Abstract
The default mode network (DMN) comprises brain structures maximally active at rest. Disturbance of network nodes or their connections occurs with some neuropsychiatric conditions and may underlie associated dysfunction. DMN connectivity has not been examined in alcoholism, which is marked by compromised DMN nodes and impaired spatial working memory. To test whether performance would be related to DMN integrity, we examined DMN functional connectivity using functional magnetic resonance imaging (fMRI) data and graph theory analysis. We assumed that disruption of short paths between network nodes would attenuate processing efficiency. Alcoholics and controls were scanned at rest and during a spatial working memory task. At rest, the spontaneous slow fluctuations of fMRI signals in the posterior cingulate and cerebellar regions in alcoholics were less synchronized than in controls, indicative of compromised functional connectivity. Graph theory analysis indicated that during rest, alcoholics had significantly lower efficiency indices than controls between the posterior cingulate seed and multiple cerebellar sites. Greater efficiency in several connections correlated with longer sobriety in alcoholics. During the task, on which alcoholics performed on par with controls, connectivity between the left posterior cingulate seed and left cerebellar regions was more robust in alcoholics than controls and suggests compensatory networking to achieve normal performance.
Keywords: alcohol, cerebellum, default mode network, fMRI, resting state
Introduction
The default mode network (DMN) is defined by intrinsic and correlated activity of selective brain regions (Raichle et al. 2001) when the brain is not involved in an externally imposed goal-directed activity. The regions of activity reliably correlated are the posterior parietal cortex, precuneus and posterior cingulate cortex, medial prefrontal cortex, cerebellar Crus I and Crus II, and cerebellar lobules VI and IX (Greicius et al. 2003; Habas et al. 2009; Andrews-Hanna et al. 2010). Compromise of this network can affect working memory performance (Sambataro et al. 2010) and may explain, in part, how some neuropsychiatric pathologies without space-occupying lesions cause such impairment (Zhou et al. 2007; Damoiseaux et al. 2008; Kennedy and Courchesne 2008; Skudlarski et al. 2010). Some of these neuropsychiatric pathologies could cause decoupling of this intrinsic connectivity, thereby disturbing function, even without local structural damage or disconnecting lesions (Nomura et al. 2010). The DMN seems to be a core feature of brain activity that defines a functionally relevant “baseline” state. Although the DMN has been studied in neuropsychiatric conditions such as schizophrenia (Skudlarski et al. 2010) and Alzheimer's disease (Greicius et al. 2004), to our knowledge, DMN functioning in chronic alcoholism, known to affect nodes of the DMN, has not been investigated.
Chronic alcoholism has been characterized by structural damage of both widespread and selective brain regions and networks (Jernigan et al. 1991; Pfefferbaum et al. 1992; Sullivan 2003; Chanraud et al. 2007; Harris et al. 2008; Cardenas et al. 2007; for review, Chanraud, Zahr et al. 2010). Relationships among pathophysiological mechanisms, clinical symptoms, and cognitive impairment, however, cannot be fully explained by local structural damage. Alcoholism-induced neuropathology does not typically eliminate a function; rather, it results in anatomically injured tissue or functionally disturbed networks that contribute to cognitive dysfunction, especially deficits of executive functions such as verbal and spatial working memory (for review, Sullivan and Pfefferbaum 2005; Oscar-Berman and Marinkovic 2007). Further, insults to subcortical nodes of the frontocerebellar circuitry can be better predictors of executive impairments than tissue loss in prefrontal regions (Sullivan 2003; Chanraud et al. 2009).
Functional magnetic resonance imaging (fMRI) studies of working memory have provided evidence for inefficient cognitive processing in alcoholism as they have revealed that alcoholics activated either a different neural network from controls (Pfefferbaum et al. 2001) or activated appropriate regions but more widely than controls (Desmond et al. 2003) to perform on par with controls in working memory tasks. The “inefficient processing” model derives from observations indicating inefficiency of allocating or sharing resources between different cognitive processes (Wagner et al. 2006) and the use of atypical neuronal networks to compensate for compromised areas (Audoin et al. 2006). In alcoholics, the concept of inefficiency includes difficulties in isolating irrelevant information (Nixon et al. 2002), which is necessary for discriminating between targets and distractors. One source of inefficient processing in chronic alcoholism could arise from disruption of functional brain networks and their dynamics, whether at rest or while engaged in a task. Thus, processing inefficiency could reflect or arise from disorganization of a network, leading to inefficiency in conveying information from place to place. Given a potential role for the DMN in working memory because of the commonality of neural systems to both processes, we hypothesized that the spatial working memory deficit of alcoholics (Chanraud, Pitel et al. 2010) could be related to disruption of the DMN.
Human brain networks, including the DMN, have been described as having “small-world” characteristics (Latora and Marchiori 2001) comprising sets of nodes connected by anatomical or functional links and used for shuttling information. Therefore, small-world organization provides components, that is, the nodes and thus a structure that maximizes functional segregation (Sporns and Zwi 2004) and facilitates the adaptation of neuronal assemblies in response to changing cognitive demands (Bassett and Bullmore 2006). This structure is characterized by short length paths that are responsible for efficient information processing. Local efficiency can be weighted via the path length connecting a node to all its neighbors within a network, such that some links are shorter than others, offering a shorter distance for information to travel. Local efficiency can be quantified by graph theory analysis, which provides a means of characterizing complex systems, as has been applied to functional imaging data (Achard and Bullmore 2007; Hua et al. 2008; Wang et al. 2009). Graph theory analysis allows detection of small-scale changes in regional organization that may underlie more global network changes (Nomura et al. 2010). Here, we used graph theory analysis for characterizing between-groups differences and determining whether connectivity alteration would be related to poor test performance on a spatial working memory task by alcoholics.
Based on anatomical evidence (Mesulam 1998) and computational analysis (Sporns et al. 2007), nodes with disproportionately numerous connections can enhance network efficiency by providing a limited number of long-distance connections that integrate local networks (Bassett and Bullmore 2006) and are considered hubs. Modulations within and between brain networks are dynamic and likely perform as such via interconnections through neural hubs. The posterior cingulate region has been described as a hub because it is activated during passive and active states, although at different levels, and because it has the highest global connectivity and perfusion of any region (Hagmann et al. 2008; Raichle et al. 2001; Pfefferbaum et al. 2010). Physiologically, this region appears as a “crossroad” of information processes, probably related to its high level of perfusion even at rest (Raichle et al. 2001; Pfefferbaum et al. 2010). Although the role of the posterior cingulate cortex is well established as a part of the DMN (Greicius et al. 2003), this brain region is also active in working memory processes involving selection, which operates to retrieve the most relevant item in an array to be remembered (Bledowski et al. 2009). Thus, involvement of the posterior cingulate cortex within the DMN has the potential to influence and predict the working memory efficiency and thus working memory performance (Esposito et al. 2009). This dual function may be particularly relevant to alcoholism where processing can be inefficient in isolating irrelevant information (Nixon et al. 2002).
Here, we tested the hypothesis that functional connectivity stemming from the posterior cingulate hub during rest and task states would be different in alcoholics and controls and that the connectivity of brain networks revealed in controls would be disturbed in alcoholics. For this purpose, we used blood oxygen level–dependent (BOLD) fMRI with one block of rest and one block of a working memory task, which has been shown to involve frontocerebellar networks in controls and alcoholics (Chanraud, Pitel et al. 2010). Specifically, we tested the hypothesis that functional connectivity in networks involving the posterior cingulate and frontocerebellar regions would differ between the groups. First, we defined networks connecting to the posterior cingulate region during rest and task in control and alcoholic groups; then, we estimated local efficiency in networks presenting different patterns of connectivity between groups. Finally, relationships between network metrics and task performance were tested in each group.
Materials and Methods
Subjects
The two subject groups comprised 15 alcohol-dependent subjects and 15 healthy controls. The alcoholics were recruited from community treatment centers, outpatient clinics, hospitals, and by word of mouth. Controls were recruited through flyers, announcements, and word of mouth. All participants provided written informed consent to participate in studies assessing the impact of alcohol on brain structure and function and received a modest stipend for study participation. All were right-handed as determined by quantitative testing (Crovitz and Zener 1962). All participants were administered the Structured Clinical Interview for DSM-IV (SCID; First et al. 1998) by a clinical research psychologist or research nurse, and diagnosis was determined by consensus of at least two calibrated interviewers. Volunteers meeting lifetime diagnostic criteria for schizophrenia or bipolar disorder were excluded. Controls did not meet criteria for any lifetime Axis I pathology or drug or alcohol use disorders. Approximate lifetime alcohol consumption was quantified using a modification (Pfefferbaum et al. 1988) of a semi-structured, timeline interview (Skinner 1982; Skinner and Sheu 1982). Drinks of each type of alcoholic beverage (wine, beer, spirits) were standardized to units containing approximately 13.6 g of alcohol and summed over the lifetime.
Alcoholics were younger than controls but did not differ significantly (t(28) = 1.775, P = 0.083) (Table 1). Compared with the controls, the alcoholics had fewer years of education (t(28) = 2.09, P = 0.046) but did not differ in general intelligence (t(28) = 0.651, P = 0.521) estimated with the National Adult Reading Test (Nelson 1982). As expected, alcoholics reported significantly higher lifetime alcohol consumption than controls (t(28) = 3.252, P = 0.003). The average length of lifetime alcohol dependence based on the SCID interview was 14.79 ± 9.0 years, the mean total lifetime alcohol consumption was 1078.2 ± 1161 kg, and the mean length of sobriety for alcoholics was 66.5 ± 32.4 days with a range of 5–126 days. The alcoholic sober for 5 days was an exception; all the others had past the early withdrawal period.
Table 1.
Demographic characteristics of subjects
| Group | Age (years) | Education (years) | NART IQ | Lifetime alcohol consumption (kg) | Duration of dependence (years) | Duration of abstinence (days) |
| Alcoholics | ||||||
| Mean | 40.1 | 13.3 | 108.9 | 1078.2 | 14.8 | 66.5 |
| Standard deviation | 10.9 | 1.6 | 8.104 | 1161 | 9 | 32.4 |
| Controls | ||||||
| Mean | 47.7 | 14.9 | 111.1 | 28.1 | NA | NA |
| Standard deviation | 12.29 | 2.4 | 9.6 | 35.9 | ||
| P value | P = .09 | P = 0.05 | P = 0.52 | P = 0.0003 | ||
Working Memory Task Conditions during Image Acquisition
Active Condition
Before going to the scanner, all the participants practiced the working memory task until reaching a satisfactory level of performance. Stimuli were presented via E-Prime software (Psychology Software Tools, Inc.). For their responses, subjects used a keypad connected to the laptop running E-prime (http://www.pstnet.com). The working memory tasks included two different interference tasks and one control task without interference. Each working memory task included two memoranda loads: 3 and 6 items. Stimuli consisted of crosses presented in the right, center, or left part of the screen.
All conditions followed the same general pattern: presentation of memoranda, imposition of a retention interval (8 s), and a period of recall (4.5 s for the 3-item sequences and 9 s for the 6-item sequences, Chanraud, Pitel et al. 2010). The sequence of either 3 or 6 items had to be recalled in the order of presentation. During the retention interval, subjects had either to wait (no interference condition) or to perform another task (interference condition: arithmetic or tracking). Participants were instructed to give equal priority to the primary and the interference task. For the arithmetic interference task, participants read 3 numbers, added them together, and then said whether the sum was less than, equal to, or greater than 15. The percentage of correctly answered problems was recorded. For the tracking interference task, participants had to press a key on a computer keyboard corresponding to the position of a target, which was a white circle, presented for 1100 ms with intervals of 280 ms during the 8s period of retention. To ensure that the participants were performing the interference task, we set a 60% level-of-accuracy response criterion for the interference task in each domain for each task (Chan and Newell 2008).
Data Acquisition
The scanning was conducted on a GE (General Electric Medical Systems) 3T whole-body MRI scanner equipped with an 8-channel head coil. A T2-weighted fast spin-echo anatomical scan (axial acquisition; time echo [TE] = 17 ms; time repetition [TR] = 5000 ms; field of view = 24 cm; 256 × 192 matrix; Number of excitations = 1.0; slice thickness = 5 mm; 36 slices) was acquired with the fMRI scans. A field map was generated from a gradient recalled echo sequence pair (TE = 3/5 ms TR = 460 ms, slice thickness = 2.5 mm, 62 slices). Whole-brain fMRI data were acquired with a gradient echo planar pulse sequence (axial, mode = 2D, scan timing: TE = 30 ms, TR = 2200 ms, flip angle = 90°, matrix = 64 × 64, slice thickness = 5 mm, 36 slices). Four blocks of 3 min and 10 s each, synchronized with the beginning of fMRI volume acquisitions were acquired. Four sessions of 3096 functional images were acquired for each subject with the following pseudorandomized conditions: 5 dummy scans + 10 pseudorandomized trials: 3 retention conditions × 2 loads = 6 trials (9 TR required for 3 memoranda and 13 TR required for 6 memoranda) + 2 interference trials without memoranda (1 block of arithmetic without any memoranda run for 5 TRs; 1 block of tracking without any memoranda run for 5 TRs) + 2 blocks of rest run for 5 TRs each. The scan for the rest condition, during which subjects were instructed to lie still with eyes closed, relaxed, and to not fall asleep, lasted 4 min and 57 s (135 TR, 4860 images) and occurred after the active task.
Image spatial preprocessing and statistical analysis were performed using SPM8 (Wellcome Department of Cognitive Neurology). Functional images were realigned for motion corrections and unwarped (correction for fields distortions) using the gradient echo field maps (constructed from the complex difference image between 2 echoes [3 and 5 ms] of the gradient-recalled echoes series). Unwarped functional images were coregistered to structural images for each subject. The anatomical volume was then segmented into gray matter, white matter, and cerebrospinal fluid. The gray matter image was used for determining the parameters of normalization onto the standard Montreal Neurological Institute gray matter template. The spatial parameters were then applied to the realigned and unwarped functional volumes that were finally resampled to voxels of 3 × 3 × 3 mm and smoothed with an 8-mm full-width at half-maximum Gaussian smoothing kernel.
Construction of Functional Connectivity at Rest and during Task
Functional Connectivity Analysis
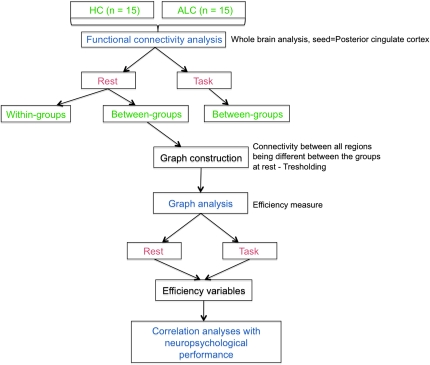
The last block of the task acquisition was used in the correlational analysis because it was the closest in time to the rest block. For rest and task separately, a connectivity analysis on preprocessed data was conducted with the “conn” toolbox, implemented in SPM (http://www.fil.ion.ucl.ac.uk/spm/ext/) (Fig. 1). Correlational analyses between the BOLD signal from an a priori selected seed, that is, the posterior cingulate region taken from the Tzourio-Mazoyer template (Tzourio-Mazoyer et al. 2002), and from every other brain voxel during the entire acquisition condition (rest and task) provided seed-to-voxel connectivity estimations. Age and education were entered as first-level covariates in the model. Before averaging individual voxel data, the waveform of each brain voxel was filtered using a bandpass filter (0.0083/s < f < Inf) to reduce the effect of low-frequency drift and high-frequency noise. Several sources of spurious variance along with their temporal derivatives were then removed from the data through linear regression: signal from ventricular regions and signal from the white matter. Global signal was not included as a regressor, given evidence that this may introduce spurious anticorrelations into the data (Murphy et al. 2009). Because further steps included between-groups comparisons, temporal connectivity maps were generated for each subject across the conditions in one-sided analyses, showing only positive correlations in order to avoid interaction effects. These images were then included in a second-level between-groups, random-effects analysis. The magnitude and extent of temporal connectivity between the groups were thresholded using a false discovery rate (FDR) correction of PFDR < 0.05 for the whole-brain volume with a minimum cluster extent of 10 contiguous voxels.
Figure 1.
Study design schematic. HC, healthy controls; ALC, alcoholics.
Graph Construction (Unweighted Network)
The above analyses resulted in a graph G that included N nodes (regions) and E edges (connections). The analyses were focused on the regions highlighted by between-groups differences at rest: cerebellar regions and seed of interest, the posterior cingulate cortex. Correlational analyses of functional time course during rest and task were run between all these regions for each subject, and an unweighted network was created by thresholding the correlation matrix considering a threshold T. We ran the analysis with a published threshold, that is, >0.2 (Dosenbach et al. 2007). When the absolute value of the correlation between two nodes was higher than the threshold value T, the connection (edge) between these two nodes was considered as part of the resulting graph (Stam and Reijneveld 2007). In other words, if a correlation between the BOLD signals of two nodes measured during a whole condition was significantly higher than the threshold used, the connection existed in the new graph. Connections between nodes, therefore, either existed or did not exist; because of the thresholding, connections lost their graded values. In our case, presence of a direct connection (statistically strong correlation) between nodes of the graph should be interpreted as the shortest distance identified in the graph. The more connections needed for linking two nodes, the longer is the distance. Here, distance does not correspond to a physical distance between nodes in the graph but rather to the number of connections between end points, that is, a node and an ultimate destination.
Graph Analysis (Estimation of Local Efficiency at Rest and during Task)
Network metrics are affected by the number of nodes in a graph. Here, we used a network that differed between groups with nodes more functionally synchronized to the posterior cingulate cortex in controls than in alcoholics. For exploring links between the network efficiency and neuropsychological performance, we focused our analysis on a local level, that is, specifically on nodes that were derived from the between-groups comparison at rest, to be part of the DMN. Using graph theory at a local level allowed addressing the question of the organization of subnetworks with and without a lesion anywhere in the network. Therefore, a basic measure for each node within the network, local efficiency (“Elocal”), was computed as a measure of the connectivity using standard graph theory (Gong et al. 2009). First, the characteristic path length L was computed; L gives the average number of connections (1 for a direct edge) that have to be crossed to travel from each node to every other node in the network (van den Heuvel et al. 2008).
Local efficiency of a given node i that measures the communication efficiency between a node i and its neighbors (Achard and Bullmore 2007) is defined as:
where is the total number of nodes in the subgraph Gi that includes all the neighbors nodes of the node i. Because efficiency is inversely related to path length, a short path length reflects a high level of local efficiency.
Statistical Analyses
Performance
We conducted a 2 group (controls vs. alcoholics) × 3 interference condition (no interference vs. arithmetic vs. tracking) × 2 memory load (3 vs. 6 items) analysis of covariance with age and education entered as confounding variables on the dual-task accuracy. Follow-up t-tests identified between-group differences for significant analysis of variance effects. Reaction time was subjected to 2 groups × 2 memory loads × 3 interference conditions ANOVA and t-tests. Mean accuracy across the interference conditions was calculated for each subject and was used in correlation analyses with local efficiency.
Network Metrics
Comparison of Elocal between the 2 groups was performed by using a 2-sample 2-tailed t-tests.
Relationship between Elocal and Performance/Clinical Variables
Pearson correlation coefficients evaluated relationships between the network metrics (Elocal) of the brain network of interest and several clinical variables (total lifetime alcohol consumption, duration of dependence, and duration of abstinence) of the alcoholic group. Because these analyses were exploratory, we used a statistical significance level of P < 0.05 (uncorrected) but expected that alcoholism severity would be related to lower efficiency between nodes. Pearson correlation analyses were also conducted in each group separately between Elocal and the mean accuracy in the working memory task.
fMRI Analysis
In the present study, we conducted between-group connectivity analysis across the whole task without regard for working memory condition (Chanraud, Pitel et al. 2010). Therefore, we first generated contrasts for each subject by comparing a block comprising all experimental conditions with a block comprising all rest runs. The results of these contrasts were submitted to a factorial analysis with the group (controls vs. alcoholics), the interference (no vs. arithmetic vs. tracking), and the factor memory load (3 vs. 6 items) entered as main factors. fMRI analyses were thresholded as in the connectivity analysis at PFDR < 0.05 for the whole-brain volume with a minimum cluster extent of 10 contiguous voxels.
Results
Table 2 displays the recall accuracy and response time for each group in each condition.
Table 2.
Descriptive statistics for accuracy scores and reaction times by condition and group
| Retention interval task | Delayed condition |
Arithmetic condition |
Tracking condition |
|||
| Memory load | 3 Items | 6 Items | 3 Items | 6 Items | 3 Items | 6 Items |
| Accuracy (%) | ||||||
| Alcoholics | ||||||
| Mean | 81.1 | 68.3 | 61.1 | 53.0 | 63.1 | 51.9 |
| Standard deviation | 22.15 | 16.65 | 27.6 | 25.18 | 30.219 | 19.27 |
| Controls | ||||||
| Mean | 95.0 | 71.7 | 70.5 | 51.1 | 76.2 | 56.8 |
| Standard deviation | 9.85 | 24.15 | 27.6 | 15.7 | 19.46 | 19.2 |
| P value | P = 0.35 | P = 0.663 | P = 0.31 | P = 0.737 | P = 0.156 | P = 0.50 |
| Reaction time (ms) | ||||||
| Alcoholics | ||||||
| Mean | 2205.5 | 5362.3 | 2905.8 | 5998.2 | 2705.5 | 5695.7 |
| Standard deviation | 650.13 | 1084.74 | 749.4 | 1539.95 | 637.28 | 1384.77 |
| Controls | ||||||
| Mean | 2938.3 | 4970.8 | 2692.6 | 5741.7 | 2626.0 | 5633.4 |
| Standard deviation | 1945.54 | 1418.61 | 858.27 | 1316.17 | 546.72 | 1337.62 |
| P value | P = 0.177 | P = 0.403 | P = 0.474 | P = 0.628 | P = 0.717 | P = 0.90 |
Accuracy and Reaction Time on the Working Memory Task
The 2 group × 3 condition (No Interference or Tracking) × 2 memory load ANOVA did not reveal any significant group differences in performance (F1,168 = 0.022, P = 0.978) or reaction time (F1,168 = 1.079, P = 0.342). Similarly, neither the 2 group × 3 condition (F2,168= 0.322; P = 0.725) nor the 2 group × 2 memory load interaction (F1,168 = 2.621; P = 0.107) was significant for performance. Nor did we find significant interactions for 2 group × 2 condition (F2,168 = 0.435; P = 0.648) or 2 group × 2 memory load (F1,168 = 1.153; P = 0.284) for reaction time.
We examined whether reaction time during the tracking task or performance during the arithmetic task differed by group. The mean time on target of the combined 3 items and 6 items conditions for the tracking task was 431.43 ± 98.89 ms for the controls and 490.12 ± 223.43 ms for the alcoholics (t(28) = −0.76; P = 0.46). The mean number of correct responses of the combined 3 items and 6 items conditions was 87% for the arithmetic task for each group. On neither performance measure did the control and alcoholic groups differ significantly.
Functional Connectivity in the DMN within and between Groups: Rest Condition
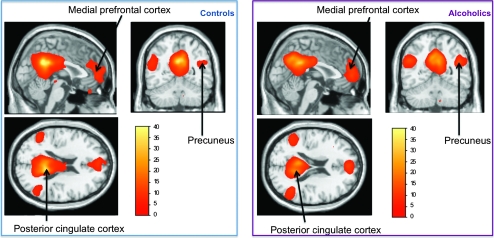
At P < 0.05 FDR corrected, both groups revealed a pattern of functional connectivity commonly described in the literature, with significant functional connectivity between the seed (bilateral posterior cingulate cortex) and the bilateral precuneus, medial prefrontal cortex, and medial temporal cortex (Fig. 2). Also in both groups, functional connectivity with cerebellar regions was also revealed at P < 0.001 uncorrected.
Figure 2.
Functional connectivity at rest using the posterior cingulate cortices as seeds. Left: control group; right: alcoholic group. P < 0.05 FDR corrected.
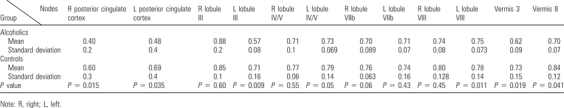
Between-groups differences in connectivity were not observed with the right posterior cingulate cortex used as a seed but were with a left posterior cingulate cortex seed. Relative to alcoholics, controls while at rest had more positive connectivity between signal in the left posterior cingulate cortex and voxels in the cerebellum, particularly, the left anterior cerebellar lobule III, left and right inferior cerebellar lobules VIIb, left inferior cerebellar lobule VIII, and vermis 3 and vermis 8.
Functional Connectivity in the DMN between Groups: Task Condition
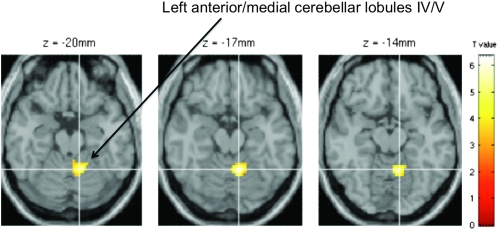
No between-group difference was found with the right posterior cingulate region used as a seed. During the task, connectivity between the left posterior cingulate region and the left anterior/medial cerebellar lobules IV/V was greater in alcoholics than controls (Fig. 3).
Figure 3.
Between-group comparison of the functional connectivity during the task with the left posterior cingulate cortex as seed. P < 0.05 FDR corrected.
Elocal Between-Groups Analysis
When local efficiency for nodes of the network of interest was evaluated during rest scans, t-tests revealed significantly higher efficiency indices in the controls than alcoholics in bilateral posterior cingulate regions, left anterior lobule cerebellum III, right inferior cerebellar lobule VIIb, and left inferior cerebellar lobule VIII, vermis 3 and vermis (Table 3). By contrast, no between-groups difference was revealed during the task.
Table 3.
Local efficiency at rest in the matrix of interest
 |
Correlation between Local Efficiency and Performance
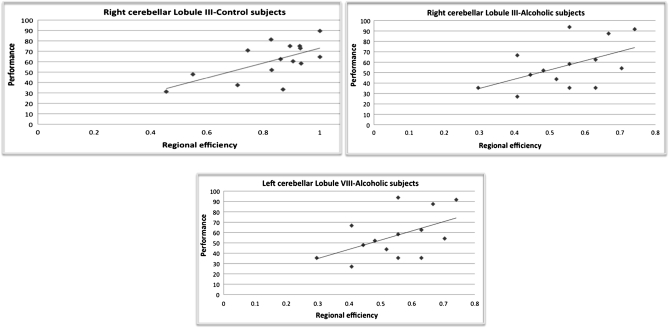
At rest, mean accuracy of the alcoholics correlated with local efficiency in the left inferior cerebellar lobule VIII (r = 0.522, P = 0.046). No significant correlation was found between performance and local efficiency at rest in controls. During the task, better performance correlated with greater efficiency in the right anterior cerebellar lobule III of the alcoholics (r = 0.52, P = 0.047) and controls (r = 0.614, P = 0.015) (Fig. 4).
Figure 4.
Regression slopes between local efficiency during the task in right cerebellar lobule III and performance for controls (top-left figure; r = 0.614, P = 0.015) and for alcoholics (top-right figure; r = 0.52, P = 0.047) and between local efficiency at rest in the left inferior cerebellar lobule VIII and performance for alcoholics (r = 0.522, P = 0.046).
fMRI Between-Groups Comparison of the Task Block
We used group-by-regional activation ANOVAs to test whether group differences in functional connectivity could be accounted for by group differences in functional activation measured by the BOLD response during the task block (Table 4). This analysis revealed a significant effect of group, where bilateral frontal cortices, bilateral insula, and the right postcentral were more activated in the alcoholic than control group, whereas the fusiform gyrus (extending to the cerebellar region VI) and the left lingual gyrus (extending to the posterior cingulate region) were more activated in the control than alcoholic group. Group differences were not present, however, in regions relevant to DMN connectivity.
Table 4.
Results of the factorial analysis
| Group effect | Brain region | Peak T value | MNI coordinates |
||
| x | y | z | |||
| Right frontal cortex | 6.05 | 48 | 41 | 13 | |
| Fusiform gyrus | 5.85 | −30 | −67 | −14 | |
| Left frontal cortex | 4.74 | −33 | 50 | 25 | |
| Right postcentral gyrus | 4.5 | 45 | −19 | 46 | |
| Left Insula | 4.38 | −42 | 11 | −14 | |
| Lingual gyrus | 4.34 | 18 | −70 | −11 | |
| Right Insula | 4.27 | 42 | −1 | −14 | |
Note: MNI, Montreal Neurological Institute.
Correlation between Performance, Connectivity, and Clinical Variables in Alcoholics
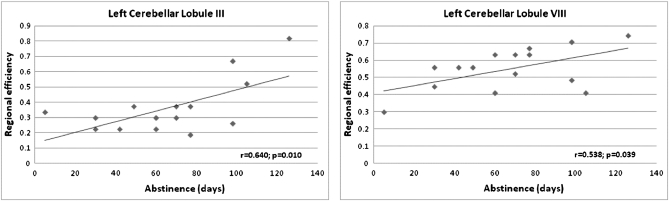
Longer abstinence from alcohol correlated with greater local efficiency at rest in the left anterior cerebellar lobule III (r = 0.640, P = 0.010) and in the left inferior cerebellar lobule VIII (r = 0.538, P = 0.039; Fig. 5). Neither total lifetime consumption nor duration of dependence correlated significantly with local efficiency between any nodes of the network, either at rest or during the task.
Figure 5.
Regression slopes between duration of abstinence (in days) for alcoholics and local efficiency at rest of left cerebellar lobules III (left figure; r = 0.640, P = 0.010) and VIII (right figure; r = 0.538, P = 0.039).
Discussion
Functional connectivity analysis conducted on the BOLD signal at rest and during a spatial working memory task enabled statistical assessment of functional brain networks in alcoholics and healthy subjects. We thresholded the correlational matrices to construct a set of unweighted binary graphs for each subject and weighted connectivity of each node of the network for which connectivity differed between the 2 groups.
At rest, the spontaneous slow fluctuations of the signal in the posterior cingulate and cerebellar regions in the alcoholics were less synchronized than in controls, indicative of compromised functional connectivity. To our knowledge, this is the first report of abnormalities in functional connectivity associated with the DMN in alcoholics. The DMN is defined as a set of functionally connected regions in the resting brain and is part of the default settings of the brain. The posterior cingulate cortex plays a key role in this network (Raichle et al. 2001; Fransson and Marrelec 2008), and the cerebellum has more recently been identified as a node in the default network (Habas et al. 2009). In the healthy brain, modulation of the DMN plays a crucial role in normal functioning. Several reports have shown, for example, that weaker suppression of the DMN while engaged in a task is associated with inferior cognitive performance, for example, inferior memory formation (Daselaar et al. 2004) and poor learning of cognitive skill (Mason et al. 2007). Also, with increasing working memory demands, greater suppression of the DMN is required (McKiernan et al. 2003). Thus, a failure in this suppression would compound a cognitive challenge (Whitfield-Gabrieli et al. 2009). In the case of alcoholics, a deficit in DMN modulation between the posterior cingulate and cerebellar regions between rest and task could reflect processing inefficiency. Compromised connectivity detected in alcoholics represents a decoupling of synchronization between regions that are functionally synchronized in controls. Corrupted synchronization could result in disturbed connectivity, precluding direct transmission between nodes and requiring longer paths to reach nodes of the graph.
Task engagement elicited another pattern of group differences in cerebellar involvement. Alcoholics showed more robust connectivity than controls between the posterior cingulate cortex and the left anterior/medial lobules IV–V of the cerebellum. The group differences in connectivity cannot be a direct result of activation differences because the groups did not show such differences.
Brain networks other than the ones invoked by controls could thus be involved when alcoholics engage in a task. The addition of new direct functional connections may have led to greater connectivity and better than expected results in this sample of alcoholics who were able to reach the same level of performance as controls (cf. Chanraud, Pitel et al. 2010). Such a mechanism, however, would involve additional connections within the network that would have been detected as an increase of the local efficiency in alcoholics. Yet, we detected an extra connection between the posterior cingulate cortex and a specific region within the cerebellum but outside of the DMN. With further probing, these results might reveal the presence of anatomical connections or functional synchronization in alcoholics not present in controls.
The presence of extra connections outside the DMN together with the observations of comparable performance of the controls and alcoholics led us to speculate that a compensatory system could operate through an alternate network, as also suggested by other studies (Pfefferbaum et al. 2001; Desmond et al. 2003; Chanraud-Guillermo et al. 2009), and counteract compromise in another network (Caplan et al. 2007). The anterior/medial cerebellar lobule IV/V complex, a region that was more correlated with the posterior cingulate region during the task in alcoholics, and thus a candidate for compensation, is usually involved in motor functions (Habas et al. 2009; Marvel and Desmond 2010). The alcoholics may have recruited the motor loop, potentially spared in alcoholism (Harper et al. 2003), to compensate for the executive one, which is likely compromised in alcoholism (Harper et al. 2003) and necessary for performing the spatial working memory task (Pfefferbaum et al. 2001). Involvement of the motor network to compensate for inefficient functioning within a closed cerebrocerebellar loop, such as the executive network, comports with previous findings (Chanraud, Pitel et al. 2010). Further to this point, the activity in the posterior cingulate cortex has been shown to be negatively correlated with the activity of nodes of the prefrontal-based motor control circuits, and its influence has been speculated to modulate the activity within this circuit involved in execution and observation of goal-directed actions (Uddin et al. 2009).
Other cerebellar regions are also relevant for performance of the working memory task used. Local efficiency of the anterior cerebellar lobule III during the task was correlated with performance in both the controls and the alcoholics. While at rest, local efficiency in the inferior cerebellar lobule VIII, which is also a node of the DMN, was related to performance only of the alcoholics. Higher efficiency value in these regions also correlated with longer sobriety in alcoholics, suggesting that whatever the compensatory processes were used by alcoholics involving these regions, sustained abstinence was associated with better performance. A further implication is that longer sobriety provides extended time for recovery and repair, and this variability contributed to performance differences within the group of alcoholics. Similarly, variability of cognitive performance has been shown to correlate with path length across a combined group of control and patients with Alzheimer's disease (Stam and Reijneveld 2007) and with network efficiency in groups of healthy subjects and schizophrenic patients (Rubinov et al. 2009). Also, intellectual performance measured with IQ correlated with path length in healthy subjects (van den Heuvel et al. 2009). In heroin users, path length in the bilateral cerebellum is linked to duration of use, suggesting that length of consumption is influential in functional connectivity alteration (Yuan et al. 2010). In alcoholics, however, working memory performance was also positively related to local efficiency in a cerebellar node measured during the rest period (the inferior cerebellar lobule VIII). One interpretation is that this region is part of a network of compensation that facilitates cognitive performance. An alternative interpretation derives from the between-groups difference at rest. Specifically, alcoholics had less connectivity than controls between the posterior cingulate cortex and the inferior cerebellar lobule VIII, the efficiency of which correlated with working memory performance and duration of abstinence in alcoholics. One interpretation of these results suggests that this region needs to be available, even at rest, for alcoholics to meet high task demands. This hypothesis is further corroborated by the significant relationship between the efficiency in the cerebellar region and the strength of functional connectivity between the posterior cingulate cortex and the inferior cerebellar lobule VIII during the task.
Anatomically, cerebrocerebellar circuits are based only on indirect projections through the thalamus and pontine nucleus (Kelly and Strick 2003). Functional connectivity reveals contralateral cerebellar correlations with cortex, consistent with polysynaptic connectivity of the cerebellum (Allen et al. 2005). Thus, unlike imaging analyses that use anatomy directly (Sporns et al. 2007; Hagmann et al. 2008), connectivity patterns defined herein could reflect both direct and indirect anatomical projections. Functionally, regions of the cerebellum are active during rest and task engagement, and patterns of BOLD activity can be modified by the order of experimental blocks, that is, whether rest follows task or task follows rest. Evidence for such modulation derives from application of a probabilistic independent components analysis (Albert et al. 2009), which identified 2 distinct resting-state networks, one of which was a cerebellar network that was modulated by learning as the network strength increased following a novel motor learning task but not following a simple motor performance task. Because the rest block followed the task block in this experiment, the modulatory process of the cerebellum may be disrupted in alcoholics and potentially underlie their spatial working memory impairment.
A principal assumption of the statistical approach taken by graph theory to model processing is that short path lengths between neural nodes can help preserve transmitted signals from decay with time and degradation over multilocal transmission. Events and conditions that disrupt short paths can impair connectivity. With respect to the untoward effects of alcoholism on the DMN, even if the principal network nodes are themselves not pathological, subtle disruption of their functional connections could result in inefficiency of information transmission and manifest as impairment (cf., Sullivan and Pfefferbaum 2005). We conclude that the results obtained in this study comport with pathophysiological models of alcoholism as a functional disconnection syndrome (Geschwind 1965; Chanraud, Zahr et al. 2010) marked by processing inefficiency arising from compromised connectivity of required functional networks.
Funding
National Institutes of Health (grants AA010723, AA012388, AA017168, and AA017923).
Acknowledgments
The authors thank Alfonso Nieto-Castanon, PhD, for helpful comments on graph theory analysis. Conflict of Interest: None of the authors has any conflicts of interest with this work, either financial or otherwise.
References
- Achard S, Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput Biol. 2007;3:e17. doi: 10.1371/journal.pcbi.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert NB, Robertson EM, Mehta P, Miall RC. Resting state networks and memory consolidation. Commun Integr Biol. 2009;2:530–532. doi: 10.4161/cib.2.6.9612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G, McColl R, Barnard H, Ringe WK, Fleckenstein J, Cullum CM. Magnetic resonance imaging of cerebellar-prefrontal and cerebellar-parietal functional connectivity. NeuroImage. 2005;28:39–48. doi: 10.1016/j.neuroimage.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audoin B, Au Duong MV, Malikova I, Confort-Gouny S, Ibarrola D, Cozzone PJ, Pelletier J, Ranjeva JP. Functional magnetic resonance imaging and cognition at the very early stage of MS. J Neurol Sci. 2006;245:87–91. doi: 10.1016/j.jns.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E. Small-world brain networks. Neuroscientist. 2006;12:512–523. doi: 10.1177/1073858406293182. [DOI] [PubMed] [Google Scholar]
- Bledowski C, Rahm B, Rowe JB. What "works" in working memory? Separate systems for selection and updating of critical information. J Neurosci. 2009;29:13735–13741. doi: 10.1523/JNEUROSCI.2547-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan JB, McIntosh AR, De Rosa E. Two distinct functional networks for successful resolution of proactive interference. Cereb Cortex. 2007;17:1650–1663. doi: 10.1093/cercor/bhl076. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Gadzinski S, Durazzo TC, Meyerhoff DJ. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage. 2007;34:879–887. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JS, Newell FN. Behavioral evidence for task-dependent "what" versus "where" processing within and across modalities. Percept Psychophys. 2008;70:36–49. doi: 10.3758/pp.70.1.36. [DOI] [PubMed] [Google Scholar]
- Chanraud-Guillermo S, Andoh J, Martelli C, Artiges E, Pallier C, Aubin HJ, Martinot JL, Reynaud M. Imaging of language-related brain regions in detoxified alcoholics. Alcohol Clin Exp Res. 2009;33:977–984. doi: 10.1111/j.1530-0277.2009.00918.x. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, Reynaud M, Martinot JL. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32:429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Pitel AL, Rohlfing T, Pfefferbaum A, Sullivan EV. Dual tasking and working memory in alcoholism: relation to frontocerebellar circuitry. Neuropsychopharmacology. 2010;35:1868–1878. doi: 10.1038/npp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Reynaud M, Wessa M, Penttila J, Kostogianni N, Cachia A, Artiges E, Delain F, Perrin M, Aubin HJ, et al. Diffusion tensor tractography in mesencephalic bundles: relation to mental flexibility in detoxified alcohol-dependent subjects. Neuropsychopharmacology. 2009;34:1223–1232. doi: 10.1038/npp.2008.101. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Zahr N, Sullivan EV, Pfefferbaum A. MR diffusion tensor imaging: a window into white matter integrity of the working brain. Neuropsychol Rev. 2010;20:209–225. doi: 10.1007/s11065-010-9129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crovitz HF, Zener KA. Group test for assessing hand and eye dominance. Am J Psychol. 1962;75:271–276. [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA. Reduced resting-state brain activity in the "default network" in normal aging. Cereb Cortex. 2008;18:1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Cabeza R. When less means more: deactivations during encoding that predict subsequent memory. Neuroimage. 2004;23:921–927. doi: 10.1016/j.neuroimage.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Chen SH, De Rosa E, Pryor MR, Pfefferbaum A, Sullivan EV. Increased fronto-cerebellar activation in alcoholics during verbal working memory: an fMRI study. Neuroimage. 2003;19:1510–1520. doi: 10.1016/s1053-8119(03)00102-2. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito F, Aragri A, Latorre V, Popolizio T, Scarabino T, Cirillo S, Marciano E, Tedeschi G, Di Salle F. Does the default-mode functional connectivity of the brain correlate with working-memory performances? Arch Ital Biol. 2009;147:11–20. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) Version 2.0. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1998. [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. Neuroimage. 2008;42:1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. Brain. 1965;88:237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Gong G, Rosa-Neto P, Carbonell F, Chen ZJ, He Y, Evans AC. Age- and gender-related differences in the cortical anatomical network. J Neurosci. 2009;29:15684–15693. doi: 10.1523/JNEUROSCI.2308-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C, Dixon G, Sheedy D, Garrick T. Neuropathological alterations in alcoholic brains. Studies arising from the New South Wales Tissue Resource Centre. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:951–961. doi: 10.1016/S0278-5846(03)00155-6. [DOI] [PubMed] [Google Scholar]
- Harris GJ, Jaffin SK, Hodge SM, Kennedy D, Caviness VS, Marinkovic K, Papadimitriou GM, Makris N, Oscar-Berman M. Frontal white matter and cingulum diffusion tensor imaging deficits in alcoholism. Alcohol Clin Exp Res. 2008;32:1001–1013. doi: 10.1111/j.1530-0277.2008.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua QP, Zeng XZ, Liu JY, Wang JY, Guo JY, Luo F. Dynamic changes in brain activations and functional connectivity during affectively different tactile stimuli. Cell Mol Neurobiol. 2008;28:57–70. doi: 10.1007/s10571-007-9228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Butters N, DiTraglia G, Schafer K, Smith T, Irwin M, Grant I, Schuckit M, Cermak L. Reduced cerebral grey matter observed in alcoholics using magnetic resonance imaging. Alcohol Clin Exp Res. 1991;15:418–427. doi: 10.1111/j.1530-0277.1991.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. Functional abnormalities of the default network during self- and other-reflection in autism. Soc Cogn Affect Neurosci. 2008;3:177–190. doi: 10.1093/scan/nsn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latora V, Marchiori M. Efficient behavior of small-world networks. Phys Rev Lett. 2001;87:198701. doi: 10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- Marvel CL, Desmond JE. Functional topography of the cerebellum in verbal working memory. Neuropsychol Rev. 2010;20:271–279. doi: 10.1007/s11065-010-9137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121(Pt 6):1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? NeuroImage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson HE. The National Adult Reading Test (NART) Windsor, Canada: Nelson Publishing Company; 1982. [Google Scholar]
- Nixon SJ, Tivis R, Ceballos N, Varner JL, Rohrbaugh J. Neurophysiological efficiency in male and female alcoholics. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:919–927. doi: 10.1016/s0278-5846(02)00206-3. [DOI] [PubMed] [Google Scholar]
- Nomura EM, Gratton C, Visser RM, Kayser A, Perez F, D'Esposito M. Double dissociation of two cognitive control networks in patients with focal brain lesions. Proc Natl Acad Sci U S A. 2010;107:12017–12022. doi: 10.1073/pnas.1002431107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Chanraud S, Pitel AL, Muller-Oehring E, Shankaranarayanan A, Alsop DC, Rohlfing T, Sullivan EV. Cerebral blood flow in posterior cortical nodes of the default mode network decreases with task engagement but remains higher than in most brain regions. Cereb Cortex. 2010;21:233–244. doi: 10.1093/cercor/bhq090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, Sullivan EV. Reorganization of frontal systems used by alcoholics for spatial working memory: an fMRI study. Neuroimage. 2001;14:7–20. doi: 10.1006/nimg.2001.0785. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, Ha CN, Sullivan EV. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcohol Clin Exp Res. 1992;16:1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Crusan K, Jernigan TL. Brain CT changes in alcoholics: the effects of age and alcohol consumption. Alcohol Clin Exp Res. 1988;12:81–87. doi: 10.1111/j.1530-0277.1988.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Raichle M, MacLeod A, Snyder A, Powers W, Gusnard D, Shulman G. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Knock SA, Stam CJ, Micheloyannis S, Harris AW, Williams LM, Breakspear M. Small-world properties of nonlinear brain activity in schizophrenia. Hum Brain Mapp. 2009;30(2):403–416. doi: 10.1002/hbm.20517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambataro F, Murty VP, Callicott JH, Tan HY, Das S, Weinberger DR, Mattay VS. Age-related alterations in default mode network: impact on working memory performance. Neurobiol Aging. 2010;31:839–852. doi: 10.1016/j.neurobiolaging.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA. Development and Validation of a Lifetime Alcohol Consumption Assessment Procedure. Toronto, Canada: Addiction Research Foundation; 1982. [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices: the lifetime drinking history and the MAST. J Stud Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Skudlarski P, Jagannathan K, Anderson K, Stevens MC, Calhoun VD, Skudlarska BA, Pearlson G. Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol Psychiatry. 2010;68:61–69. doi: 10.1016/j.biopsych.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, Honey CJ, Kotter R. Identification and classification of hubs in brain networks. PLoS One. 2007;2:e1049. doi: 10.1371/journal.pone.0001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, Zwi JD. The small world of the cerebral cortex. Neuroinformatics. 2004;2:145–162. doi: 10.1385/NI:2:2:145. [DOI] [PubMed] [Google Scholar]
- Stam CJ, Reijneveld JC. Graph theoretical analysis of complex networks in the brain. Nonlinear Biomed Phys. 2007;1:3. doi: 10.1186/1753-4631-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV. Compromised pontocerebellar and cerebello thalamocortical systems: speculations on their contributions to cognitive and motor impairment in nonamnesic alcoholism. Alcohol Clin Exp Res. 2003;27:1409–1419. doi: 10.1097/01.ALC.0000085586.91726.46. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Xavier Castellanos F, Milham MP. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp. 2009;30:625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Stam CJ, Kahn RS, HulshoffPol HE. Efficiency of functional brain networks and intellectual performance. J Neurosci. 2009;29:7619–7624. doi: 10.1523/JNEUROSCI.1443-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G, Sinsel E, Sobanski T, Kohler S, Marinou V, Mentzel HJ, Sauer H, Schlosser RG. Cortical inefficiency in patients with unipolar depression: an event-related FMRI study with the Stroop task. Biol Psychiatry. 2006;59:958–965. doi: 10.1016/j.biopsych.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang L, Zang Y, Yang H, Tang H, Gong Q, Chen Z, Zhu C, He Y. Parcellation-dependent small-world brain functional networks: a resting-state fMRI study. Hum Brain Mapp. 2009;30:1511–1523. doi: 10.1002/hbm.20623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K, Qin W, Liu J, Guo Q, Dong M, Sun J, Zhang Y, Liu P, Wang W, Wang Y, et al. Altered small-world brain functional networks and duration of heroin use in male abstinent heroin-dependent individuals. Neurosci. Lett. 2010;477:37–42. doi: 10.1016/j.neulet.2010.04.032. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Tian L, Wang K, Hao Y, Liu H, Liu Z, Jiang T. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007;97:194–205. doi: 10.1016/j.schres.2007.05.029. [DOI] [PubMed] [Google Scholar]