Abstract
Different cortical regions within the ventral occipitotemporal junction have been reported to show preferential responses to particular objects. Thus, it is argued that there is evidence for a left-lateralized visual word form area and a right-lateralized fusiform face area, but the unique specialization of these areas remains controversial. Words are characterized by greater power in the high spatial frequency (SF) range, whereas faces comprise a broader range of high and low frequencies. We investigated how these high-order visual association areas respond to simple sine-wave gratings that varied in SF. Using functional magnetic resonance imaging, we demonstrated lateralization of activity that was concordant with the low-level visual property of words and faces; left occipitotemporal cortex is more strongly activated by high than by low SF gratings, whereas the right occipitotemporal cortex responded more to low than high spatial frequencies. Therefore, the SF of a visual stimulus may bias the lateralization of processing irrespective of its higher order properties.
Keywords: faces, fusiform gyrus, object recognition, reading, ventral visual stream
Introduction
Written words and faces are highly important visual stimuli that are processed through occipital (visual), unimodal, and heteromodal temporal association cortices and paralimbic and limbic structures (Goodale and Milner 1992; Ungerleider and Haxby 1994; Mesulam 1998). The so-called ventral visual stream is an important component of this pathway, and neuroimaging studies have identified discrete areas of cortex that are activated by particular types of visual object. The “visual word form area” (VWFA, Cohen et al. 2000) in the left posterior fusiform gyrus and the “fusiform face area” ([FFA], Kanwisher et al. 1997) in the right posterior fusiform gyrus have received the most attention.
The lateralization of word and face processing to left and right hemispheres, respectively, has been demonstrated in a number of neuroimaging studies (Sergent et al. 1992; Kanwisher et al. 1997; McCarthy et al. 1997; Cohen et al. 2000, 2002; Hasson et al. 2002). Further evidence for this hemispheric dissociation comes from neuropsychological studies, which consistently show that pure alexia (an acquired deficit in word recognition) is caused in the vast majority of cases by left hemisphere lesions (Damasio and Damasio 1983; Binder and Mohr 1992; Leff et al. 2006) and prosopagnosia (an acquired deficit in face recognition) is more strongly associated with right hemisphere lesions (Meadows 1974; Damasio et al. 1982; Benton 1990; Barton 2008b). Although there are exceptions to this lateralization (Hirose et al. 1977; Erkulvrawatr 1978; Winkelman and Glasson 1984; Pillon et al. 1987; Mattson et al. 2000; Barton 2008a), these cases are normally attributed to atypical lateralization in left-handed individuals.
Cai et al. (2008) demonstrated that the lateralization of visual word recognition is strongly related to the lateralization of speech production. Speech, which is innate, precedes reading, which is acquired, and the inference is that top-down signal from anterior speech production regions influences the lateralization of the VWFA, thereby minimizing the need for callosal transfer of information within the reading network. What directs the lateralization of the FFA is less clear (Cantlon et al. 2010). Willems et al. (2010) recently showed that lateralization of the FFA depended on handedness: In right-handed participants, the FFA was right lateralized, whereas left-handed participants showed no reliable lateralization. Given that right handedness also predicts left hemisphere language dominance, it is unclear whether lateralization of word and face processing operate independently or are mutually dependent on a more general, heritable trait.
It should be noted that although pure alexia and prosopagnosia are commonly conceived of as category-specific visual agnosias, this view is not universally held. Recent studies have demonstrated category-general visual deficits in patients with pure alexia (Mycroft et al. 2009; Starrfelt et al. 2009), suggesting that lateralization of function in the fusiform may be attributed to more basic perceptual processes rather than higher-order object recognition as such.
A number of studies have questioned whether basic perceptual features such as eccentricity may influence the organization of the ventral occipitotemporal cortex. Malach and colleagues (Levy et al. 2001; Hasson et al. 2002) showed that retinotopic eccentricity mapping in the visual cortex extends into ventral occipitotemporal cortex, with central vision represented laterally and peripheral vision represented medially. This mapping may explain why words and faces (which are typically viewed in foveal vision) activate regions in the lateral bank of the fusiform gyrus, whereas the parahippocampal place area ([PPA]; Epstein and Kanwisher 1998) is located more medially, reflecting the greater reliance on peripheral vision for viewing buildings and landscapes. Although this factor may influence intrahemispheric organization of visual association cortex, it may also have an influence on interhemispheric organization.
Although words and faces are similar in their reliance on high acuity foveal vision, they differ in terms of spatial frequency (SF) content, another basic perceptual feature. SF is defined as the change in luminance across space and can be quantified as the number of cycles per degree (cpd) of visual angle. As written words are composed of high-contrast edges, high spatial frequencies predominate; whereas as faces have a combination of sharp edges and smooth contrast gradients, they comprise a broader range of frequencies, with lower SF power than words. The object of this study was to explore whether SF differences between words and faces correlated with the hemispheric dominance for the VWFA and the FFA.
The idea that there might be a dissociation between visual processing in the left and right hemispheres, rather than a within-hemisphere dissociation between medial and lateral cortex, was first explored in the late 1970s in behavioral studies using split-field presentation. These studies indicated a left visual field, and therefore right hemisphere (LVF/RH), advantage for large stimuli or stimuli presented to the periphery of the visual field, and a right visual field or left hemisphere (RVF/LH) advantage for small stimuli or stimuli presented foveally (Polich 1978; Marzi et al. 1979; Sergent 1982, 1983).
More recent behavioral studies have found similar effects using high-pass or low-pass filtering to remove low or high SF information from images, respectively. Using this technique, Peyrin et al. (2006) demonstrated an RVF/LH advantage for perceptual decisions about highpass filtered images of natural scenes and an LVF/RH advantage for low-pass filtered images. Furthermore, imaging studies have shown that spatial filtering can modulate lateralization of activations. Iidaka et al. (2004) showed that high-pass filtered images of houses and faces preferentially activated an area of the left occipitotemporal cortex compared with low-pass filtered images. In a study using electroencephalography, Mercure et al. (2008) showed that the N170 response for normal and high-pass filtered words was left lateralized, but it was bilateral when the high-frequency information was removed using low-pass filtering. By comparison, the N170 for unfiltered images of faces was bilateral, and filtering had no effect on lateralization. These studies consistently indicate a bias toward high SF processing in the left hemisphere, but this interpretation is limited due to the use of filtered images so that it remains unclear whether the results are driven by stimulus legibility rather than by the manipulation of frequency content per se. If the effects are truly related to SF, then lateralization should be apparent for simple stimuli that vary only in SF.
The design of the present study, which was to assess the influence of SF on the lateralization of ventral occipitotemporal activations in the absence of any intelligibility effects, employed simple sine-wave gratings rather than filtered images. It was predicted that left ventral occipitotemporal cortex would be preferentially activated by high spatial frequencies and right ventral occipitotemporal cortex by low spatial frequencies. Word or face processing areas in ventral occipitotemporal cortex were identified using appropriate functional localizers, and the preferred SF within these regions of interest was directly compared.
Materials and Methods
Participants
Twelve healthy volunteers (6 female, mean age 31.5 years) participated in this functional magnetic resonance imaging (fMRI) study. Participants were right-handed healthy volunteers, with normal or corrected-to-normal vision. They had no history of neurological illness or developmental reading or face recognition deficits. All participants gave written, informed consent, and ethical approval for the experimental procedures was granted by the local research ethics committee.
Materials
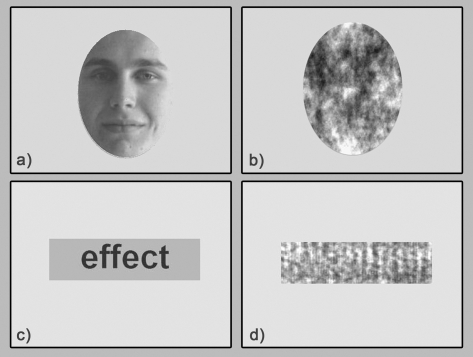
Stimuli for the functional localizer scan (used to define word and face preferential regions) comprised grayscale word and face images and scrambled versions of the same stimuli (Fig. 1). Words were selected from the Medical Research Council Psycholinguistic Database (Coltheart 1981). The words were familiar (Kucera–Francis written frequency >200) and imageable (imageability rating >250), 3–7 letters long. They were presented in black Arial font on a gray rectangular background. The face stimuli consisted of greyscale frontal photographs of faces with neutral expressions taken from the Computer Vision Laboratory Face Database (Solina et al. 2003). The images were cropped with an oval mask of fixed dimensions to remove hair, clothing, or background details. Face and word stimuli were luminance matched using a custom-written algorithm in Matlab (MathWorks).
Figure 1.
Examples of stimuli used in the functional localizer scan, from the conditions (a) faces, (b) scrambled faces, (c) words, and (d) scrambled words.
Scrambled versions of the word and face images were created by taking the Fourier transform of the word or face images, randomly permutating the phase information, and inverting the Fourier transform (after Eger et al. 2005). These scrambled images had similar SF content to the conditions of interest but without any meaningful structure or semantic content.
Stimuli for the SF mapping scans were whole-screen grayscale sine-wave gratings, ranging from 0.05 to 7 cpd. Gratings were presented in smoothly ascending or descending sequence over a 64-s period. Grating orientation changed by random increments approximately every 800 ms throughout the cycle.
Experimental Procedure
The localizer scan was acquired first and comprised 1 fMRI run lasting approximately 8 min. Within the run, there were 6 repetitions of the 4 experimental conditions: words, faces, scrambled words, and scrambled faces. A block design was used, with 12.6 s per block and a 6-s fixation period between blocks. Block order was pseudorandomized to avoid order effects between conditions and to maximize the number of transitions between conditions. Within each block, there were 14 trials. In each trial, a stimulus was presented centrally on the screen for 300 ms, followed by a fixation cross for 600 ms. A dot-detection task was used to maintain participants’ attention during the run: They were instructed to respond by button press whenever a red dot appeared on a stimulus, which occurred once or twice within each block.
The SF mapping data were acquired in 2 runs: In one, the 64-s SF cycle ranged from low to high, and in the other, it ranged from high to low. Run order was counterbalanced between participants. In each run, 8 immediately consecutive repetitions of the 64-s SF sweep were presented. There were occasional catch trials (ca. 24 per run) where participants were required to detect a rapid doubling or halving of SF and respond with a button press. This ensured that attention to the stimuli was maintained throughout the run.
Image Acquisition and Analysis
Data were acquired on a Siemens Avanto 1.5 T MRI scanner with a 32-channel head coil. A whole-brain T1-weighted structural scan was acquired for registration purposes. The functional runs used a T2*-weighted echo planar imaging sequence with time repetition (TR) = 2 s and time echo = 39 ms. The field of view was 205 mm, and the slab consisted of 24 slices of 3.2-mm thickness acquired in an interleaved order. Partial field-of-view acquisition allowed a fast repetition time without compromising on voxel size. The slab covered the occipital and ventral occipitotemporal areas of interest but not the dorsal parietal or frontal lobes or cerebellum. Two hundred and fifty-six volumes were acquired for each run.
Data were analyzed using tools from the Oxford Centre for Functional MRI of the Brain's Software Library (FSL) (Smith et al. 2004). A 2-stage linear registration was used to align functional data to structural images and structural images to Montreal Neurological Institute (MNI) standard templates. Functional data preprocessing included high-pass filtering, motion correction, and smoothing using a 7-mm full-width at half-maximum (FWHM) gaussian kernel.
For the functional localizer scan, the general linear model (GLM) modeled the face, word, scrambled face or scrambled word stimuli as explanatory variables (EVs), convolved with a hemodynamic response function (HRF). Within the timecourse of each EV, the onset of every stimulus was modeled. The temporal derivative of each EV was modeled to improve the sensitivity of the model. Motion parameters were entered into the GLM as confounding variables of no interest. Statistical contrasts of faces versus scrambled faces and words versus scrambled words were evaluated. Single subject data were analyzed at the first level, and the resulting contrasts were passed forward to a mixed-effects group-level analysis. Z statistic images were thresholded at z > 2.3 and a cluster-corrected significance level threshold of P < 0.05 (Worsley 2001). A mask of the fusiform gyrus in both hemispheres was created based on the Harvard–Oxford Cortical Structural Atlas. Voxels within this mask that were statistically significant in either of the 2 contrasts were combined to produce a binary mask spanning word/face preferential fusiform areas (hereafter called the functional localizer mask).
For the SF scans, the GLM modeled TRs acquired during high and low SF presentations, using a median split of the full 64-s cycle. The low SF half of the cycle was modeled as one EV, convolved with the HRF. As the SF stimuli were continuous (with no rest between cycles), the remaining data not modeled by this EV represent the high SF half of the cycle. Motion parameters were entered as confounding variables of no interest. Statistical contrasts of high versus low SF, and vice versa, were evaluated. Single session data were analyzed at the first level, and the 2 runs from each subject were combined using a fixed-effects analysis at the second level. The group-level analysis and statistical thresholds (using cluster-based correction for multiple comparisons) were the same as for the functional localizer analysis but using the functional localizer mask as a prethreshold mask to reduce multiple comparison correction.
In addition to the FSL analysis, the data were analyzed using a phase-encoded mapping approach (Sereno et al. 1995) on the reconstructed cortical surface. Surface-based analysis of fMRI data allows accurate registration of activation onto the cortical surface, rather than smoothing across cortical folds. It can also enhance statistical power by improving intersubject registration. Cortical reconstruction was performed with the Freesurfer image analysis suite (Dale et al. 1999; Fischl et al. 1999). Phase-encoded mapping calculates the SF that maximally activates each voxel, thereby building a map of SF sensitivity on the cortical surface. The time series for each voxel was motion corrected using the 3dvolreg tool from the Analysis of Functional NeuroImages software package (Cox and Jesmanowicz 1999), detrended, and analyzed with a Fourier transform. The phase angle at the stimulus frequency of 8 cycles per scan corresponds to the preferred SF for that voxel and was used in surface analyses and subsequent region of interest analyses.
The surface-based regions of interest were created by repeating the functional localizer analysis in FSL but with no spatial smoothing in order to minimize blurring across cortical folds. The resulting contrasts for each subject were transformed onto surface space, then smoothed on the surface to 5-mm FWHM, and averaged across the group to create a surface-based group map of the face versus scrambled face and word versus scrambled word contrasts. Again, areas of significant activation from the 2 contrasts (words vs. scrambled words and faces vs. scrambled faces) were combined to form the functional localizer mask. The mean phase angle across all voxels was calculated within the functional localizer mask for each hemisphere of each individual to create an average SF response for the left and right hemisphere word or face preferential areas. These were then compared across subjects with a paired t-test to see if there was a lateralization bias in preferred SF.
Results
Functional Localizer
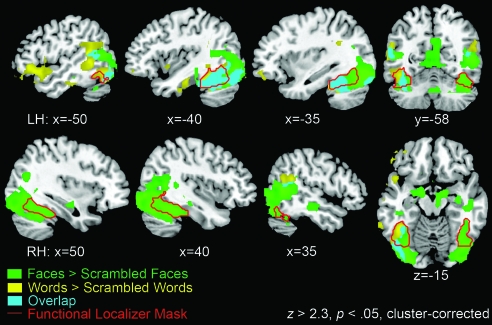
A mixed-effects group analysis GLM identified face preferential activation (Z > 2.3, cluster-corrected threshold P < 0.05) in bilateral occipital and occipitotemporal fusiform gyrus, extending into more anterior midline and temporal lobe regions (Fig. 2). A post hoc analysis of activity in the left and right posterior fusiform gyrus on the data from the present study demonstrated a greater response on the right relative to the left (t(11) = 3.2, P < 0.01). Word preferential activation was identified in left occipitotemporal fusiform gyrus, again with more anterior frontotemporal activity. Significant voxels from the word and face activation maps that fell within the fusiform gyri were combined to make the functional localizer mask—a bilateral, word or face sensitive area of interest for the subsequent SF analysis.
Figure 2.
Statistical maps of the functional localizer results, using a cluster-corrected threshold of Z > 2.3, P < 0.05. Yellow areas were significantly more active during presentation of words than scrambled words, including the left fusiform gyrus and (predominantly left) temporal and frontotemporal cortex. Green areas were significantly more active for faces than for scrambled faces, including bilateral fusiform gyrus, extending into more anterior temporal cortex. Blue areas demonstrate where the contrasts of words versus scrambled words and faces versus scrambled faces overlapped. The red outline shows areas within the fusiform gyrus (as defined by the Harvard–Oxford Cortical Structural Atlas) that were significantly active in either contrast—this combined area across both hemispheres was used as the ‘functional localizer mask’ in subsequent analyses.
SF Analysis
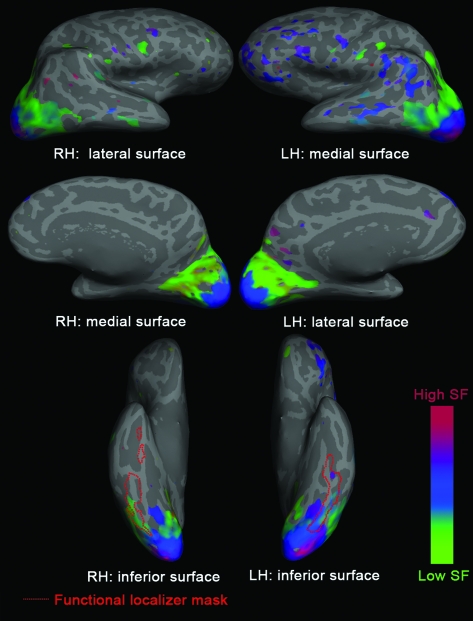
Phase-encoded mapping (Sereno et al. 1995) was used to identify the preferred SF of each voxel. To demonstrate the broad pattern of SF sensitivity across the cortex, Figure 3 presents surface maps for a representative subject. SF sensitivity was observed extending from primary occipital cortex into more anterior ventral occipitotemporal and dorsal occipitoparietal areas. There was a gradient of high to low SF preference moving from the occipital pole outward. Qualitatively similar results were observed in all subjects. These findings are in agreement with the broad pattern found in previous mapping studies of SF (Sasaki et al. 2001; Henriksson et al. 2008).
Figure 3.
Surface maps of preferred SF for a representative subject. A liberal threshold was used for demonstrative purposes.
To directly test the hypothesis that there are differences in SF processing between word or face processing areas of the left and right fusiform gyri, a group-level region of interest analysis was performed. The phase angle of each voxel in the functional localizer mask was averaged for each hemisphere for each subject. A paired t-test comparing average phase angle in the left and right hemispheres showed that the left hemisphere preferred significantly higher spatial frequencies than the right hemisphere (t(11) = 2.7; P < 0.05).
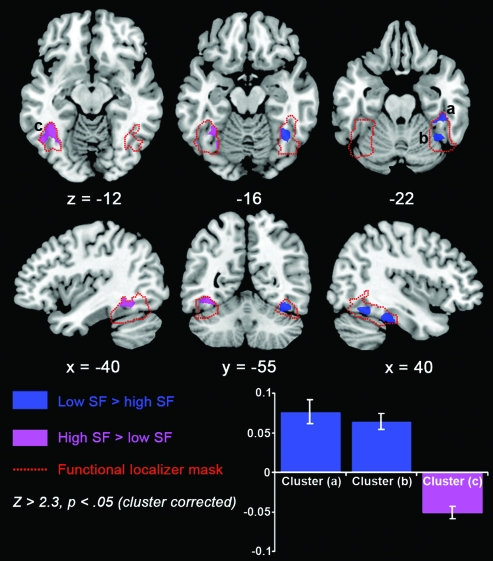
In order to confirm this result on the volumetric brain data, a mixed-effects GLM analysis using the fMRI Expert Analysis Tool from FSL (Smith et al. 2004) assessed whether any voxels identified within the functional localizer mask were activated significantly more strongly by low versus high SF or vice versa. The results are shown in Figure 4. There was greater activation for low than high SF in the right fusiform gyrus (2 significant clusters, Z > 2.3, cluster-corrected threshold of P < 0.05, centered on MNI stereotactic coordinates 40 −34 −26, Z = 4.0 and 38 −54 −19, Z = 3.9). In contrast, there was greater activation for high than low SF in the left posterior fusiform gyrus (center of gravity located at −40 −55 −13, Z = 4.0), at coordinates consistent with the location of the VWFA (Jobard et al. 2003).
Figure 4.
Statistical maps of the SF analysis results, using a cluster-corrected threshold of Z > 2.3, P < 0.05. The functional localizer mask (outlined in red) was used to constrain the area of interest. The contrast of low versus high SF, shown in blue, resulted in 2 significant clusters (a,b), both in the right occipitotemporal fusiform gyrus. The opposite contrast of high versus low SF, shown in purple, revealed one significant cluster (c) in the left occiptotemporal fusiform gyrus. The plot shows the mean percentage change in parameter estimate for the contrast of low versus high SF for the 3 clusters a, b and c. Error bars represent 95% confidence intervals.
A further analysis was performed using subject-specific functional localizer masks (as displayed in Supplementary Fig. 1) in order to investigate how reliable these effects were across individuals. These individual masks were split into left and right halves and used to mask the contrast of low SF versus high SF for each participant. In this contrast, higher z-statistics indicated a stronger preference for low than for high SF. Ten out of 12 subjects showed higher average z-statistics in their right hemisphere voxels of interest than in the left hemisphere. A paired-subjects t-test demonstrated a significant difference in SF preference in the left and right hemisphere regions (t(11) = 2.73, P < 0.02). These data were also used to investigate the proportion of voxels that were preferential for low and high SFs in the left and right hemisphere masks. A repeated-measures analysis of variance confirmed that there were significantly more low SF preferential voxels in the right hemisphere regions (8.5% on average) than high SF preferential voxels (1.8% on average; t(11) = 2.4, P < 0.05). In the left hemisphere, this comparison was not significant—on average, 4.5% of voxels were preferential for high SF and 3.9% were preferential for low SF. Analyzing differences based on activation extent alone is inherently less sensitive and asking a different question than analyses that take both extent and strength into account—hence, the lack of a difference observed in the left hemisphere should not take precedence over the significant activation cluster depicted in Figure 4.
Discussion
Using simple sine-wave gratings, we have demonstrated differences in SF sensitivity in the fusiform gyrus: object-processing areas in the left fusiform gyrus were more strongly activated by high than low spatial frequencies, and areas in the right fusiform gyrus were more strongly activated by low than by high spatial frequencies. This finding demonstrates that SF, a low-level visual property, modulates activity in high-order ventral visual association cortex in the adult literate brain. Furthermore, the hemispheric asymmetry of high SF and low SF processing is concordant with the known lateralization of word and face processing. Therefore, the original hypothesis that motivated this study was confirmed.
The demonstration that an important low-level visual feature associated with written words, namely, high SF, is lateralized to the left hemisphere raises issues about when in development this occurs—and what drives this hemispheric bias. Both clinical (Rasmussen and Milner 1977) and functional imaging (Indefrey and Levelt 2004) studies clearly indicate that language-related systems are lateralized to the left hemisphere, and so, in that sense, the VWFA ends up on the ‘correct’ side of the brain. There are 3 possibilities: 1) There is an innate hemispheric bias for high and low SF, which provides the fertile ground for the development of word and face processing areas in development; 2) specialization for word and face processing in the left and right fusiform gyri causes neurons to become tuned to visual stimuli with similar perceptual characteristics; or 3) the lateralization of both SF processing and category-specific processing are driven by a third factor, for example, the lateralization of speech production to left frontotemporal regions may drive, through top-down processes, a preference for high SF in the left posterior fusiform gyrus. Whether innate or acquired, we predict that sensitivity to SF effectively channels the processing of particular classes of objects along visual pathways that are best adapted for their efficient processing: this might be tested by predicting the lateralization of occipitotemporal activations following training with novel object categories with high or low SF characteristics.
Our results are in agreement with those of a recent study by Andrews et al. (2010), which demonstrated preferential activation of the FFA and PPA to images of faces and places, respectively. This study revealed that the FFA was more strongly activated by Fourier-scrambled faces than scrambled places and vice versa for the PPA. This suggests that the FFA and PPA are sensitive to low-level visual features (such asSF) that remain intact after Fourier scrambling has removed all meaningful structure from the image.
SF and retinal eccentricity are not independent. Visual stimuli with high SF are normally viewed in high acuity foveal vision in order to resolve fine-grained detail; by contrast, stimuli with low SF tend to be larger and extend further into peripheral vision. As a result, retinotopic maps of eccentricity and SF in early visual cortex are very similar (Sasaki et al. 2001). Therefore, the results of the present study may not be the consequence of SF alone. Levy et al. (2001) and Hasson et al. (2002) have proposed a medial-to-lateral gradient in the fusiform gyri, depending on retinal eccentricity, and our results are compatible with a bias for left hemisphere processing of objects that depend most strongly on foveal vision for their recognition.
Our findings have potential implications for the understanding and treatment of conditions such as pure alexia and prosopagnosia, where unilateral damage to occipitotemporal cortex causes a selective impairment in recognition of words or faces, respectively. Recent studies have shown that patients with pure alexia have general visual deficits that are not limited to perception of orthographic stimuli (Mycroft et al. 2009; Starrfelt et al. 2009). SF sensitivity may be a potential locus of this impairment. There have also been suggestions that, compared with children with normal reading development, children with dyslexia may have impaired contrast sensitivity at SFs of around 2–8 cpd (Cornelissen 1993; Skottun 2000), and it has been demonstrated that contrast sensitivity around 2–4 cpd in preliterate children is a significant predictor of subsequent reading ability 2 years later (Lovegrove et al. 1986). These findings suggest that there is an association between the development of SF sensitivity and the acquisition of reading expertise. Further studies are needed to address the causal nature of this association.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
This work was supported by funding from the Medical Research Council (MRC) to Z.W. and from Research Councils UK (RCUK) to R.L.
Supplementary Material
Acknowledgments
We would like to thank the participants for taking part in the study and Fred Dick and Iead Rezek for their help with the data analysis. Conflict of Interest: None declared.
References
- Andrews TJ, Clarke A, Pell P, Hartley T. Selectivity for low-level features of objects in the human ventral stream. NeuroImage. 2010;49:703–711. doi: 10.1016/j.neuroimage.2009.08.046. [DOI] [PubMed] [Google Scholar]
- Barton JJ. Prosopagnosia associated with a left occipitotemporal lesion. Neuropsychologia. 2008a;46:2214–2224. doi: 10.1016/j.neuropsychologia.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Barton JJ. Structure and function in acquired prosopagnosia: lessons from a series of 10 patients with brain damage. J Neuropsychol. 2008b;2:197–225. doi: 10.1348/174866407x214172. [DOI] [PubMed] [Google Scholar]
- Benton A. Facial recognition 1990. Cortex. 1990;26:491–499. doi: 10.1016/s0010-9452(13)80299-7. [DOI] [PubMed] [Google Scholar]
- Binder JR, Mohr JP. The topography of callosal reading pathways: a case-control analysis. Brain. 1992;115:1807–1826. doi: 10.1093/brain/115.6.1807. [DOI] [PubMed] [Google Scholar]
- Cai Q, Lavidor M, Brysbaert M, Paulignan Y, Nazir TA. Cerebral lateralization of frontal lobe language processes and lateralization of the posterior visual word processing system. J Cogn Neurosci. 2008;20:672–681. doi: 10.1162/jocn.2008.20043. [DOI] [PubMed] [Google Scholar]
- Cantlon J, Pinel P, Dehaene S, Pelphrey K. Cortical representations of symbols, objects, and faces are pruned back during early childhood. Cereb Cortex. 2010;21:191–199. doi: 10.1093/cercor/bhq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehéricy S, Dehaene-Lambertz G, Hénaff MA, Michel F. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123:291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the visual word form area. Brain. 2002;125:1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Coltheart M. The MRC psycholinguistic database. Q J Exp Psychol A. 1981;33:497–505. [Google Scholar]
- Cornelissen PL. Fixation, contrast sensitivity and children’s reading. In: Wright SF, Groner R, editors. Facets of dyslexia and its remediation. Amsterdam (Netherlands): Elsevier; 1993. pp. 139–162. [Google Scholar]
- Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magn Reson Med. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I: segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Damasio H, Van Hoesen GW. Prosopagnosia: anatomic basis and behavioral mechanisms. Neurology. 1982;32:331–341. doi: 10.1212/wnl.32.4.331. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Damasio H. The anatomic basis of pure alexia. Neurology. 1983;33:1573–1583. doi: 10.1212/wnl.33.12.1573. [DOI] [PubMed] [Google Scholar]
- Eger E, Schweinberger SR, Dolan RJ, Henson RN. Familiarity enhances invariance of face representations in human ventral visual cortex: fMRI evidence. NeuroImage. 2005;26:1128–1139. doi: 10.1016/j.neuroimage.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Erkulvrawatr S. Alexia and left homonymous hemianopia in a non-right-hander. Ann Neurol. 1978;3:549–552. doi: 10.1002/ana.410030617. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Goodale M, Milner A. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Hasson U, Levy I, Behrmann M, Mendler T, Malach R. Eccentricity bias as an organizing principle for human high-order object areas. Neuron. 2002;34:479–490. doi: 10.1016/s0896-6273(02)00662-1. [DOI] [PubMed] [Google Scholar]
- Henriksson L, Nurminen L, Hyvarinen A, Vanni S. Spatial frequency tuning in human retinotopic areas. J Vis. 2008;8:1–13. doi: 10.1167/8.10.5. [DOI] [PubMed] [Google Scholar]
- Hirose G, Kin T, Murakami E. Alexia without agraphia associated with right occipital lesion. J Neurol Neurosurg Psychiatry. 1977;40:225–227. doi: 10.1136/jnnp.40.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iidaka T, Yamashita K, Kashikura K, Yonekura Y. Spatial frequency of visual image modulates neural responses in the temporo-occipital lobe: an investigation with event-related fMRI. Cognitive Brain Res. 2004;18:196–204. doi: 10.1016/j.cogbrainres.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJ. The spatial and temporal signatures of word production components. Cognition. 2004;92:101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: a metanalysis of 25 neuroimaging studies. NeuroImage. 2003;20:693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff AP, Spitsyna G, Plant GT, Wise RJ. Structural anatomy of pure and hemianopic alexia. J Neurol Neurosurg Psychiatry. 2006;77:1004–1007. doi: 10.1136/jnnp.2005.086983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy I, Hasson U, Avidan G, Hendler T, Malach R. Center-periphery organization of human object areas. Nat Neurosci. 2001;4:533–539. doi: 10.1038/87490. [DOI] [PubMed] [Google Scholar]
- Lovegrove W, Slaghuis W, Bowling A, Nelson P, Greeves E. Spatial frequency processing and the prediction of reading ability: a preliminary investigation. Percept Psychophys. 1986;40:440–444. doi: 10.3758/bf03208204. [DOI] [PubMed] [Google Scholar]
- Marzi CA, Distefano M, Tassinari G, Crea F. Iconic storage in the 2 hemispheres. J Exp Psychol Hum Percept Perform. 1979;5:31–41. doi: 10.1037//0096-1523.5.1.31. [DOI] [PubMed] [Google Scholar]
- Mattson AJ, Levin HS, Grafman J. A case of prosopagnosia following moderate closed head injury with hemisphere focal lesion. Cortex. 2000;36:125–137. doi: 10.1016/s0010-9452(08)70841-4. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Puce A, Gore J, Allison T. Face-specific processing in the human fusiform gyrus. J Cogn Neurosci. 1997;9:605–610. doi: 10.1162/jocn.1997.9.5.605. [DOI] [PubMed] [Google Scholar]
- Meadows JC. The anatomical basis of prosopagnosia. J Neurol Neurosurg Psychiatry. 1974;37:489–501. doi: 10.1136/jnnp.37.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercure E, Dick F, Halit H, Kaufman J, Johnson MH. Differential lateralization for words and faces: category or psychophysics? J Cogn Neurosci. 2008;20:2070–2087. doi: 10.1162/jocn.2008.20137. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Mycroft RH, Behrmann M, Kay J. Visuoperceptual deficits in letter-by-letter reading? Neuropsychologia. 2009;47:1733–1744. doi: 10.1016/j.neuropsychologia.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Peyrin C, Chokron S, Guyader N, Gout O, Moret J, Marendaz C. Neural correlates of spatial frequency processing: a neuropsychological approach. Brain Res. 2006;1073:1–10. doi: 10.1016/j.brainres.2005.12.051. [DOI] [PubMed] [Google Scholar]
- Pillon B, Bakchine S, Lhermitte F. Alexia without agraphia in a left-handed patient with a right occipital lesion. Arch Neurol. 1987;44:1257–1262. doi: 10.1001/archneur.1987.00520240039009. [DOI] [PubMed] [Google Scholar]
- Polich JM. Hemispheric differences in stimulus identification. Percept Psychophys. 1978;24:49–57. doi: 10.3758/bf03202973. [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Milner B. The role of early left-brain injury in determining lateralization of cerebral speech functions. Ann N Y Acad Sci. 1977;299:355–359. doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Hadjikhani N, Fischl B, Liu AK, Marrett S, Dale AM, Tootell RB, Marret S. Local and global attention are mapped retinotopically in human occipital cortex. Proc Natl Acad Sci USA. 2001;98:2077–2082. doi: 10.1073/pnas.98.4.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RB. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- Sergent J. Theoretical and methodological consequences of variations in exposure duration in visual laterality studies. Percept Psychophys. 1982;31:451–461. doi: 10.3758/bf03204855. [DOI] [PubMed] [Google Scholar]
- Sergent J. Role of the input in visual hemispheric asymmetries. Psychol Bull. 1983;93:481–512. [PubMed] [Google Scholar]
- Sergent J, Ohta S, MacDonald B. Functional neuroanatomy of face and object processing: a positron emission tomography study. Brain. 1992;115:15–36. doi: 10.1093/brain/115.1.15. [DOI] [PubMed] [Google Scholar]
- Skottun BC. The magnocellular deficit theory of dyslexia: the evidence from contrast sensitivity. Vision Res. 2000;40:111–127. doi: 10.1016/s0042-6989(99)00170-4. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Solina F, Peer P, Batagelj B, Juvan S, Kovac J. Color-based face detection in the ‘5 seconds of fame’ art installation. 2003 Paper presented at the Mirage 2003 Conference on Computer Vision/Computer Graphics Collaboation for Model-based Imaging, Rendering, Image Analysis and Graphical Special Effects, Rocquencourt, France. [Google Scholar]
- Starrfelt R, Habekost T, Leff AP. Too little, too late: reduced visual span and speed characterize pure alexia. Cereb Cortex. 2009;19:2880–2890. doi: 10.1093/cercor/bhp059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Haxby JV. ‘What’ and ‘where’ in the human brain. Curr Opin Neurobiol. 1994;4:157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Willems RM, Peelen MV, Hagoort P. Cerebral lateralization of face-selective and body-selective visual areas depends on handedness. Cereb Cortex. 2010;20:1719–1725. doi: 10.1093/cercor/bhp234. [DOI] [PubMed] [Google Scholar]
- Winkelman MD, Glasson CC. Unilateral right cerebral representation of reading in a familial left-hander. Neuropsychologia. 1984;22:621–626. doi: 10.1016/0028-3932(84)90027-7. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: an introduction to methods. Oxford (UK): Oxford University Press; 2001. Chapter 14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.