Abstract
Callosal volume reduction has been observed in patients with bipolar disorder, but whether these deficits reflect genetic vulnerability to the illness remains unresolved. Here, we used computational methods to map corpus callosum abnormalities in a population-based sample of twin pairs discordant for bipolar disorder. Twenty-one probands with bipolar I disorder (mean age 44.4 ± 7.5 years; 48% female), 19 of their non-bipolar co-twins, and 34 demographically matched control twin individuals underwent magnetic resonance imaging. Three-dimensional callosal surface models were created to visualize its morphologic variability and to localize group differences. Neurocognitive correlates of callosal area differences were additionally investigated in a subsample of study participants. Bipolar (BPI) probands, but not their co-twins, showed significant callosal thinning and area reduction, most pronounced in the genu and splenium, relative to healthy twins. Altered callosal curvature was additionally observed in BPI probands. In bipolar probands and co-twins, genu and splenium midsagittal areas were significantly correlated with verbal processing speed and response inhibition. These findings suggest that aberrant connections between cortical regions—possibly reflecting decreased myelination of white matter tracts—may be involved in bipolar pathophysiology. However, findings of callosal thinning appear to be disease related, rather than reflecting genetic vulnerability to bipolar illness.
Keywords: magnetic resonance imaging, mood disorders, myelination, processing speed, twin study
Introduction
Several lines of evidence suggest that abnormal white matter connectivity may be relevant to the pathophysiology of bipolar disorder. Traditional volumetric magnetic resonance imaging (MRI) studies report nonspecific white matter volume deficits (Strakowski et al. 2002; Kieseppa et al. 2003; Davis et al. 2004). Elevated rates of white matter hyperintensities have been widely observed in bipolar disorder (Aylward et al. 1994; Altshuler et al. 1995; Dupont et al. 1995; Videbech 1997). More recently, diffusion tensor imaging (DTI) studies have observed microstructural changes consistent with lower white matter integrity in bipolar patients (Adler et al. 2004; Regenold et al. 2006), suggesting a loss of bundle coherence in white matter tracts.
As the largest white matter fiber tract in the brain, the corpus callosum (CC) is a critical component of the biological infrastructure that allows communication among brain regions. Abnormal callosal structure may lead to altered interhemispheric communication, which may underlie some of the cognitive impairments in bipolar patients (Wilder-Willis et al. 2001; Brambilla et al. 2004). A recent meta-analysis of 5 case–control studies concluded that bipolar I patients showed lower callosal area than healthy volunteers (effect size of 0.52), with no evidence of heterogeneity across studies (Arnone et al. 2008). Brambilla et al. (2004) found lower CC signal intensity (a putative index of callosal myelination) in bipolar patients relative to healthy controls, while unipolar depressed patients did not differ from healthy subjects.
Although there are few neuroanatomic studies of relatives of bipolar patients to date, white matter may be particularly affected in those at genetic risk for the illness. In an extended pedigree with a strong family history of bipolar disorder, Ahearn et al. (1998) found lesions of white matter and subcortical gray nuclei in affected and unaffected family members. In a twin sample partially overlapping with the one studied here, Kieseppa et al. (2003) previously found decreased left hemisphere white matter volume in both bipolar probands and their non-bipolar co-twins. Similarly, McDonald et al. (2004) found that genetic risk for both schizophrenia and bipolar disorder was associated with white matter reduction in left frontal and temporoparietal regions, suggesting that left frontotemporal disconnectivity may be a genetically controlled neuroanatomic abnormality common to both disorders.
To our knowledge, only 2 other studies of twin pairs discordant for bipolar disorder have been published (Noga et al. 2001; van der Schot et al. 2009). The first focused on medial temporal structures and included just 6 twin pairs discordant for bipolar disorder and 6 normal twins; increased left caudate volume was observed in both bipolar probands and their well co-twins, suggesting a genetic contribution. In a larger Dutch twin sample, van der Schot et al. (2009) found that reduction in total white matter volume was related to genetic risk for bipolar disorder, whereas significant environmental correlations were observed for cortical gray matter.
However, the familial aggregation of callosal abnormalities in twin pairs discordant for bipolar illness has not been investigated. Thus, it is not yet known whether such abnormalities may represent genetic vulnerability markers, as may be the case in schizophrenia (e.g., Narr, Cannon, et al. 2002), or a disease-related trait. Furthermore, geometric anatomic models and statistical mapping approaches have not previously been employed in studies of callosal abnormalities in patients with bipolar disorder.
Here, we used computational mapping methods to visualize callosal morphometry in a nationwide sample of twins with bipolar disorder, their co-twins, and a demographically balanced sample of control twin subjects. A novel measurement of callosal thickness (radial distance) was combined with a shape-averaging technique to create models of the CC used to visualize differences in callosal size and shape between groups.
Our first goal was to confirm prior findings of callosal abnormalities in patients with bipolar disorder, using these novel methods. Our primary aim, however, was to assess genetic and/or disease-related contributions to altered midsagittal callosal morphometry in bipolar disorder. We hypothesized that we would detect localized thinning in the genu and the splenium in patients with bipolar disorder, as these interconnect cortical regions previously implicated in bipolar pathophysiology (Strakowski et al. 2005; Cerullo et al. 2009; Walterfang, Malhi, et al. 2009; Walterfang, Wood, et al. 2009).
Materials and Methods
Study Participants
Study participants were ascertained via the National Hospital Discharge Register of Finland, which identified all patients with a mood disorder diagnosis (ICD-8; World Health Organization codes 296.10 or 296.30 or DSM-III-R (American Psychiatric Association 1987) codes 296.4, 296.5, or 296.6) during 1969–1991. The National Population Register and the Finnish Twin Cohorts (Kaprio et al. 1978, 1990) were used to locate twins born between 1940 and 1969. This comprehensive search identified 59 twins, who were invited to participate in the study with their co-twin. After diagnostic ascertainment, only twins with BPI and their co-twins were included in the MRI study. Other psychotic disorders, neurologic disorders affecting the brain, or brain injuries were criteria for exclusion (for further details, see Kieseppa et al. 2003, 2004). A control group of 34 twin subjects without any psychotic or mood disorder was recruited from the same Finnish twin cohorts and matched to the age, gender, and zygosity distribution of the patient sample. Zygosity determination was based on genetic marker analysis in all control and index twin pairs (Cannon et al. 2000).
In total, 21 bipolar probands, 19 of their non-bipolar co-twins and 34 healthy control twins were included in the current analysis (see Table 1). Four (19%) of the bipolar twins were from monozygotic (MZ) pairs. Because the sample size was relatively modest, and we wished to maximize the utility of the information available to us, we included some probands for which co-twin data were not available.
Table 1.
Demographic and clinical features of the 3 study groups
| Characteristics | Bipolar I disorder twins | Non-BPI co-twins | Control twin subjects | Difference between groups |
| n = 21 | n = 19 | n = 34 | ||
| Age in years | ||||
| Mean (±SD) | 44.4 (7.5) | 45.1 (8.1) | 46.2 (5.4) | F2,71 = 0.52, P = 0.60 |
| Gender | ||||
| Female, n (%) | 10 (48) | 11 (58) | 17 (50) | χ2 == 0.47, P = 0.79 |
| Education (SCID category) | ||||
| Mean (±SD) | 11.1 (4.9) | 10.9 (4.1) | 11.2 (4.2) | F2,71 = 0.01, P = 0.95 |
| Zygosity | ||||
| MZ, n (%) | 4 (19) | 2 (10.5) | 8 (23.5) | χ2 == 1.34, P = 0.51 |
| Handedness | ||||
| Right, n (%) | 21 (100) | 18 (95) | 33 (97) | χ2 == 1.06, P = 0.59 |
| Lifetime alcohol | ||||
| Abuse/dependence, n (%) | 5 (23.8) | 2 (10.5) | 2 (5.9) | χ2 == 3.97, P = 0.14 |
| Current alcohol | ||||
| Abuse/dependence, n (%) | 1 (4.8) | 0 (0) | 1 (2.9) | χ2 == 0.87, P = 0.65 |
| Lifetime anxiety | ||||
| Disorder, n (%) | 7 (33) | 3 (15.8) | 2 (5.9) | χ2 == 7.20, P = 0.03 |
| Lithium (mg/day) | ||||
| Mean (±SD) | 927 (249.4) | 0 | 0 | — |
| Any psychotropic | ||||
| Medication, n (%) | 15 (71) | 0 | 0 | — |
| Length of use of medication in years | ||||
| Mean (±SD) | 13.1 (9.6) | 0 | 0 | — |
| TBV, cc3 (±SD) | 1298.2 (125.6) | 1307.5 (140.5) | 1244.9 (125.6) | F2,71 = 1.5, P = 0.23 |
| Gray matter volume, cc3 (±SD) | 722.2 (83.3) | 725.3 (74.3) | 694.1 (62.5) | F2,71 = 1.6, P = 0.22 |
| White matter volume, cc3 (±SD) | 446.5 (87.8) | 461.7 (62.9) | 450.3 (64.5) | F2,71 = 0.25, P = 0.78 |
| Ventricular volume, cc3 (±SD) | 22.5 (14.0) | 19.8 (9.2) | 16.2 (5.6) | F2,71 = 2.9, P = 0.06* |
*BP < CTL, P = 0.07; BP = CO-TWIN; CO-TWIN = CTL; TBV = Total Brain Volume.
One BPI patient was hypomanic during the MRI session; no BP1 patients fulfilled criteria for a current manic or major depressive episode at the time of the scan. Five BPI patients (24%) were unmedicated, whereas 11 patients (52%) were taking lithium, at a mean daily dose of 927 mg (range 600–1200 mg). Thirteen patients (62%) were taking neuroleptics, at a mean daily dose of 15 mg haloperidol equivalents (range 2–48 mg). The mean duration of medication was 13.1 years (±9.6 years). Those without current medication usage had all used lithium or neuroleptics during at least one period in their life; however, the mean time without medication prior to the MRI scan was 10 years (±8 years). None of the co-twins or control twin subjects was taking psychotropic medication.
The study was approved by the Ministry of Social Affairs and Health, the Ethics Committee of the National Institute for Health and Welfare, and the UCLA Institutional Review Board. Written informed consent was obtained from all subjects after they had received a complete description of the study.
Diagnostic and Cognitive Assessment
All the probands, co-twins, and control twin subjects were interviewed using the Structured Clinical Interview for DSM-IV diagnoses (SCID; Spitzer et al. 1994). One investigator (T.K.) interviewed the probands and co-twins and field workers of the schizophrenia twin study (Cannon et al. 2000) interviewed the control twin subjects. All interviews were made blind to zygosity. The diagnostic and case consensus procedures are described in detail elsewhere (Cannon et al. 2000; Kieseppa et al. 2004).
Bipolar probands and their co-twins also completed a neuropsychological battery, which included measures of verbal processing speed (verbal category and letter fluency), auditory attention/working memory (Digit Span), and response inhibition (Stroop paradigm, interference condition). In order to reduce the risk of Type I error, we examined only those cognitive measures expected a priori to be related to callosal white matter integrity, that is, measures of processing speed, attention, and inhibition (Turken et al. 2008; Madsen et al. 2010).
Imaging Procedures
Image acquisition procedures are described in detail in Kieseppa et al. (2003). Briefly, T1-weighted magnetization prepared rapid gradient echo scans (time repetition/time echo = 11.4/4.4 ms, sagittal orientation, matrix size = 256 × 256 × 128, Field of View = 250 mm, in-plane resolution = 0.98 × 0.98 × 1.2) were acquired with a 1.0-T scanner (Siemens Medical Systems) in a private medical center (Teslamed).
Image Processing and Analysis
Each brain volume was corrected for field inhomogeneties (Sled et al. 1998) and resliced into a standard orientation. First, image volumes were placed into the standard coordinate system of the ICBM-305 average brain (Mazziotta et al. 1995), using a 3-translation and 3-rotation rigid-body transformation in order to correct for differences in head alignment between subjects (Woods et al. 1998). This procedure ensures that callosal measurements are not influenced by differences in brain orientation. Each brain volume was resampled to 1.0-mm isotropic voxels using trilinear interpolation and a 6-parameter Procrustes fit.
Brain and Ventricle Volumes
Brain tissue volumes and lateral ventricle volumes were obtained after classifying each image set into gray matter, white matter, and cerebrospinal fluid, after removal of extracortical tissue, as previously described (Narr, Cannon, et al. 2002). Total brain volume (TBV) was determined in centimeters cubed as the sum of voxels representing gray matter, white matter, and cerebrospinal fluid (CSF) (including ventricular CSF).
Corpus Callosum Delineation and Analysis
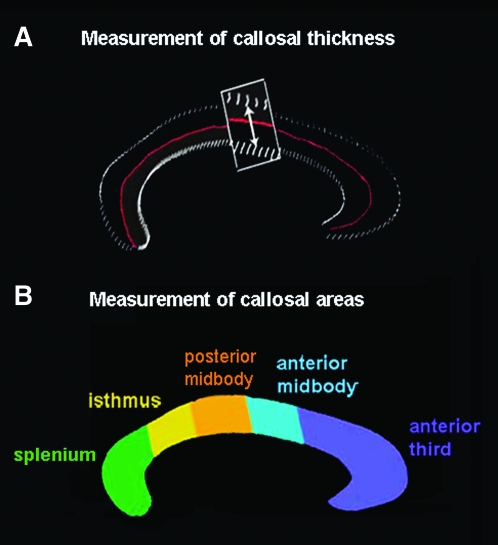
One rater (C.E.B.), who was blind to group status and demographic variables, identified the CC in midsagittal sections and delineated the upper (top) and lower (bottom) callosal boundaries in each magnified brain volume, using a mouse-driven cursor (Fig. 1). Midsagittal sections were defined by identifying the interhemispheric fissure in native contiguous 1.5-mm coronal slices and again in the sagittal plane when volumes were resliced at 0.5 mm after AC–PC scaling. The anterior and posterior splitting points for the upper and lower callosal boundaries were defined as in prior publications (e.g., Luders et al. 2007, 2009); specifically, we used the tip of the rostrum as the anterior splitting point, and for the posterior splitting point, the most inferior point of the splenium (see Supplementary Fig. 1). Reliability of callosal tracing was evaluated by repeatedly outlining the CC (6 times) in a randomly chosen brain (root mean square [r.m.s.] error < 1 mm).
Figure 1.
(a) Pointwise distances between the medial curve (in red) and the callosal top and bottom surfaces are calculated to obtain a thickness measure at each point on the callosal surface. (b) Subdivision of the CC into 5 areas according to the modified Witelson scheme (Clarke and Zaidel 1994; adapted from Ballmaier et al. 2008).
Callosal thickness measurements were performed after correcting brains for head position and tilts but preserving original brain size, as in Ballmaier et al. (2008). Callosal top and bottom sections were redigitized, resulting in 100 equidistant points along the midsagittal callosal curve per section (see Fig. 1). Then a new callosal outline (medial curve) was created by computing the spatial mean curve from surface points representing the top and bottom traces, followed by calculating the pointwise distances from the medial curve to the callosal top and bottom curves. The resulting distance values in each participant are color coded and superimposed onto each individual's callosal model. Subsequently, individual callosal surfaces and pointwise distance values were averaged within groups in order to create maps of callosal mean thickness, as well as group-specific profiles of average callosal shape. Next, we tested for group differences in callosal thickness and generated color-coded maps illustrating localized regions in which bipolar probands differ from healthy individuals and/or their unaffected co-twins, as well as differences between co-twins and healthy control twins. Finally, measures of dorsal and ventral callosal curvature were obtained from the uniformly redigitized grid points representing the callosal surfaces in pixel coordinates in the coordinate space of the ICBM-305 average brain, as described in detail in Narr et al. (2000).
Area Measurement
To obtain measures of CC morphology consistent with regional measurements typically reported in the literature, callosal renderings were divided into 5 partitions using a modification of the Witelson partitioning scheme (Witelson 1989; Clarke and Zaidel 1994) into 5 discrete partitions representing the 1) splenium, 2) isthmus, 3) posterior midbody, 4) anterior midbody, and 5) anterior third (see Fig. 1b). Area measures were computed in square millimeters for each callosal segment, as described previously (Narr, Cannon, et al. 2002; Vidal et al. 2006).
Statistical Analysis
Differences in callosal areas and morphometric shape parameters (i.e., curvature) were analyzed using mixed model regression analyses, treating each twin pair as a cluster in modeling (Laird and Ware 1982). To take into account the correlated nature of twin data (and to account for the fact that not all twins had a co-twin included in the analysis), twin pair was included as a random effect in the model. Risk status (patient, co-twin, control twin subject) as well as the 2/3 root of TBV was included in the model as fixed effects (covariates). Significant main effects were followed up with post hoc contrasts, using Scheffé's correction for multiple comparisons (Scheffé 1953). Secondary analyses examined the effects of medication on callosal measures.
Thickness measures allow greater localization of deficits but may be less sensitive to differences in anterior–posterior length of the CC. Thus, volumetric or regional cross-sectional area measures may in some cases be more sensitive than thickness maps for detecting group differences and vice versa. To compare both methods, we used both traditional volumetric methods and statistical maps. Given that comparisons of callosal thickness were made at hundreds of equidistant callosal surface points and adjacent data points may be correlated, a false discovery rate (FDR) of 0.05 was employed to control for multiple comparisons (Benjamini and Hochberg 1995; Storey et al. 2002). Significant group differences in callosal thickness, corrected using FDR, were color coded and mapped on the callosal surface model.
Brain Size Correction
Given that relationships between callosal size and brain size may differ across biological risk groups and our goal was to target differences specific to the CC, we chose to assess callosal parameters after brain volumes were aligned into a standard orientation with no scaling and therefore to use a brain size correction for the derived parameters in statistical analyses (e.g., Narr, Cannon, et al. 2002; Narr, van Erp, et al. 2002).
Correlations with Cognitive Measures
To assess the relationship between callosal area measures and cognitive measures, we used Spearman's rank correlational analyses to examine only those callosal areas corresponding to regions for which significant area and thickness reduction were observed in bipolar probands. Spearman's correlations are not influenced by outliers in small samples; bivariate analyses were deemed appropriate as the diagnostic groups did not differ in terms of age, education, or gender. To control for Type I error, Bonferroni correction was applied and the threshold of significance was set at P < 0.01.
Results
Subjects
The 3 groups (bipolar probands, non-bipolar co-twins, and control twins) were similar in terms of age, education, handedness, gender, and TBV (see Table 1). The groups did not differ with regard to frequency of lifetime alcohol abuse/dependence, but there was a significant difference in the frequency of lifetime anxiety disorder diagnoses, with bipolar I probands having a higher rate of anxiety disorder diagnoses (χ2 = 7.20, P = 0.03). For this reason, secondary analyses included lifetime anxiety disorder as a covariate.
Bipolar probands and co-twins did not significantly differ from each other on verbal fluency, auditory attention, and Stroop measures (FAS: F = 1.96, P = 0.18; animal naming: F = 3.8, P = 0.07; Digit Span: F = 0.64, P = 0.43; Stroop Interference condition, response time: F = 0.28, P = 0.60; Stroop Interference condition, errors: F = 1.95, P = 0.18).
Group Differences in Callosal Area Measurements
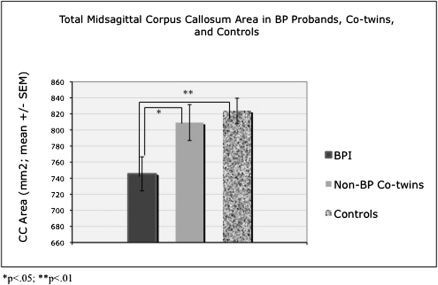
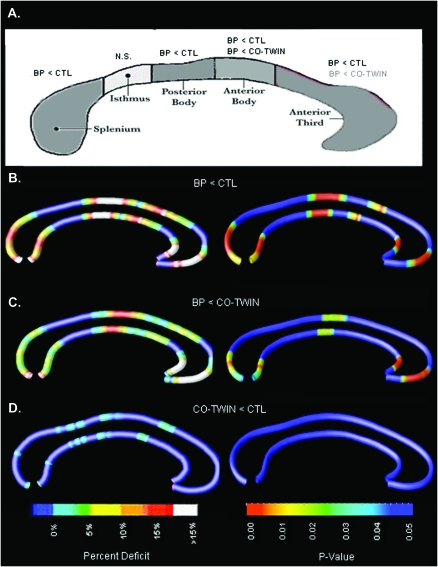
Traditional morphometric methods detected a significant reduction in callosal area in bipolar probands relative to control twins but not their co-twins. Mixed model analyses indicated a main effect of diagnosis on total callosal area (F2,70 = 4.01, P = 0.022; see Fig. 2). Post hoc pairwise contrasts revealed that bipolar probands differed significantly from both their co-twins (t = 1.97, P < 0.05) and control twins (t = 2.78, P = 0.007), but the co-twins and control twins did not differ significantly from each other (P = 0.61). Post hoc contrasts, displayed in Figure 3A, indicated significant main effects for CC area differences in the anterior third (genu) (F2,70 = 2.96, P < 0.05; P = 0.023 for BP vs. controls (CTL); P = 0.07 for BP vs. co-twins), anterior midbody (F2,70 = 6.66, P = 0.002; P = 0.001 for BP vs. controls; P = 0.024 for BP vs. co-twins), posterior midbody (F2,70 = 3.47, P = 0.037; P = 0.014 and P = 0.095 for BP vs. control twins and BP vs. co-twins, respectively), and splenium (F2,70 = 2.76, P < 0.05; P = 0.02 and P = 0.29 for BP vs. control twins and BP vs. co-twins, respectively). There were no group differences in the isthmus (F2,70 = 0.78, P = 0.46). Including lifetime anxiety disorder as a covariate did not alter the significance of these results. Finally, these analyses were reconducted excluding the 2 bipolar probands without co-twins; the pattern of results remained the same.
Figure 2.
Callosal area differences. Bipolar probands differed significantly from both their co-twins (P < 0.05) and control twins (P = 0.007); callosal area in non-bipolar co-twins was intermediate between that of bipolar probands and controls but was not significantly different from that of controls (P = 0.61). Statistical analyses covary for 2/3 root of TBV, but raw values are presented in figure for illustrative purposes.
Figure 3.
Group differences in callosal thickness. CC maps comparing BP probands, their non-bipolar co-twins, and control twin subjects. (A) Modified Witelson partitioning scheme. The anterior midbody of the CC showed a significant area reduction in BP1 probands relative to both control twins and their non-bipolar co-twins. Posterior midbody and anterior third (genu) areas were significantly reduced in BP probands relative to healthy control twins, with a similar trend toward area reduction relative to their non-ill co-twins. Splenium area was also significantly reduced in BP versus control twins. (B) Left panel depicts reduction of callosal thickness in BPI probands, expressed as a percentage relative to healthy control twin subjects. Right panel depicts corrected statistical maps (FDR) showing P values for group differences (BPI vs. controls) in callosal thickness. (C) Reduced callosal thickness in BPI probands, expressed as a percentage (left) and map of P values (right), relative to their non-bipolar co-twins. (D) Nonsignificant reduction of callosal thickness in co-twins of BP probands, expressed as a percentage (left) and map of P values (right), relative to healthy control twin subjects.
Computational Mapping
Maps of callosal thickness were consistent with results of area analyses, revealing regions of localized thinning of greatest magnitude in the genu and splenium in BPI probands, relative to both their non-bipolar co-twins and control twins. Bipolar probands also demonstrated significant thinning in the midbody of the CC. The significance of these results was confirmed via FDR correction for multiple comparisons (Pcritical = 0.012, BP < CTL; Pcritical = 0.0064, BPI vs. co-twins; see Fig. 3, below). Control subjects did not show significantly reduced callosal thickness relative to bipolar subjects or co-twins in any region of the CC. Consistent with area analyses, callosal thickness in co-twins of bipolar probands did not differ from control twins.
Shape Parameters
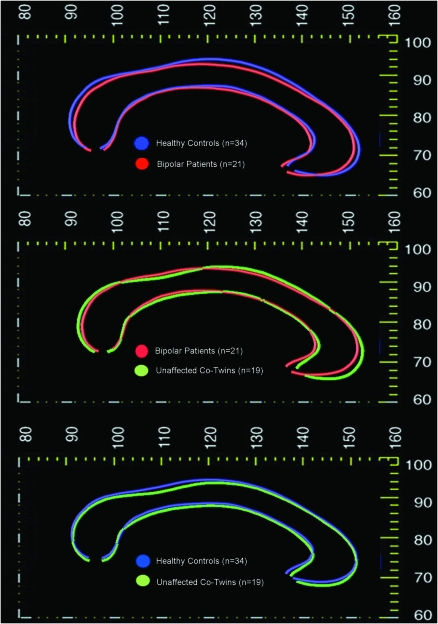
Mixed model regression analysis was performed to determine whether group differences existed in parameters characterizing the shape of the CC (i.e., dorsal and ventral surface curvature). This analysis revealed a significant main effect of diagnosis for ventral CC curvature (F2,73 = 3.63, P = 0.03) and a trend toward main effect of diagnosis for dorsal CC curvature (F2,73 = 2.94, P = 0.059; see Fig. 4). Univariate post hoc contrasts revealed that ventral curvature significantly differed in the bipolar probands compared with both their non-bipolar co-twins (P = 0.017) and normal control twins (P = 0.028). Dorsal curvature significantly differed in the BPI probands versus their non-BP co-twins (P = 0.03), with a trend toward difference between BPI probands and controls (P = 0.054). However, callosal curvature in co-twins did not differ from that of normal control twins (P > 0.20 for both top and bottom curvature).
Figure 4.
Differences in callosal morphology. Callosal shape profiles mapped in groups defined by biological risk for bipolar disorder. Average anatomical mesh models of the CC are shown in different colors to illustrate differences between groups, indicating genetic and disease-related effects on callosal morphology. From the top, average callosal shape profiles are mapped in the following: 1) bipolar probands versus healthy control twins; 2) bipolar probands versus non-bipolar co-twins; and 3) unaffected co-twins of bipolar probands versus control twins.
Medication Effects
There were no differences in callosal measures between BPI probands with and without lithium treatment (P = 0.90) nor was there a correlation between lithium dosage and/or chlorpromazine equivalents with callosal area or thickness (P > 0.30 for all comparisons). This is consistent with prior studies that have found no effect of lithium or antipsychotic medications on white matter volumes (Kieseppa et al. 2003; Bearden et al. 2007).
Relationship to Neurocognitive Measures
In BP1 probands and co-twins, significant correlations were observed between verbal fluency (FAS and animal naming) and callosal area in the splenium (r = 0.63, P = 0.001; r = 0.61, P = 0.002, respectively). There was a significant inverse relationship between number of errors on Stroop Interference and area of the genu (r = −0.55, P = 0.005) and splenium (r = −0.63, P = 0.001). There was a trend toward relationship in the same direction for response time on the Stroop Interference task, with both the genu (r = −0.43, P = 0.035) and the splenium (r = −0.36, P = 0.088) that did not survive Bonferroni correction. Similarly, there was a trend toward correlation of auditory attention (Digit Span) with callosal area in the splenium (r = 0.46, P = 0.024). Correlations between verbal fluency and auditory attention measures and area of the genu were also positive but not significant (r = 0.27, P = 0.20; r = 0.35, P = 0.10, r = 0.33, P = 0.12, respectively).
Discussion
To our knowledge, this is the first study to use computational mesh-based mapping methods to investigate callosal structure in twins discordant for bipolar disorder. Four key findings emerged from this study: 1) Localized regions of callosal thinning, most pronounced in the genu and splenium, were observed in bipolar probands, relative to both healthy control twins and to their non-bipolar co-twins. Corresponding reductions in callosal area were identified in bipolar probands using traditional morphometric methods; 2) Significantly altered callosal curvature was found in bipolar I probands, relative to both healthy control twins and their non-bipolar co-twins. 3) Within bipolar patients and their co-twins, callosal measures were significantly correlated with cognitive performance on measures of verbal processing speed and response inhibition, indicating that quantitative variation in CC size has functional significance. And finally, 4) no differences could be detected between co-twins and controls on measures of callosal structure, suggesting that callosal alterations in bipolar patients are disease related.
Callosal aberrations in bipolar probands were most pronounced in the genu and splenium and also observed within the callosal midbody but were not present within the isthmus, a callosal region containing fibers mainly projecting to parietal regions (Hofer and Frahm 2006). Our findings are highly consistent with those of a recent study using similar methods to map the CC in elderly patients with major depressive disorder, in which significant callosal thinning was restricted to the genu in early-onset depressed patients but evident in both the genu and the splenium in patients with late-onset depression (Ballmaier et al. 2008). Similarly, in patients with established bipolar illness, Walterfang, Malhi, et al. (2009a) found evidence for disproportionately reduced callosal thickness in the splenium. Notably, this pattern is also consistent with that seen in patients with Alzheimer disease, who tend to show the greatest callosal atrophy in the rostrum and splenium, with relative sparing of the callosal body, a pattern which has been proposed to reflect specific loss of large pyramidal neurons in cortical layers III and V of frontal and parietooccipital association areas (Hampel et al. 1998).
Although no previous studies have examined callosal structure in twins discordant for bipolar I disorder, a prior study—using a different methodology—examined callosal size and shape in BPI patients and their first-degree relatives (both siblings and adult offspring of bipolar patients). Consistent with our findings, Walterfang, Wood, et al. (2009b) found significant global and regional reductions in callosal thickness in BD patients, but first-degree relatives did not differ in callosal size or shape from controls. This pattern of findings provides further support for our conclusion that CC abnormalities may be linked to disease expression in bipolar disorder and thus do not appear to represent a marker of familial predisposition. These investigators also found that BD patients on lithium treatment showed a thicker anterior midbody than those on other psychotropics. While we did not find a medication effect in our sample, the majority of BPI patients in our sample were on lithium, thus limiting our power to compare the effects of lithium to those of other medications. However, it is important to note that the effect of lithium detected in the study by Walterfang and colleagues was in the opposite direction of the effect of diagnosis; this is consistent with prior studies showing volumetric increases associated with lithium treatment (Moore et al. 2000; Bearden et al. 2007, 2008). Thus, effects of lithium in our patient sample would be likely to result—if anything—in attenuation of the observed callosal reductions.
A variety of methods can be used to normalize raw neuroimaging data, and the results from these methodologies are not always interchangeable (Bermudez and Zatorre 2001). Due to concerns that the relationship between callosal structure and overall brain size is not necessarily linear and also because the relationships between callosal size and brain size may differ across biological risk groups, we chose—for our primary analysis— not to use a global scaling transform but rather to adjust for brain size statistically in our analyses. As shown in Supplementary Figure 2, the group differences observed for our “unscaled” callosal thickness maps remained significant when a scaling parameter is used.
Decreased callosal size may be associated with a reduction in fibers or decreased myelination of fibers connecting the prefrontal and parietal cortices. This might interfere with interhemispheric communication required for sustained attention and response inhibition, thus contributing to symptoms of distractibility, impulsivity, and inattention, frequently observed in patients with bipolar disorder. Supporting this view, we found significant associations of genu and splenium midsagittal areas with verbal processing speed and response inhibition, and a similar trend for a measure of auditory attention. While these tasks cannot be considered specific to interhemispheric connectivity, they are nonetheless measures requiring integrated, efficient information processing and thus are likely to be disrupted by abnormalities of major white matter fiber tracts (Karlsgodt et al. 2008; Madsen et al. 2010).
At present the cellular changes leading to the observed callosal alterations in bipolar patients are not known. Although postmortem neuropathology studies have reported decreased neuronal and glial density in the dorsolateral prefrontal cortex in bipolar patients (Rajkowska et al. 2001). Beasley et al. (2002) found no differences in the spatial pattern distribution or density of interstitial white matter neurons in frontal cortex. A follow-up study (Beasley et al. 2005) demonstrated that postmortem brain cholesterol (a major component of myelin and of cell membranes) was reduced by 10% in individuals with bipolar disorder compared with controls, suggesting that reduced brain cholesterol levels and/or a reduction in synapses may underlie findings of white matter volume reduction in bipolar disorder. Loss of NRG1-erbB signaling in oligodendrocytes in the adult brain has been shown to lead to reduced myelin thickness and increased levels of dopamine (D1-like and D2-like) receptors and transporter (dopamine active transporter), and behavioral alterations that are consistent with mental disorders, for example, heightened anxiety-like behavior and increased amphetamine sensitization. This signaling pathway may also contribute to mental disorders through nongenetic mechanisms, as both NRG1 and erbB4 expression can be altered by environmental stressors such as forced locomotion (Roy et al. 2007). Further studies are needed to determine whether the observed regional callosal thinning reflects neurodevelopmental alterations and/or whether bipolar disorder is characterized by progressive callosal atrophy.
Our findings of abnormal callosal structure in bipolar I probands are also supported by recent DTI studies of white matter microstructure (Bellani et al. 2009). Wang et al. (2008) recently observed reduced fractional anisotropy (FA) in the anterior and middle CC in bipolar patients relative to healthy controls, suggesting reduced myelination and/or axonal damage in these regions. Only one study, to our knowledge, has applied DTI methods to first-degree relatives of bipolar patients (Chaddock et al. 2009); these investigators found lower FA in bipolar patients compared with controls in the genu of the CC and in major intrahemispheric white matter tracts (i.e., right inferior longitudinal fasciculus and left superior longitudinal fasciculus). Unaffected relatives did not show significant FA differences relative to controls in a straightforward group-level comparison, but they did show intermediate FA values in the same clusters that showed reduced FA in bipolar patients. Increasing genetic liability for bipolar disorder was significantly associated with lower FA across distributed white matter regions.
Similar to what has been reported in schizophrenia (e.g., Casanova et al. 1990), we did find evidence for altered morphology of the CC in bipolar patients. These disorders may therefore involve common processes such as deficient myelination and abnormal white matter integrity (Casanova et al. 1990; Narr et al. 2000). Nevertheless, the pattern of shape alteration observed in bipolar probands was not identical to that typically seen in schizophrenia patients, involving an upward bowing of the callosum (which may reflect ventricular enlargement). Here, we observed differences in posterior CC curvature in bipolar probands, a finding which is unlikely to result from enlarged lateral ventricles.
In contrast to findings in relatives of schizophrenia patients (Casanova et al. 1990; Narr, Cannon, et al. 2002), we did not find evidence that these alterations are a result of genetic liability to bipolar disorder, as non-bipolar co-twins of bipolar probands did not differ from controls on any callosal measures. Overall callosal area in co-twins was intermediate between bipolar probands and control twins, but this difference was nonsignificant and the effect size was small (eta-squared = 0.005). Genetic effects on callosal structure—if present—may be very subtle, and thus a much larger twin sample would be needed to detect such effects. Thus, while global decreases in white matter volume may be related to genetic risk for bipolar disorder (Kieseppa et al. 2003; van der Schot et al. 2009), to our knowledge, no studies have yet identified genetic contributions to regionally specific white matter variation in bipolar disorder.
Certain limitations of this study should be noted. In particular, the limited number of MZ twin pairs in our sample precluded comparison of MZ with dizygotic twin pairs. Nevertheless, groups were very well matched on demographic variables, and the correlative nature of the data from twin pairs was taken into account in the statistical analyses. Second, in order to maximize our sample, we included some BP1 probands without a matching co-twin. Nevertheless, excluding these 2 subjects from the analysis did not substantively alter our results. Also, despite the field strength of 1.0 T, we were able to get good gray–white contrast for the images (see Supplementary Fig. 1). While this field strength could potentially be a limitation for imaging smaller structures (e.g., amygdala), the CC was clearly visible in our images, and our intrarater reliability was excellent (r.m.s. error < 1 mm). Finally, our study was limited to structural MR methodology; future studies employing multimodel neuroimaging techniques will be important to determine the direct relationship between regional callosal thickness and white matter integrity.
In summary, this was the first MRI study to examine the integrity of the CC and its functional correlates in twins with bipolar I disorder, their co-twins, and appropriate control twin subjects. We found evidence for structural alteration in the CC associated with bipolar illness, which appeared to be a disease-specific, rather than genetically mediated, effect. Callosal reduction in the genu and splenium was significantly associated with measures of cognitive processing speed (verbal fluency) and response inhibition in bipolar probands and co-twins. Our findings further implicate disrupted interhemispheric connectivity in bipolar disorder. Further studies are needed to determine the etiology of callosal alterations and when in the course of illness they develop.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
National Institutes of Health (grants K23MH74644 to C.E.B., MH48207, MH43880, 1 U54 GM072978-01) and a gift to the UCLA Foundation from the International Mental Health Research Organization. The Finnish Twin Cohort study forms part of the Academy of Finland Centre of Excellence in Complex Disease Genetics.
Supplementary Material
Acknowledgments
We thank Ulla Mustonen for her role in recruitment and clinical evaluation as well as the twins who participated in the study. Conflict of Interest: None declared.
References
- Adler CM, Holland SK, Schmithorst V, Wilke M, Weiss KL, Pan H, Strakowski SM. Abnormal frontal white matter tracts in bipolar disorder: a diffusion tensor imaging study. Bipolar Disord. 2004;6:197–203. doi: 10.1111/j.1399-5618.2004.00108.x. [DOI] [PubMed] [Google Scholar]
- Ahearn EP, Steffens DC, Cassidy F, Van Meter SA, Provenzale JM, Seldin MF, Weisler RH, Krishnan KR. Familial leukoencephalopathy in bipolar disorder. Am J Psychiatry. 1998;155:1605–1607. doi: 10.1176/ajp.155.11.1605. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Curran JG, Hauser P, Mintz J, Denicoff K, Post R. T2 hyperintensities in bipolar disorder: magnetic resonance imaging comparison and literature meta-analysis. Am J Psychiatry. 1995;152:1139–1144. doi: 10.1176/ajp.152.8.1139. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 3rd ed. Washington (DC): Author; 1987. [Google Scholar]
- Arnone D, McIntosh AM, Chandra P, Ebmeier KP. Meta-analysis of magnetic resonance imaging studies of the corpus callosum in bipolar disorder. Acta Psychiatr Scand. 2008;118:357–362. doi: 10.1111/j.1600-0447.2008.01229.x. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Roberts-Twillie JV, Barta PE, Kumar AJ, Harris GJ, Geer M, Peyser CE, Pearlson GD. Basal ganglia volumes and white matter hyperintensities in patients with bipolar disorder. Am J Psychiatry. 1994;151:687–693. doi: 10.1176/ajp.151.5.687. [DOI] [PubMed] [Google Scholar]
- Ballmaier M, Kumar A, Elderkin-Thompson V, Narr KL, Luders E, Thompson PM, Hojatkashani C, Pham D, Heinz A, Toga AW. Mapping callosal morphology in early- and late-onset elderly depression: an index of distinct changes in cortical connectivity. Neuropsychopharmacology. 2008;33:1528–1536. doi: 10.1038/sj.npp.1301538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, Thompson PM, Dalwani M, Hayashi KM, Lee AD, Nicoletti M, Trakhtenbroit M, Glahn DC, Brambilla P, Sassi RB, et al. Greater cortical gray matter density in lithium-treated patients with bipolar disorder. Biol Psychiatry. 2007;62:7–16. doi: 10.1016/j.biopsych.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, Thompson PM, Dutton RA, Frey BN, Peluso MA, Nicoletti M, Dierschke N, Hayashi KM, Klunder AD, Glahn DC, et al. Three-dimensional mapping of hippocampal anatomy in unmedicated and lithium-treated patients with bipolar disorder. Neuropsychopharmacology. 2008;33:1229–1238. doi: 10.1038/sj.npp.1301507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley CL, Cotter DR, Everall IP. Density and distribution of white matter neurons in schizophrenia, bipolar disorder and major depressive disorder: no evidence for abnormalities of neuronal migration. Mol Psychiatry. 2002;7:564–570. doi: 10.1038/sj.mp.4001038. [DOI] [PubMed] [Google Scholar]
- Beasley CL, Honer WG, Bergmann K, Falkai P, Lütjohann D, Bayer TA. Reductions in cholesterol and synaptic markers in association cortex in mood disorders. Bipolar Disord. 2005;7(5):449–455. doi: 10.1111/j.1399-5618.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- Bellani M, Yeh PH, Tansella M, Balestrieri M, Soares JC, Brambilla P. DTI studies of corpus callosum in bipolar disorder. Biochem Soc Trans. 2009;37:1096–1098. doi: 10.1042/BST0371096. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J. R. Stat. Soc. B Met. 1995;57:289–300. [Google Scholar]
- Bermudez P, Zatorre RJ. Sexual dimorphism in the corpus callosum: methodological considerations in MRI morphometry. Neuroimage. 2001;13:1121–1130. doi: 10.1006/nimg.2001.0772. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Nicoletti M, Sassi RB, Mallinger AG, Frank E, Keshavan MS, Soares JC. Corpus callosum signal intensity in patients with bipolar and unipolar disorder. J Neurol Neurosurg Psychiatry. 2004;75:221–225. [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Huttunen MO, Lonnqvist J, Tuulio-Henriksson A, Pirkola T, Glahn D, Finkelstein J, Hietanen M, Kaprio J, Koskenvuo M. The inheritance of neuropsychological dysfunction in twins discordant for schizophrenia. Am J Hum Genet. 2000;67:369–382. doi: 10.1086/303006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, Sanders RD, Goldberg TE, Bigelow LB, Christison G, Torrey EF, Weinberger DR. Morphometry of the corpus callosum in monozygotic twins discordant for schizophrenia: a magnetic resonance imaging study. J Neurol Neurosurg Psychiatry. 1990;53:416–421. doi: 10.1136/jnnp.53.5.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerullo MA, Adler CM, Delbello MP, Strakowski SM. The functional neuroanatomy of bipolar disorder. Int Rev Psychiatry. 2009;21:314–322. doi: 10.1080/09540260902962107. [DOI] [PubMed] [Google Scholar]
- Chaddock CA, Barker GJ, Marshall N, Schulze K, Hall MH, Fern A, Walshe M, Bramon E, Chitnis XA, Murray R, et al. White matter microstructural impairments and genetic liability to familial bipolar I disorder. Br J Psychiatry. 2009;194:527–534. doi: 10.1192/bjp.bp.107.047498. [DOI] [PubMed] [Google Scholar]
- Clarke JM, Zaidel E. Anatomical-behavioral relationships: corpus callosum morphometry and hemispheric specialization. Behav Brain Res. 1994;64:185–202. doi: 10.1016/0166-4328(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Davis KA, Kwon A, Cardenas VA, Deicken RF. Decreased cortical gray and cerebral white matter in male patients with familial bipolar I disorder. J Affect Disord. 2004;82:475–485. doi: 10.1016/j.jad.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Dupont RM, Butters N, Schafer K, Wilson T, Hesselink J, Gillin JC. Diagnostic specificity of focal white matter abnormalities in bipolar and unipolar mood disorder. Biol Psychiatry. 1995;38:482–486. doi: 10.1016/0006-3223(95)00100-u. [DOI] [PubMed] [Google Scholar]
- Hampel H, Teipel SJ, Alexander GE, Horwitz B, Teichberg D, Schapiro MB, Rapoport SI. Corpus callosum atrophy is a possible indicator of region- and cell type-specific neuronal degeneration in Alzheimer disease: a magnetic resonance imaging analysis. Arch Neurol. 1998;55(2):193–198. doi: 10.1001/archneur.55.2.193. [DOI] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited—comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Kaprio J, Koskenvuo M, Rose RJ. Population-based twin registries: illustrative applications in genetic epidemiology and behavioral genetics from the Finnish Twin Cohort Study. Acta Genet Med Gemellol (Roma) 1990;39:427–439. doi: 10.1017/s0001566000003652. [DOI] [PubMed] [Google Scholar]
- Kaprio J, Sarna S, Koskenvuo M, Rantasalo I. The Finnish Twin Registry: formation and compilation, questionnaire study, zygosity determination procedures, and research program. Prog Clin Biol Res. 1978;24(Pt B):179–184. [PubMed] [Google Scholar]
- Karlsgodt KH, van Erp TG, Poldrack RA, Bearden CE, Nuechterlein KH, Cannon TD. Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biol Psychiatry. 2008;63:512–518. doi: 10.1016/j.biopsych.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Kieseppa T, Partonen T, Haukka J, Kaprio J, Lonnqvist J. High concordance of bipolar I disorder in a nationwide sample of twins. Am J Psychiatry. 2004;161:1814–1821. doi: 10.1176/ajp.161.10.1814. [DOI] [PubMed] [Google Scholar]
- Kieseppa T, van Erp TG, Haukka J, Partonen T, Cannon TD, Poutanen VP, Kaprio J, Lonnqvist J. Reduced left hemispheric white matter volume in twins with bipolar I disorder. Biol Psychiatry. 2003;54:896–905. doi: 10.1016/s0006-3223(03)00373-1. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Luders E, Narr KL, Bilder RM, Thompson PM, Szeszko PR, Hamilton L, Toga AW. Positive correlations between corpus callosum thickness and intelligence. Neuroimage. 2007;37:1457–1464. doi: 10.1016/j.neuroimage.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Narr KL, Hamilton LS, Phillips OR, Thompson PM, Valle JS, Del'Homme M, Strickland T, McCracken JT, Toga AW, et al. Decreased callosal thickness in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2009;65:84–88. doi: 10.1016/j.biopsych.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen KS, Baare WF, Vestergaard M, Skimminge A, Ejersbo LR, Ramsoy TZ, Gerlach C, Akeson P, Paulson OB, Jernigan TL. Response inhibition is associated with white matter microstructure in children. Neuropsychologia. 2010;48:854–862. doi: 10.1016/j.neuropsychologia.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J. A probabilistic atlas of the human brain: theory and rationale for its development. The International Consortium for Brain Mapping (ICBM) Neuroimage. 1995;2:89–101. doi: 10.1006/nimg.1995.1012. [DOI] [PubMed] [Google Scholar]
- McDonald C, Zanelli J, Rabe-Hesketh S, Ellison-Wright I, Sham P, Kalidindi S, Murray RM, Kennedy N. Meta-analysis of magnetic resonance imaging brain morphometry studies in bipolar disorder. Biol Psychiatry. 2004;56:411–417. doi: 10.1016/j.biopsych.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Moore GJ, Bebchuk JM, Wilds IB, Chen G, Manji HK. Lithium-induced increase in human brain grey matter. Lancet. 2000;356:1241–1242. doi: 10.1016/s0140-6736(00)02793-8. [DOI] [PubMed] [Google Scholar]
- Narr KL, Cannon TD, Woods RP, Thompson PM, Kim S, Asunction D, van Erp TG, Poutanen VP, Huttunen M, Lonnqvist J, et al. Genetic contributions to altered callosal morphology in schizophrenia. J Neurosci. 2002;22:3720–3729. doi: 10.1523/JNEUROSCI.22-09-03720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr KL, Thompson PM, Sharma T, Moussai J, Cannestra AF, Toga AW. Mapping morphology of the corpus callosum in schizophrenia. Cereb Cortex. 2000;10:40–49. doi: 10.1093/cercor/10.1.40. [DOI] [PubMed] [Google Scholar]
- Narr KL, van Erp TG, Cannon TD, Woods RP, Thompson PM, Jang S, Blanton R, Poutanen VP, Huttunen M, Lonnqvist J, et al. A twin study of genetic contributions to hippocampal morphology in schizophrenia. Neurobiol Dis. 2002;11:83–95. doi: 10.1006/nbdi.2002.0548. [DOI] [PubMed] [Google Scholar]
- Noga JT, Vladar K, Torrey EF. A volumetric magnetic resonance imaging study of monozygotic twins discordant for bipolar disorder. Psychiatry Res. 2001;106:25–34. doi: 10.1016/s0925-4927(00)00084-6. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Halaris A, Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biol Psychiatry. 2001;49:741–752. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- Regenold WT, D'Agostino CA, Ramesh N, Hasnain M, Roys S, Gullapalli RP. Diffusion-weighted magnetic resonance imaging of white matter in bipolar disorder: a pilot study. Bipolar Disord. 2006;8:188–195. doi: 10.1111/j.1399-5618.2006.00281.x. [DOI] [PubMed] [Google Scholar]
- Roy K, Murtie JC, El-Khodor BF, Edgar N, Sardi SP, Hooks BM, Benoit-Marand M, Chen C, Moore H, O'Donnell P, et al. Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proc Natl Acad Sci U S A. 2007;104(19):8131–8136. doi: 10.1073/pnas.0702157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffé H. A method for judging all contrasts in the analysis of variance. Biometrika. 1953;40(1–2):87–110. [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. Nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med. Imag. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M. Structured clinical interview for DSM-IV. New York: Biometrics Research Department, New York State Psychiatric Institute; 1994. [Google Scholar]
- Storey JD. A direct approach to false discovery rates under dependence. J. R. Stat. Soc. Ser. B. 2002;64:479–498. [Google Scholar]
- Strakowski SM, Adler CM, DelBello MP. Volumetric MRI studies of mood disorders: do they distinguish unipolar and bipolar disorder? Bipolar Disord. 2002;4:80–88. doi: 10.1034/j.1399-5618.2002.01160.x. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Delbello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- Turken A, Whitfield-Gabrieli S, Bammer R, Baldo JV, Dronkers NF, Gabrieli JD. Cognitive processing speed and the structure of white matter pathways: convergent evidence from normal variation and lesion studies. Neuroimage. 2008;42:1032–1044. doi: 10.1016/j.neuroimage.2008.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schot AC, Vonk R, Brans RG, van Haren NE, Koolschijn PC, Nuboer V, Schnack HG, van Baal GC, Boomsma DI, Nolen WA, et al. Influence of genes and environment on brain volumes in twin pairs concordant and discordant for bipolar disorder. Arch Gen Psychiatry. 2009;66:142–151. doi: 10.1001/archgenpsychiatry.2008.541. [DOI] [PubMed] [Google Scholar]
- Vidal CN, Nicolson R, DeVito TJ, Hayashi KM, Geaga JA, Drost DJ, Williamson PC, Rajakumar N, Sui Y, Dutton RA, et al. Mapping corpus callosum deficits in autism: an index of aberrant cortical connectivity. Biol Psychiatry. 2006;60:218–225. doi: 10.1016/j.biopsych.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Videbech P. MRI findings in patients with affective disorder: a meta-analysis. Acta Psychiatr Scand. 1997;96:157–168. doi: 10.1111/j.1600-0447.1997.tb10146.x. [DOI] [PubMed] [Google Scholar]
- Walterfang M, Malhi GS, Wood AG, Reutens DC, Chen J, Barton S, Yucel M, Velakoulis D, Pantelis C. Corpus callosum size and shape in established bipolar affective disorder. Aust N Z J Psychiatry. 2009;43:838–845. doi: 10.1080/00048670903107534. [DOI] [PubMed] [Google Scholar]
- Walterfang M, Wood AG, Barton S, Velakoulis D, Chen J, Reutens DC, Kempton MJ, Haldane M, Pantelis C, Frangou S. Corpus callosum size and shape alterations in individuals with bipolar disorder and their first-degree relatives. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1050–1057. doi: 10.1016/j.pnpbp.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Wang F, Kalmar JH, Edmiston E, Chepenik LG, Bhagwagar Z, Spencer L, Pittman B, Jackowski M, Papademetris X, Constable RT, et al. Abnormal corpus callosum integrity in bipolar disorder: a diffusion tensor imaging study. Biol Psychiatry. 2008;64:730–733. doi: 10.1016/j.biopsych.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder-Willis KE, Sax KW, Rosenberg HL, Fleck DE, Shear PK, Strakowski SM. Persistent attentional dysfunction in remitted bipolar disorder. Bipolar Disord. 2001;3:58–62. doi: 10.1034/j.1399-5618.2001.030202.x. [DOI] [PubMed] [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112(Pt 3):799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration: II. Intersubject validation of linear and nonlinear models. J Comput Assist Tomogr. 1998;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.