Abstract
A large-scale study of 484 elementary school children (6–10 years) performing word repetition tasks in their native language (L1-Japanese) and a second language (L2-English) was conducted using functional near-infrared spectroscopy. Three factors presumably associated with cortical activation, language (L1/L2), word frequency (high/low), and hemisphere (left/right), were investigated. L1 words elicited significantly greater brain activation than L2 words, regardless of semantic knowledge, particularly in the superior/middle temporal and inferior parietal regions (angular/supramarginal gyri). The greater L1-elicited activation in these regions suggests that they are phonological loci, reflecting processes tuned to the phonology of the native language, while phonologically unfamiliar L2 words were processed like nonword auditory stimuli. The activation was bilateral in the auditory and superior/middle temporal regions. Hemispheric asymmetry was observed in the inferior frontal region (right dominant), and in the inferior parietal region with interactions: low-frequency words elicited more right-hemispheric activation (particularly in the supramarginal gyrus), while high-frequency words elicited more left-hemispheric activation (particularly in the angular gyrus). The present results reveal the strong involvement of a bilateral language network in children’s brains depending more on right-hemispheric processing while acquiring unfamiliar/low-frequency words. A right-to-left shift in laterality should occur in the inferior parietal region, as lexical knowledge increases irrespective of language.
Keywords: foreign language, functional near-infrared spectroscopy (fNIRS), learning, native language, phonology
Introduction
Native, or first, language (L1) acquisition is a natural phenomenon, and it occurs even without intervention. Skinner (1957) suggested that a child acquires L1 through imitating the language of its parents or caregivers. Children do imitate adults, and repetition of new words and phrases is a basic feature of a child’s speech. A body of studies in various research domains, including psychology, linguistics, and anthropology, has intensively discussed the role of repetition (often referred to as imitation) in language acquisition, reporting that repetition facilitates grammatical and lexical development (Corrigan 1980; Snow 1981, 1983; Kuczaj 1982; Speidel and Nelson 1989; Perez-Pereira 1994).
On the other hand, learning a nonnative, or second, language (L2) is not always as easy as acquiring L1. Repetition in a foreign language is a more difficult task than that in L1 as it requires learners to process unfamiliar speech sounds. Particularly, it entails auditory perception skills as well as memory and articulation skills. Previous studies suggest that the ability to replicate unfamiliar foreign pronunciation and intonation is associated with the capacity to learn foreign languages (Tahta et al. 1981; Service 1992). Therefore, the ability to repeat unfamiliar foreign sounds can be considered an indicator of foreign language learning predisposition and also of the robustness of some neurofunctional processes involved in speech.
In recent years, a large body of neuroimaging and neurophysiological studies has been devoted to the study of the neural organization of language (Hickok and Poeppel 2000; Kutas and Federmeier 2000; Ullman 2001; Friederici 2002; Kaan and Swaab 2002). Such neuroimaging studies have not only converged with the findings of clinical aphasiology but have also started to broaden our understanding of the neural basis of language processing. The left perisylvian region of the human cortex is known to play a major role in language processing (Galaburda et al. 1978; Caplan and Waters 1999; Geschwind and Miller 2001). On the other hand, we have progressively learned that respective brain regions within or outside of the traditional left perisylvian areas and the language processing networks encompassing frontal, temporal, and/or parietal regions differentially contribute to or are involved in specific aspects of linguistic computation, such as syntax, semantics, and phonology, from the word level to sentence processing (Grodzinsky 2000; Price 2000; Friederici 2002; Indefrey and Levelt 2004; Poeppel and Hickok 2004; Szaflarski et al. 2006).
Recent research has also demonstrated that both the left and right hemispheres (LH, RH) contribute to varying aspects of language processing in the normal brain (Beeman and Chiarello 1998; Gandour et al. 2000; Friederici 2002; Zatorre et al. 2002; Friederici and Alter 2004) even though historical and current works still regard the LH as having a primary and significant role in language processing. As previous neuroimaging work has indicated that word repetition tasks elicit widespread bilateral activation in areas associated with auditory processing of speech (Howard et al.1992; Castro-Caldas et al. 1998; McCrory et al. 2000; Price 2000; Liégeois et al. 2003), in the present study, we employed a word repetition task as a robust predictor of language learning ability in children (Tahta et al. 1981; Service 1992) and explored its neural substrate.
To date, positron emission tomography (PET), functional magnetic resonance imaging (fMRI), event-related potential, and magnetoencephalography have been used extensively to elucidate detailed pictures of the brain–language relationship. In addition, a relatively new brain imaging technique, functional near-infrared spectroscopy (fNIRS), has been demonstrated to be an effective tool for monitoring local changes in cerebral oxygenation and hemodynamics during functional brain activation. Functional NIRS has a major advantage in developmental studies with children, especially for large-scale studies: Unlike PET, which uses injections of a radioactive substance, or fMRI, which uses strong magnetic fields and is physically restrictive, fNIRS is a fully noninvasive and unrestrictive neuroimaging technique that enables the real-time monitoring of brain hemodynamics of children (Hoshi and Chen 2002), infants (Meek et al. 1998; Taga et al. 2003; Homae et al. 2006, 2007; Bortfeld et al. 2007, 2009; Minagawa-Kawai et al. 2007, 2009), and even neonates (Sakatani et al. 1999; Peña et al. 2003), as well as adults (Maki et al. 1995; Watanabe et al. 1998). Its components and setup are compact compared with fMRI and PET, and the application of the measurement probes is also quick and easy, allowing the effective acquisition of mass data. In addition, a participant’s motion during measurement is tolerated to a higher degree than in fMRI and PET (Watanabe et al. 1998; Ikegami and Taga 2008; Hull et al. 2009), in which the head position must be strictly fixed and vocalization may induce severe motion artifacts (Hinke et al. 1993; Yetkin et al. 1995; Birn et al. 1998, 1999; Barch et al. 1999; Wilson et al. 2004). Given that elicited imitation is necessarily accompanied by articulation and small motions of the participant’s head, this advantage makes fNIRS a primary candidate for the language task employed in the current study.
In general, functional neuroimaging studies of children pose unique scientific, ethical, and technical challenges. Although there are numerous lesion and neuroimaging studies on the brain–language relationship, most of them are small in size. In addition, the inevitable differences in age, tasks, culture, L2-learning environments, and so on, make it difficult to see the overall picture of the study results. Studies with small sample pools also tend to result in reduced statistical power, limiting the interpretation of their results. In reality, however, it is often difficult for researchers to recruit participants and acquire data, especially in studies of children. Recruiting participants is especially challenging in the study of normally developing children as they do not receive any direct benefits from the research, and this difficulty increases for longitudinal studies. Moreover, acquisition of data for child subjects is restricted by many factors including restlessness, motion, lack of child-friendly language tasks, and so on, as children are unable to comply with complicated tasks for long periods of time. For these reasons, most studies focus on adults, infants, or patients. As language skills continue to develop rapidly in children during the school-age years, systematic observation of functional brain development (in both L1 and L2) is crucial. While behavioral studies are abundant, there are only a few studies dealing with normally developing school-aged children (ca., 6–12 years) using neuroimaging techniques (Gaillard et al. 2003a, 2003b; Sachs and Gaillard 2003; Szaflarski et al. 2006), and literature dealing with L2 acquisition is even more unobtainable, although studies dealing with older children have been conducted (Sakai 2005; Tatsuno and Sakai 2005). Furthermore, previous neuroimaging studies regarding children focused mainly on perception or comprehension rather than articulation or production because of instrumental limitations including articulation-induced motion artifacts.
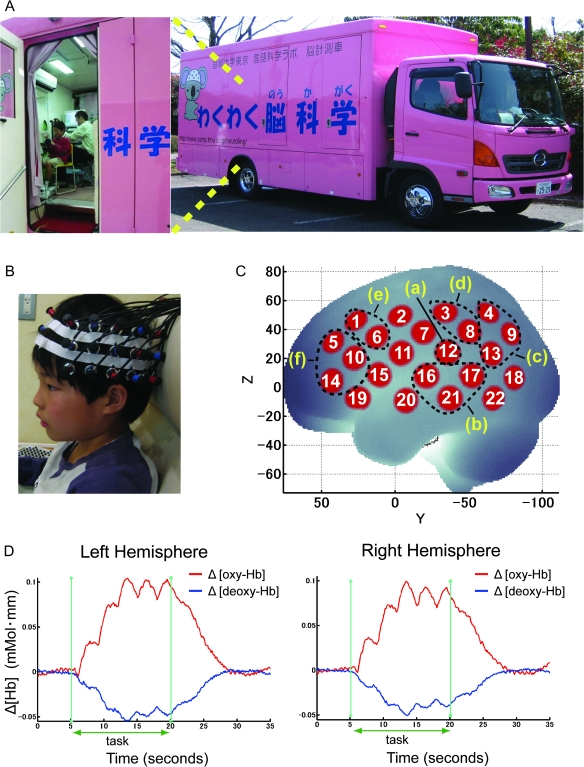
In order to overcome these limitations, we have conducted a large-scale 3-year cohort study enrolling approximately 500 normally developing elementary school children (6–10 years of age) per year in Japan. In this paper, we report the results of a cross-sectional examination of the data obtained from the middle year of the cohort study. We investigated children in 3 age groups in the initial analyses, and put them together in the subsequent analyses. We utilized fNIRS as a data acquisition tool and a basic word repetition task as a predictor of language learning ability. To fully exploit the merits of fNIRS while performing a massive neuroimaging analysis of elementary school children, we installed an fNIRS system in a mobile laboratory, shown in Figure 1A, so that the neuroimaging facility could be transported to the elementary schools.
Figure 1.
fNIRS measurements. (A) Our original neuroimaging vehicle. (B) Closeup view of the fNIRS equipment. fNIRS data were obtained using a 44-channel spectrometer (Hitachi ETG-4000). A 3 × 5 array of 8 laser diodes and 7 light detectors was applied, resulting in 22 channels on each side of the participant’s head. (C) Cortical projection points of fNIRS measurements (location of 22 channels) and the 6 defined ROIs for language processing are mapped onto the MNI standard brain coordinate system by spatial registration. This figure shows the left hemisphere. The locations of the 22 channels and 6 ROIs on the RH are symmetrical to those of the left hemisphere. The 6 defined ROIs: (a) the primary and auditory association cortices consisting of BAs (BA 41, 42) with channel 12, (b) the vicinity of Wernicke’s area, the posterior part of the superior/middle temporal gyri (BA 21, 22) with channels 16, 17, and 21, (c) the angular gyrus (BA 39) with channels 4, 9, and 13, (d) the supramarginal gyrus (BA 40) with channels 3 and 8, (e) the pars opercularis, part of Broca’s area, (BA 44) with channels 1 and 6, and (f) the pars triangularis, part of Broca’s area, (BA 45) with channels 5, 10, and 14. (D) An example of the time course in [oxy-Hb] and [deoxy-Hb] of grand-averaged data of the 392 participants for the channel that showed the highest t-value in [oxy-Hb] signals during word repetition tasks. (Channel 6 on the LH showed the highest t-value. The time course of the hemodynamic response at the same channel on the right homolog is also shown.) Red line: Δ [oxy-Hb]; blue line: Δ [deoxy-Hb]; vertical green line: task onset and end timing. Increases in [oxy-Hb] and decreases in [deoxy-Hb] indicate brain activations.
As the language system dramatically develops during childhood, we expect that brain functions and structures do as well. With this in mind, we first investigated whether developmental changes in cortical activation during a word repetition task exist or not. Following the results that age variances among our subjects produced no salient differences, in the present study, we aimed to investigate the factors (language: L1/L2 and word frequency: high/low), which would influence cortical representation. We also explored the different characteristics of language-related regions of interest (ROIs) and hemispheric laterality with respect to L1 and L2 processing in developing brains of school-age children. In addition, the characteristics of [oxy-Hb] and [deoxy-Hb] signals were compared. It should be noted here that, at this stage, presenting a broad view is of great importance because previous studies on this age group have been small in size. Consequently, creating a systematic picture based on various studies is difficult due to different task employment, differences in neuroimaging methods, cultural differences of participants, and differences in languages or L2-learning environments. Hence, we have chosen to omit the details of group and/or individual differences, which will be presented in subsequent reports. Unlike previous studies, which focused on either L1 or L2, but not both at the same time, this study addresses both L1 and L2 processing by the same individuals at the same time, enabling us to compare different facets of language processing in the young developing brain.
Materials and Methods
Participants
The present study was carried out on 484 children (248 girls and 236 boys) from 7 different elementary schools in Japan. Their mean age was 8.93 ± 0.89 (mean ± standard deviation [SD]) with an age range of 6–10 years. All participants completed a questionnaire before commencing this study. Nonnationals and participants with psychiatric disorders are excluded from the analyses. The Edinburgh Handedness Inventory (Oldfield 1971) was used to determine hand dominance. The left-handed (8) and ambidextrous (38) were excluded from the analyses and only right-handed participants (438) were further analyzed. Each participant’s parent gave written informed consent before their child’s participation in this study, and each participant was given a token of gratitude for his/her involvement after the experiment. All the procedures in this study were approved by the Human Subject Ethics Committee of Tokyo Metropolitan University.
Children’s Exposure to English
We had participants of the same age with different levels of English proficiency as they had had different levels of exposure to L2. Some public schools provided 45-min English lessons (11–35 school h/year), while others did not. The children who went to public schools that did not provide English lessons had been exposed to English through commercial language schools and/or home study. The frequency of the English lessons provided by commercial language schools did not differ much from those provided by public schools. As for home study, the parents/caretakers provided their children with exposure to English, using videos, CDs, and other learning materials. A few children who had at least one parent who was a native English speaker took part in our project, but their data were excluded from the analyses because English was not a foreign language for them. Our study also included some children who went to a private school which ran an immersion program, where English was not the subject of study but the language through which other subjects, such as arithmetic, were taught. Immersion programs are often associated with bilingual societies such as the Province of Quebec in Canada, but this Japanese private school is located in a monolingual city and is not an international school; hence, these children were not excluded.
As described above, we had participants with different levels of exposure to L2. However, before analyzing the effect of L2 proficiency or exposure, which will be presented in a subsequent paper, we have attempted to obtain an overall view of the cortical representation of L1 and L2 in the present report.
Experimental Tasks
We employed a word repetition task: recordings of speech samples from a female native speaker of Japanese and from one of English were used for the experimental stimuli. We used 120 single words: 30 Japanese high-frequency words (Jpn_HF), 30 Japanese low-frequency words (Jpn_LF), 30 English high-frequency words (Eng_HF), and 30 English low-frequency words (Eng_LF). High-frequency words are defined as words that have >50 occurrences per million while the low-frequency words have <5 occurrences per million. All words used in this experiment were emotionally neutral and taken from 2 corpora: one by Amano and Kondo (2000) for Japanese and the other by Kučera and Francis (1967) for English. A list of all the words used in this study is provided in Supplementary Table 1. All Japanese words contained 4 morae (Japanese syllabic unit), and English words consisted of 2 syllables. The length of Japanese and English words was kept approximately equal (within ±10% difference). The mean durations of Japanese and English words used in each task (30 words for each task) were 643.0 ms (Jpn_HF), 648.4 ms (Jpn_LF), 737.5 ms (Eng_HF), and 725.9 ms (Eng_LF).
After the procedure was described to the children, they were seated in a chair and given instructions to repeat the words presented from a loud speaker. They were asked to overtly repeat the words as they heard them. The children heard the stimuli through the loud speaker at a comfortable volume (around 65 dB SPL). The order of the 4 tasks (Jpn_HF, Jpn_LF, Eng_HF, and Eng_LF) was counterbalanced, and the stimuli within each task were presented in blocks of 5 words. One task consisted of 6 blocks, presented in random order while stimuli in each block were kept in the same sequence. One block was 35 s: a 5-s prestimulus period, 15-s stimulus period, and 10-s recovery period, followed by a 5-s poststimulus period. Children were asked to do a brief practice session of the word repetition task before the experiment. The word stimuli used for the practice session were not used for the experiment. Each experimental stimulus was presented only once per participant. During fNIRS measurement, children were instructed to look at a fixation point. In order to minimize head motion, children were asked to hold their body as still as possible during the tasks. Their oral repetition responses were recorded. An experimenter checked the children’s performance during the practice session for whether their utterance was clear and their head movement was within tolerance. When a participant’s utterance was so loud that his/her vocalization might induce severe motion artifacts, or so soft that his/her voice data may fail to record, the participant was asked to change his/her behavior until his/her performance level fell within tolerance. Children took a short rest between tasks.
As for the behavioral data, whether the words were correctly repeated or not was evaluated phoneme by phoneme by a native Japanese and bilingual (Japanese and English) speaker. Repetition success rates were calculated and statistical comparisons were made between the 4 tasks (Jpn_HF, Jpn_LF, Eng_HF, and Eng_LF) using a 2 × 2 repeated-measures analysis of variance (ANOVA) (2 languages × 2 word-frequencies). Children were also asked to judge whether they knew the words heard in the 4 repetition tasks or not, according to the following criteria: 1) I know the word and its meaning, 2) the word is familiar but its meaning is not known, or 3) the word is not familiar at all. Statistical comparisons of the children’s ratings of their semantic knowledge of the word stimuli (i.e., the relative frequencies of ratings of 1) in the above criteria) were conducted between the 4 tasks using a 2 × 2 repeated-measures ANOVA (2 languages × 2 word-frequencies). Details of the ANOVA main-effect results were investigated using paired t-tests when a significant interaction was found.
It is possible that duration and intensity of children’s utterances were different between L1 and L2, and it is conceivable that a longer and stronger utterance may lead to greater brain activation. In order to clarify this point, acoustic analysis was conducted, and the results were compared between the 4 tasks. For the acoustic analysis, the root mean square (RMS; an estimate of sound intensity) was calculated from the amplitude of the speech signal. The RMS amplitude is the square root of the average (mean) of the square of the distance of the sound curve (waveform) from the baseline. The amount of sound to which a child was exposed is not just a matter of sound intensity but also of the duration involved. Therefore, the total sound exposure during the word repetition period (6 blocks) was integrated and defined as TASK-RMS. The total of the rest period (7 rest blocks between 6 task blocks) was integrated in the same way and defined as REST-RMS. The ratios between TASK-RMS and REST-RMS were calculated for all the children and were represented in decibels (dB). Thus, the temporal integration of acoustic intensity (which represents intensity × duration of speech sound, that is, TASK-RMS/REST-RMS in decibels) during task periods and its statistics between the 4 tasks (Jpn_HF, Jpn_LF, Eng_HF, and Eng_LF) were determined. A 2 × 2 repeated-measures ANOVA (2 languages × 2 word frequencies) was performed.
Data Acquisition—fNIRS
Functional NIRS data were obtained using a multichannel spectrometer (ETG-4000, Hitachi Medical Co., Tokyo, Japan). A 3 × 5 array of optodes consisting of 8 laser diodes and 7 light detectors, alternately placed at an interoptode distance of 3 cm to yield 22 channels, was applied on each side of the participant’s head (Fig. 1B). The middle column of the 3 × 5 array was placed along the coronal reference curve (T3-C3-Cz-C4-T4) of the international 10/20 system (Jurcak et al. 2005, 2007) so that the lower edge of the array was placed directly above the ear. The highest sensitivity of hemodynamic changes in the lateral cortical region encompassing a pair of optodes is expected to be localized at the midpoint between the optodes (Okada et al. 1997), and this point is the location of a channel. Optical data from individual channels were collected at 2 different wavelengths (695 and 830 nm) and analyzed using the modified Beer–Lambert Law for a highly scattering medium (Cope et al. 1988). Changes in oxygenated ([oxy-Hb]), deoxygenated ([deoxy-Hb]), and total hemoglobin ([total-Hb]) signals were calculated in units of millimolar–millimeter (Maki et al. 1995). Optical signals were sampled at a rate of 10 Hz.
Spatial Registration
After going through all 4 tasks, the positions of optodes and scalp landmarks (i.e., nasion, right and left preauricular points, and Oz and Cz of the international 10–20 system) were measured for each participant using an electromagnetic 3D digitizer system (ISOTRAK II, Polhemus Inc.).
We employed virtual registration (Tsuzuki et al. 2007) to register fNIRS data to Montreal Neurological Institute (MNI) standard brain space (Brett et al. 2002). Briefly, utilizing the positional information of a particular channel relative to the anatomical landmarks, this method enables the placement of a virtual probe holder on the scalp by simulating the holder’s deformation and thereby registering probes and channels onto the reference brains, in place of a participant’s brain, in a probabilistic manner. The optodes and channels were registered onto the surface of an averaged reference brain in MNI space (Okamoto et al. 2004), and the most likely coordinates for the channels were subjected to anatomical labeling using a Matlab function (Okamoto et al. 2009; available at http://brain.job.affrc.go.jp. Last accessed date: February 18, 2011), which reads anatomical labeling information coded in a macroanatomical brain atlas constructed by Tzourio-Mazoyer et al. (2002) and the Brodmann cytoarchitectonic area atlas available in the MRIcro program (Rorden and Brett 2000). Specifically, for each surface voxel of the atlas brains, the function scanned anatomical labels of surface voxels located within a sphere with a radius of 10 mm from a given voxel corresponding to a channel location and reassigned the most frequent labels to that voxel.
Referring to thus-acquired macroanatomical labels, we combined the channels to set ROIs based on the mode macroanatomical label in each channel (Fig. 1C). For example, channels 3 and 8 with the mode anatomical label on the left supramarginal gyrus at 67% and 85%, respectively, were combined to generate the left supramarginal gyrus ROI. Since a recent study clarified that optical properties including optical path length between corresponding channels on the RH and LH do not differ significantly (Katagiri et al. 2010), brain activation in both hemispheres was compared.
Verification of Anatomical Information for Representative Data with MRI
Although Okamoto’s method is based on the adult brain, it was used for the children in our study, as there is evidence indicating minimal anatomical differences between children, ages 7 and 8, and adults relative to the resolution of fMRI data (Burgund et al. 2002) and minimal difference in functional foci between adults and children (Kang et al. 2003). Some other research has also indicated that adult standard brain atlases are valid for children over 6 years of age (Talaraich and Tournoux 1988; Muzik et al. 2000; Schlaggar et al. 2002). For confirmation, the positions of the probes from 30 representative cases (10 representative participants × 3 images, one from each of the 3 years of our cohort study) were measured using a 3D digitizer and translated to participants’ MRI images using a 3D Composite Display Unit (Hitachi Medical Co., Japan). The probe positions and the MR images compared are from the same children. Probe and channel positions were projected onto the cortical surface of individual participants, to examine cortical structures underlying each measuring position. Anatomical information obtained by spatial registration and by MR images was compared, and it was confirmed that the outcome was consistent.
fNIRS Data Analysis
First, the participants whose task performance or behavior did not meet our criteria were excluded from further analyses. We evaluated whether the words were correctly repeated or not phoneme by phoneme for each participant. The participants with a repetition success rate of less than 70% were excluded from the analyses. Note that the present study focused on the difference in cortical representation of L1 and L2, and whether the words were correctly pronounced or not was not a main issue here. A repetition was considered complete when we are able to evaluate a subject’s performance (pronunciation) from the oral recording. No repetition at all or vocalization that was too soft or not clear enough to evaluate, were considered repetition failures. fNIRS data were preprocessed using the Platform for Optical Topography Analysis Tools (Adv. Res. Lab., Hitachi Ltd.), a plug-in-based analysis platform that runs on Matlab (The MathWorks, Inc.). To remove components originating from slow fluctuations of cerebral blood flow and heartbeat noise, the Hb signals were bandpass filtered between 0.02 and 1 Hz, and, by detecting rapid changes in [total-Hb] signal (signal variations >0.1 mmol·mm over 2 consecutive samples), all blocks that had been affected by movement artifacts were subsequently identified and removed. Following this elimination process, participant data that contained a minimum of 3 of 6 data blocks for each task were used. In addition, by visual inspection, we discarded an entire task when there was insufficient optical signal (i.e., when the peak signal of [oxy-Hb] during the task period was lower than approximately 0.01 mmol·mm as determined with reference to the SD of the rest period) due to obstruction by hair or for other reasons. We utilized the channels that had >60% survival rate of data after the motion check. As channels 15 and 20 did not reach the criterion due to movement in the temporal muscles, they were not used for further analyses.
In each individual set of hemoglobin data, we extracted data blocks from time course data. Each data block consisted of 5 s prior to stimulus onset, 15 s of stimulus, 10 s of recovery, and a 5 s poststimulus period. For each channel in nonrejected blocks, a first-degree baseline fit to the mean of the 5 s prestimulus period and 5 s of the poststimulus period was performed.
For statistical analyses, we opted to focus on the [oxy-Hb] signal because it is more sensitive to changes in cerebral blood flow than are [deoxy-Hb] and [total-Hb] signals (Hoshi et al. 2001; Strangman et al. 2002; Hoshi 2003), has a higher signal-to-noise ratio (Strangman et al. 2002), and also has a higher retest reliability (Plichta et al. 2006). On the other hand, it has been indicated that [oxy-Hb] signal is sensitive to extracerebral blood volume changes and is more prone to contamination from extracerebral artifacts (Boden et al. 2007). Moreover, a recent fNIRS study revealed that the word-frequency effect elicited significant differences between low and high-frequency words for decreases in [deoxy-Hb], while [oxy-Hb] changes only showed a nonsignificant trend (Hofmann et al. 2008). Thus, we also examined [deoxy-Hb] for the main analyses (whole-group analyses). To apply [deoxy-Hb] changes to the analyses also has merit in linking the fNIRS studies to fMRI-based imaging literature, as a decrease in [deoxy-Hb] corresponds well to an increase in blood oxygen level–dependent contrast (Kleinschmidt et al. 1996). For each child, the mean change in concentration of [oxy-Hb] and [deoxy-Hb] over 25 s after the onset of stimulus was calculated for each task and for each channel.
All statistical analyses were carried out using the SPSS statistical package (SPSS Inc.). First, Student’s t-tests (P < 0.05 Bonferroni corrected for familywise errors) were conducted to examine the activation of each independent channel (22 channels in the LH and 22 channels in the RH) for each of the 4 tasks. Activity during the stimulus and recovery periods (25 s) was compared with that from the baseline periods (5 s prestimulus and 5 s poststimulus).
Second, we defined appropriate ROIs for language processing according to the results of spatial registration. Six ROIs were selected bilaterally referring to an MNI-compatible macroanatomical atlas (Automatic Anatomical Label) from the channels that showed a statistically significant increase of [oxy-Hb] for at least one out of 4 tasks (Jpn_HF, Jpn_LF, Eng_HF, and Eng_LF) in either the LH or the RH. We did this because even if [oxy-Hb] did not show significant activation for 3 of 4 tasks, there is value in comparing the 1 task that did show significant [oxy-Hb] increase with the other 3 tasks. The overall [oxy-Hb] signal level in a single ROI was obtained by calculating the unweighted mean [oxy-Hb] signal level of all the channels within the ROI. Figure 1C shows the location of the channels and the 6 defined ROIs for language processing mapped onto the MNI standard brain: a) the primary and auditory association cortices consisting of Brodmann areas (BA 41, 42) with channel 12; b) the vicinity of Wernicke’s area, the posterior part of the superior/middle temporal gyri (BA 21, 22) with channels 16, 17, and 21; c) the angular gyrus (BA 39) with channels 4, 9, and 13; d) the supramarginal gyrus (BA 40) with channels 3 and 8; e) the pars opercularis, part of Broca’s area (BA 44), with channels 1 and 6; and f) the pars triangularis, part of Broca’s area (BA 45), with channels 5, 10, and 14. MNI coordinates of the estimated cortical projection points for all the channels are also shown in Table 1.
Table 1.
MNI coordinates of the estimated cortical projection points for the channels used for ROIs
| LH | MNI coordinate |
RH | MNI coordinate |
Brain area | ||||
| X | Y | Z | X | Y | Z | |||
| CH01 | –45 | 26 | 45 | CH01 | 48 | 27 | 43 | POP (BA 44) |
| CH03 | –60 | –34 | 50 | CH03 | 64 | –31 | 49 | SMG (BA 40) |
| CH04 | –51 | –62 | 50 | CH04 | 56 | –57 | 48 | AG (BA 39) |
| CH05 | –45 | 41 | 30 | CH05 | 48 | 42 | 29 | PTR (BA 45) |
| CH06 | –58 | 12 | 34 | CH06 | 60 | 13 | 33 | POP (BA 44) |
| CH08 | –63 | –50 | 38 | CH08 | 66 | –45 | 37 | SMG (BA 40) |
| CH09 | –48 | –76 | 36 | CH09 | 52 | –72 | 34 | AG (BA 39) |
| CH10 | –56 | 27 | 20 | CH10 | 58 | 29 | 19 | PTR (BA 45) |
| CH12 | –68 | –35 | 24 | CH12 | 71 | –32 | 24 | PAAC (BA 41, 42) |
| CH13 | –60 | –64 | 23 | CH13 | 63 | –60 | 22 | AG (BA 39) |
| CH14 | –52 | 42 | 4 | CH14 | 54 | 44 | 4 | PTR (BA 45) |
| CH16 | –69 | –21 | 8 | CH16 | 72 | –19 | 8 | Posterior part of SMTG (BA 21, 22) |
| CH17 | –67 | –51 | 8 | CH17 | 70 | –48 | 8 | Posterior part of SMTG (BA 21, 22) |
| CH21 | –70 | –37 | –7 | CH21 | 72 | –34 | –7 | Posterior part of SMTG (BA 21, 22) |
Note: Names of the channels shown in Figure 1C are indicated in the first (LH) and fifth (RH) columns. All values are in millimeters. PAAC = primary and auditory association cortices, SMTG = superior/middle temporal gyri, AG = angular gyrus, SMG = supramarginal gyrus, POP = pars opercularis, part of Broca’s area, and PTR = pars triangularis, part of Broca’s area.
We first investigated the relationship between age and brain response during L1 task performance for the 6 ROIs. We opted to analyze the Japanese task data, as we had participants of the same age with different levels of English proficiency (exposure to L2), as mentioned above, and it is hard to observe developmental effects with L2 tasks. The total of 392 children who satisfied all our entry criteria were used as described earlier, and divided into 3 age groups (age 8 group = 130, mean age ± SD: 8.0 ± 0.4; age 9 group = 130, 8.9 ± 0.2; age 10 group = 132, 9.9 ± 0.4). 2 × 2 × 3 3-way ANOVAs were performed for each defined ROI with the within-subject effects of hemisphere (LH and RH) and word frequency (high and low), and the between-subject effect of age group (age 8–10). Familywise errors were Bonferroni-corrected for 6 tests. A significance level of P < 0.05 was applied after correction for multiple testing. For confirmation, we also conducted regression analyses of the relation between age and brain activation (relative [oxy-Hb] changes) during L1 frequent-word repetition tasks. The results were Bonferroni corrected for 12 tests with a significance level of P < 0.05.
Next, whole-study group analyses were conducted to produce an overall view of L1 and L2 processing. Statistical analyses using a 3-way repeated-measures ANOVA were conducted for each ROI to evaluate the effects of 3 within-subject factors: the 2 languages (Japanese: L1, and English: L2), 2 word-frequencies (high and low), and 2 hemispheres (LH and RH). P values were Bonferroni corrected for 6 tests with a significance level of P < 0.05 after correction for multiple testing.
Results
Behavioral Results
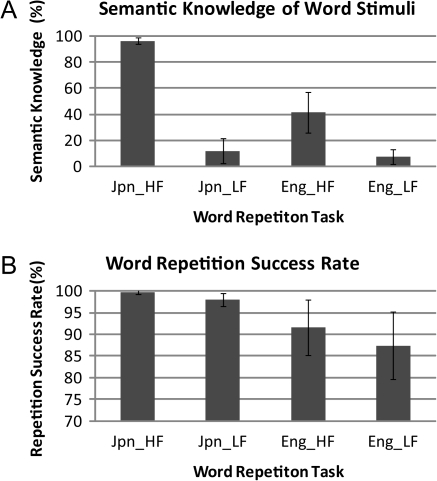
A comparison of the children’s ratings of their semantic knowledge of the word stimuli among the 4 repetition tasks is shown in Figure 2A. As exhibited in the figure, mean semantic knowledge of the Japanese high-frequency words (96%) was much higher than that of the Japanese low-frequency words (12%), the English high-frequency words (42%) and the English low-frequency words (8%). It was also revealed that children rarely have semantic knowledge of low-frequency words irrespective of language. Statistical comparisons of the children’s ratings of their semantic knowledge of the word stimuli between the 4 tasks using a 2 × 2 repeated-measures ANOVA revealed highly significant main effects of language (F [1, 29] = 253.76, P < 0.001), word frequency (F [1, 29] = 1492.75, P < 0.001), and a language × word-frequency interaction (F [1, 29] = 209.79, P < 0.001). Appropriate post hoc pairwise comparisons using paired t-tests showed significant differences in children’s ratings of their semantic knowledge between the tasks: Jpn_HF > Eng_HF, Jpn_HF > Jpn_LF, and Eng_HF > Eng_LF (corrected P < 0.001), but the difference between Jpn_LF & Eng_LF failed to reach significance.
Figure 2.
Behavioral results. (A) A comparison of the semantic knowledge between the 4 tasks (Jpn_HF, Jpn_LF, Eng_HF, and Eng_LF) and (B) a comparison of word repetition success rate between the 4 tasks.
A comparison of word repetition success rates between the 4 tasks is shown in Figure 2B. A 2 × 2 repeated-measures ANOVA on repetition success rates showed highly significant main effects of language (F [1, 391] = 853.62, P < 0.001), word frequency (F [1, 391] = 398.73, P < 0.001), and a language × word–frequency interaction (F [1, 391] = 63.74, P < 0.001). Appropriate post hoc pairwise comparisons using paired t-tests (Jpn_HF > Eng_HF, Jpn_LF > Eng_LF, Jpn_HF > Jpn_LF, and Eng_HF > Eng_LF) showed significant differences in rates between all pairs (corrected P < 0.001).
As is clear from comparison of Figure 2A,B, semantic knowledge did not strongly associate with word repetition success rate. Rather, language familiarity (difference in phonological familiarity between L1 and L2) is likely to be the dominant factor.
In order to clarify whether a longer and stronger utterance during repetition could lead to greater brain activation, statistical analyses were conducted to compare the children’s oral responses (see the Material and Methods section) between the 4 tasks. The 2 × 2 repeated-measures ANOVA between the 4 tasks showed significant main effects of language (F [1, 391] = 199.96, P < 0.001), word frequency (F [1, 391] = 21.71, P < 0.001), and a language × word-frequency interaction (F [1, 391] = 49.77, P < 0.001). Post hoc pairwise comparisons using paired t-tests showed significant differences in children’s oral responses between the tasks: Jpn_HF < Eng_HF, Jpn_LF < Eng_LF, and Jpn_HF > Jpn_LF (corrected P < 0.001) but not for Eng_HF & Eng_LF.
Functional Imaging Results
An example of the time course in [oxy-Hb] and [deoxy-Hb] of grand-averaged data for the 392 participants is given in Figure 1D. It is the channel that showed the highest t-value in [oxy-Hb] signals (channel 6) and in which we found an increase in [oxy-Hb] and a decrease in [deoxy-Hb] indicating brain activations similar to response patterns reported in a number of previous studies.
A Developmental Perspective (Age Factor)
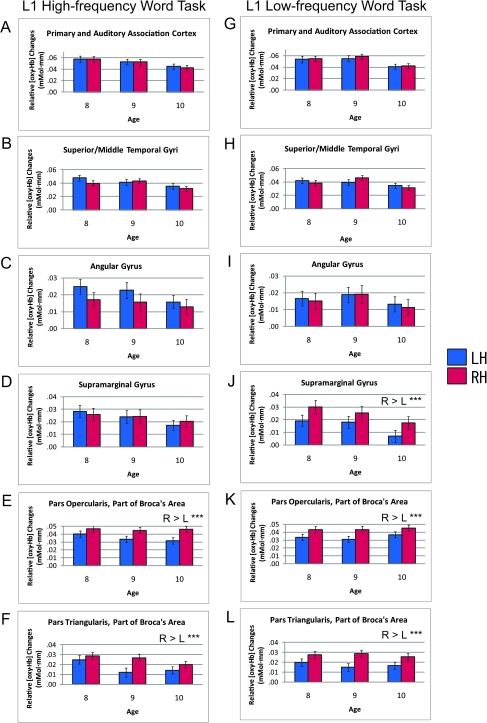
The differences in brain activations between the 3 age groups were derived from the results of descriptive statistics (Fig. 3). In general, increase in age was associated with decrease in brain activation, especially in the high-frequency word task, in all 6 ROIs. In order to examine the quantitative difference in brain activation between the 3 groups, a 2 × 2 × 3 3-way mixed-effects ANOVA was carried out for each defined ROI with the within-subject effects of hemisphere (LH and RH) and word frequency (high and low) and the between-subject effect of age group (age 8–10). The results of the 3-way ANOVAs for L1 word repetition tasks grouped by age are shown in Supplementary Table 2. Although a main effect of age group was found before correction for multiple comparisons (age 8 > age 9 > age 10) for the brain regions at an early stage of cortical auditory processing (i.e., the primary and auditory association cortices, and the superior/middle temporal gyri), none of the 6 ROIs reached significance after Bonferroni correction.
Figure 3.
Bar graphs of average brain activations of children during L1 word repetition tasks. ROI analyses were employed. The results of the L1 high-frequency word task are shown in (A–F) and those of the L1 low-frequency word task in (G–L): (A,G) primary and auditory association cortices (BA 41, 42), (B,H) superior/middle temporal gyri (BA 21, 22), (C,I) angular gyrus (BA 39), (D,J) supramarginal gyrus (BA 40), (E,K) pars opercularis, part of Broca’s area (BA 44), and (F,L) pars triangularis, part of Broca’s area (BA 45). Comparisons of 3 different age groups, 8–10, can be seen in each figure. The bar graphs show the relative changes in [oxy-Hb], and error bars indicate standard error. Asterisks indicate statistically significant results (*** corrected P < 0.001).
As for within-subject factors, the ANOVA revealed a main effect of hemisphere for the supramarginal gyrus (corrected P < 0.05, RH > LH) and with a nonsignificant trend of opposite dominance for the angular gyrus (LH > RH). There were also significant frequency and hemisphere interactions for the supramarginal gyrus, and, intriguingly, a higher activation in the right supramarginal gyrus was prominent for the low-frequency L1 word task (Fig. 3D,J), and an insignificant trend of higher activation in the left angular gyrus was observed for the high-frequency L1 word task (Fig. 3C,I). A post hoc simple main-effect analysis for the supramarginal gyrus applying the Bonferroni correction revealed a significant effect of hemisphere for low-frequency L1 words (corrected P < 0.001, RH > LH) but not for high-frequency L1 words. As for the Broca’s area, the ANOVA revealed a main effect of hemisphere for the pars opercularis (F [1,381] = 33.527, corrected P < 0.001, RH > LH) and for the pars triangularis (F [1,381] = 34.969, corrected P < 0.001, RH > LH).
Although there were moderate downward trends in brain activations as age increased and the linearization coefficients were negative in all 6 ROIs in the high-frequency word task, statistical analyses showed no significant difference in brain activation between age groups.
For confirmation, we also conducted regression analyses of the relation between age and brain activation (relative [oxy-Hb] changes) during high-frequency L1 word repetition tasks. Results of the regression analyses are shown in Supplementary Table 3. As with the age group analyses, the regression coefficients of the lines are negative in most of the brain regions (all the regions in the LH), but the decrease in brain activation was small and none of the 6 ROIs reached significance after Bonferroni correction for 12 tests.
Whole-Group Analyses
Followed by the observation that the age difference did not show any significance in our study group, whole-group analyses were conducted to achieve an overall view of neural substrates during L1 and L2 processing and their comparison in the young population as a whole.
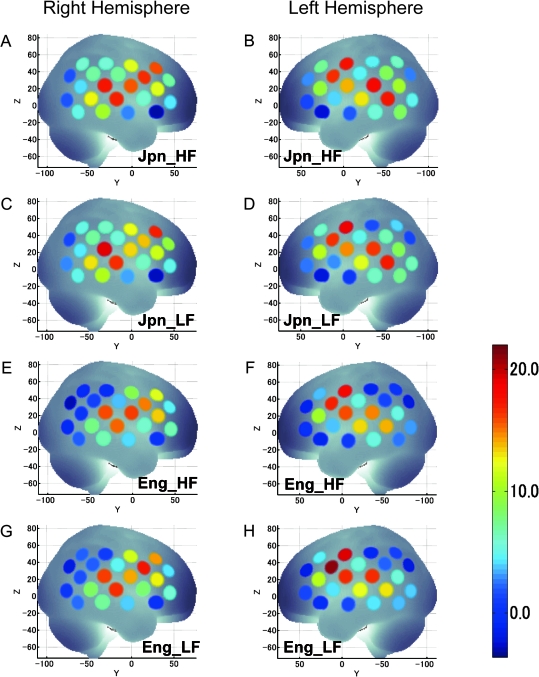
The positions of the measurement channels together with mean cortical activation in t-values (uncorrected) of both RH and LH are shown in Figure 4. The results show, in broad terms, that the overall activation patterns encompassing frontal, temporal, and parietal lobes were similar both in L1 and L2 irrespective of word frequency. The activated regions included the primary auditory area, classical Wernicke’s and Broca’s areas, the angular gyrus, and the supramarginal gyrus. These similar, widespread activation patterns indicate that children used largely overlapping neural substrates when processing words in both L1 and L2, irrespective of word frequency.
Figure 4.
Cortical activations during word repetition tasks. Average fNIRS data obtained from 392 participants were projected onto the MNI standard brain space by spatial registration. The position of the measurement channels together with cortical activation of both RH and LH are shown in the figures: high-frequency Japanese (Jpn_HF) RH (A) and LH (B); low-frequency Japanese (Jpn_LF) RH (C) and LH (D); high-frequency English (Eng_HF) RH (E) and LH (F); and low-frequency English (Eng_LF) RH (G) and LH (H). The color scale indicates t-values (uncorrected).
Cortical activations during word repetition tasks for each ROI are shown in Figure 5. Since a basic assumption of fNIRS measurements is that an increase in the [oxy-Hb] signal and a decrease in the [deoxy-Hb] signal indicate cortical activation (Villringer and Chance 1997; Obrig et al. 2000; Seiyama et al. 2004), relative changes in the [deoxy-Hb] signals are also indicated as positive values in the figure for comparison with [oxy-Hb] signals. In broad terms, the results of [oxy-Hb] and [deoxy-Hb] are quite similar as demonstrated in the figure. Three-way repeated-measure ANOVAs using within-subject factors (language [L1 and L2] × word frequency [low and high] × hemisphere [LH and RH]) were conducted for both [oxy-Hb] and [deoxy-Hb] in order to reveal different characteristic features for each defined ROI, and the results are summarized in Table 2.
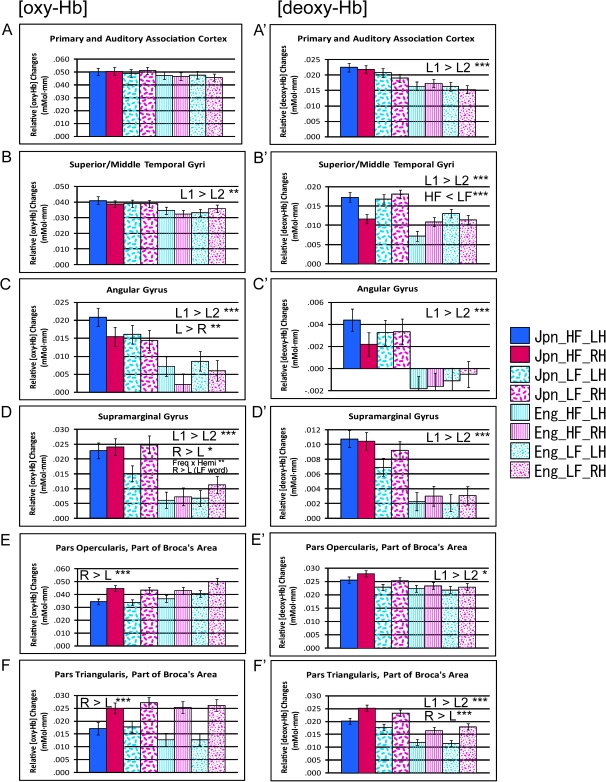
Figure 5.
Average brain activation in children during word repetition tasks. ROI analyses were employed: primary and auditory association cortices (BA 41, 42) [oxy-Hb] (A) and [deoxy-Hb] (A′); superior/middle temporal gyri (BA 21, 22) [oxy-Hb] (B) and [deoxy-Hb] (B′), angular gyrus (BA 39) [oxy-Hb] (C) and [deoxy-Hb] (C′), supramarginal gyrus (BA 40) [oxy-Hb] (D) and [deoxy-Hb] (D′), pars opercularis, part of Broca’s area (BA 44) [oxy-Hb] (E) and [deoxy-Hb] (E′), and pars triangularis, part of Broca’s area (BA 45) [oxy-Hb] (F) and [deoxy-Hb] (F′). The bar graphs show the relative changes in [oxy-Hb] and [deoxy-Hb]. Error bars indicate standard error. Since an increase in the [oxy-Hb] signal and a decrease in the [deoxy-Hb] signal indicate cortical activation, the relative changes in the [deoxy-Hb] signals are indicated as positive values in the figure for comparison with [oxy-Hb] signals. Abbreviations: Jpn_HF_LH = Japanese high-frequency words (LH), Jpn_HF_RH = Japanese high-frequency words (RH), Jpn_LF_LH = Japanese low-frequency words (LH), Jpn_LF_RH = Japanese low-frequency words (RH), Eng_HF_LH = English high-frequency words (LH), Eng_HF_RH = English high-frequency words (RH), Eng_LF_LH = English low-frequency words (LH), Eng_LF_RH = English low-frequency words (RH). Abbreviations in the bar graphs: L1 = native language (Japanese), L2 = second language (English), L = left hemisphere, R = right hemisphere, Freq = word frequency, Hemi = hemisphere. Asterisks indicate statistically significant results (* P < 0.05, **P < 0.01, ***P < 0.001; all corrected).
Table 2.
ANOVA results
| Brain area | Source of variation | df | F | Puncorrected | Multiple comparison | Remarks |
| (a) Oxy-Hb | ||||||
| PAAC (BA 41,42) | Language | 1, 352 | 3.190 | 0.075 | ||
| Word frequency | 1, 352 | 0.027 | 0.870 | |||
| Hemisphere | 1, 352 | 0.000 | 0.988 | |||
| Language × frequency | 1, 352 | 0.004 | 0.950 | |||
| Language × hemisphere | 1, 352 | 1.974 | 0.161 | |||
| Frequency × hemisphere | 1, 352 | 0.019 | 0.891 | |||
| Language × frequency × hemisphere | 1, 352 | 0.523 | 0.470 | |||
| SMTG (BA 21,22) | Language | 1, 376 | 11.658 | 0.001 | <0.005** | L1 > L2 |
| Word frequency | 1, 376 | 0.000 | 0.997 | |||
| Hemisphere | 1, 376 | 0.083 | 0.774 | |||
| Language × frequency | 1, 376 | 0.270 | 0.604 | |||
| Language × hemisphere | 1, 376 | 0.504 | 0.478 | |||
| Frequency × hemisphere | 1, 376 | 3.232 | 0.073 | |||
| Language × frequency × hemisphere | 1, 376 | 0.510 | 0.476 | |||
| AG (BA 39) | Language | 1, 389 | 23.660 | 0.000 | <0.001*** | L1 > L2 |
| Word frequency | 1, 389 | 0.004 | 0.951 | |||
| Hemisphere | 1, 389 | 10.003 | 0.002 | <0.01** | L > R | |
| Language × frequency | 1, 389 | 1.515 | 0.219 | |||
| Language × hemisphere | 1, 389 | 0.055 | 0.815 | |||
| Frequency × hemisphere | 1, 389 | 4.265 | 0.040 | cf. captiona | ||
| Language × frequency × hemisphere | 1, 389 | 0.202 | 0.654 | |||
| SMG (BA 40) | Language | 1, 383 | 35.164 | 0.000 | <0.001*** | L1 > L2 |
| Word frequency | 1, 383 | 0.069 | 0.793 | |||
| Hemisphere | 1, 383 | 8.483 | 0.004 | <0.05* | R > L | |
| Language × frequency | 1, 383 | 1.682 | 0.195 | |||
| Language × hemisphere | 1, 383 | 2.822 | 0.094 | |||
| Frequency × hemisphere | 1, 383 | 12.813 | 0.000 | <0.005** | R > L (LF word) | |
| Language × frequency × hemisphere | 1, 383 | 2.201 | 0.139 | |||
| POP (BA 44) | Language | 1, 380 | 3.732 | 0.054 | ||
| Word frequency | 1, 380 | 2.214 | 0.138 | |||
| Hemisphere | 1, 380 | 26.347 | 0.000 | <0.001*** | R > L | |
| Language × frequency | 1, 380 | 3.760 | 0.053 | |||
| Language × hemisphere | 1, 380 | 1.853 | 0.174 | |||
| Frequency × hemisphere | 1, 380 | 0.605 | 0.437 | |||
| Language × frequency × hemisphere | 1, 380 | 1.721 | 0.190 | |||
| PTR (BA 45) | Language | 1, 378 | 2.056 | 0.152 | ||
| Word frequency | 1, 378 | 0.358 | 0.550 | |||
| Hemisphere | 1, 378 | 60.631 | 0.000 | <0.001*** | R > L | |
| Language × frequency | 1, 378 | 0.082 | 0.774 | |||
| Language × hemisphere | 1, 378 | 5.691 | 0.018 | |||
| Frequency × hemisphere | 1, 378 | 0.513 | 0.474 | |||
| Language × frequency × hemisphere | 1, 378 | 0.042 | 0.839 | |||
| (b) Deoxy-Hb | ||||||
| PAAC (BA 41,42) | Language | 1, 342 | 28.500 | 0.000 | <0.001*** | L1 > L2 |
| Word frequency | 1, 342 | 4.267 | 0.040 | HF > LF | ||
| Hemisphere | 1, 342 | 0.318 | 0.573 | |||
| Language × frequency | 1, 342 | 0.466 | 0.495 | |||
| Language × hemisphere | 1, 342 | 1.778 | 0.183 | |||
| Frequency × hemisphere | 1, 342 | 3.431 | 0.065 | |||
| Language × frequency × hemisphere | 1, 342 | 0.260 | 0.610 | |||
| SMTG (BA 21,22) | Language | 1, 372 | 43.317 | 0.000 | <0.001*** | L1 > L2 |
| Word frequency | 1, 372 | 15.561 | 0.000 | <0.001*** | HF < LF | |
| Hemisphere | 1, 372 | 0.469 | 0.494 | |||
| Language × frequency | 1, 372 | 0.027 | 0.869 | |||
| Language × hemisphere | 1, 372 | 8.474 | 0.004 | <0.05* | ||
| Frequency × hemisphere | 1, 372 | 0.572 | 0.450 | |||
| Language × frequency × hemisphere | 1, 372 | 32.913 | 0.000 | <0.001*** | ||
| AG (BA 39) | Language | 1, 386 | 28.607 | 0.000 | <0.001*** | L1 > L2 |
| Word frequency | 1, 386 | 0.336 | 0.563 | |||
| Hemisphere | 1, 386 | 0.331 | 0.565 | |||
| Language × frequency | 1, 386 | 0.241 | 0.624 | |||
| Language × hemisphere | 1, 386 | 4.770 | 0.030 | |||
| Frequency × hemisphere | 1, 386 | 4.053 | 0.045 | cf. captiona | ||
| Language × frequency × hemisphere | 1, 386 | 2.196 | 0.139 | |||
| SMG (BA 40) | Language | 1, 377 | 53.687 | 0.000 | <0.001*** | L1 > L2 |
| Word frequency | 1, 377 | 2.250 | 0.134 | |||
| Hemisphere | 1, 377 | 1.597 | 0.207 | |||
| Language × frequency | 1, 377 | 1.700 | 0.193 | |||
| Language × hemisphere | 1, 377 | 0.020 | 0.887 | |||
| Frequency × hemisphere | 1, 377 | 3.323 | 0.069 | |||
| Language × frequency × hemisphere | 1, 377 | 1.950 | 0.163 | |||
| POP (BA44) | Language | 1, 374 | 9.146 | 0.003 | <0.05* | L1 > L2 |
| Word frequency | 1, 374 | 3.703 | 0.055 | HF > LF | ||
| Hemisphere | 1, 374 | 4.430 | 0.036 | R > L | ||
| Language × frequency | 1, 374 | 1.194 | 0.275 | |||
| Language × hemisphere | 1, 374 | 2.742 | 0.099 | |||
| Frequency × hemisphere | 1, 374 | 0.001 | 0.978 | |||
| Language × frequency × hemisphere | 1, 374 | 0.002 | 0.964 | |||
| PTR (BA 45) | Language | 1, 374 | 64.363 | 0.000 | <0.001*** | L1 > L2 |
| Word frequency | 1, 374 | 0.945 | 0.332 | |||
| Hemisphere | 1, 374 | 58.095 | 0.000 | <0.001*** | R > L | |
| Language × frequency | 1, 374 | 2.151 | 0.143 | |||
| Language × hemisphere | 1, 374 | 0.080 | 0.777 | |||
| Frequency × hemisphere | 1, 374 | 2.199 | 0.139 | |||
| Language × frequency × hemisphere | 1, 374 | 0.564 | 0.453 | |||
Note: Statistical analyses using a 3-way repeated-measure ANOVAs were conducted for 6 ROIs to evaluate the effects of 3 within-subject factors: the 2 languages (Japanese: L1 and English: L2), 2 word frequencies (high: HF and low: LF) and 2 hemispheres (left: L and right: R). df = degree of freedom, PAAC = primary and auditory association cortices, SMTG = superior/middle temporal gyri, AG = angular gyrus, SMG = supramarginal gyrus, POP = pars opercularis, part of Broca’s area, and PTR = pars triangularis, part of Broca’s area.
aCf., Compare the laterality (hemisphere effects) for the angylar gyrus with that for the supramarginal gyrus: Additional paired t-test analyses for the [oxy-Hb] signals showed LH > RH for the high-frequency L1 (corrected P < 0.01) and L2 (corrected P < 0.05) word tasks, and for the [deoxy-Hb] signals showed LH > RH for the high-frequency L1 word task (corrected P < 0.05).
P values were Bonferroni corrected for 6 tests with a significance level of P < 0.05 after correction for multiple testing. Asterisks indicate statistically significant results (*P < 0.05, **P < 0.01, ***P < 0.001).
In the primary and auditory association cortices, the ANOVA for [oxy-Hb] showed neither a significant main effect nor an interaction after the conservative Bonferroni correction (Fig. 5A). However, the ANOVA for [deoxy-Hb] demonstrated a significant main effect of language (F [1,342] = 28.500, corrected P < 0.001, L1 > L2) (Fig. 5A′).
In the superior/middle temporal gyri, the ANOVA for [oxy-Hb] exhibited a significant main effect of language (F [1,376] = 11.658, corrected P < 0.01, L1 > L2) (Fig. 5B). The ANOVA for [deoxy-Hb] also demonstrated a significant main effect of language (F [1,372] = 43.317, corrected P < 0.001, L1 > L2) (Fig. 5B′). In addition, there was a significant main effect of word frequency (F [1,372] = 15.561, corrected P < 0.001, HF < LF), and there were also significant interactions between language and hemisphere (F [1,372] = 8.474, corrected P < 0.05) and language × frequency × hemisphere (F [1,372] = 32.913, corrected P < 0.001). A post hoc simple main-effect analysis applying the Bonferroni correction revealed a significant effect of hemisphere for L1 tasks (P < 0.05), but opposite trends of LH >> RH for HF task and RH > LH for LF task were observed.
In the angular gyrus, the ANOVA for [oxy-Hb] exhibited significant main effects of language (F [1,389] = 23.660, corrected P < 0.001, L1 > L2) and hemisphere (F [1,389] = 10.003, corrected P < 0.01, LH > RH) (Fig. 5C). In addition, there was a marginal interaction between frequency and hemisphere, which failed to reach significance after Bonferroni correction. Similarly, the statistical analyses for [deoxy-Hb] also revealed a significant main effect of language (F [1,386] = 28.607, corrected P < 0.001, L1 > L2; increase in [deoxy-Hb] for L2 was not statistically significant) and a marginal interaction between frequency and hemisphere, which also failed to reach significance after Bonferroni correction. As the omnibus ANOVA results comparing all 3 conditions for both [oxy-Hb] and [deoxy-Hb] for the angular gyrus (as well as the [oxy-Hb] within-subject ANOVA results for age group shown in Supplementary Table 2) revealed marginal interactions between frequency and hemisphere, we further explored the details to better characterize the hemisphere effect for the 4 tasks by conducting additional paired t-tests. The [oxy-Hb] results showed significant difference in activation (LH > RH) for both the high-frequency L1 (corrected P < 0.01) and L2 (corrected P < 0.05) word tasks (Fig. 5C), and the [deoxy-Hb] results showed significant difference in activation (LH > RH) for the high-frequency L1 word task (corrected p < 0.05) (Fig. 5C′). In contrast, neither the [oxy-Hb] nor the [deoxy-Hb] results showed significant hemispheric difference for the low-frequency word tasks.
In the supramarginal gyrus, the ANOVA for [oxy-Hb] demonstrated significant main effects of language (F [1,383] = 35.164, corrected P < 0.001, L1 > L2) and hemisphere (F [1,383] = 8.483, corrected P < 0.05, RH > LH), and a significant interaction between frequency and hemisphere (F [1,383] = 12.813, corrected P < 0.01) (Fig. 5D). A post hoc simple main-effect analysis applying the Bonferroni correction revealed a significant effect of hemisphere for low-frequency words (corrected P < 0.001, RH > LH). As for the [deoxy-Hb] analyses, there was only a significant main effect of language (F [1,377] = 53.687, corrected P < 0.001, L1 > L2), but, as shown in Figure 5D′, RH > LH activations similar to those seen in [oxy-Hb] were observed for the low-frequency word tasks.
In the pars opercularis, part of Broca’s area, the ANOVA for [deoxy-Hb] exhibited a significant main effect of language (F [1,374] = 9.146, corrected P < 0.05, L1 > L2), while that for [oxy-Hb] did not. On the other hand, while the ANOVA for [oxy-Hb] showed a significant main effect of hemisphere (F [1,380] = 26.347, corrected P < 0.001, RH > LH), that for [deoxy-Hb] did not survive Bonferroni correction (Fig. 5E,E′).
The results for the pars triangularis, part of Broca’s area, are similar to those of the pars opercularis: The ANOVA for [deoxy-Hb] only exhibited a significant main effect of language (F [1,374] = 64.363, corrected P < 0.001, L1 > L2), while that for [oxy-Hb] did not (Fig. 5F,F′). A significant main effect of hemisphere was demonstrated for both [oxy-Hb] (F [1,378] = 60.631, corrected P< 0.001, RH > LH) and [deoxy-Hb] changes (F [1,374] = 58.095, corrected P < 0.001, RH > LH) (Fig. 5F,F′).
Discussion
In this study, we revealed that the cortical activation pattern associated with language processing in elementary school children involves a bilateral network of regions in the frontal, temporal, and parietal lobes.
Here, we list the major findings:
Though not statistically significant, a trend toward lower hemodynamic responses with increasing age from 6 to 10 was observed, especially in the auditory and temporal regions.
L2 words were processed like nonword auditory stimuli in the brain as indicated by lower activation than that elicited by L1 words in the superior/middle temporal and inferior parietal regions.
Low-frequency words elicited more right-hemispheric activation (particularly in the supramarginal gyrus and high-frequency) words elicited more left-hemispheric activation (particularly in the angular gyrus).
The importance of the RH temporo-parieto-frontal network as well as the traditional LH language network was suggested especially at the early stages of language acquisition/learning both in L1 and L2.
Differences in sensitivity between [oxy-Hb] and [deoxy-Hb] signals for detecting language, frequency, and hemisphere effects were observed.
Details of the major findings are described in subsequent sections. We will first discuss the age factor in L1 tasks. Next, we will move on to the whole-group analyses to explore language difference (language effect), then we will examine the function of each ROI in relation to phonological versus semantic processing and LH versus RH, and, finally, we will note the different characteristics of [oxy-Hb] and [deoxy-Hb] signals.
A Developmental Perspective (Age Factor)
To summarize the results, both the age group and regression analyses between age and cortical activation revealed weak trends of decreasing cortical activation with age in L1 tasks, but such changes were not statistically significant. As the human language system dramatically develops during childhood, one might expect that brain functions and structures change dramatically in this period. However, we did not detect significant differences in brain response. This is probably because the age range of our participants was small (6–10 years, with very few 6-year-olds among our participants).
Alternatively, the absence of significant differences in cortical activation may be due to the tasks we employed. Since we focused on L2 learning in children, the single-word repetition task we employed was tailored to measure the level of L2 acquisition rather than to detect developmental changes in the mother tongue. Thus, the mere repetition of L1 words was presumed to be easy for elementary school children regardless of their semantic knowledge of the presented words. It is assumed that the repetition of L1 words by elementary school children occurs automatically. The “dual-process” information-processing model of Schneider and Shiffrin (1977) and Shiffrin and Schneider (1977) offered compelling evidence for the distinction between “automatic detection” and “controlled search,” 2 qualitatively different human information-processing operations. In their view, the execution of cognitive tasks changes with training. Acquiring a new skill primarily requires a controlled search operation. Gradually, as the skill is mastered, it becomes more automatic, enabling the participant to carry out another task simultaneously (dual-task performance). In fact, the cortical activations observed during L1 tasks in our study showed marginal decrease with age. Indeed, this may suggest that the repetition of L1 words is performed more automatically as age increases.
Whole-Group Analyses
To summarize the language effect, there was less overall cortical activation for L2 than for L1, but the statistical significance differed between [oxy-Hb] and [deoxy-Hb] analyses. The [deoxy-Hb] analysis showed greater sensitivity for detecting language effects than the widely used [oxy-Hb] analysis in our study, and this will be discussed later.
We have considered whether a longer and stronger utterance during word repetition would lead to greater hemodynamic response by acoustic analysis. While children’s brain responses during word repetition were significantly greater for L1 than L2, children’s oral responses were greater for L2 than L1. Therefore, it was confirmed that the greater brain responses for L1 than L2 were not because utterances during L1 tasks were longer or stronger than those during L2 tasks.
As for the hemisphere effect, while the [oxy-Hb] analyses showed no significant differences in brain activation between the LH and RH in the superior/middle temporal gyri, nor in the primary or auditory association cortices, significant differences in activation were found in the angular/supramarginal gyri. Interestingly, the LH showed greater activation than the right in the angular gyrus, whereas the RH showed greater activation than the left in the supramarginal gyrus. While the statistical analyses of [oxy-Hb] detected activation laterality in the angular/supramarginal gyri and roughly similar trends in both [oxy-Hb] and [deoxy-Hb] results were observed in the bar graphs (Fig. 5), the statistical analyses of [deoxy-Hb] did not detect critical differences in activations between LH and RH. In contrast to the language effect mentioned above, the [oxy-Hb] analysis showed better sensitivity for detecting language effects than did the [deoxy-Hb] analysis.
Language Difference and Lexicality
We detected equivalent bilateral activation in the primary and auditory association cortices but found less activation for L2 tasks than for L1 tasks in the superior/middle temporal gyri and in the inferior parietal region (angular/supramarginal gyri). Language processing involves lexical versus nonlexical processing, and phonological versus semantic processing. For auditory processing, the bilateral primary auditory cortex, the anterior superior temporal region, and the left-lateralized inferior parietal region near the angular and supramarginal gyri have been reported to be activated by lexical processing (Petersen et al. 1988), while presentation of nonword auditory stimuli failed to activate the anterior superior temporal and the inferior parietal regions (Roland et al. 1980; Mazziotta et al. 1982; Lauter et al. 1985). It is also reported that the human superior temporal region, consisting primarily of the auditory sensory cortex, is activated bilaterally and symmetrically by a variety of speech and nonspeech auditory stimuli (Binder et al. 2000; Patterson et al. 2002). The response at the level of the superior temporal sulcus is not considered to be speech specific but rather arises from the complex frequency and amplitude modulations that characterize speech, whereas speech-specific lexical and semantic processing is thought to be a function of the cortex ventral to the superior temporal sulcus (Binder et al. 1996; Binder and Frost 1998). As for the inferior parietal region (angular/supramarginal gyri), a greater response to words than to pseudowords during a feature detection task has been also shown in PET studies (Brunswick et al. 1999; Price 2000). A recent fNIRS study revealed the lexicality effect, in which words elicited a larger focal hyperoxygenation in comparison to pseudowords in the left inferior parietal gyrus (Hofmann et al. 2008). Taken all together, the superior/middle temporal gyri and the inferior parietal region are presumed to be associated with lexicality.
Considering our results together with previous findings, the language effect is likely to correspond to the lexicality effect (word or nonword). In other words, L2 words were processed like nonword auditory stimuli. As the children are at the very early stages of L2 learning so that the L2 words were not all familiar to them, the lexicality effect should be more pronounced in L1 than in the unfamiliar L2, regardless of whether or not subjects have semantic knowledge of the words. Cortical activations in the superior/middle temporal gyri and angular/supramarginal gyri may not simply depend on the acoustic complexity of speech sounds, but also reflect processes tuned to the phonology of the native language, suggesting that the activations in these brain regions are stronger for L1 than for L2.
Whether the superior/middle temporal gyri and angular/supramarginal gyri are related to phonological or semantic processing will be discussed in subsequent sections.
Phonological Versus Semantic Processing: Temporal Region (Superior/Middle Temporal Gyri)
Although the superior/middle temporal gyri and the inferior parietal region were revealed to be associated with lexicality, whether the observed lexicality effect arose from the semantic and/or the phonological content of words is worthy of intensive discussion.
As for the superior/middle temporal gyri, the [oxy-Hb] analyses did not reveal any significant difference in activations except for the language. In contrast, the [deoxy-Hb] analyses revealed a word-frequency effect (HF < LF) in addition to a language effect. Post hoc simple main-effect analyses revealed left-hemispheric dominance for high-frequency words and right-hemispheric dominance for low-frequency words for the L1 tasks. As revealed in a recent fNIRS study (Hofmann et al. 2008), the [deoxy-Hb] signal may be more sensitive for detecting the word-frequency effect than the [oxy-Hb] signal. Based on both [oxy-] and [deoxy-Hb] results, in the superior/middle temporal gyri, the lexcicality effect should be more pronounced in L1 than in the unfamiliar L2 since L1 words are expected to be perceived more lexically than L2 words regardless of the participants’ semantic knowledge of the words. More specifically, phonological processing is more likely to be executed than semantic processing. Importantly, as [deoxy-Hb] results revealed, during the repetition of L1 words, significantly greater activation was observed in the LH for high-frequency words (96% semantic knowledge) whereas greater activation was observed in the RH for low-frequency words (12% semantic knowledge) (Fig. 5B′). These results suggest that the left temporal region is engaged in semantic processing to some extent, whereas unknown words elicit more activation in the RH.
Phonological Versus Semantic Processing: Inferior Parietal Region (Angular/Supramarginal Gyri)
Another interesting observation was that the LH showed greater activation than the right in the angular gyrus, whereas the RH showed greater activation than the left in the supramarginal gyrus. The right-hemispheric dominance in the supramarginal gyrus was prominent for low-frequency word tasks in both L1 and L2.
An intriguing issue is roles of the angular gyrus and the supramarginal gyrus in relation to semantic and phonological processing. While lesion studies have reported that the inferior parietal region is associated with phonological deficits (Shallice 1981; Roeltgen et al. 1983), and is a good candidate for a phonological coding region, the role of the angular gyrus in semantic processing that supports “word meanings” has been identified (Mesulam 1990; Binder et al. 1997; Inui et al. 1998; Niznikiewicz et al. 2000; Obleser et al. 2007).
The roles of the LH and RH of these regions are also worth discussing. Activation of the left angular gyrus has, for many years, been reported to be associated with reading (Dejerine 1892; Damasio AR and Damasio H 1983; Henderson 1986; Horwitz et al. 1998). Horwitz et al. (1998) found a functional connectivity of the angular gyrus during single-word reading in normal readers, as found in lesion studies (Dejerine 1892; Damasio AR and Damasio H 1983; Henderson 1986). In particular, they have demonstrated strong functional linkages of the left angular gyrus with areas of the visual association cortex in the occipital and temporal lobes known to be activated by words and word-like stimuli (Petersen et al. 1989; Howard et al. 1992; Price et al. 1994; Bookheimer et al. 1995; Rumsey et al. 1997). They also reported that the left angular gyrus is functionally linked to a region in the left superior and middle temporal gyri that is part of Wernicke’s area, and to an area in the frontal region in or near Broca’s area during pseudoword reading, where explicit grapheme-to-phoneme conversions are required. This finding suggests that the left angular gyrus is involved not only in semantic processing, but also in phonological processing.
In the present study, by far the highest activation was observed in the left angular gyrus when children performed the high-frequency L1 task, in which children knew the meanings of an average of 96% of the words, and, correspondingly, this rating is much higher than those of the other 3 tasks as shown in Figure 2A. This result is relevant to previous findings that the left angular gyrus is involved in semantic processing. On the other hand, the mean ratings of semantic knowledge were much lower for low-frequency L1 words (12%), high-frequency L2 words (42%), and low-frequency L2 words (8%) than those for high-frequency L1 words (96%); however, the magnitude of brain activation during both high- and low-frequency L1 tasks was much higher than that during L2 tasks regardless of semantic knowledge of words. Similarly, the mean ratings of semantic knowledge were significantly low for both low-frequency L1 (12%) and L2 words (8%), and, importantly, the statistical analysis did not show significant difference in the ratings between these 2 tasks. Nevertheless, cortical activations during low-frequency L1 and L2 word tasks elicited significant differences. These facts suggest that the left angular gyrus is involved not only in semantic processing but also in phonological processing. In other words, processing familiar phonology in L1 induces higher brain activation than processing unfamiliar phonology in a foreign language, independent of semantic knowledge.
With respect to the word repetition tasks we employed, we postulate that phonological processing is the main process and that the activations in the angular gyrus arose mainly from phonological familiarity (phonological analysis of novel relative to familiar stimuli), which is relevant to differences in word repetition success rates. If we compare the bar graphs for semantic knowledge of words (Fig. 2A), repetition success rate (Fig. 2B), and brain activations in the angular gyrus (Fig. 5C,C′), brain activation in the angular gyrus is obviously associated with word repetition success rate rather than semantic knowledge of words. More specifically, we observed significant differences in brain activations between L1 and L2 tasks regardless of the children’s semantic knowledge of the words; accordingly, we observed significant differences in the word repetition success rates between L1 and L2 tasks, which would reflect differences in phonological familiarity. In addition, a complex interaction of semantic and phonological information processing was observed. Although clear separation of semantic and phonological processing is difficult, considering the relationship between the protruding semantic knowledge of high-frequency L1 words and corresponding cortical activation in the left angular gyrus, it is possible that semantic processing is also involved to some extent, especially in the left angular gyrus.
In contrast to the angular gyrus, the supramarginal gyrus exhibited a rather different feature. We did not observe a pronounced cortical activation in the LH in this region as we did for the left angular gyrus during the L1 high-frequency word task. Instead, right-dominant activation was noticeable, especially for low-frequency word tasks.
Lesions to the left supramarginal gyrus are often associated with conduction aphasia (Green and Howes 1978), characterized by relatively preserved comprehension, impaired repetition, and paraphasic and otherwise disordered speech. The results of an MRI study of lesions in aphasic patients by Caplan et al. (1995) indicated that the left supramarginal gyrus is the principal site of phonemic processing in speech perception. Moreover, previous studies show that the left supramarginal gyrus is strongly activated by phonological tasks relative to semantic tasks, supporting its role in phonological processing (Démonet et al. 1994; Caplan et al.1995; Celsis et al. 1999).
Specifically, Binder et al. (1996) demonstrated in their fMRI study that the left supramarginal gyrus was more strongly activated by nonlinguistic stimuli (tone sequences) than by words when subjects performed active listening tasks involving tone sequence analysis in comparison to analysis of words. Although the ROIs were only defined in the LH, and no information about the RH was mentioned, they reported in another paper that the supramarginal gyrus was activated bilaterally by the tone decision task relative to the semantic decision task, and, importantly, this activation was much more extensive in the RH than in the left (Binder et al. 1997).
Furthermore, lesions of the left inferior parietal lobe in the region of the supramarginal gyrus have been reported to give rise to deficit in auditory–verbal short-term memory (Shallice and Vallar 1990; Vallar et al. 1997). Also, specific activation of the left supramarginal gyrus by a short-term memory task was demonstrated by Paulesu et al. (1993), who considered this region to be the location of the phonological store; similar results were obtained by Salmon et al. (1996), Smith and Jonides (1998), and Smith et al. (1998). Studies with normal and brain-damaged subjects have indicated that there are semantic as well as phonological contributions to verbal short-term memory. Combined, it is likely that the supramarginal gyrus is involved in phonological processing in linguistic stimuli as well as nonlinguistic stimuli, and is the principal site of phonological representation and phonological store (verbal short-term memory).
Given the observation that the children in our study did not know the meanings of an average of 88% and 92% of low-frequency Japanese and English words, respectively, and that the supramarginal gyrus was activated by all the tasks irrespective of language or semantic knowledge, bilateral right-dominant activation in the supramarginal gyrus is likely to reflect phonological processing and storage. Moreover, given the fact that repetition success rates significantly differed between L1 and L2 tasks and that phonologically familiar words are easier to memorize than phonologically unfamiliar words, the greater brain activation during auditory word processing in L1 than in L2, regardless of semantic knowledge level, is likely related to phonological familiarity, which is relevant to the phonological store.
Taken together, it could be explained that both linguistic (mainlyLH) and nonlinguistic (mainly RH) processing, including the phonological store, can be executed in parallel, and that the children would depend more on nonlinguistic processing for unfamiliar or low-frequency words in repetition tasks.
In sum, a complex interaction of semantic and phonological information processing was observed, especially in the angular gyrus. As the repetition task employed in this study strongly demands phonological and prosodic analyses rather than semantic analyses, a clear separation of semantic and phonological processing is difficult. However, the present results clarify that left-hemispheric activation is dominant for high-frequency tasks especially in the angular gyrus, while right-hemispheric activation is dominant for low-frequency tasks in the supramarginal gyrus. These results suggest that a right-to-left shift in laterality occurs in the inferior parietal region as lexical knowledge increases, irrespective of language.
Inferior Frontal Region
With respect to the inferior frontal region, the statistical results of [oxy-Hb] changes demonstrate that brain activation in the RH was significantly greater than that in the LH in both the pars opercularis and the pars triangularis, and the statistics of the [deoxy-Hb] changes also confirmed the same results although with slightly lower statistical significance.
Price et al. (1996) demonstrated that Broca’s area is involved in both auditory word perception and repetition. The peak of frontal activation in response to hearing words is anterior to that associated with repeating words: Roughly, the former corresponds to the pars triangularis and the latter to the pars opercularis and the adjacent precentral sulcus. We observed greater activation in the RH, and the right-hemispheric dominance was more prominent in the pars triangularis than in the pars opercularis (Fig. 5E,E′,F,F′), which may indicate that right-hemispheric asymmetry is more pronounced when hearing words (auditory word perception) than when repeating words.
Broca’s area in the left inferior frontal gyrus has been traditionally considered a language area. However, there is not yet a consensus on the anatomical demarcation of this region, and its functional characterization remains a matter of debate. This region is often discussed in the context of language, working memory, episodic memory, or implicit memory. It is suggested that the left inferior prefrontal region serves as a crossroad between meaning in language and memory (for a review, see Gabrieli et al. 1998). In the adult brain, syntactic and working memory-related functions may be more pronounced in superior portions of the inferior frontal lobe (pars opercularis), whereas the inferior portions including pars triangularis may be more involved in lexicosemantic function (Dapretto and Bookheimer 1999; Friederici 2002).
Our repetition tasks undoubtedly incorporated aspects of working memory. Generally speaking, activation in the inferior frontal gyrus during memory tasks appears to be lateralized to the left (Broca’s area) and to be associated with subvocal speech approaches to the tasks (for a review, see Fletcher and Henson 2001). If we try to explain the present results regarding the inferior frontal gyrus primarily with the working memory functions, we notice some inconsistencies. First of all, we observed significant right-hemispheric activation in this region, even though the observed activation in the LH might be partly elicited by working memory function. Moreover, we expected L2 words to elicit more activation than L1 words since repetition of unknown/unfamiliar words with unfamiliar phonology is expected to require a higher working memory load than that of known/familiar words. Nevertheless, statistical results of [deoxy-Hb] changes revealed greater activation for L1 than L2 word repetition. In a review, Cabeza and Nyberg (2000) accord some spatial, object, and problem-solving working memory localization to the right inferior frontal gyrus. However, none of this seems to relate to the word repetition task. Thus, the function of working memory is not sufficient enough to account for the present results regarding the inferior frontal gyrus.
Hence, it may be worthwhile to view the function of the inferior frontal region from different perspectives, for example with emphasis on phonological versus semantic processing. Although the [deoxy-Hb] results revealed differences in brain activation between L1 and L2 tasks both in the pars opercularis and the pars triangularis, we did not observe differences in brain activation between high- and low-frequency word tasks nor a relationship between semantic knowledge and brain activation in this region. Thus, even though the left inferior frontal gyrus has been reported to be associated with semantic analysis of words (Dapretto and Bookheimer 1999; Poldrack et al. 1999; Friederici 2002), this function is unlikely to be required for simple word repetition tasks. Rather, our results support the other possibility that phonological analysis provokes left inferior frontal activation (Demonet et al. 1992; Zatorre et al. 1992; Fiez et al. 1995). Poldrack et al. (1999) reported that activation for phonological processing was centered on the dorsal aspect of Broca’s area (including pars opercularis), whereas that for semantic processing was on the ventral aspect (including pars triangularis), and that activation of the right Broca homolog was greater for phonological than semantic processing. Petersen et al. (1988) also reported that simple repetition of presented words failed to activate the left-frontal semantic area. Given that our repetition task required phonological processing rather than semantic processing, the right-dominant activation and the smaller degree of activation in the pars triangularis that we observed seem reasonable.
Another important point that needs to be considered is prosody, referring to the suprasegmental features of natural speech including rhythm, intonation, and stress. One major hypothesis is that the RH is related to emotional or paralinguistic prosody. Recent studies have confirmed the involvement of the RH in at least some aspects of pitch processing (Zatorre et al. 1992; Johnsrude et al. 2000; Meyer et al. 2004), and the role of the right inferior frontal gyrus (pars opercularis) in prosodic processing has been demonstrated in pitch assessment (Pugh et al. 1996; Celsis et al. 1999; Zatorre et al. 1999) and sentence melody processing (Meyer et al. 2002). A recent fNIRS study also demonstrated that in accordance with the imaging data reported in adults, processing prosody in isolation elicits a larger right frontotemporal activation whereas a larger left-hemispheric activation is elicited by the perception of normal language with full linguistic content in 4-year-olds (Wartenburger et al. 2007). Furthermore, the most recent fNIRS study suggests that even a newborn’s brain shows functional asymmetry for processing slow acoustic modulations, such as prosodic information, predominantly in the RH. However, that experiment was dedicated solely to auditory input (perception) and not to output (articulation/production) as their subjects were newborns so that they did not investigate the function of the frontal area (Telkemeyer et al. 2009). In addition, Langheim et al. (2002) have suggested that the right superior parietal lobule, bilateral lateral cerebellum, and right inferior frontal gyrus are integral components in musical rehearsal.
Adults may rely on lexical knowledge when learning a new language, whereas such information is not available for neonates, infants, or for young children. Therefore, the speech signal must contain some prelexical cues that enable language discrimination. Indeed, several studies have established that neonates and infants have an early sensitivity to the prosodic properties of natural languages, and sentential prosody is considered to be essential for them to acquire their native languages (Fernald and Kuhl 1987; Mehler et al. 1988; Mandel et al. 1994). Homae et al. (2006) suggested that prosodic processing in the RH may facilitate the acquisition of lexical or syntactic knowledge in the early stages of language development. This infant dependency on prosodic cues is not only the case with acquiring a native language but is also equally valid for acquiring nonnative languages. Mehler et al. (1996) hypothesized that infants use rhythm to discriminate languages when they are exposed to languages of different rhythmic classes. This hypothesis was supported by the findings of Bahrick and Pickens (1988), Mehler et al. (1988), Christophe and Morton (1998), Dehaene-Lambertz and Houston (1998), and Moon et al. (1993), who showed that young infants, including newborns (Mehler et al. 1988; Moon et al. 1993), can discriminate between sentences drawn from their native language and sentences from a language belonging to another rhythmic class. In other words, speakers of stress-timed languages segment speech in feet, speakers of syllable-timed languages in syllables, and speakers of mora-timed languages in morae (Cutler et al. 1986; Otake et al. 1993; Mehler et al. 1996).
Our results of greater activation in the right Broca’s area compared to the left are in accordance with the findings of previous studies. In fact, a growing body of studies has advocated the importance of the right Broca homolog exemplified by phonological and prosodic processing, although numerous historical and current works still regard the left Broca homolog as having a primary and significant role in language production. On the basis of previous studies, our results of bilateral activation in Broca’s areas are presumed to be due to parallel processing, that is, left-hemispheric segmental and right-hemispheric suprasegmental information processing. Although a word repetition task is basically segmental, the right dominance can be accounted for provided that children made an effort to repeat words as accurately as possible and depended more on the suprasegmental information processing because they are still in the process of learning language, even L1, and have little lexical knowledge compared with adults.
Oxy-Hb Versus Deoxy-Hb Signals
Another challenging point clarified in the present study is the difference in sensitivity between [oxy-Hb] and [deoxy-Hb] signals for detecting language, frequency, and hemisphere effects. Although both [oxy-] and [deoxy-Hb] analyses showed similar results, greater statistical significance was observed in the analyses of [deoxy-Hb] changes for language and frequency effects, while greater statistical significance was observed in the analyses of [oxy-Hb] changes for the hemisphere effect. The vast majority of fNIRS researchers opt to analyze [oxy-Hb] data rather than [deoxy-Hb] data, since the [oxy-Hb] signal is more sensitive to changes in cerebral blood flow than are [deoxy-Hb] and [total-Hb] signals (Hoshi et al. 2001; Strangman et al. 2002; Hoshi 2003) and has a higher signal-to-noise ratio (Strangman et al. 2002). On the other hand, regarding linguistic studies, Hofmann et al. (2008) reported that [deoxy-Hb] was a more sensitive parameter for a lexical decision task, and word-frequency effects occurred only in [deoxy-Hb], not in [oxy-Hb]. In addition, it has recently been argued that extracerebral (i.e., systemic) hemoglobin changes particularly affect [oxy-Hb] (Boden et al. 2007).
The current results are consistent with the previous reports as to a stronger [oxy-Hb] signal than [deoxy-Hb] as seen in Figure 5. A plausible reason for the [deoxy-Hb] sensitivity in the present study may be the smaller intersubject variability of [deoxy-Hb] changes. Indeed, the error mean squares (which are subject to between-subjects variances) were about 3–7 times greater for [oxy-Hb] than for [deoxy-Hb] in the our study. Differences in sensitivity (i.e., the higher sensitivity of [deoxy-Hb] signals for language and word-frequency effects, and the higher sensitivity of [oxy-Hb] signals for hemisphere effect) should be attributed to the nature of the contrasts assessed. The language and word-frequency effects were obtained from the same region, and thus the contrast between 2 conditions should genuinely reflect the difference in cortical responses. In this case, smaller intersubject variability of [deoxy-Hb] changes, which might reflect less extracerebral (i.e., systemic) effect (Boden et al. 2007), could yield stable measures, given a large enough sample size. On the other hand, the hemisphere effect was obtained from corresponding but different regions. Thus, the contrast between 2 conditions should not only include the difference in cortical responses between hemispheres but variability in tissue properties and measurement conditions in the 2 different regions. In this case, the higher signal-to-noise ratio of [oxy-Hb] signals would give rise to stable results. In fact, extraordinarily large differences in the means were obtained when detecting the effect of hemisphere in the angular and supramarginal gyri. Considering the present results, it is appropriate to analyze both [oxy-Hb] and [deoxy-Hb] to obtain an unbiased view of cortical events.
Conclusions
This cross-sectional large-scale neuroimaging study with fNIRS enrolling approximately 500 normally developing Japanese elementary school children revealed differential cortical organization for processing L1 and L2 words when performing word repetition tasks. While a trend toward lower hemodynamic responses was observed for L1 tasks with increasing age from 6 to 10, the present results revealed greater brain activation with L1 than with L2 overall, regardless of semantic knowledge. Significantly greater activation in the superior/middle temporal and inferior parietal regions to L1 words suggests that they are phonological loci. In these regions, cortical responses are likely tuned to the native-language phonology, while phonologically unfamiliar L2 words were processed like nonword auditory stimuli. The statistical difference in cortical activation between L1 and L2 was enhanced from the primary auditory area to the posterior language regions. At the same time, while bilateral activation was observed in the auditory and the temporal regions, hemispheric asymmetry was observed in the posterior language and the inferior frontal regions. These results suggest that small differences in acoustic processing initially derived from low-level non–domain-specific processing in the auditory region are enhanced at subsequent stages of language processing in the superior/middle temporal and inferior parietal regions, which exhibit higher-level functional specializations. A strong involvement of a bilateral language network in children’s brains was demonstrated at the early stages of language acquisition/learning both in L1 and L2. Considering the present results together with previous literature, left-hemispheric segmental and right-hemispheric suprasegmental information processing are presumed to be executed in parallel, and children seem to depend more on the right-hemispheric suprasegmental processing while acquiring unfamiliar or low-frequency words, which might be an important skill for foreign language learning. The left dominance in the angular gyrus for high-frequency words, and right dominance in the supramarginal gyrus for low-frequency words suggest that a right-to-left shift in laterality might occur in the inferior parietal region as lexical knowledge increases, irrespective of language.
Exploring whether the right-hemispheric asymmetry seen in this study only occurs in the early developmental stage, whether it depends on language proficiency (if so, the degree of lateralization may change), or whether it is a task-specific effect (i.e., it occurred because our tasks strongly demanded phonological and prosodic analyses) would be of great interest. Analyses of language proficiency in relation to cerebral specifications are currently underway, and other questions will be explored in our future study or elsewhere.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
This work was supported by a Grant-in-Aid for the promotion of “Brain Science and Education, Type II” from the Research Institute of Science and Technology for Society, Japan Science and Technology Agency (RISTEX, JST) to H.H. Preparation of the revised manuscript was partly supported by a Grant-in-Aid for Scientific Research (A) (No. 22242012) from Japan Society for the Promotion of Science to H.H.
Supplementary Material
Acknowledgments
We thank all the children, and their families, who participated in this study and also the elementary school teachers for their support. We are grateful to Naoko Nakamura for her help in the earliest stage of data acquisition, to Dr Fumitaka Homae for his helpful advice in the data preprocessing and discussion, to Dr Masako Okamoto for her helpful advice in the data preprocessing, to Dr Yasushi Kyotoku for his statistical advice, and to Dr Atsushi Maki for his technical support on the analysis tool. We appreciate Dr Hideaki Koizumi for his encouragement and continuous support. Conflict of Interest: None declared.
References
- Amano S, Kondo T. NTT Database Series, Nihongo-no Goitokusei: lexical properties of Japanese. Vol. 7. Tokyo: Sanseido-shoten; 2000. [Google Scholar]
- Bahrick LE, Pickens JN. Classification of bimodal English and Spanish language passages by infants. Infant Behav Dev. 1988;11:277–296. [Google Scholar]
- Barch DM, Sabb FW, Carter CS, Braver TS, Noll DC, Cohen JD. Overt verbal responding during fMRI scanning: empirical investigations of problems and potential solutions. Neuroimage. 1999;10:642–657. doi: 10.1006/nimg.1999.0500. [DOI] [PubMed] [Google Scholar]
- Beeman M, Chiarello C. Right hemisphere language comprehension: perspectives from cognitive neuroscience. Mahwah, NJ: Lawrence Erlbaum Associates; 1998. [Google Scholar]
- Binder JR, Frost JA. Functional MRI studies of language processes in the brain. Neurosci News. 1998;1:15–23. [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PSF, Springer JA, Kaufman JN, Possing ET. Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex. 2000;10:512–528. doi: 10.1093/cercor/10.5.512. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. J Neurosci. 1997;17:353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Rao SM, Cox RW. Function of the left planum temporale in auditory and linguistic processing. Brain. 1996;119:1239–1247. doi: 10.1093/brain/119.4.1239. [DOI] [PubMed] [Google Scholar]
- Birn RM, Bandettini PA, Cox RW, Jesmanowicz A, Shaker R. Magnetic field changes in the human brain due to swallowing or speaking. Magn Reson Med. 1998;40:55–60. doi: 10.1002/mrm.1910400108. [DOI] [PubMed] [Google Scholar]
- Birn RM, Bandettini PA, Cox RW, Shaker R. Event-related fMRI of tasks involving brief motion. Hum Brain Mapp. 1999;7:106–114. doi: 10.1002/(SICI)1097-0193(1999)7:2<106::AID-HBM4>3.0.CO;2-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden S, Obrig H, Koehncke C, Benav H, Koch SP, Steinbrink J. The oxygenation response to functional stimulation: is there a physiological meaning to the lag between parameters? Neuroimage. 2007;36:100–107. doi: 10.1016/j.neuroimage.2007.01.045. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Zeffiro TA, Blaxton T, Gaillard W, Theodore W. Regional cerebral blood flow changes during object naming and word reading. Hum Brain Mapp. 1995;3:93–106. [Google Scholar]
- Bortfeld H, Fava E, Boas DA. Identifying cortical lateralization of speech processing in infants using near-infrared spectroscopy. Dev Neuropsychol. 2009;34:52–65. doi: 10.1080/87565640802564481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortfeld H, Wruck E, Boas DA. Assessing infants’ cortical response to speech using near-infrared spectroscopy. Neuroimage. 2007;34:407–415. doi: 10.1016/j.neuroimage.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Johnsrude IS, Owen AM. The problem of functional localization in the human brain. Nat Rev Neurosci. 2002;3:243–249. doi: 10.1038/nrn756. [DOI] [PubMed] [Google Scholar]
- Brunswick N, McCrory E, Price CJ, Frith CD, Frith U. Explicit and implicit processing of words and pseudowords by adult developmental dyslexics. Brain. 1999;122:1901–1917. doi: 10.1093/brain/122.10.1901. [DOI] [PubMed] [Google Scholar]
- Burgund ED, Kang HC, Kelly JE, Buckner RL, Snyder AZ, Petersen SE, Schlaggar BL. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. Neuroimage. 2002;17:184–200. doi: 10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Caplan D, Gow D, Makris N. Analysis of lesions by MRI in stroke patients with acoustic-phonetic processing deficits. Neurology. 1995;45:293–298. doi: 10.1212/wnl.45.2.293. [DOI] [PubMed] [Google Scholar]
- Caplan D, Waters GS. Verbal working memory and sentence comprehension. Behav Brain Sci. 1999;22:114–126. doi: 10.1017/s0140525x99001788. [DOI] [PubMed] [Google Scholar]
- Castro-Caldas A, Petersson KM, Reis A, Stone-Elander S, Ingvar M. The illiterate brain: learning to read and write during childhood influences the functional organization of the adult brain. Brain. 1998;121:1053–1063. doi: 10.1093/brain/121.6.1053. [DOI] [PubMed] [Google Scholar]
- Celsis P, Boulanouar K, Doyon B, Ranjeva JP, Berry I, Nespoulous JL, Chollet F. Differential fMRI responses in the left posterior superior temporal gyrus and left supramarginal gyrus to habituation and change detection in syllables and tones. Neuroimage. 1999;9:135–144. doi: 10.1006/nimg.1998.0389. [DOI] [PubMed] [Google Scholar]
- Christophe A, Morton J. Is Dutch native English? Linguistic analysis by 2-month-olds. Dev Sci. 1998;1:215–219. [Google Scholar]
- Cope M, Delpy DT, Reynolds EOR, Wray S, Wyatt J, Van der Zee P. Methods of quantitating cerebral near infrared spectroscopy data. Adv Exp Med Biol. 1988;222:183–189. doi: 10.1007/978-1-4615-9510-6_21. [DOI] [PubMed] [Google Scholar]
- Corrigan R. Use of repetition to facilitate spontaneous language acquisition. J Psycholinguist Res. 1980;9:231–241. [Google Scholar]
- Cutler A, Mehler J, Norris D, Segui J. The syllable’s differing role in the segmentation of French and English. J Mem Lang. 1986;25:385–400. [Google Scholar]
- Damasio AR, Damasio H. The anatomic basis of pure alexia. Neurology. 1983;33:1573–1583. doi: 10.1212/wnl.33.12.1573. [DOI] [PubMed] [Google Scholar]
- Dapretto M, Bookheimer SY. Form and content: dissociating syntax and semantics in sentence comprehension. Neuron. 1999;24:427–432. doi: 10.1016/s0896-6273(00)80855-7. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Houston D. Faster orientation latencies toward native language in two-month-old infants. Lang Speech. 1998;41:21–43. [Google Scholar]
- Dejerine J. Contribution a l’étude anatomo-pathologique et clinique des differentes variétés de cécité-verbale. Mem Soc Biol. 1892;4:61–90. [Google Scholar]
- Démonet JF, Chollet F, Ramsay S, Cardebat D, Nespoulous JL, Wise R, Rascol A, Frackowiak R. The anatomy of phonological and semantic processing in normal subjects. Brain. 1992;115:1753–1768. doi: 10.1093/brain/115.6.1753. [DOI] [PubMed] [Google Scholar]
- Démonet JF, Price C, Wise R, Frackowiak RS. Differential activation of right and left posterior sylvian regions by semantic and phonological tasks: a positron-emission tomography study in normal human subjects. Neuroscience. 1994;182:25–28. doi: 10.1016/0304-3940(94)90196-1. [DOI] [PubMed] [Google Scholar]
- Fernald A, Kuhl P. Acoustic determinants of infant preference for motherese speech. Infant Behav Dev. 1987;10:279–293. [Google Scholar]
- Fiez JA, Tallal P, Raichle ME, Miezin FM, Katz WF, Petersen SE. PET studies of auditory and phonological processing: effects of stimulus characteristics and task demands. J Cogn Neurosci. 1995;7:357–375. doi: 10.1162/jocn.1995.7.3.357. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Henson RN. Frontal lobes and human memory: insights from functional neuroimaging. Brain. 2001;124:849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Friederici AD. Towards a neural basis of auditory sentence processing. Trends Cogn Sci. 2002;6:78–84. doi: 10.1016/s1364-6613(00)01839-8. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Alter K. Lateralization of auditory language functions: a dynamic dual pathway model. Brain Lang. 2004;89:267–276. doi: 10.1016/S0093-934X(03)00351-1. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE, Poldrack RA, Desmond JE. The role of left prefrontal cortex in language and memory. Proc Natl Acad Sci USA. 1998;95:906–913. doi: 10.1073/pnas.95.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard WD, Balsamo LM, Ibrahim Z, Sachs BC, Xu B. fMRI identifies regional specialization of neural networks for reading in young children. Neurology. 2003a;60:94–100. doi: 10.1212/wnl.60.1.94. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Sachs BC, Whitnah JR, Ahmad Z, Balsamo LM, Petrella JR, Braniecki SH, McKinney CM, Hunter K, Xu B, et al. Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Hum Brain Mapp. 2003b;18:176–185. doi: 10.1002/hbm.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaburda AM, LeMay M, Kemper TL, Geschwind N. Right–left asymmetry in the brain. Science. 1978;199:852–856. doi: 10.1126/science.341314. [DOI] [PubMed] [Google Scholar]
- Gandour J, Wong D, Hsieh L, Weinzapfel B, Van Lancker D, Hutchins GD. A cross-linguistic PET study of tone perception. J Cogn Neurosci. 2000;12:207–222. doi: 10.1162/089892900561841. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Miller BL. Molecular approaches to cerebral laterality: development and neurodegeneration. Am J Med Genet A. 2001;101:370–381. [PubMed] [Google Scholar]
- Green E, Howes DH. The nature of conduction aphasia: a study of anatomic and clinical features and of underlying mechanisms. In: Whitaker A, Whitaker HA, editors. Studies in neurolinguistics. San Diego, CA: Academic Press; 1978. pp. 123–156. [Google Scholar]
- Grodzinsky Y. The neurology of syntax: language use without Broca’s area. Behav Brain Sci. 2000;23:1–71. doi: 10.1017/s0140525x00002399. [DOI] [PubMed] [Google Scholar]
- Henderson VW. Anatomy of posterior pathways in reading: a reassessment. Brain Lang. 1986;29:119–133. doi: 10.1016/0093-934x(86)90037-4. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Towards a functional neuroanatomy of speech perception. Trends Cogn Sci. 2000;4:131–138. doi: 10.1016/s1364-6613(00)01463-7. [DOI] [PubMed] [Google Scholar]
- Hinke R, Hu X, Stillman A, Kim SG, Merkle H, Salmi R, Ugurbil K. Functional magnetic resonance imaging of Broca’s area during internal speech. Neuroreport. 1993;4:675–678. doi: 10.1097/00001756-199306000-00018. [DOI] [PubMed] [Google Scholar]
- Hofmann MJ, Herrmann MJ, Dan I, Obrig H, Conrad M, Kuchinke L, Jacobs AM, Fallgatter AJ. Differential activation of frontal and parietal regions during visual word recognition: an optical topography study. Neuroimage. 2008;40:1340–1349. doi: 10.1016/j.neuroimage.2007.12.037. [DOI] [PubMed] [Google Scholar]
- Homae F, Watanabe H, Nakano T, Asakawa K, Taga G. The right hemisphere of sleeping infant perceives sentential prosody. Neurosci Res. 2006;54:276–280. doi: 10.1016/j.neures.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Homae F, Watanabe H, Nakano T, Taga G. Prosodic processing in the developing brain. Neurosci Res. 2007;59:29–39. doi: 10.1016/j.neures.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Rumsey JM, Donohue BC. Functional connectivity of the angular gyrus in normal reading and dyslexia. Proc Natl Acad Sci USA. 1998;95:8939–8944. doi: 10.1073/pnas.95.15.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi Y. Functional near-infrared optical imaging: utility and limitations in human brain mapping. Psychophysiology. 2003;40:511–520. doi: 10.1111/1469-8986.00053. [DOI] [PubMed] [Google Scholar]
- Hoshi Y, Chen SJ. Regional cerebral blood flow changes associated with emotions in children. Pediatr Neurol. 2002;27:275–281. doi: 10.1016/s0887-8994(02)00432-0. [DOI] [PubMed] [Google Scholar]
- Hoshi Y, Kobayashi N, Tamura M. Interpretation of near-infrared spectroscopy signals: a study with a newly developed perfused rat brain model. J Appl Physiol. 2001;90:1657–1662. doi: 10.1152/jappl.2001.90.5.1657. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson K, Wise R, Brown WD, Friston K, Weiller C, Frackowiak R. The cortical localization of the lexicons: positron emission tomography evidence. Brain. 1992;115:1769–1782. doi: 10.1093/brain/115.6.1769. [DOI] [PubMed] [Google Scholar]
- Hull R, Bortfeld H, Koons S. Near-infrared spectroscopy and cortical responses to speech production. Open Neuroimag J. 2009;3:26–30. doi: 10.2174/1874440000903010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami T, Taga G. Decrease in cortical activation during learning of a multi-joint discrete motor task. Exp Brain Res. 2008;191:221–236. doi: 10.1007/s00221-008-1518-2. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJM. The spatial and temporal signatures of word production components. Cognition. 2004;92:101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Inui T, Otsu Y, Tanaka S, Okada T, Nishizawa S, Konishi J. A functional MRI analysis of comprehension processes of Japanese sentences. Neuroreport. 1998;9:3325–3328. doi: 10.1097/00001756-199810050-00032. [DOI] [PubMed] [Google Scholar]
- Johnsrude IS, Penhune VB, Zatorre RJ. Functional specificity in the right human auditory cortex for perceiving pitch direction. Brain. 2000;123:155–163. doi: 10.1093/brain/123.1.155. [DOI] [PubMed] [Google Scholar]
- Jurcak V, Okamoto M, Singh A, Dan I. Virtual 10–20 measurement on MR images for inter-modal linking of transcranial and tomographic neuroimaging methods. Neuroimage. 2005;26:1184–1192. doi: 10.1016/j.neuroimage.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Jurcak V, Tsuzuki D, Dan I. 10/20, 10/10, and 10/5 systems revisited: their validity as relative head-surface-based positioning systems. Neuroimage. 2007;34:1600–1611. doi: 10.1016/j.neuroimage.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Kaan E, Swaab TY. The brain circuitry of syntactic comprehension [review] Trends Cogn Sci. 2002;6:350–356. doi: 10.1016/s1364-6613(02)01947-2. [DOI] [PubMed] [Google Scholar]
- Kang HC, Burgund ED, Lugar HM, Petersen SE, Schlaggar BL. Comparison of functional activation foci in children and adults using a common stereotactic space. Neuroimage. 2003;19:16–28. doi: 10.1016/s1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- Katagiri A, Dan I, Tuzuki D, Okamoto M, Yokose N, Igarashi K, Hoshino T, Fujiwara T, Katayama Y, Yamaguchi Y, et al. Mapping of optical pathlength of human adult head at multi-wavelengths in near infrared spectroscopy. Adv Exp Med Biol. 2010;662:205–212. doi: 10.1007/978-1-4419-1241-1_29. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A, Obrig H, Requardt M, Merboldt KD, Dirnagl U, Villringer A, Frahm J. Simultaneous recording of cerebral blood oxygenation changes during human brain activation by magnetic resonance imaging and near-infrared spectroscopy. J Cereb Blood Flow Metab. 1996;16:817–826. doi: 10.1097/00004647-199609000-00006. [DOI] [PubMed] [Google Scholar]
- Kučera H, Francis WN. Computational analysis of present-day American English. Providence, RI: Brown University Press; 1967. [Google Scholar]
- Kuczaj SA. Language play and language acquisition. In: Reese HW, editor. Advances in child development and behavior. Vol. 17. New York: Academic Press; 1982. pp. 197–232. [DOI] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Electrophysiology reveals semantic memory use in language comprehension. Trends Cogn Sci. 2000;4:463–470. doi: 10.1016/s1364-6613(00)01560-6. [DOI] [PubMed] [Google Scholar]
- Langheim FJ, Callicott JH, Mattay VS, Duyn JH, Weinberger DR. Cortical systems associated with covert music rehearsal. Neuroimage. 2002;16:901–908. doi: 10.1006/nimg.2002.1144. [DOI] [PubMed] [Google Scholar]
- Lauter JL, Herscovitch P, Formby C, Raichle ME. Tonotopic organization in human auditory cortex revealed by positron emission tomography. Hearing Res. 1985;20:199–205. doi: 10.1016/0378-5955(85)90024-3. [DOI] [PubMed] [Google Scholar]
- Liégeois FJ, Baldeweg T, Connelly A, Gadian DG, Mishkin M, Vargha-Khadem F. Language fMRI abnormalities associated with FOXP2 gene mutation. Nat Neurosci. 2003;6:1230–1237. doi: 10.1038/nn1138. [DOI] [PubMed] [Google Scholar]
- Maki A, Yamashita Y, Ito Y, Watanabe E, Mayanagi Y, Koizumi H. Spatial and temporal analysis of human motor activity using noninvasive NIR topography. Med Phys. 1995;22:1997–2005. doi: 10.1118/1.597496. [DOI] [PubMed] [Google Scholar]
- Mandel DR, Jusczyk PW, Nelson DGK. Does sentential prosody help infants organize and remember speech information? Cognition. 1994;53:155–180. doi: 10.1016/0010-0277(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Mazziotta JC, Phelps ME, Carson RE, Kuhl DE. Tomographic mapping of human cerebral metabolism: auditory stimulation. Neurology. 1982;32:921–937. doi: 10.1212/wnl.32.9.921. [DOI] [PubMed] [Google Scholar]
- McCrory E, Frith U, Brunswick N, Price C. Abnormal functional activation during a simple word repetition task: a PET study of adult dyslexics. J Cogn Neurosci. 2000;12:753–762. doi: 10.1162/089892900562570. [DOI] [PubMed] [Google Scholar]
- Meek JH, Firbank M, Elwell CE, Atkinson J, Braddick O, Wyatt JS. Regional hemodynamic responses to visual stimulation in awake infants. Pediatr Res. 1998;43:840–843. doi: 10.1203/00006450-199806000-00019. [DOI] [PubMed] [Google Scholar]
- Mehler J, Dupoux E, Nazzi T, Dehaene-Lambertz G. Coping with linguistic diversity: the infant’s viewpoint. In: Morgan JL, Demuth K, editors. Signal to syntax: bootstrapping from speech to grammar in early acquisition. Mahwah, NJ: Lawrence Erlbaum Associates; 1996. pp. 101–116. [Google Scholar]
- Mehler J, Jusczyk P, Lambertz G, Halsted N, Bertoncini J, Amiel-Tison C. A precursor of language acquisition in young infants. Cognition. 1988;29:143–178. doi: 10.1016/0010-0277(88)90035-2. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Meyer M, Alter K, Friederici AD, Lohmann G, von Cramon DY. Functional MRI reveals brain regions mediating slow prosodic manipulations of spoken sentences. Hum Brain Mapp. 2002;17:73–88. doi: 10.1002/hbm.10042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Steinhauer K, Alter K, Friederici AD, von Cramon DY. Brain activity varies with modulation of dynamic pitch variance in sentence melody. Brain Lang. 2004;89:277–289. doi: 10.1016/S0093-934X(03)00350-X. [DOI] [PubMed] [Google Scholar]
- Minagawa-Kawai Y, Matsuoka S, Dan I, Naoi N, Nakamura K, Kojima S. Prefrontal activation associated with social attachment: facial-emotion recognition in mothers and infants. Cereb Cortex. 2009;19:284–292. doi: 10.1093/cercor/bhn081. [DOI] [PubMed] [Google Scholar]
- Minagawa-Kawai Y, Mori K, Naoi N, Kojima S. Neural attunement processes in infants during the acquisition of a language-specific phonemic contrast. J Neurosci. 2007;27:315–321. doi: 10.1523/JNEUROSCI.1984-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon C, Cooper RP, Fifer WP. Two-day-olds prefer their native language. Infant Behav Dev. 1993;16:495–500. [Google Scholar]
- Muzik O, Chugani DC, Juhász C, Shen C, Chugani HT. Statistical parametric mapping: assessment of application in children. Neuroimage. 2000;12:538–549. doi: 10.1006/nimg.2000.0651. [DOI] [PubMed] [Google Scholar]
- Niznikiewicz M, Donnino R, McCarley RW, Nestor PG, Iosifescu DV, O’Donnell B, Levitt J, Shenton ME. Abnormal angular gyrus asymmetry in schizophrenia. Am J Psychiatry. 2000;157:428–437. doi: 10.1176/appi.ajp.157.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obleser J, Wise RIS, Dresner MA, Scott SK. Functional integration across brain regions improves speech perception under adverse listening conditions. J Neurosci. 2007;27:2283–2289. doi: 10.1523/JNEUROSCI.4663-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrig H, Wenzel R, Kohl M, Horst S, Wobst P, Steinbrink J, Thomas F, Villringer A. Near-infrared spectroscopy: does it function in functional activation studies of the adult brain? Int J Psychophysiol. 2000;35:125–142. doi: 10.1016/s0167-8760(99)00048-3. [DOI] [PubMed] [Google Scholar]
- Okada E, Firbank M, Schweiger M, Arridge SR, Cope M, Delpy D. Theoretical and experimental investigation of near infrared light propagation in a model of the adult head. Appl Opt. 1997;36:21–31. doi: 10.1364/ao.36.000021. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Dan H, Sakamoto K, Takeo K, Shimizu K, Kohno S, Oda I, Isobe S, Suzuki T, Kohyama K, et al. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. Neuroimage. 2004;21:99–111. doi: 10.1016/j.neuroimage.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Tsuzuki D, Clowney L, Dan H, Singh AK, Dan I. Structural atlas-based spatial registration for functional near-infrared spectroscopy enabling inter-study data integration. Clin Neurophysiol. 2009;120:1320–1328. doi: 10.1016/j.clinph.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Otake T, Hatano G, Cutler A, Mehler J. Mora or syllable? Speech segmentation in Japanese. J Mem Lang. 1993;32:258–278. [Google Scholar]
- Patterson R, Uppenkamp S, Johnsrude I, Griffiths T. The processing of temporal pitch and melody information in auditory cortex. Neuron. 2002;36:767–776. doi: 10.1016/s0896-6273(02)01060-7. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RS. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Peña M, Maki A, Kovačić D, Dehaene-Lambertz G, Koizumi H, Bouquet F, Mehler J. Sounds and silence: an optical topography study of language recognition at birth. Proc Natl Acad Sci USA. 2003;100:11702–11705. doi: 10.1073/pnas.1934290100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Pereira M. Imitations, repetitions, routines, and the child’s analysis of language: insights from the blind. J Child Lang. 1994;21:317–337. doi: 10.1017/s0305000900009296. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331:585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies of the processing of single words. J Cogn Neurosci. 1989;1:153–170. doi: 10.1162/jocn.1989.1.2.153. [DOI] [PubMed] [Google Scholar]
- Plichta MM, Herrmann MJ, Baehne CG, Ehlis AC, Richter MM, Pauli P, Fallgatter AJ. Event-related functional near infrared spectroscopy (fNIRS): are the measurements reliable? Neuroimage. 2006;31:116–124. doi: 10.1016/j.neuroimage.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Poeppel D, Hickok G. Towards a new functional anatomy of language. Cognition. 2004;92:1–12. doi: 10.1016/j.cognition.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JDE. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: contributions from functional neuroimaging. J Anat. 2000;197:335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Wise RJS, Warburton EA, Moore CJ, Howard D, Patterson K, Frackowiak RSJ, Friston KJ. Hearing and saying: the functional neuro-anatomy of auditory word processing. Brain. 1996;119:919–931. doi: 10.1093/brain/119.3.919. [DOI] [PubMed] [Google Scholar]
- Price CJ, Wise RJS, Watson JDG, Patterson K, Howard D, Frackowiak RSJ. Brain activity during reading: the effects of exposure duration and task. Brain. 1994;117:1255–1269. doi: 10.1093/brain/117.6.1255. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Shaywitz BA, Shaywitz SE, Fulbright RK, Byrd D, Skudlarski P, Shankweiler DP, Katz L, Constable RT, Fletcher J, et al. Auditory selective attention: an fMRI investigation. Neuroimage. 1996;4:159–173. doi: 10.1006/nimg.1996.0067. [DOI] [PubMed] [Google Scholar]
- Roeltgen DP, Sevush S, Heilman KM. Phonological agraphia: writing by the lexical-semantic route. Neurology. 1983;33:755–765. doi: 10.1212/wnl.33.6.755. [DOI] [PubMed] [Google Scholar]
- Roland PE, Larsen B, Lassen NA, Skinhoj E. Supplementary motor area and other cortical areas in organization of voluntary movements in man. J Neurophysiol. 1980;43:118–136. doi: 10.1152/jn.1980.43.1.118. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Horwitz B, Donohue BC, Nace K, Maisog JM, Andreason P. Phonological and orthographic components of word recognition: a PET-rCBF study. Brain. 1997;120:739–759. doi: 10.1093/brain/120.5.739. [DOI] [PubMed] [Google Scholar]
- Sachs BC, Gaillard WD. Organization of language networks in children: functional magnetic resonance imaging studies. Curr Neurol Neurosci Rep. 2003;3:157–162. doi: 10.1007/s11910-003-0068-z. [DOI] [PubMed] [Google Scholar]
- Sakai KL. Language acquisition and brain development. Science. 2005;310:815–819. doi: 10.1126/science.1113530. [DOI] [PubMed] [Google Scholar]
- Sakatani K, Chen S, Lichty W, Zuo H, Wang YP. Cerebral blood oxygenation changes induced by auditory stimulation in newborn infants measured by near infrared spectroscopy. Early Hum Dev. 1999;55:229–236. doi: 10.1016/s0378-3782(99)00019-5. [DOI] [PubMed] [Google Scholar]
- Salmon E, Van der Linden M, Collette F, Delfiore G, Maquet P, Degueldre C, Luxen A, Franck G. Regional brain activity during working memory tasks. Brain. 1996;119:1617–1625. doi: 10.1093/brain/119.5.1617. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE. Functional neuroanatomical differences between adults and school-age children in the processing of single words. Science. 2002;296:1476–1479. doi: 10.1126/science.1069464. [DOI] [PubMed] [Google Scholar]
- Schneider W, Shiffrin RM. Controlled and automatic human information processing: I. detection, search, and attention. Psychol Rev. 1977;84:1–66. [Google Scholar]
- Seiyama A, Seki J, Tanabe HC, Sase I, Takatsuki A, Miyauchi S, Eda H, Hayashi S, Imaruoka T, Iwakura T, et al. Circulatory basis of fMRI signals: relationship between changes in the hemodynamic parameters and BOLD signal intensity. Neuroimage. 2004;21:1204–1214. doi: 10.1016/j.neuroimage.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Service E. Phonology, working memory, and foreign-language learning. Q J Exp Psychol. 1992;45A:21–50. doi: 10.1080/14640749208401314. [DOI] [PubMed] [Google Scholar]
- Shallice T. Phonological agraphia and the lexical route in writing. Brain. 1981;104:413–429. doi: 10.1093/brain/104.3.413. [DOI] [PubMed] [Google Scholar]
- Shallice T, Vallar G. The impairment of auditory-verbal short-term storage. In: Vallar G, Shallice T, editors. Neuropsychological impairments of short-term memory. Cambridge, MA: Cambridge University Press; 1990. pp. 11–53. [Google Scholar]
- Shiffrin RM, Schneider W. Controlled and automatic human information processing: II. Perceptual learning, automatic attending and a general theory. Psychol Rev. 1977;84:127–190. [Google Scholar]
- Skinner BF. Verbal behavior. New York: Appleton-Century-Crofts; 1957. [Google Scholar]
- Smith EE, Jonides J. Neuroimaging analyses of human working memory. Proc Natl Acad Sci USA. 1998;95:12061–12068. doi: 10.1073/pnas.95.20.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Marshuetz C, Koeppe RA. Components of verbal working memory: evidence from neuroimaging. Proc Natl Acad Sci USA. 1998;95:876–882. doi: 10.1073/pnas.95.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow CE. The uses of imitation. J Child Lang. 1981;8:205–212. doi: 10.1017/s0305000900003111. [DOI] [PubMed] [Google Scholar]
- Snow CE. Saying it again: the role of expanded and deferred imitations in language acquisition. In: Nelson KE, editor. Children’s language. Vol. 4. Hillsdale, NJ: Erlbaum (Lawrence Erlbaum Associates; 1983. pp. 29–58. [Google Scholar]
- Speidel GE, Nelson KE. The many faces of imitation in children. New York: Springer-Verlag; 1989. [Google Scholar]
- Strangman G, Culver JP, Thompson JH, Boas DA. A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. Neuroimage. 2002;17:719–731. [PubMed] [Google Scholar]
- Szaflarski JP, Schmithorst VJ, Altaye M, Byars AW, Ret J, Plante E, Holland SK. A longitudinal functional magnetic resonance imaging study of language development in children 5 to 11 years old. Ann Neurol. 2006;59:796–807. doi: 10.1002/ana.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taga G, Asakawa K, Maki A, Konishi Y, Koizumi H. Brain imaging in awake infants by near-infrared optical topography. Proc Natl Acad Sci USA. 2003;100:10722–10727. doi: 10.1073/pnas.1932552100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahta S, Wood M, Loewenthal K. Age changes in the ability to replicate foreign pronunciation and intonation. Lang Speech. 1981;24:363–372. [Google Scholar]
- Talaraich J, Tournoux P. Co-planar stereotaxic atlas of the human brain: three dimensional proportional system: an approach to cerebral imaging. New York: Thieme; 1988. [Google Scholar]
- Tatsuno Y, Sakai KL. Language-related activations in the left prefrontal regions are differentially modulated by age, proficiency, and task demands. J Neurosci. 2005;25:1637–1644. doi: 10.1523/JNEUROSCI.3978-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telkemeyer S, Rossi S, Koch SP, Nierhaus T, Steinbrink J, Poeppel D, Obrig H, Wartenburger I. Sensitivity of newborn auditory cortex to the temporal structure of sounds. J Neurosci. 2009;29:14726–14733. doi: 10.1523/JNEUROSCI.1246-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzuki D, Jurcak V, Singh AK, Okamoto M, Watanabe E, Dan I. Virtual spatial registration of stand-alone fNIRS data to MNI space. Neuroimage. 2007;34:1506–1518. doi: 10.1016/j.neuroimage.2006.10.043. 15:273–289. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Ullman MT. A neurocognitive perspective on language: the declarative/procedural model. Nat Rev Neurosci. 2001;2:717–726. doi: 10.1038/35094573. [DOI] [PubMed] [Google Scholar]
- Vallar G, Di Betta AM, Silveri MC. The phonological short-term store-rehearsal system: patterns of impairment and neural correlates. Neuropsychologia. 1997;35:795–812. doi: 10.1016/s0028-3932(96)00127-3. [DOI] [PubMed] [Google Scholar]
- Villringer A, Chance B. Non-invasive optical spectroscopy and imaging of human brain function. Trends Neurosci. 1997;20:435–442. doi: 10.1016/s0166-2236(97)01132-6. [DOI] [PubMed] [Google Scholar]
- Wartenburger I, Steinbrink J, Telkemeyer S, Friedrich M, Friederici AD, Obrig H. The processing of prosody: evidence of interhemispheric specialization at the age of four. Neuroimage. 2007;34:416–425. doi: 10.1016/j.neuroimage.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Watanabe E, Maki A, Kawaguchi F, Takashiro K, Yamashita Y, Koizumi H, Mayanagi Y. Non-invasive assessment of language dominance with near-infrared spectroscopic mapping. Neurosci Lett. 1998;256:49–52. doi: 10.1016/s0304-3940(98)00754-x. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Saygin AP, Sereno MI, Iacoboni M. Listening to speech activates motor areas involved in speech production. Nat Neurosci. 2004;7:701–702. doi: 10.1038/nn1263. [DOI] [PubMed] [Google Scholar]
- Yetkin FZ, Hammeke TA, Swanson SJ, Morris GL, Mueller WM, McAuliffe TL, Haughton VM. A comparison of functional MR activation patterns during silent and audible language tasks. AJNR Am J Neuroradiol. 1995;16:1087–1092. [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Belin P, Penhune VB. Structure and function of auditory cortex: music and speech. Trends Cogn Sci. 2002;6:37–46. doi: 10.1016/s1364-6613(00)01816-7. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Evans AC, Meyer E, Gjedde A. Lateralization of phonetic and pitch discrimination in speech processing. Science. 1992;256:846–849. doi: 10.1126/science.1589767. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Mondor TA, Evans AC. Auditory attention to space and frequency activates similar cerebral systems. Neuroimage. 1999;10:544–554. doi: 10.1006/nimg.1999.0491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.