ASD might be considered misleading because it focuses on task-specific synchronization between brain regions that may or may not reflect underlying connectivity. However, we do not believe that the term “functional connectivity” should be unilaterally usurped by adherents of one or another methodological approach. What is crucial, in our view, is awareness of the impact of methodological choices and a refined interpretation of fcMRI studies in ASD that reconciles diverse findings. While methodological differences between fcMRI studies are surely multifactorial, it appears that the fcMRI literature in ASD can be broadly divided into two main approaches: one that focuses on task-driven effects, which could be called “activation fcMRI,” and one that strives to remove activation effects, which we will call “intrinsic fcMRI.”
Note that intrinsic fcMRI is not synonymous with resting-state fcMRI (Van Dijk et al. 2010). First, as mentioned above, intrinsic BOLD fluctuations can be extracted from data acquired during task performance (i.e., not rest) through task modeling and low-pass filtering (Fair et al. 2007; Fox and Raichle, 2007). Second, the resting state is in reality a highly active state (Mason et al. 2007) and BOLD fluctuations observed for this state may be compounded by cognitive events unless measures (such as low-pass filtering) are taken to minimize their effects.
A second aspect related to the distinction between activation and intrinsic fcMRI concerns temporal filtering of BOLD time series. Low-pass filtering or band-pass filtering at about 0.01 < f < 0.1 Hz can serve two complementary purposes. First, it will further reduce components in BOLD time series related to task processing (i.e., activation effects). This applies even to data sets acquired with blocked designs (as in most current ASD fcMRI studies), where task-control cycles occur at frequencies <0.1 Hz, thus passing typical low-pass filters. However, any fluctuations related to individual trials within blocks or to event-related trials presented at a higher frequency may be attenuated or removed through low-pass filtering. Second, and more crucially, low-pass filtering will isolate or accentuate frequency fluctuations considered to reflect network-specific intrinsic functional connectivity most prominently (Cordes et al. 2001; Fox and Raichle 2007). Although the nature of these slow fluctuations is not completely understood, recent evidence suggests that they may reflect history of regional coactivation and Hebbian effects of plastic changes in network organization (Lewis et al. 2009). They have been furthermore found to coincide with phase-locked oscillations in local field potentials (Leopold et al. 2003; Schölvinck et al. 2010), which may in turn reflect slow fluctuations in spontaneous neurotransmitter release (Fox and Raichle 2007). Electrical recording in nonhuman primates suggests that low-frequency fluctuations correspond to network-specific modulations of higher frequency oscillations (delta, theta, gamma), which implies a hierarchical temporal organization linking low and high frequencies (Lakatos et al. 2005).
A third aspect related to the distinction between activation and intrinsic fcMRI deals with the selection of seeds or ROIs. When fMRI data acquired during task performance are used, a simple and straightforward solution is to identify seeds based on activation clusters. Assuming that the task is designed to tap into the network of interest, the seed can then be considered to reflect a node in this network. This approach may be susceptible to circular logic (Kriegeskorte et al. 2009). Regions that strongly activate together in a TD group for a specific task will trivially tend to be highly correlated with each other. For such ROIs, the activation effects in an ASD group may be less robust. It is therefore possible that the activation-specific components in ROI time series are less distinctive and more variable in an ASD group than in their controls. Such greater variability will in turn most likely result in reduced time series correlations between ROIs.
While the impact of an explicit TD bias on seed selection is thus transparent, it is more debatable in the more common case of seed selection based on activation effects for TD and ASD groups combined (coded aCOMB in Table 1). This was the case in 15 of 22 GU, but only 3 of 11 NGU studies. In this approach, seeds or ROIs are determined either based on activation analyses for both ASD and TD groups pooled together, on activation sites shared by both groups on within-group analyses, or by combining activation clusters seen either in one or the other group. While this procedure does not appear to imply any explicit TD bias that may result in GU-type findings, subtle biases may nonetheless be at work in some instances. Few imaging studies have focused on interindividual variability of activation effects in ASD. Consistent with an earlier fMRI study suggesting atypical spatial variability of activation for a simple motor task in ASD (Müller et al., 2001), Hasson et al. (2009) observed normal intra-individual, but increased interindividual variability of activation associated with complex stimulation (viewing a movie clip) in adult men with ASD. More variable (or otherwise noisy) effects in ASD compared with TD groups may result in overall activation results (from pooled analyses for both groups) reflecting patterns for the TD group more closely than those for the ASD group. In this context, it is further relevant that most fcMRI studies applied tasks in domains suspected to be impaired in ASD. Based on these considerations, a less stringent definition of TD bias (including studies coded as aCOMB) was additionally used in Figure 1. However, we consider the question whether or not such studies may truly imply a TD bias that will affect fcMRI results as unresolved. While it is generally clear from our results shown in Figure 1 that TD-biased seeds may play some role in predicting GU findings, we consider this finding less robust compared with those on low-pass filtering and task regression.
Looking for fcMRI Effects in the Whole Brain
A further methodological parameter that was found to have potential impact on overall patterns of fcMRI findings was the FOV, that is, the search space for the detection of BOLD time series correlations. GU studies almost never reported whole-brain results and instead focused on ROIs, which were usually regions of expected or empirically detected domain-specific activation. In contrast, a whole-brain FOV was used in 7 of 11 NGU studies. Differences in FOV affect the probability of type II error (because of increased need for multiple comparison correction in whole-brain analyses), which could in principle account for a greater likelihood of detecting GU effects in studies limited to ROIs. However, differences in correction factors cannot provide a complete explanation for differences in findings because they cannot account for increased detection of greater functional connectivity in ASD groups in NGU studies with whole brain FOV (and thus higher correction factors).
While the rationale for taking a limited FOV to focus on specific ROIs may be justified by a priori hypotheses, failure to examine whole-brain effects may impede a comprehensive understanding of connectivity. The theoretical importance of using a whole-brain FOV will be discussed in detail below in the context of potentially divergent connectivity patterns within and outside functional networks.
Functional and Anatomical Connectivity: A Developmental Scenario
From the methodological perspective, the above discussion suggests that differential findings of reduced or increased functional connectivity relate to several crucial choices in data processing pathways. However, this does not imply that one pattern of findings is more ‘correct’ than another. Instead, it becomes necessary to integrate differential findings into a theoretical framework that may account for them. We will therefore turn to findings from studies of anatomical connectivity in ASD. Cooperation between distal nodes in functional networks relies on axonal connections. DTI, the most common method for examining anatomical connectivity in vivo, opens a window into white matter microarchitecture by detecting the diffusion of water molecules along axonal tracts (Mori and Zhang 2006). DTI studies for age groups comparable to those studied with fcMRI (i.e., older children, adolescents, and adults) have quite consistently reported reduced fractional anisotropy (FA) in comparison with TD individuals in a variety of white matter regions (Barnea-Goraly et al., 2004; Alexander et al. 2007; Keller et al., 2007; Lee et al. 2007; Cheung et al. 2009), reflecting reduced coherence of axonal tracts or other types of white matter damage. A few studies that also examined other DTI indices found complementary evidence of white matter compromise by detecting increased mean diffusivity and/or radial diffusivity in corpus callosum, arcuate fasciculus, and other regions (Alexander et al. 2007; Lee et al. 2007; Fletcher et al. 2010; Shukla et al., 2010). Converse findings of enhanced FA and reduced MD or radial diffusivity in ASD have been virtually nonexistent in these studies, with one recent exception (Cheng et al. 2010).
Evidence from participants with ASD ages 8 years and upwards therefore overall appears to support the GU hypothesis. However, it is remarkable that a few DTI studies that included younger children reported partially divergent results. Ben Bashat et al. (2007) found increased FA for a number of tracts (including corpus callosum) in a small sample of toddlers with ASD (ages 1.8–3.3 years). Focusing on frontal lobe tracts in children around age 5 years, Sundaram et al. (2008) found reduced FA only for short-range fibers. Long-range fibers, on the contrary, appeared to be intact and greater in length in children with ASD compared with TD children. These divergent findings may be related to brain growth anomalies in ASD. As first reported by Courchesne et al. (2001), brain volume is atypically enlarged in children with ASD around age 2–4 years. This early overgrowth affects both gray and white matter, indicating abnormal trajectories in the development of connectivity early in life. The limited DTI evidence for young children may suggest that early white matter overgrowth could at least in part reflect precocious maturation of axonal fibers. Atypically flat white matter growth at later ages, as also seen by Courchesne et al. (2001), may correspond to diminished myelination, which typically occurs in tandem with mechanisms for cortical maturation, such as synaptic stabilization and pruning (Quartz and Sejnowski, 1997). The DTI evidence, albeit incomplete thus far, suggests that abnormalities of white matter architecture in ASD may differ between early years of overgrowth and older childhood and adulthood. Such a dichotomy would be consistent with a model of precocity of white matter development early in life, followed by an impairment of differentiation of functional networks, which in the TD brain relies on synaptic stabilization and pruning (Kandel et al. 2000).
Projected onto the issue of divergent results in the fcMRI literature, this developmental model generates the following working hypothesis. Reduced network differentiation would be expected to be associated with reduced functional connectivity within neurotypical networks (reflecting diminished constructive processes, such as synaptic stabilization and axonal myelination), which is, however, accompanied by diffusely increased connectivity outside neurotypical networks (reflecting diminished regressive processes, such as synaptic pruning). This working hypothesis, which is overall consistent with the results of our survey, reconciles GU findings from studies that focus on ROIs within networks of interest with apparently divergent NGU findings from studies that included the whole brain in their FOV and therefore also tested for fcMRI effects outside neurotypical networks. Two recent studies (Agam et al. 2010; Ebisch et al. 2010) serve as telling examples. Each of these focused on domain-specific circuits (anterior cingulate cortex/frontal eye fields and insula network, respectively) and detected GU-type findings for these. However, in both studies, figures were presented that actually showed inverse findings of greater connectivity in ASD groups as well (figure 5A in Agam et al. 2010 and figures 2–5 in Ebisch et al. 2010), which were not mentioned in the publications themselves (but confirmed in personal communication with the lead authors). Although these reports were thus presented as GU studies, they belonged in fact to the NGU type (and were assigned correspondingly). The absence of any mention of inverse fcMRI effects (ASD > TD) in these studies may reflect a preconception that only underconnectivity effects are of interest to the field.
In summary, our survey of fcMRI studies in ASD suggests that different methodological approaches may be partly responsible for inconsistent findings. Studies reporting findings in agreement with GU tend to refrain from low-pass filtering and statistical removal of task-related activation effects and to focus on ROIs that are often based on activation sites. On the other hand, studies examining fcMRI effects in the whole brain after implementing low-pass filtering and removal of task-driven variance from BOLD time series tend to have more mixed results, often identifying regions of atypically increased functional connectivity in ASD. Both approaches, here called activation fcMRI and intrinsic fcMRI, may reveal different aspects of abnormal functional networks in ASD. Atypical fcMRI results in ASD—both of the GU and the NGU type—may be outcomes of early aberrations of white matter development and disturbances in experience-driven network formation through regressive and constructive processes, such as synaptic pruning and stabilization, as well as myelination.
Challenges and Perspectives
The wealth of ASD studies published in the past decades has failed to produce a comprehensive understanding of the neurobiological causes of the disorder, which would provide a firm basis for informed therapeutic interventions. Many findings from an overwhelming abundance of studies have remained isolated, unreplicated, or otherwise questionable. Among the few neuroscientific findings that appear solid are those of abnormal white matter growth trajectories and impaired connectivity. However, acceptance of an underconnectivity theory, as widely found in the field, appears primarily based on the sheer number of supportive fcMRI studies in high-impact journals, rather than a careful assessment of the underlying methods and their limitations. Our survey aims to highlight that the findings are more complex and related to methodological choices. The question of functional connectivity in ASD, rather than being definitively answered, as some may believe, still remains to be posed in a clearly defined way. If the question concerns how distal brain regions cooperate during activation in response to a task, a technique we called activation fcMRI would be appropriate. If we are instead asking how spontaneous BOLD fluctuations are synchronized across distal brain regions, presumably as a reflection of stable networks emerging from long-term effects of Hebbian plasticity, the distinctly different approach of intrinsic fcMRI will be required. Finally, if our question regards the anatomical pathways of interconnecting networks, DTI and tractography would be the methods of choice to examine axonal fibers in vivo. None of these approaches is “right” or “wrong,” but awareness of their strengths and weaknesses—and in particular their differential sensitivities (i.e., precisely which neurobiological processes and entities they can or cannot detect)—is needed today in functional connectivity studies of ASD. What is needed for the future will be the methodologically informed use of combined approaches, taking advantage of their partly complementary strengths and weaknesses for a more comprehensive description of connectivity in ASD.
Supplementary Material
Supplementary material can be found at: http://www.cercor. oxfordjournals.org/
Funding
National Institutes of Health (R01-DC006155, R01-MH081023) with additional funding for B.K. (1T32-DC007361-03).
Notes
Conflict of Interest : None declared.
Supplementary Material
References
- Agam Y, Joseph RM, Barton JJ, Manoach DS. Reduced cognitive control of response inhibition by the anterior cingulate cortex in autism spectrum disorders. Neuroimage. 2010;52:336–347. doi: 10.1016/j.neuroimage.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Boudos R, Dubray MB, Oakes TR, Miller JN, Lu J, Jeong EK, McMahon WM, et al. Diffusion tensor imaging of the corpus callosum in Autism. Neuroimage. 2007;34:61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders-IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Anderson JS, Druzgal TJ, Froehlich A, Dubray MB, Lange N, Alexander AL, Abildskov T, Nielsen JA, Cariello AN, Cooperrider JR, et al. Decreased interhemispheric functional connectivity in Autism. Cereb Cortex. 2010 doi: 10.1093/cercor/bhq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer DP. Spontaneous low-frequency blood oxygenation level-dependent fluctuations and functional connectivity analysis of the ‘resting’ brain. Magn Reson Imaging. 2008;26:1055–1064. doi: 10.1016/j.mri.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry. 2004;55:323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24:9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Bashat D, Kronfeld-Duenias V, Zachor DA, Ekstein PM, Hendler T, Tarrasch R, Even A, Levy Y, Ben Sira L. Accelerated maturation of white matter in young children with autism: a high b value DWI study. Neuroimage. 2007;37:40–47. doi: 10.1016/j.neuroimage.2007.04.060. [DOI] [PubMed] [Google Scholar]
- Bird G, Catmur C, Silani G, Frith C, Frith U. Attention does not modulate neural responses to social stimuli in autism spectrum disorders. Neuroimage. 2006;31:1614–1624. doi: 10.1016/j.neuroimage.2006.02.037. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Brieber S, Herpertz-Dahlmann B, Fink GR, Kamp-Becker I, Remschmidt H, Konrad K. Coherent motion processing in autism spectrum disorder (ASD): an fMRI study. Neuropsychologia. 2010;48:1644–1651. doi: 10.1016/j.neuropsychologia.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Casanova M, Trippe J. Radial cytoarchitecture and patterns of cortical connectivity in autism. Philos Trans R Soc Lond B Biol Sci. 2009;364:1433–1436. doi: 10.1098/rstb.2008.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Chou KH, Chen IY, Fan YT, Decety J, Lin CP. Atypical development of white matter microstructure in adolescents with autism spectrum disorders. Neuroimage. 2010;50:873–882. doi: 10.1016/j.neuroimage.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17:1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Cheung C, Chua SE, Cheung V, Khong PL, Tai KS, Wong TK, Ho TP, McAlonan GM. White matter fractional anisotrophy differences and correlates of diagnostic symptoms in autism. J Child Psychol Psychiatry. 2009;50:1102–1112. doi: 10.1111/j.1469-7610.2009.02086.x. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in "resting-state" data. AJNR Am J Neuroradiol. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Damarla SR, Keller TA, Kana RK, Cherkassky VL, Williams DL, Minshew NJ, Just MA. Cortical underconnectivity coupled with preserved visuospatial cognition in autism: evidence from an fMRI study of an embedded figures task. Autism Res. 2010;5:273–279. doi: 10.1002/aur.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisch SJ, Gallese V, Willems RM, Mantini D, Groen WB, Romani GL, Buitelaar JK, Bekkering H. Altered intrinsic functional connectivity of anterior and posterior insula regions in high-functioning participants with autism spectrum disorder. Hum Brain Mapp. 2010 doi: 10.1002/hbm.21085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE. A method for using blocked and event-related fMRI data to study "resting state" functional connectivity. Neuroimage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PT, Whitaker RT, Tao R, Dubray MB, Froehlich A, Ravichandran C, Alexander AL, Bigler ED, Lange N, Lainhart JE. Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. Neuroimage. 2010;51:1117–1125. doi: 10.1016/j.neuroimage.2010.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Avidan G, Gelbard H, Vallines I, Harel M, Minshew N, Behrmann M. Shared and idiosyncratic cortical activation patterns in autism revealed under continuous real-life viewing conditions. Autism Res. 2009;2:220–231. doi: 10.1002/aur.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, Gilmore J, Piven J. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry. 2005;62:1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Autism: the first firm finding = underconnectivity? Epilepsy Behav. 2007;11:20–24. doi: 10.1016/j.yebeh.2007.03.010. [DOI] [PubMed] [Google Scholar]
- ICD-10. International Classification of Diseases. World Health Organization, Geneva; 1994. [Google Scholar]
- Jones TB, Bandettini PA, Kenworthy L, Case LK, Milleville SC, Martin A, Birn RM. Sources of group differences in functional connectivity: an investigation applied to autism spectrum disorder. Neuroimage. 2010;49:401–414. doi: 10.1016/j.neuroimage.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Adam Just M. Atypical frontal-posterior synchronization of Theory of Mind regions in autism during mental state attribution. Soc Neurosci. 2009;4:135–152. doi: 10.1080/17470910802198510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129:2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Minshew NJ, Just MA. Inhibitory control in high-functioning autism: decreased activation and underconnectivity in inhibition networks. Biol Psychiatry. 2007;62:198–206. doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, Jessell TM, Sanes JR. Sensory experience and the fine tuning of synaptic connections. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. New York: Elsevier; 2000. pp. 1115–1130. [Google Scholar]
- Keller TA, Kana RK, Just MA. A developmental study of the structural integrity of white matter in autism. Neuroreport. 2007;18:23–27. doi: 10.1097/01.wnr.0000239965.21685.99. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008;39:1877–1885. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proc Natl Acad Sci U S A. 2006;103:8275–8280. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, Greenson J, Dawson G, Aylward E. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131:1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage. 2005;24:810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Koshino H, Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. fMRI investigation of working memory for faces in autism: visual coding and underconnectivity with frontal areas. Cereb Cortex. 2008;18:289–300. doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos P, Shah AS, Knuth KH, Ulbert I, Karmos G, Schroeder CE. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J Neurophysiol. 2005;94:1904–1911. doi: 10.1152/jn.00263.2005. [DOI] [PubMed] [Google Scholar]
- Lee JE, Bigler ED, Alexander AL, Lazar M, DuBray MB, Chung MK, Johnson M, Morgan J, Miller JN, McMahon WM, et al. Diffusion tensor imaging of white matter in the superior temporal gyrus and temporal stem in autism. Neurosci Lett. 2007;424:127–132. doi: 10.1016/j.neulet.2007.07.042. [DOI] [PubMed] [Google Scholar]
- Lee L, Friston K, Horwitz B. Large-scale neural models and dynamic causal modelling. Neuroimage. 2006;30:1243–1254. doi: 10.1016/j.neuroimage.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Lee PS, Yerys BE, Della Rosa A, Foss-Feig J, Barnes KA, James JD, Vanmeter J, Vaidya CJ, Gaillard WD, Kenworthy LE. Functional connectivity of the inferior frontal cortex changes with age in children with autism spectrum disorders: a fcMRI study of response inhibition. Cereb Cortex. 2009;19:1787–1794. doi: 10.1093/cercor/bhn209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold DA, Murayama Y, Logothetis NK. Very slow activity fluctuations in monkey visual cortex: implications for functional brain imaging. Cereb Cortex. 2003;13:422–433. doi: 10.1093/cercor/13.4.422. [DOI] [PubMed] [Google Scholar]
- Lewis CM, Baldassarre A, Committeri G, Romani GL, Corbetta M. Learning sculpts the spontaneous activity of the resting human brain. Proc Natl Acad Sci U S A. 2009;106:17558–17563. doi: 10.1073/pnas.0902455106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, Sadek SA, Pasco G, Wheelwright SJ, Suckling J, Baron-Cohen S. Atypical neural self-representation in autism. Brain. 2010;133:611–624. doi: 10.1093/brain/awp306. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S. Autism diagnostic observation schedule. Los Angeles (CA): Western Psychological Services; 1999. [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RA, Williams DL, Kana RK, Minshew N, Just MA. Theory of Mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia. 2008;46:269–280. doi: 10.1016/j.neuropsychologia.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno A, Villalobos ME, Davies MM, Dahl BC, Müller R- A. Partially enhanced thalamo-cortical functional connectivity in autism. Brain Res. 2006;1104:160–174. doi: 10.1016/j.brainres.2006.05.064. [DOI] [PubMed] [Google Scholar]
- Monk CS, Peltier SJ, Wiggins JL, Weng SJ, Carrasco M, Risi S, Lord C. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage. 2009;47:764–772. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51:527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Powell SK, Simmonds DJ, Goldberg MC, Caffo B, Pekar JJ. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain. 2009;132:2413–2425. doi: 10.1093/brain/awp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R-A. The study of autism as a distributed disorder. Ment Retard Dev Disabil Res Rev. 2007;13:85–95. doi: 10.1002/mrdd.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R-A, Pierce K, Ambrose JB, Allen G, Courchesne E. Atypical patterns of cerebral motor activation in autism: a functional magnetic resonance study. Biol Psychiatry. 2001;49:665–676. doi: 10.1016/s0006-3223(00)01004-0. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan SK, Haist F, Müller R-A. Aberrant functional connectivity in autism: evidence from low-frequency BOLD signal fluctuations. Brain Res. 2009;1262:48–63. doi: 10.1016/j.brainres.2008.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartz SR, Sejnowski TJ. The neural basis of cognitive development: a constructivist manifesto. Behav Brain Sci. 1997;20:537–596. doi: 10.1017/s0140525x97001581. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippon G, Brock J, Brown C, Boucher J. Disordered connectivity in the autistic brain: challenges for the "new psychophysiology". Int J Psychophysiol. 2007;63:164–172. doi: 10.1016/j.ijpsycho.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, LeCouteur A, Lord C. Autism Diagnostic Interview - R. Los Angeles (CA): Wester Psychological Services; 2003. [Google Scholar]
- Schölvinck ML, Maier A, Ye FQ, Duyn JH, Leopold DA. Neural basis of global resting-state fMRI activity. Proc Natl Acad Sci U S A. 2010;107:10238–10243. doi: 10.1073/pnas.0913110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopler E, Reichler RJ, DeVellis RF, Daly K. Toward objective classification of childhood autism: childhood Autism Rating Scale CARS. J Autism Dev Disord. 1980;10:91–103. doi: 10.1007/BF02408436. [DOI] [PubMed] [Google Scholar]
- Shih P, Shen M, Öttl B, Keehn B, Gaffrey MS, Müller R-A. Atypical network connectivity for imitation in autism spectrum disorder. Neuropsychologia. 2010;48:2931–2939. doi: 10.1016/j.neuropsychologia.2010.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla DK, Keehn BM, Müller R-A. Tract-specific analyses of diffusion tensor imaging data show widespread white matter compromise in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2011;52:286–295. doi: 10.1111/j.1469-7610.2010.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Ozonoff SJ, Ursu S, Ravizza S, Cummings N, Ly S, Carter CS. The neural substrates of cognitive control deficits in autism spectrum disorders. Neuropsychologia. 2009;47:2515–2526. doi: 10.1016/j.neuropsychologia.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, Maravilla KR, Giedd JN, Munson J, Dawson G, et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- Sundaram SK, Kumar A, Makki MI, Behen ME, Chugani HT, Chugani DC. Diffusion tensor imaging of frontal lobe in autism spectrum disorder. Cereb Cortex. 2008;18:2659–2665. doi: 10.1093/cercor/bhn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai NJ, Longe O, Rippon G. Disconnected brains: what is the role of fMRI in connectivity research? Int J Psychophysiol. 2009;73:27–32. doi: 10.1016/j.ijpsycho.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Turner KC, Frost L, Linsenbardt D, McIlroy JR, Müller R-A. Atypically diffuse functional connectivity between caudate nuclei and cerebral cortex in autism. Behav Brain Funct. 2006;2:34–45. doi: 10.1186/1744-9081-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos ME, Mizuno A, Dahl BC, Kemmotsu N, Müller R-A. Reduced functional connectivity between V1 and inferior frontal cortex associated with visuomotor performance in autism. Neuroimage. 2005;25:916–925. doi: 10.1016/j.neuroimage.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage. 2009;47:1408–1416. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Welchew DE, Ashwin C, Berkouk K, Salvador R, Suckling J, Baron-Cohen S, Bullmore E. Functional disconnectivity of the medial temporal lobe in Asperger's syndrome. Biol Psychiatry. 2005;57:991–998. doi: 10.1016/j.biopsych.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Weng SJ, Wiggins JL, Peltier SJ, Carrasco M, Risi S, Lord C, Monk CS. Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Res. 2010;1313:202–214. doi: 10.1016/j.brainres.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker B, Fonlupt P, Hubert B, Tardif C, Gepner B, Deruelle C. Abnormal cerebral effective connectivity during explicit emotional processing in adults with autism spectrum disorder. Soc Cogn Affect Neurosci. 2008;3:135–143. doi: 10.1093/scan/nsn007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.