Abstract
Purpose
To compare macroporous alginate scaffolds with Matrigel for culturing frozen-thawed human primordial follicles in organ culture.
Methods
Twelve girls/women donated ovarian tissue. One tissue sample was fixed immediately after thawing (uncultured samples). Slices were cultured for 2 weeks on either Matrigel or on alginate scaffolds with a serum-free culture medium. Growth evaluation consisted of follicular counts and classification, immunohistochemistry and measurement of 17β-Estradiol (E2) production.
Results
The number of developing follicles was significantly higher in alginate scaffold-cultured samples than on Matrigel with a concomitant decrease in the number of primordial follicles in alginate scaffold-cultured samples than uncultured samples. The number of atretic follicles after 1 week was significantly higher in the Matrigel-cultured samples than in the alginate scaffold cultured samples. E2 production was similar in both groups.
Conclusions
Three dimensional alginate scaffolds are a promising putative in vitro technology for developing human primordial follicles.
Keywords: Alginate scaffold, 17β-Estradiol (E2), Human primordial follicles, Matrigel, Proliferating cell nuclear antigen (PCNA)
Introduction
As cancer treatment improves, more young women of reproductive age survive [1]. However, many have ovarian failure and premature menopause as a consequence of the radiation and chemotherapy [1]. Among the limited options currently available for fertility preservation in these patients is cryopreservation of ovarian cortical tissue containing immature primordial follicles followed by autotransplantation [2–5]. However, this procedure carries the risk of reintroducing hematological or other malignancies [6–9]. This drawback could be overcome by embryo transfer after in vitro fertilization of oocytes from primordial follicles matured in vitro. Researchers are, therefore, seeking to improve techniques of culturing primordial follicles [10, 11].
Two main approaches to culturing primary and primordial follicles have been attempted [10, 11]. One is to culture whole slices of ovarian tissue (organ culture) in order to retain the interactions between the follicles and the surrounding stroma cells. Using this method, primordial follicles from cows [12] and humans [13–18] have been grown to secondary stages [13]. Matrigel is often used as a supporting matrix for human primordial follicles because it was found to increase their survival [13, 19]. However, Telfer et al. [20, 21] succeeded to activate human and bovine primordial follicles without Matrigel.
The second approach involves the use of isolated primordial and primary follicles [22, 23]. By employing this method researchers can directly monitor follicular growth during the culture period, a particularly important consideration given the poorly populated state of human ovarian tissue in adults. Recent advances in biomaterials scaffolds have led to the development of three-dimensional (3D) culturing systems that mimic the physiological environment and imitate the internal architecture of the living tissue [24–32]. Several teams have applied hydrogel beads composed of alginate, a biocompatible polysaccharide widely utilized for cell transplantation [24, 25], to encapsulate and culture isolated ovarian secondary follicles from mice [26], rats [27], nonhuman primates [28] and humans [29]. The follicles grew to antral stages [26–30]. Moreover, the oocytes from mice were successfully fertilized and implanted to yield live births [26]. This culture system was also used for smaller human follicles, but they only survived in culture [31].
The aim of the present study was to investigate for the first time, the effectiveness of a 3D macroporous alginate scaffold for culturing human primordial follicles in organ culture. We chose alginate for its mechanical stability, biocompatibility, and hydrophilicity, in addition to its high porosity (>90%) and large pore size of 80–150 μm which allow for efficient mass transport [24, 25]. The porous structure can be easily manipulated by controlling various parameters during fabrication of the scaffold, such as cross-linker concentration, solution viscosity, and freezing regimen. The alginate scaffold has been successfully used to cultivate hepatocytes [24], cardiomyocytes [25] and human embryonic stem cells [32], while preserving their functional activities. Given these unique features we hypothesized that it would enhance the diffusion rate of the culture media to the tissue and generate an extracellular matrix-like environment adjacent to the tissue. The follicular growth findings were compared to tissue slices cultured on Matrigel.
Materials and methods
Sample sources and retrieval
The Ethics Committee of Rabin Medical Center approved the study protocol, and written consent was obtained from every adult patient or the parents of all minors. The study included 12 frozen-thawed ovarian samples from girls and women (aged 10–27 years, mean±standard deviation, SD = 16 ± 6 years). All these patients underwent Gynecological laparoscopies. All operations were performed for ovarian biopsy cryopreservation before chemotherapy [1–3, 5].
All samples were handled in our laboratory within 1 h of surgery. One slice from every patient (measuring 1–2 mm) was fixed in Bouin’s solution (components purchased from BDH Chemicals Ltd., Poole, England, and Sigma, St. Louis, MO, USA) immediately after ovarian dissection (uncultured fresh control), and the remaining ovarian slices were frozen.
Cryopreservation and thawing of ovarian tissue
Tissue slices were frozen with a 1.5 M dimethylsulfoxide (DMSO) (Sigma) solution [19, 33–35]. Before freezing, the samples were kept on ice with DMSO. All samples were frozen slowly in a programmable freezer (Kryo 10; series 10/20, Planer Biomed, Sunbury on Thames, UK), and immediately placed in liquid nitrogen.
The tissue slices were thawed by three washouts with alpha minimal essential medium (αMEM) (Biological Industries, Beit Ha’emek, Israel), 5% human serum albumin (HSA) (Irvine Scientific, Santa Ana, CA, USA), followed by incubation at 37°C [34]. One slice of every thawed ovarian sample (similar in size to the uncultured fresh control) was fixed in Bouin’s solution immediately after thawing (uncultured thawed control).
Alginate scaffold preparation
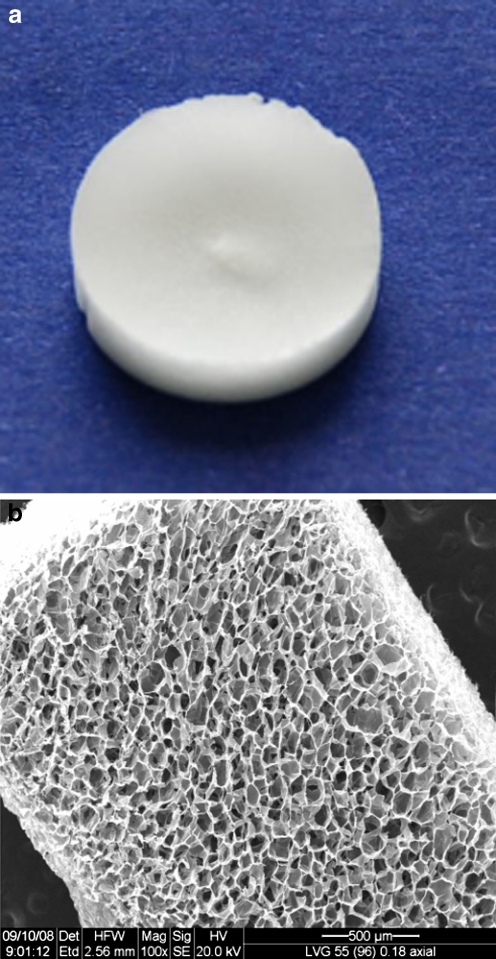
Alginate scaffolds, 10 mm in diameter and 2 mm thickness (Fig. 1) were prepared from alginate with a high guluronic (G) acid concentration (LVG; 65%G monomer content, molecular weight = ~ 100 kDa, NovaMatrix FMC Biopolymers, Drammen, Norway) using a freeze-dry technique [36]. In brief, alginate was dissolved in double distilled water (1.2% w/v) and calcium cross-linked by adding a solution of D-gluconic acid (Sigma) and hemicalcium salt (Sigma) (1.32% w/v); the solution was homogenized to obtain homogenous calcium ion distribution and cross-linking. The final component concentrations in the cross-linked solution were 1.0% for the polymer and 0.22% (w/v) for the cross-linker. The cross-linked solution was poured into 48-well plates (200 μl/well), frozen at −20°C for 24 h and lyophilized. The scaffolds were sterilized by exposure to ultraviolet light in a biological hood for 1 h. Scanning electron microscope (SEM) inspection revealed 90% scaffold porosity and a pore diameter of 80–150 μm.
Fig. 1.
Photographs of the macroporous alginate scaffolds used in the study. a An alginate scaffold with a diameter of 10 mm and thickness of 2 mm. b Scanning electron microscope micrograph of the alginate scaffold showing its porous structure and large pore size. Original magnification X100, scale bar = 500 μm
Matrigel preparation
Millicell CM inserts (Millipore, Bedford, MA, USA) fitted into 24-well plates (Nalge Nunc International) were precoated with an extracellular matrix gel (Matrigel reduced in growth factors; BD Bioscience, Franklin Lakes, NJ, USA) [19, 35].
Culturing methods
Only frozen-thawed samples were used for incubation so that follicular density could be studied histologically prior to culture [19, 35]. This practice eliminated the risk of utilizing poorly populated ovarian tissue. Moreover, previous studies in which we participated showed no differences in in vitro development of fresh and frozen-thawed isolated human ovarian cortical follicles [23], and no morphological differences between follicles from frozen-thawed and fresh human ovarian cortical tissue [34]. Because the ultimate fertility preservation and restoration technique for cancer patients will require the use of frozen-thawed follicles [10], studies using frozen-thawed ovaries are crucial.
Each ovarian sample was cut to thin ovarian cortical slices (1–2 mm, width = ~1 mm, as uniform in size as possible and similar in dimensions to the uncultured controls) and divided into two study groups. Group 1 was placed on the alginate scaffolds (47 slices, at least three slices per patient) and group 2, on the Matrigel precoated inserts (52 slices, at least three slices per patient). The slices were placed in every well and were covered with medium. The basic culture medium consisted of αMEM (Biological Industries), human recombinant follicle stimulating hormone (FSH, 1 IU/ml) (Gonal-F, Serono, Aubonne, Switzerland), 10% HSA (Irvine Scientific), insulin, transferrin, and selenium (Sigma) [19, 35]. The samples were incubated for 2 weeks in a standard incubator (95% air, 5% CO2), and the culture media were changed every second day. Once a week, at least one slice (on average two slices) was removed from culture and fixed immediately in Bouin’s solution (BDH Chemicals and Sigma), and the spent medium samples were collected for 17β-estradiol (E2) measurement [19, 35, 37, 38].
Histological preparation
The fixed specimens from both groups were prepared for paraffin embedding, sectioning, and staining with hematoxylin and eosin (H & E) [19, 35, 37, 38]. The number of follicles in the uncultured and cultured samples was counted with an image analyzer in two different section levels per sample (50 μm between sections, to avoid counting the same follicle twice) (analySIS Soft Imaging System, Digital Solutions for Imaging and Microscopy System, GmbH, Munster, Germany), and the follicles were classified according to Gougeon [39] as follows: primordial (with a single flat layer of granulosa cells-GCs surrounding the oocyte), primary (with a single cuboidal GC layer surrounding the oocyte), secondary (with at least two GC layers and a theca layer surrounding the oocyte) and antral (with a fluid filled cavity within the GCs). Developing follicles were defined as primary and secondary [19, 35]; atretic follicles were characterized by pyknotic cells, eosinophilia of the ooplasm, and clumping of the chromatin material [39]. Unstained sections were placed on OptiPlus positive charged microscope slides (BioGenex Laboratories, San Ramon, CA, USA) for immunohistochemistry assay.
Immunohistochemistry for proliferating cell nuclear antigen (PCNA)
PCNA is a nuclear protein that plays an essential role in cell-cycle regulation [19, 35, 37]. It serves as a useful marker of proliferating cells such as GCs. The sections were first microwaved with citrate buffer at pH 6.0 (Dako Corporation, Santa Barbara, CA, USA) to enhance antigen retrieval. They were then incubated with the primary antibody for PCNA, a monoclonal mouse anti-PCNA antibody (1: 70 and 1: 100, Dako). Sections were incubated with two negative control solutions that replaced the primary antibody. The first was prepared with a normal mouse IgGa2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted to the same concentrations as the primary antibody and the second included phosphate buffered saline. Solutions from a Dako EnVision System, horseradish peroxidase–3-amino-9-ethylcarbazole (AEC) kit (Dako Corporation) were used for immunostaning: red-brown AEC (Zymed Laboratories Inc., Invitrogen, Carlasbad, CA, USA) staining indicated PCNA expression. We defined a follicle as being positively stained if at least one of its GCs expressed PCNA.
E2 accumulation in the culture medium
E2 concentrations were measured by a double antibody radioimmunoassay kit (Diagnostic Products Corporation, Los Angeles, CA, USA), which has a detection limit of 1.4 pg/mL [19, 35, 37, 38].
Statistical analysis
Data were statistically analyzed by analysis of variance, chi-square test and Fisher’s exact test, as required. P values of less then 0.05 were considered significant.
Results
Follicular counts and classification
The distribution of follicles by group is presented in Table 1. We preferred to refer to developing follicles rather than to primary and secondary follicles separately as very few secondary follicles were identified in the sections. Figure 2 presents photographs of ovarian sections containing follicles at various stages of the experiment. There were no morphological differences between follicles in the uncultured fresh (as presented in Fig. 2a) controls and the uncultured thawed controls (as presented in Fig. 2b).
Table 1.
Follicular counts and classification in ovarian tissue slices cultures on alginate scaffold or Matrigel and uncultured controls for a two-week period
| Follicular classes | Alginate scaffold | Matrigel | Uncultured samples | ||
|---|---|---|---|---|---|
| Week 1 | Week 2 | Week 1 | Week 2 | ||
| aPrimordial | 138 (36%) | 121 (33%) | 231 (46%) | 120 (46%) | 445 (78%)1 |
| 12±9 | 15±8 | 38±15 | 15±11 | 31±13 | |
| Developing | 190 (51%)2 | 205 (58%)2 | 94 (18%) | 92 (34%) | 135 (22%) |
| 13±9 | 17±9 | 11±5 | 11±9 | 12±5 | |
| Atretic | 48 (13%) | 31 (9%) | 183 (36%)3 | 51 (20%) | 0 |
| 5±2 | 3±1 | 22±10 | 6±2 | 0 | |
aFollicular numbers are given as sum of follicles per group and week (percents were calculated from total number of follicles in group per week) and as average±standard deviation
1Significantly higher in uncultured group than in alginate scaffold group (p < 0.003 for week 1, p < 0.002 for week 2)
2Significantly higher in alginate scaffold group than in Matrigel after one and 2 weeks (p < 0.03 for both weeks)
3Significantly higher in Matrigel group than in alginate scaffold group for week 1 and for both weeks together (p < 0.005 for week 1, p < 0.008 for both weeks together)
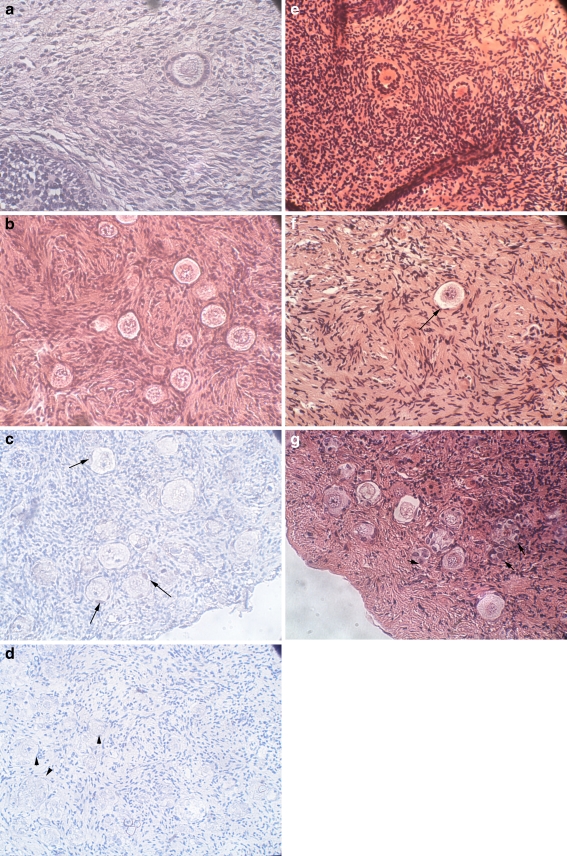
Fig. 2.
Histological and PCNA immunostained sections of the uncultured and cultured ovarian samples. a Uncultured freshly dissected ovarian section from a 19-year-old woman. Note the primary follicle. Hematoxylin and eosin (H & E) staining, original magnification X400. b Uncultured thawed ovarian section from a 13-year-old girl. Note the primordial follicles. H & E staining, original magnification X400. c Section of ovarian sample with PCNA immunohistochemical staining from a 17-year-old girl cultured on an alginate scaffold for 1 week. Note the primordial and primary follicles, with the light red-brown staining indicating PCNA expression in a portion of the oocytes and the GCs (arrows). Original magnification X400. d Negative control for PCNA from the same ovarian sample as in Panel C. Note the primordial and primary follicles (arrow heads) with overall blue staining and lack of red-brown PCNA staining. Original magnification X400. e Section of ovarian sample from the same woman as in panel A cultured on alginate scaffold for 2 weeks. Note the two primary-secondary follicles. H & E staining, original magnification X400. f Section of ovarian sample from ovarian sample from the same patient as in panels C and D cultured on Matrigel for 1 week. Note the primordial follicle (arrow). H & E staining, original magnification X400. g Section of ovarian sample from a 20-year-old woman cultured on Matrigel for 2 weeks. Note the primordial, primary and atretic follicles (small arrows). H & E staining, original magnification X400
The number of developing follicles was significantly higher (p < 0.03) in samples cultured on macroporous alginate scaffolds than in samples cultured on Matrigel. After 1 week, there was a 51% developing follicles in the alginate group compared to 18% in the Matrigel group; corresponding rates at 2 weeks were 58% and 34% (p < 0.03 for both weeks). In addition, the proportion of primordial follicles decreased significantly in the alginate scaffold group compared to the uncultured samples group to 36% after 1 week and 33% after 2 weeks compared to 78% (p < 0.003 for week 1, p < 0.002 for week 2). The number of atretic follicles was significantly higher in the Matrigel group than the alginate scaffold group accounting for 30% and 10% of all follicles, respectively for both weeks together (p < 0.008); and 36% and 13%, respectively, after 1 week (p < 0.005).
Immunohistochemistry for PCNA expression
An example of a cultured ovarian section after PCNA staining is shown in Fig. 2c. In most cases PCNA staining was weak. PCNA staining was identified in a portion of the GC and oocytes from primordial-primary and secondary follicles. After 1 week the number of PCNA-stained follicles was significantly higher in the alginate scaffold group (79.7% of all follicles) than in the uncultured controls (35.7%) (p < 0.0001). In the second week, the rate of PCNA-stained follicles was significantly higher in the alginate scaffold group (82.2%) (p < 0.0001) and the Matrigel group (66.1%) (p < 0.0004) than in the uncultured controls. Moreover, in the second week the rate of PCNA-stained follicles was also significantly higher in the alginate group than in the Matrigel group (p < 0.04). It is noteworthy that there were no follicles in any of PCNA-stained sections from the Matrigel group in the first week despite their presence in the corresponding H & E stained sections (because of different tissue sectioning levels). The negative controls did not stain positively (blue) (Fig. 2d).
E2 accumulation in the medium
E2 levels were detectable in spent media samples after culture on either alginate scaffold or Matrigel throughout the culture period. E2 concentrations were significantly higher in week 1 (mean±SD = 152±208 pg/ml/slice for alginate; mean±SD = 81±147 for Matrigel) than week 2 (mean±SD = 264±326 for alginate; mean±SD = 33±47 for Matrigel) in both groups (p < 0.003).
Discussion
The present study examined for the first time the feasibility of culturing human cortical ovarian slices on macroporous alginate scaffolds. The study suggests certain trends regarding improvements in follicular culture on alginate scaffolds than Matrigel. The proportion of developing follicles after culture on alginate scaffolds was significantly higher than after culturing on Matrigel and compared to uncultured slices, with a concomitant decline in primordial follicles. In parallel, there were more atretic follicles in the Matrigel group than the alginate scaffold group. Detectable E2 levels were measured in spent media samples after culture in both systems, although these levels decreased during the second week.
A limitation of the present study, similar to other studies on human ovarian tissue [5, 10, 13–20, 22, 23, 29, 31, 34, 35, 40], is the heterogenecity in follicular numbers between samples. Young age is strongly correlated with high follicular density, and therefore, we chose samples from young patients. However, the availability of human ovaries for research is limited and therefore, by contrast to rodents [26, 27], selection of tissue from human subjects of uniform ages is unpractical. Moreover, as follicular distribution is uneven within individual human ovaries [5, 40], this might have led to our culturing ovarian slices with lower follicular numbers than in the uncultured controls.
The lower number of primordial follicles in the alginate scaffold group than the Matrigel group can be due to higher activation rates of primordial follicles or to more atretic follicles in the Matrigel group. This is in line also with more PCNA stained follicles, indicating better GC proliferation rates, in the alginate scaffold group than in the Matrigel group. Alternatively, similar to other studies with organ culture of human ovarian tissue [13–20], it is possible that we placed tissue slices that initially contained less primordial follicles on alginate scaffolds.
PCNA oocyte staining was observed not only in the current study but also in various previous studies [19, 35, 37, 41]. It cannot be attributed to cell division, since the oocyte is not engaged in mitotic activity [37, 42]. However, the mammalian oocyte is not quiescent. Although no new DNA is created, DNA polymerase delta might be activated to repair possible damage to the nuclear and mitochondrial DNA in the oocyte, and during its activity, it or possibly the proliferating mitochondrial DNA itself might incorporate PCNA [42]. This might account for the positive PCNA staining.
As E2 is secreted only from secondary follicular stages onwards [39], study of its production in culture allows for the evaluation of growth to secondary stages [19, 35, 37]. Since our histological studies identified very few secondary follicles, the E2 production might have been due to secondary follicles in tissue levels that were not examined histologically, or to follicles between primary and secondary stages or to E2 secretion from primary follicles, although to the best of our knowledge this has not been reported previously. Moreover, by contrast to the histological findings suggesting a benefit for alginate scaffold on Matrigel, the lack of significant difference in E2 production in the spent media samples between the two groups might have been due to the sharp variability in E2 secretion levels, as represented by the high SD values.
The significant decrease in E2 after 2 weeks in both groups may be attributable to either a deterioration in follicular quality during culture or by intersample variation [5, 19, 35, 40]. Although we evaluated different ovarian slices from individual patients at various time points (prior to culture, week 1 of culture and week 2 of culture), it is possible that the initial follicular density differed among the individual samples. Moreover, in an earlier study, Telfer et al. [20] reported a decline in E2 secretion after 4 days of culture. These findings suggest that while surviving follicles seem to continue growth in culture their hormonal function in vitro needs to be further optimized.
In previous studies, isolated ovarian follicles were encapsulated in alginate hydrogel beads and cultured [26–31, 43–45]. Culturing of isolated follicles in this manner removes them from their natural environment, thereby limiting the interactions between neighboring follicles and most importantly limiting the diffusion rate of large proteins in the hydrogels [46] and restricting follicular growth [47]. Moreover, culturing follicles in alginate hydrogel beads might potentially restrict their ability to grow to large antral sizes. Therefore, we suggest that culturing isolated follicles in alginate macroporous scaffolds similar to those used in the present study would provide a new alternative. This scaffold has a high porosity and large pore size providing a suitable culture environment for numerous isolated follicles, while imitating and maintaining follicle-follicle communication. In addition, both the porous structure and the pore size of the alginate scaffold can be easily manipulated by judiciously selecting various parameters during fabrication [36, 48], so it can be adjusted to follicular size.
The development of a successful culture system for primordial follicles poses a technological and scientific challenge, and studies are only at the initial stages. It is possible that a two-step culturing system is the optimal strategy for human follicle culture [20]. This approach has been utilized for murine [43, 49], bovine [21] and human [20] primordial follicles. The primordial follicles were initially cultured in organ culture without a supporting matrix [20, 21]; and thereafter secondary follicles were isolated and further cultured. So far the in vitro follicular growth from primordial stages to the development of functioning oocytes has only been achieved in mice [43, 49] with production of live young [49].
Telfer et al. [20] showed that human primordial follicles within cortical pieces could undergo activation to secondary stages within 6 days, as the physical setting of the follicles within the cortical tissue seems to influence the growth capacity [21]. Taking the present promising results into account, we propose that the activation of primordial follicles might be enhanced in the first culture step (organ culture) if the tissue is cultured on alginate scaffolds combined with improvements in their culture medium [10, 19, 35]. The macroporous alginate scaffolds would maximize the tissue’s surface area for gas exchange and the supply of nutrients, while providing a support system that more closely resembles the ovary, such that the intact follicle is maintained and its contact with the surrounding stroma cells is preserved. Further follicular growth might then be achieved by isolating the secondary follicles followed by their culture in alginate hydrogel beads [26–31], collagen gels [22, 23], or other systems [20, 38]. Moreover, the alginate scaffold culturing system might be improved in future studies by use of fresh rather than cryopreserved-thawed tissue for culture and by incorporating growth factors in its backbone or in special controlled release microspheres [24, 50, 51].
Acknowledgment
The authors are greatly indebted to Ms. Gloria Ganzach from the Editorial Board of Rabin Medical Center, Beilinson Hospital for the English editing.
Footnotes
Capsule Culturing human ovarian cortical tissue on a macroporous alginate scaffold seems to promote better follicular development than culturing on Matrigel
References
- 1.Abir R, Fisch B, Raz A, Nitke S, Ben-Rafael Z. Preservation of fertility in women undergoing chemotherapy: current approach and future prospects. J Assist Reprod Genet. 1998;15:469–77. doi: 10.1023/A:1022578303272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–10. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 3.Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353:318–21. doi: 10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- 4.Silber SJ, Lenahan KM, Levine DJ, Pineda JA, Gorman KS, Friez MJ, et al. Ovarian transplantation between monozygotic twins discordant for premature ovarian failure. N Engl J Med. 2005;353:58–63. doi: 10.1056/NEJMoa043157. [DOI] [PubMed] [Google Scholar]
- 5.Gook DA, Edgar DH. Ovarian tissue cyopreservation. In: Donnez J, Kim SS, editors. Principles and practice of fertility preservation. UK: Cambridge University Press; 2011. pp. 342–56. [Google Scholar]
- 6.Shaw J, Trounson A. Oncological implications in the replacement of ovarian tissue. Hum Reprod. 1997;12:403–5. doi: 10.1093/humrep/12.3.403. [DOI] [PubMed] [Google Scholar]
- 7.Meirow D, Hardan I, Dor J, Fridman E, Elizur S, Ra'anani H, et al. Searching for evidence of disease and malignant cell contamination in ovarian tissue stored from hematologic cancer patients. Hum Reprod. 2008;23:1007–13. doi: 10.1093/humrep/den055. [DOI] [PubMed] [Google Scholar]
- 8.Rosendahl M, Andersen MT, Ralfkiaer E, Kjeldsen L, Andersen MK, Andersen CY. Evidence of residual disease in cryopreserved ovarian cortex from female patients with leukemia. Fertil Steril. 2010;94:2186–90. [DOI] [PubMed]
- 9.Abir R, Feinmesser M, Yaniv I, Fisch B, Cohen IJ, Ben-Haroush A, et al. Occasional involvement of the ovary in Ewing sarcoma. Hum Reprod. 2010;25:1708–12. doi: 10.1093/humrep/deq121. [DOI] [PubMed] [Google Scholar]
- 10.Abir R, Nitke S, Ben-Haroush A, Fisch B. In vitro maturation of human primordial ovarian follicles: clinical significance, progress in mammals, and methods for growth evaluation. Histol Histopathol. 2006;21:887–98. doi: 10.14670/HH-21.887. [DOI] [PubMed] [Google Scholar]
- 11.Hurk R, Abir R, Telfer EE, Bevers MM. Primate and bovine immature oocytes and follicles as sources of fertilizable oocytes. Hum Reprod Update. 2000;6:457–74. doi: 10.1093/humupd/6.5.457. [DOI] [PubMed] [Google Scholar]
- 12.Braw-Tal R, Yossefi S. Studies in vivo and in vitro on the initiation of follicle growth in the bovine ovary. J Reprod Fertil. 1997;109:165–71. doi: 10.1530/jrf.0.1090165. [DOI] [PubMed] [Google Scholar]
- 13.Hovatta O, Silye R, Abir R, Krausz T, Winston RM. Extracellular matrix improves survival of both stored and fresh human primordial and primary ovarian follicles in long-term culture. Hum Reprod. 1997;12:1032–6. doi: 10.1093/humrep/12.5.1032. [DOI] [PubMed] [Google Scholar]
- 14.Wright CS, Hovatta O, Margara R, Trew G, Winston RM, Franks S, et al. Effects of follicle-stimulating hormone and serum substitution on the in-vitro growth of human ovarian follicles. Hum Reprod. 1999;14:1555–62. doi: 10.1093/humrep/14.6.1555. [DOI] [PubMed] [Google Scholar]
- 15.Louhio H, Hovatta O, Sjoberg J, Tuuri T. The effects of insulin, and insulin-like growth factors I and II on human ovarian follicles in long-term culture. Mol Hum Reprod. 2000;6:694–8. doi: 10.1093/molehr/6.8.694. [DOI] [PubMed] [Google Scholar]
- 16.Zhang P, Louhio H, Tuuri T, Sjoberg J, Hreinsson J, Telfer EE, et al. In vitro effect of cyclic adenosine 3′, 5′-monophosphate (cAMP) on early human ovarian follicles. J Assist Reprod Genet. 2004;21:301–6. doi: 10.1023/B:JARG.0000043704.10845.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott JE, Carlsson IB, Bavister BD, Hovatta O. Human ovarian tissue cultures: extracellular matrix composition, coating density and tissue dimensions. Reprod Biomed Online. 2004;9:287–93. doi: 10.1016/S1472-6483(10)62143-8. [DOI] [PubMed] [Google Scholar]
- 18.Scott JE, Zhang P, Hovatta O. Benefits of 8-bromo-guanosine 3′,5′-cyclic monophosphate (8-br-cGMP) in human ovarian cortical tissue culture. Reprod Biomed Online. 2004;8:319–24. doi: 10.1016/S1472-6483(10)60912-1. [DOI] [PubMed] [Google Scholar]
- 19.Garor R, Abir R, Erman A, Felz C, Nitke S, Fisch B. Effects of basic fibroblast growth factor on in vitro development of human ovarian primordial follicles. Fertil Steril. 2009;91:1967–75. doi: 10.1016/j.fertnstert.2008.04.075. [DOI] [PubMed] [Google Scholar]
- 20.Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23:1151–8. doi: 10.1093/humrep/den070. [DOI] [PubMed] [Google Scholar]
- 21.McLaughlin M, Telfer EE. Oocyte development in bovine primordial follicles is promoted by activin and FSH within a two-step serum-free culture system. Reproduction. 139: 971–8 [DOI] [PubMed]
- 22.Abir R, Roizman P, Fisch B, Nitke S, Okon E, Orvieto R, et al. Pilot study of isolated early human follicles cultured in collagen gels for 24 h. Hum Reprod. 1999;14:1299–301. doi: 10.1093/humrep/14.5.1299. [DOI] [PubMed] [Google Scholar]
- 23.Abir R, Fisch B, Nitke S, Okon E, Raz A, Ben Rafael Z. Morphological study of fully and partially isolated early human follicles. Fertil Steril. 2001;75:141–6. doi: 10.1016/S0015-0282(00)01668-X. [DOI] [PubMed] [Google Scholar]
- 24.Kedem A, Perets A, Gamlieli-Bonshtein I, Dvir-Ginzberg M, Mizrahi S, Cohen S. Vascular endothelial growth factor-releasing scaffolds enhance vascularization and engraftment of hepatocytes transplanted on liver lobes. Tissue Eng. 2005;11:715–22. doi: 10.1089/ten.2005.11.715. [DOI] [PubMed] [Google Scholar]
- 25.Dvir T, Kedem A, Ruvinov E, Levy O, Freeman I, Landa N, et al. Prevascularization of cardiac patch on the omentum improves its therapeutic outcome. Proc Natl Acad Sci U S A. 2009;106:14990–5. doi: 10.1073/pnas.0812242106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006;12:2739–46. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heise M, Koepsel R, Russell AJ, McGee EA. Calcium alginate microencapsulation of ovarian follicles impacts FSH delivery and follicle morphology. Reprod Biol Endocrinol. 2005;3:47. doi: 10.1186/1477-7827-3-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, Zelinski MB. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod. 2009;81:587–94. doi: 10.1095/biolreprod.108.074732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu M, Barrett SL, West-Farrell E, Kondapalli LA, Kiesewetter SE, Shea LD, et al. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod. 2009;24:2531–40. doi: 10.1093/humrep/dep228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pangas SA, Saudye H, Shea LD, Woodruff TK. Novel approach for the three-dimensional culture of granulosa cell-oocyte complexes. Tissue Eng. 2003;9:1013–21. doi: 10.1089/107632703322495655. [DOI] [PubMed] [Google Scholar]
- 31.Amorim CA, Langendonckt A, David A, Dolmans MM, Donnez J. Survival of human pre-antral follicles after cryopreservation of ovarian tissue, follicular isolation and in vitro culture in a calcium alginate matrix. Hum Reprod. 2009;24:92–9. doi: 10.1093/humrep/den343. [DOI] [PubMed] [Google Scholar]
- 32.Leor J, Gerecht S, Cohen S, Miller L, Holbova R, Ziskind A, et al. Human embryonic stem cell transplantation to repair the infarcted myocardium. Heart. 2007;93:1278–84. doi: 10.1136/hrt.2006.093161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newton H, Fisher J, Arnold JR, Pegg DE, Faddy MJ, Gosden RG. Permeation of human ovarian tissue with cryoprotective agents in preparation for cryopreservation. Hum Reprod. 1998;13:376–80. doi: 10.1093/humrep/13.2.376. [DOI] [PubMed] [Google Scholar]
- 34.Hovatta O, Silye R, Krausz T, Abir R, Margara RA, Trew G, Lass A, Winston RML. Cryopreservation of human ovarian tissue by using dimethylsulphoxide and propandiol-sucrose as cryoprotectants. Hum Reprod. 1996;11:1268–72. doi: 10.1093/oxfordjournals.humrep.a019370. [DOI] [PubMed] [Google Scholar]
- 35.Kedem A, Fisch B, Garor R, Ben-Zaken A, Gizunterman T, Felz C, Ben-Haroush A, Kravarusic D, Abir R. Growth differentiating factor 9 (GDF9) and bone morphogenetic protein 15 both activate development of human primordial follicles in vitro, with seemingly more beneficial effects of GDF9. J Clin Endocrinol Metab. 2011; electronic publication ahead of print [DOI] [PubMed]
- 36.Shapiro L, Cohen S. Novel alginate sponges for cell culture and transplantation. Biomaterials. 1997;18:583–90. doi: 10.1016/S0142-9612(96)00181-0. [DOI] [PubMed] [Google Scholar]
- 37.Biron-Shental T, Fisch B, Hurk R, Felz C, Feldberg D, Abir R. Survival of frozen-thawed human ovarian fetal follicles in long-term organ culture. Fertil Steril. 2004;81:716–9. doi: 10.1016/j.fertnstert.2003.06.034. [DOI] [PubMed] [Google Scholar]
- 38.Abir R, Franks S, Mobberley MA, Moore PA, Margara RA, Winston RM. Mechanical isolation and in vitro growth of preantral and small antral human follicles. Fertil Steril. 1997;68:682–8. doi: 10.1016/S0015-0282(97)00264-1. [DOI] [PubMed] [Google Scholar]
- 39.Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17:121–55. doi: 10.1210/edrv-17-2-121. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt KLT, Byskov AG, Nyobe Andersen A, Muller J, Andersen CY. Density and distribution of primordial follicles in single pieces of cortex from 21 patients and in individual pieces of cortex from three entire human ovaries. Hum Reprod. 2003;18:1158–64. doi: 10.1093/humrep/deg246. [DOI] [PubMed] [Google Scholar]
- 41.Wandji SA, Srsen V, Nathanielsz PW, Eppig JJ, Fortune JE. Initiation of growth of baboon primordial follicles in vitro. Hum Reprod. 1997;12:1993–2001. doi: 10.1093/humrep/12.9.1993. [DOI] [PubMed] [Google Scholar]
- 42.Jewgenow K. Role of media, protein and energy supplements on maintenance of morphology and DNA-synthesis of small secondary domestic cat follicles during short-term culture. Theriogenology. 1998;49:1567–77. doi: 10.1016/S0093-691X(98)00102-2. [DOI] [PubMed] [Google Scholar]
- 43.Jin SY, Lei L, Shikanov A, Shea LD, Woodruff TK. A novel two-step strategy for in vitro culture of early-stage ovarian follicles in the mouse. Fertil Steril. 93: 2633–9 [DOI] [PMC free article] [PubMed]
- 44.Xu M, Banc A, Woodruff TK, Shea LD. Secondary follicle growth and oocyte maturation by culture in alginate hydrogel following cryopreservation of the ovary or individual follicles. Biotechnol Bioeng. 2009;103:378–86. doi: 10.1002/bit.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kreeger PK, Fernandes NN, Woodruff TK, Shea LD. Regulation of mouse follicle development by follicle-stimulating hormone in a three-dimensional in vitro culture system is dependent on follicle stage and dose. Biol Reprod. 2005;73:942–50. doi: 10.1095/biolreprod.105.042390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka H, Matsumura M, Veliky IA. Diffusion characteristics of substrates in Ca-alginate gel beads. Biotechnol Bioeng. 1984;26:53–8. doi: 10.1002/bit.260260111. [DOI] [PubMed] [Google Scholar]
- 47.Rowghani NM, Heise MK, McKeel D, McGee EA, Koepsel RR, Russell AJ. Maintenance of morphology and growth of ovarian follicles in suspension culture. Tissue Eng. 2004;10:545–52. doi: 10.1089/107632704323061906. [DOI] [PubMed] [Google Scholar]
- 48.Zmora S, Glicklis R, Cohen S. Tailoring the pore architecture in 3-D alginate scaffolds by controlling the freezing regime during fabrication. Biomaterials. 2002;23:4087–94. doi: 10.1016/S0142-9612(02)00146-1. [DOI] [PubMed] [Google Scholar]
- 49.O'Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod. 2003;68:1682–6. doi: 10.1095/biolreprod.102.013029. [DOI] [PubMed] [Google Scholar]
- 50.Freeman I, Kedem A, Cohen S. The effect of sulfation of alginate hydrogels on the specific binding and controlled release of heparin-binding proteins. Biomaterials. 2008;29:3260–8. doi: 10.1016/j.biomaterials.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 51.Perets A, Baruch Y, Weisbuch F, Shoshany G, Neufeld G, Cohen S. Enhancing the vascularization of three-dimensional porous alginate scaffolds by incorporating controlled release basic fibroblast growth factor microspheres. J Biomed Mater Res A. 2003;65:489–97. doi: 10.1002/jbm.a.10542. [DOI] [PubMed] [Google Scholar]