Abstract
Purpose
The number of women attempting to conceive between the ages of 36 and 44 has increased significantly in the last decade. While it is well established that women’s reproductive success dramatically declines with age, the underlying physiological changes responsible for this phenomenon are not well understood. With assisted reproductive technologies, it is clear that oocyte quality is a likely cause since women over 40 undergoing in vitro fertilization (IVF) with oocytes donated by younger women have success rates comparable to young patients. Apart from oocyte donation, there is no known intervention to improve the pregnancy outcome of older patients. The aim of this paper was the review the relevant data on the potential role of mitochondria in reproductive aging.
Method
Review of current literature on the subject.
Results
We present the current evidence that associate mitochondrial dysfunction with age related decrease in female reproductive outcome.
Conclusions
The aging process is complex, driven by a multitude of factors thought to modulate cellular and organism life span. Although the factors responsible for diminished oocyte quality remain to be elucidated, the present review focuses on the potential role of impaired mitochondrial function.
Keywords: Reproductive aging, Oocyte, Embryo, Aneuploidy, Mitochondria, Mitochondrial DNA
Introduction
Current societal trends have resulted in women delaying pregnancy to the later part of their childbearing years. Between 1991 and 2001 in the United States, the percentage of first births for women 35–39 years of age increased by 36% and that for women 40–44 years of age increased by 70% [1]. This trend is even more apparent among patients who attend infertility clinics since these couples tend to be older than couples who conceive spontaneously [2]. It is well known that fertility decreases with age and that pregnancy rates for women over the age of 35 are significantly lower both naturally and with assisted reproduction, compared to those in women under the age of 35.
The Centers for Disease Control (CDC) in Atlanta reports results for 90% of the infertility clinics in the USA. For the year 2005, the average live-birth rate for ART fresh embryo transfer procedures was 34%. The live birth rate sharply declined with age, from 43% among women aged <35 years to 6% among women aged >42 years [3]. The decline in live birth rate reflects a decline in response to ovarian stimulation, reduced embryo quality and pregnancy rate, as well as an increased incidence of miscarriages and fetal aneuploidy. To date there is no known intervention to improve the pregnancy outcome of older patients.
One of the main reasons for the poor performance of embryos from older patients is an increased rate of chromosomal aberrations [4]. For example, the estimated incidence of trisomy 21 at age 25 is 1/1500, at age 40 is 1/60, and at age 49 is 1/11 [5]. However, including both sex chromosomes, there are 23 other chromosomes that could contribute to the rate of trisomy and aneuploidy during embryo development. Sher et al. performed oocyte and embryo numeric karyotyping using the recently developed technique of comparative genomic hybridization (CGH) [6]. This method enables determination of the number of copies of all the chromosomes. Their findings indicated that the incidence of oocyte aneuploidy for women at a mean age of 27.0 ± 2.5 years was 65% and would presumably be even higher in older women. In this study, euploid embryos were far more likely to survive and develop to blastocyst stage by day 5 than were aneuploid embryos (93% vs.21%). In addition, oocytes with proper chromosomal number almost always retained correct ploidy after fertilization, as 87% of the euploid oocytes developed into euploid embryos. These findings show that embryo ploidy is linearly propagated after fertilization and underscores the immense importance of oocyte euploidy in early embryo survival. These reproductive changes associated with aging are also accompanied by a decreased ovarian reserve [7], thought to be due to increased follicle atresia as a result of programmed cell death. When ovarian reserve reaches its nadir with menopause, approximately at age 50, infertility is absolute.
Oogenesis, energy and mitochondria
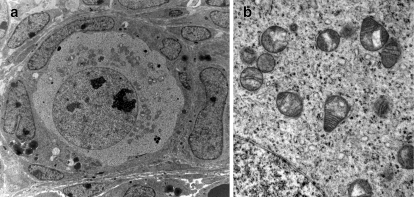
Oogenesis as well as the formation of the ovarian follicles starts in fetal life. It involves a transition of the primordial germ cells into dividing oogonia that produce the primary oocytes. Both the oocyte and the primordial follicle may reside within the ovary for as long as 50 years before growth and development into mature oocytes. Immature oocytes in the ovarian cortex are diploid, containing 46 chromosomes arrested in prophase of the first meiotic division. After follicle growth and maturation, the onset of the LH surge or the hCG trigger in ART leads to resumption of meiosis in the oocyte. During this process the chromosomes condense, align in pairs and then separate via pull of the chromosomes by the spindle fibers resulting in a mature oocyte that contains 23 chromosomes. The other set of chromosomes are isolated outside the oolema in the first polar body. The second meiotic division commences with the penetration of a viable sperm. The oocyte then extrudes 23 sister chromatids resulting in a second polar body and a fertilized zygote having a normal diploid complement of 46 chromosomes. The process of pulling chromosomes outside the egg to form the first and second polar bodies requires a significant amount of energy, which is provided by ATP from oxidative phosphorylation in the mitochondria. Perhaps reflecting these unique metabolic demands, oocyte mitochondria are immature, with round or pear-like shapes, possess few cristae and have an electron dense matrix (Fig. 1). Unlike their fully differentiated counterparts, which are polyploid, each mitochondrion in the oocyte is thought to contain only one to two mtDNA nucleoids.
Fig. 1.
Mitochondrial morphology in oocytes of primordial follicles. a Primordial follicle of neonatal (day 4) murine ovary. Oocytes of primordial follicle, showing clustering of mitochondria in a half/moon shape around nucleus. b Magnified image of mitochondrial ultrastructure with loosely defined cristae and round or pear like mitochondrial shape
The oocyte has by far, the largest number of mitochondria, and mtDNA copies, of any cell, (approximately 2x105 copies) [8], at least an order of magnitude more than somatic cells like muscle and neurons that have high energy requirements. Data from mtDNA segregation in pedigrees affected by mtDNA mutations show that a complete switch of the mtDNA may occur in a single generation, leading researchers to postulate the bottleneck theory of mitochondrial inheritance [9]. According to this theory, the primordial germ cell contains only a few copies of a founder mitochondrial genome enclosed in immature mitochondria that replicate and eventually populate the new organism. It was originally thought that the bottleneck resulted in selection of mitochondria with the best mtDNA while eliminating possibly mutated mtDNA, and resulting in a more homogenous mtDNA population in primordial germ cells. However, a recent study [10] showed that selection of mitochondria and mtDNA in the oocyte is a random process that does not screen for the intact wild type mitochondrial genome. Therefore, mitochondria with abnormal mtDNA are just as likely to be inherited by the offspring as normal mitochondria. During the process of mitochondrial replication and expansion, oocytes will dramatically amplify their population of mitochondria, thereby supplying each gamete with a large copy number of both normal and abnormal mtDNA. The selection of mtDNA nucleoids preferentially amplified during replication, or the so called genetic bottleneck, appears to occur during oocyte growth and not during primordial germ cell expansion as originally thought. From longitudinal developmental murine studies performed in Eric Shoubridge’s laboratory [11] it was determined that mtDNA replication is initiated during primordial germ cell formation, which in mice contain ~200 mitochondrial genomes. This number steadily increases so by the time the primordial follicles form during oogenesis, each oocyte contains about ten thousand mtDNA copies. At this stage TFAM positive mtDNA nucleoids were often observed near the Balbiani body, a structure around which organelles often cluster in immature oocytes of various species including mammals [12] (Fig. 2). Shortly after formation of primordial follicles, the Balbiani body disappears, and mitochondria continue to replicate, so by the time the oocyte is fully grown, it contains over 100.000 mtDNA copies. There is a large inter-oocyte variability in mtDNA content [13]. However, the relationship between mtDNA copy number and the mitochondrial dowry is still unclear. Data from transmission electron microscopy and extrapolated predictions of actual mitochondrial numbers do not always correlate with mtDNA copy number estimates (reviewed in [14]), questioning whether each organelle truly contains only a single/double nucleoid.
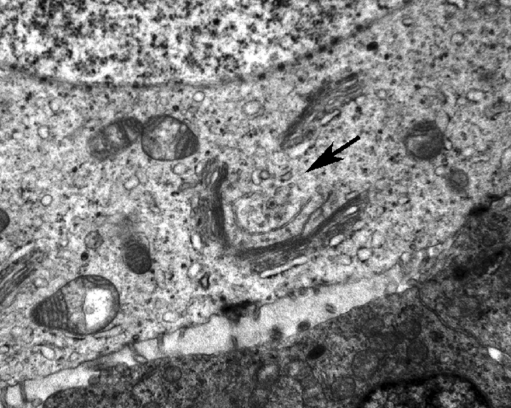
Fig. 2.
Breakdown of Balbiani body (arrow) observed in neonatal (day 4) murine oocyte. Golgi apparatus is surrounding centrally located vesicles,which are components of Balbiani body
In somatic cells replication and transcription of the mtDNA is independent of the cell cycle and occurs in organelles residing near the nucleus ([15]). This process is also regulated by metabolic demands and the differentiation status of cells ([16]). Mitochondrial replication is controlled by several nuclear encoded transcription factors which stabilize (TFAM) and unwind mtDNA (Peo1/SSbp1), and its integrity is maintained by mtDNA polymerase Polga/b (reviewed in [17]). These factors are mainly involved with modulation of mtDNA copy number rather than mitochondrial function. There are additional factors that coordinate the metabolic demands of the cell with mitochondrial biogenesis. These involve nuclear-encoded transcription factors PGC-1 (PPARγ coactivator-1 α/β), Nrf-1/2, as well as sensors such as AMPK (AMP-activated kinase) that respond to cellular caloric status, obesity and diabetes (reviewed in [18]). Expression of TFAM and Nrf1 in oocytes has been shown to be affected by obesity in the mother [19] .MtDNA copy number and embryo developmental competence have been a subject of study for many years. The total number of mtDNA copies in the developing embryo does not change from fertilization until the blastocyst stage, despite numerous cell divisions, resulting in a progressively diluted mtDNA content in each of the blastomeres [20]. With progressive development, mitochondria mature and regain their classical shape with well-defined cristae and elongated shape (reviewed in [14]).
An association between low mtDNA copy number and ability of the oocyte to become fertilized has been described [21]. In addition, oocytes of women with ovarian insufficiency have been reported to contain a lower mtDNA copy number than women with a normal ovarian profile [22]. Based on this evidence, and the fact that impairment of fertilization and embryo development are associated with insufficient energy production, it is tempting to speculate that the failure of the oocyte to sufficiently increase the number of mitochondria during maturation would lead to a poor embryo outcome. This in turn would mean that there is a certain threshold amount of functional mitochondria required for normal development. To establish whether such a threshold exists, a mouse line with a germline-specific deletion of Tfam, which has on average only 11% of mtDNA copy number of controls, was used to study reproductive performance [23]. Interestingly, this dramatic reduction of mtDNA did not compromise ovulation and fertilization, but post-implantation development was impeded when embryos started with fewer than 50,000 mtDNA copies. The above results are an interesting extension of the finding from crosses of mice heterozygous for mutant Tfam, where homozygous mutant pups died at midgestation, due to depletion of mtDNA [24]. A similar outcome was observed in PolgA deficient animals [25], which also caused death at mid-gestation due to mitochondrial depletion. Embryonic lethality has been observed even earlier due to disruption of Nrf-1 [26] and Nrf-2 [27]. Evidently, normal preimplantation development is rather resistant to the decreases in mtDNA content. Also, while disruption of nuclear genes governing mitochondrial activity is useful for understanding the regulatory pathways, it does not necessarily reflect the actual changes that occur with aging.
Energy Production in the oocyte
Oocytes mostly rely on energy (e.g. ATP) produced by mitochondria via oxidative phosphorylation (OXPHOS), a process that relies on the oxidation of nutrients to phosphorylated ADP [28]. OXPHOS involves the action of the mitochondrial respiratory chain consisting of four complexes located on the inner mitochondrial membrane. Complexes I and II oxidize products of the tricarboxylic acid cycle (TCA) or Krebs cycle, nicotinamide dinucleotide (NADH) and flavine adenine dinucleotide (FADH2), and then transfers the electrons to ubiquinone, also known as coenzyme Q10 (CoQ10). CoQ10 transfers the electrons to complex III. Complex III reduces cytochrome c and transfers the electrons to complex IV with reduction of O2 to produce H2O. The energy produced by the transfer of electrons along the respiratory chain is used to eject protons to the space between the inner and outer mitochondrial membranes. The process of proton transfer to intermembrane space is the result of the action of complexes I, III, IV and CoQ10 [29]. The accumulation of protons creates a gradient composed both of a charge and pH imbalance. Complex V, ATP synthase, provides a channel for the entrance of protons back into the mitochondrial matrix and this influx of protons supplies the energy needed for the phosphorylation of ADP to ATP. In addition to catabolism of nutrients and provision of energy, mitochondria play an important role in cell survival and steroid biosynthesis. Although mitochondria produce ROS, they also participate in ROS detoxification through a specific ROS-detoxifying enzymatic pathway. Mitochondria also play an essential role in thermogenesis and apoptosis [8].
Mitochondrial function in the mature egg and early embryo
Mitochondria are already active in the immature egg in which a low but steady ATP production from metabolism of pyruvate [30] is required to meet the demand of a slow respiratory rate. The mammalian egg and early embryo are dependent on mitochondrial OXPHOS for their supply of energy since the alternative energetic process of glycolysis is blocked due to suppression of the regulatory glycolytic enzyme, phosphofructokinase [31]. However, as oocytes proceed through their maturation, they become able to utilize beta-oxidation of lipids [32], an ATP producing process that also occurs in the mitochondria. There is an increased rate of ATP consumption in the mature oocyte that is essential for its ability to undergo normal fertilization. The processes that follow the fertilization of the egg, cortical granule exocytosis, and chromosome disjunction for second polar body extrusion all increase the energy demand even more. Therefore, it is possible that an age related decrease in mitochondrial function impairs the process of oocyte maturation, especially nuclear spindle activity and chromosomal segregation [4, 33]. The outcome of these changes is the increased rate of aneuploidy, especially trisomy that is observed in older women. Supporting data for this hypothesis includes the observation that mitochondrial mutations in follicular cells increase with age, leading to impaired follicular oxidative phosphorylation and ATP production in older women [34]. Furthermore, it has been demonstrated that the potential for embryo implantation is correlated with the ATP content of the embryo [35]. Wilding et al., [36] measured changes in mitochondrial inner membrane potential (ΔΨm) of 2–3 day old fresh human embryos. The ΔΨm is highly correlated with mitochondrial capacity to produce ATP. They found a strong correlation between low ΔΨm and a state of chaotic mosaics in which there was random segregation of chromosomes between the blastomeres. The embryos that had chaotic mosaicism were significantly slower in their rate of cleavage and significantly more common among the older group of patients.
Due to the lack of mitochondrial replication until the blastocyst stage [37], the initial population of mitochondria in the oocyte must be split into each of the blastomeres of the developing embryo and the metabolic activity of the shrinking population of mitochondria per cell must escalate to meet the increasing demands of cellular activity. This in turn would require recruitment of some quiescent mitochondria into an active subset leading to an overall increase in the ratio of high to low ΔΨm. Many mitochondrial functions, including protein import, ATP generation and lipid biogenesis, depend on the maintenance of ΔΨm [38] and had been previously shown to be elevated in embryos with compromised development [39].
The oocyte maintains cytoplasm connections with the cumulus cells that surround it. These connections allow for transport of nutrients to support the metabolic needs of the oocyte and are disrupted when the oocyte matures. Therefore, mitochondrial dysfunction in the oocyte might only be expressed after the onset of oocyte maturation [40]. In order to prove that mitochondrial dysfunction can have negative impact on oocyte quality, mitochondrial damage was induced by photosensitized rhodamine-123 fluorophore, which resulted in a decrease in mitochondrial potential, ATP production, and an increase in production of reactive singlet oxygen intermediate [41]. This caused compromised meiotic spindle configuration, chromosomal misalignment and eventually caused oocyte death. However, transfer of the photosensitized nucleus to an intact ooplasm containing healthy mitochondria resulted in successful maturation, fertilization and delivery of healthy offspring [42]. When young and aged murine oocytes were compared, it appeared that a shorter duration of photosensitization was required to completely inhibit the development past blastocyst stage in aged oocytes [43]. Similarly, maturation arrest, aberrant spindles and an increase in polyploidy were observed with exposure of maturing mouse oocytes to diazepam that reduces ATP production in the mitochondria by binding to its benzodiazepine receptor [44]. Therefore, it is reasonable to suggest that the documented relationship between maternal age and chromosomal abnormalities in human embryos is likely a result of diminished mitochondrial activity in the oocyte.
Mitochondrial biogenesis and clearance
Constant maintenance of a healthy mitochondrial pool is necessary for normal aging and depends on a precisely coordinated balance of mitochondrial biogenesis and selective cellular degradation. While too few mitochondria impede generation of energy, too many mitochondria also compromise cellular function [45]. Morphometric analyses of human oocytes showed an increase in mitochondrial numerical density, size, and volume fraction with increasing age [28]. This increase in mitochondrial size and number could either suggest a compensatory expansion in order to correct for decreased energy production experienced by aging oocytes, or alternatively, impaired mitochondrial clearance, or both. In response to increased metabolic needs, mitochondrial biogenesis is triggered by PPARγ coactivator1 (PGC-1α/β), which in turn triggers expression of genes regulating mitochondrial fusion (Mfn2) and metabolism (AMPK). These gene targets have been shown to be decreased in muscle of diabetic patients [46] and are directly linked with age-related insulin resistance [47]. In addition, mitochondria have developed a surveillance mechanism to ensure efficient removal of damaged, misfolded or aggregated mitochondrial proteins. Under conditions of cellular stress or aging, the activity of mitochondrial chaperons and proteases, needed for efficient ATP-dependent removal of damaged proteins is compromised, leading to the formation of intra-mitochondrial protein aggregates (reviewed in [48]). Once these aggregates reach a critical threshold, defective mitochondria must either fuse with healthy mitochondria, or they are recycled by mitophagy. Mitochondrial fusion, which involves docking, fusion and mixing of inner mitochondrial matrix components is regulated by Mitofusin 1,2 and OpaI [18]. This process results in maintenance of mtDNA integrity and repair [49]. If the process of fusion fails, dysfunctional mitochondria need to be cleared by an autophagocytic process called mitophagy. Mitophagy is a molecularly controlled process designed for selective removal of damaged mitochondria via lysosomal degradation [50]. Clearance of damaged organelles and unnecessary protein complexes is required for proper cell survival. Excessive loss of mitochondria via mitophagy has been linked to a variety of neurodegenerative diseases [51]. On the other hand, ineffective mitophagy can lead to accumulation of damaged organelles, contributing to a global cellular metabolic dysfunction. The aging process is known to impair mitochondrial turnover via both reduced biogenesis and inefficient clearance [52]. It should be noted that the process of mitophagy, being energy dependent, relies on proper functioning of a certain minimal number of mitochondria.
Aging and mtDNA mutations
Senescence is often associated with loss of mitochondrial function as a result of the accumulation of mtDNA mutations and deletions. This process of cumulative DNA damage is attributed to the continuous exposure to ROS generated through normal metabolism by the mitochondria themselves [53, 54].
Resting follicles maintain a low level of mitochondrial oxidative phosphorylation. This process may occur for up to 40 years prior to follicle maturation and ovulation. The decades long constant exposure of the mtDNA to ROS produced as a by-product of the OXPHOS system together with the lack of protection from histone proteins, and the lack of non-coding introns, make the mtDNA much more prone to functional mutations [55]. Maintaining and repairing damaged mtDNA relies upon the same enzymes that maintain the nuclear genome. These enzymes are, however, not suitable for the repair of mtDNA, making it less effective. This mechanism was suggested by a recent publication demonstrating that the high rate of mutation in mtDNA may in fact be the result of the faulty action of the DNA repair mechanism on the mtDNA [56]. The “common deletion” of 4977 base pairs, almost a third of the whole mtDNA genome is one of the more frequent mtDNA deletions. There are several theories regarding the formation of the common mtDNA deletions and all of them point to the unique structure of the mtDNA, with two coding strands having identical replication initiation sites at the two ends of the deleted section [56]. This deletion is common in unfertilized eggs and eggs from older patients [57, 58]. Since the mtDNA contains genes encoding proteins of the respiratory chain, a significant loss of this DNA will ultimately result in dysfunctional OXPHOS that may be detected by the absence of cytochrome c oxidase activity.
The presentation of mitochondrial dysfunction secondary to mtDNA deletions is tissue specific and has been linked to many pathologies such as diabetes, heart failure, neurodegenerative diseases and even cancer [59]. Since mitochondria are maternally inherited, mitochondrial abnormalities due to ageing could theoretically result in pathology in the offspring. However, as mtDNA mutations effect only a subset of organells, usually 60% or more of the mtDNA copies need to contain deletions before significant biochemical defect would be apparent. Since the accumulation of mutations in mtDNA is often blamed for the reproductive decline with age, some studies have tried to determine the connection between mtDNA mutations and reproductive performance. For example, polymorphism in mitochondrial ATP synthase 8 (mtAtp8), characterized by distortion in mitochondrial shape and an increase in H2O2 production was associated with reduced litter size [60]. A very promising approach for studying mitochondrial mutations is the use of the mtDNA-mutator mice, which have a proofreading-deficient PolgA [61]. Unlike mutant animals that completely lack Polg activity and die mid-gestation, these animals exhibit a large increase in mtDNA point mutations and deletions, reduced life span, and signs of premature aging, including failure to produce offspring when females are older than 20 weeks of age. It would be interesting to determine in this model if the frequency of mitochondrial mutations in oocytes also increases with age and if mutational load is elevated in the offspring of older dams.
Since mitochondria in oocytes from older women are thought to contain increased mtDNA mutations, deletions and dysfunction, and since mitochondria are maternally inherited, researchers began searching for adult phenotypic expression of mitochondrial abnormalities [62]. Aegesen et al. [63] compared the incidence of Down’s syndrome (DS) among infants of age-matched mothers based on the age of the grandmother when the mother was born. They found that advanced grand-maternal age at the birth of the mother was associated with an increased risk of Down's syndrome. This observation was verified by Malini et al. [64] in India where delivery of newborns with Down’s syndrome (DS) is common since prenatal screening is unusual. Young mothers (18 to 29 years) born to women over age 30 years produced 91% of the children with DS. Logistic regression of this case–control study revealed that the age of the grandmother was the most significant of all the variables studied for DS birth. For every year of age of the maternal grandmother over age 30, the risk (odds) of birth of a DS baby increased by 30%!
In addition, offspring of older mothers (≥ 40 years of age) were observed to have a significantly higher rate of male infertility due to asthenozoospermia [65] and female subfertility associated with oligomenorrhea and amenorrhea, likely representing polycystic ovary syndrome (PCOS) [66], both conditions associated with mitochondrial dysfunction [67, 68]. Diabetes, Alzheimer's disease and the metabolic syndrome are examples of other common problems that are now regarded as diseases with mitochondrial involvement.
Possible role of mitochondria in the Barker Hypothesis
The developmental origins of health and disease (DOHaD) was originally conceptualized by Barker and Osmond in 1986 [69] based on the observation of a geographical correlation between adult coronary heart disease and infant mortality. This and several other geographical studies led to the hypothesis that undernutrition in utero and during infancy affected developmental plasticity resulting in permanent physiologic, metabolic, and morphologic changes in the infant to allow adaptation to an unfavorable environment. This adjustment in physiology and metabolism teleologically might enhance survival under times of stress but later, under more favorable conditions, may lead to adult disease. A pivotal study done in 1989 [70] confirmed a significant decrease in hazard ratios for death from CHD with increasing birth weight, and with increasing infant weight at 1 year of age. Therefore, the DOHaD hypothesis is likely correct but the molecular mechanisms responsible for DOHaD have not been not delineated. It is possible, that not only the intrauterine environment, but also the status of mitochondria inherited from the mother could contribute to DOHaD. With maternal ageing, mitochondrial dysfunction, inherited from the mother, could contribute to the onset of metabolic syndrome including obesity, insulin resistance, abnormal lipid profile and an increased risk of CHD later in life [71].
Previous attempts to improve embryo development
Cytoplasmic transfer between oocytes was initially developed in order to treat infertility patients exhibiting persistent poor embryonic development and recurrent implantation failure after IVF. The technique was based on the assumption that the ooplasm of eggs, particularly from older women, could be rescued by the introduction of cytoplasm from eggs of younger donors. The procedure involved microinjection of 5–15% of the ooplasm from a young presumably fertile donor oocyte into a putative defective recipient oocyte [72]. This treatment was based on results of earlier animal experiments involving mouse embryos from strains that experience a developmental block. Injection of cytoplasm from an oocyte of a non-blocking into a blocking strain increased cleavage rates of the recipient embryos compared with non-injected controls, suggesting the presence of an ooplasmic factor capable of rescuing the developmental block [73]. Transfer of ooplasm from healthy fertile donors into oocytes of patients with repeated embryonic developmental failure has been used clinically, resulting with the birth of several children worldwide [72–75]. Despite the fact that several cytoplasmic components are injected it is commonly believed that the beneficial effects are derived from the mitochondria. To investigate whether mitochondrial dysfunction is a factor in ooplasmic insufficiency, bovine oocytes were exposed to ethidium bromide, an inhibitor of mitochondrial DNA replication and transcription, during in-vitro maturation (IVM). Exposure of immature oocytes to ethidium bromide for 24 h during IVM hampered meiotic resumption and the migration of cortical granules. However, a shorter treatment with ethidium bromide during the last 4 h of IVM led to partial arrest of preimplantation development without affecting oocyte maturation. Ooplasm transfer was then performed to rescue the oocytes with impaired development. In spite of this developmental hindrance, transfer of normal ooplasm into ethidium bromide-treated oocytes resulted in a complete rescue of embryonic development and the birth of heteroplasmic calves [76].
Children born following cytoplasmic transfer have demonstrated persistent mitochondrial heteroplasmy [77]. The long term health effect of induced mitochondrial heteroplasmy in these children is as yet unknown, although we have demonstrated that a mouse model of persistent neutral heteroplasmy resulted in a phenotype which is consistent with early onset of the metabolic syndrome [78].
Clinical Implications and Potential Treatment
We have reviewed evidence that dysfunction of oocyte mitochondria is a possible mechanism involved in poor developmental competence of oocytes in older infertility patients. The meiotic spindle, crucial for normal chromosome segregation, may not be formed properly in the absence of appropriate mitochondrial activity. This hypothesis is based on findings of abnormal spindles and chromosomal scattering in oocytes deficient in the pyruvate metabolising enzyme, Pdha1 [40]. Altered spindles may result in aneuploid embryos, leading to failed implantation or early miscarriage. Abnormal mitochondrial activity reduces the production of ATP, which in turn may interfere not only with efficient chromosomal disjunction, but may also limit cell division and embryo development. Mitochondrial function also plays a major role in the initiation and control of apoptosis of oocytes and embryos.
Since mitochondria are transmitted through the oocyte to the next generation, we hypothesize that the offspring of older women are more likely to inherit a greater proportion of abnormal mitochondria leading in some cases to pathophysiologic conditions such as insulin resistance or full blown metabolic syndrome. In this regard, maternal aging and mitochondrial dysfunction may be contributors to the “Barker hypothesis” or the developmental origins of health and disease (DOHaD).
Can the aging process in reproductive function be altered? We believe the observations presented in this review point to the conclusion that a mechanism that will increase mitochondrial function and energy production should have a positive impact on pregnancy outcome in older women. In this regard, supplementing the diets of older women with mitochondrial nutrients may potentially be beneficial. Mitochondrial nutrients are naturally occurring chemicals that have been used successfully to treat conditions associated with diminished energy production from mitochondria, and appear to be very safe for both mother and fetus. Supplementation with mitochondrial nutrients, by increasing energy for chromosomal disjunction, may be able to reduce the risk of trisomy and other types of chromosomal aneuploidies related to oocyte aging. This strategy may lead to improvement in oocyte and embryo quality, and subsequently, a healthy pregnancy outcome for older women.
Mitochondrial nutrients
α Lipoic acid (ALA) ALA or thioctic acid is a coenzyme involved in mitochondrial metabolism. ALA is found in virtually all plant and animal species and is a natural part of our diet. ALA readily crosses the blood–brain barrier and is accepted by human cells as a mitochondrial substrate. The reduced form of ALA, dihydrolipoic acid, is a powerful mitochondrial antioxidant formed by nicotinamide adenine dinucleotide (NADH)-dependent mitochondrial dihydrolipoamide dehydrogenase [79]. ALA was shown to be essential for normal embryonic development in mice[80].
Coenzyme Q10 CoQ10 is a lipid soluble electron transporter, that transports electrons from complexes I and II to complex III in the mitochondrial respiratory chain and is essential for the stability of complex III [81]. It also participates in the transport of protons in the mitochondria to maintain the membrane potential and drive ATP formation through ATP synthetase [82]. CoQ10 is a major cellular antioxidant [83]. CoQ10 is naturally present in the fetal circulation [84] and in breast milk [85]. There is a gradual, age relate decline in the tissue levels of CoQ10 [86] and with certain drugs such as statins which block its synthesis [87]. Mutations of genes involved in coQ10 synthesis may lead to CoQ10 Deficiency, characterized by clinical disorders involving mitochondrial dysfunction in the nervous system, skeletal muscles and endocrine glands [88].One study examined the use of CoQ10 in the in vitro culture of bovine embryos and found a superior rate of early embryo cleavage, blastocyst formation, percentage of expanding blastocysts and a larger inner cell mass. These changes were associated with an increased ATP content in the group of embryos cultured with CoQ10. Other investigators have demonstrated an increased ATP production in lymphocytes supplemented with CoQ10 [89].
Resveratrol Resveratrol is a polyphenol that belongs to the stilbene family of phytoalexins, which are defense molecules produced by plants in response to infection. Grapes, red wine and peanuts are the richest dietary sources of resveratrol [90]. Resveratrol has received media and scientific attention recently due to its widely reported ability to delay aging and prevent age related diseases. In addition, resveratrol is one of the chemicals present in red wine that has been implicated in the so called “French Paradox”.The known mechanisms by which resveratrol induce its health promoting effects are through potent antioxidant capabilities, the activation of SIRT1, and as an aryl hydrocarbon receptor antagonist [91]. Mice treated with resveratrol lived longer and displayed enhanced insulin sensitivity and increased mitochondrial number, similar to calorie restricted animals [92]. Transgenic mice over-expressing SIRT1, display most of the beneficial metabolic effects of resveratrol, suggesting that this gene is its main mediator [93].
Conclusion
The aging process is complex and includes genetic predisposition, impaired mitochondrial function, genomic instability, oxidative stress, caloric intake and subsequent metabolic activity, and the activity of several cell-signaling systems. Mitochondrial abnormalities or mutations are believed to contribute to reproductive aging and also to age-related pathologies such as insulin resistance and type II diabetes, lipid abnormalities, and hypertension. Mitochondria in the offspring are exclusively inherited from the mother, while paternal mitochondria are actively targeted and destroyed by ubiquitination. It is possible that mitochondrial problems can contribute to poor reproductive outcome. In addition, inheritance of dysfunctional maternal mitochondria by the next generation could increase the risk of developing adult diseases later in life. The molecular factors responsible for oocyte mitochondrial abnormalities and diminished oocyte quality remain to be elucidated. If mitochondrial abnormalities occur in oocytes of older mothers, as our review suggests, inherited mitochondrial dysfunction in the offspring may partially explain the epidemic of obesity, insulin resistance and type 2 diabetes afflicting our modern society.
Acknowledgments
Disclosure YB, TY, AJ, NE and RFC have nothing to disclose.
Footnotes
Capsule
We describe the fundamental importance of mitochondria in the oocyte and their potential role in reproductive senescence
Contributor Information
Yaakov Bentov, Email: yaakovb@tcartonline.com.
Tetyana Yavorska, Email: yavorska@lunenfeld.ca.
Navid Esfandiari, Email: nesfand@yahoo.com.
Andrea Jurisicova, Email: jurisicova@lunenfeld.ca.
Robert F. Casper, Phone: +1-416-9720777, FAX: +1-416-9720036, Email: casper@lunenfeld.ca
References
- 1.Huang L, Sauve R, Birkett N, Fergusson D, Walraven C. Maternal age and risk of stillbirth: a systematic review. Cmaj. 2008;178:165–72. doi: 10.1503/cmaj.070150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergh T, Ericson A, Hillensjo T, Nygren KG, Wennerholm UB. Deliveries and children born after in-vitro fertilisation in Sweden 1982–95: a retrospective cohort study. Lancet. 1999;354:1579–85. doi: 10.1016/S0140-6736(99)04345-7. [DOI] [PubMed] [Google Scholar]
- 3.Wright VC, Chang J, Jeng G, Macaluso M. Assisted reproductive technology surveillance–United States, 2005. MMWR Surveill Summ. 2008;57:1–23. [PubMed] [Google Scholar]
- 4.Bartmann AK, Romao GS, Ramos Eda S, Ferriani RA. Why do older women have poor implantation rates? A possible role of the mitochondria. J Assist Reprod Genet. 2004;21:79–83. doi: 10.1023/B:JARG.0000027018.02425.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hook EB. Rates of chromosome abnormalities at different maternal ages. Obstet Gynecol. 1981;58:282–5. [PubMed] [Google Scholar]
- 6.Sher G, Keskintepe L, Keskintepe M, Ginsburg M, Maassarani G, Yakut T, et al. Oocyte karyotyping by comparative genomic hybridization [correction of hybrydization] provides a highly reliable method for selecting "competent" embryos, markedly improving in vitro fertilization outcome: a multiphase study. Fertil Steril. 2007;87:1033–40. doi: 10.1016/j.fertnstert.2006.08.108. [DOI] [PubMed] [Google Scholar]
- 7.Freeman SB, Yang Q, Allran K, Taft LF, Sherman SL. Women with a reduced ovarian complement may have an increased risk for a child with Down syndrome. Am J Hum Genet. 2000;66:1680–3. doi: 10.1086/302907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.May-Panloup P, Chretien MF, Malthiery Y, Reynier P. Mitochondrial DNA in the oocyte and the developing embryo. Curr Top Dev Biol. 2007;77:51–83. doi: 10.1016/S0070-2153(06)77003-X. [DOI] [PubMed] [Google Scholar]
- 9.Ashley MV, Laipis PJ, Hauswirth WW. Rapid segregation of heteroplasmic bovine mitochondria. Nucleic Acids Res. 1989;17:7325–31. doi: 10.1093/nar/17.18.7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue K, Nakada K, Ogura A, Isobe K, Goto Y, Nonaka I, et al. Generation of mice with mitochondrial dysfunction by introducing mouse mtDNA carrying a deletion into zygotes. Nat Genet. 2000;26:176–81. doi: 10.1038/82826. [DOI] [PubMed] [Google Scholar]
- 11.Wai T, Teoli D, Shoubridge EA. The mitochondrial DNA genetic bottleneck results from replication of a subpopulation of genomes. Nat Genet. 2008;40:1484–8. doi: 10.1038/ng.258. [DOI] [PubMed] [Google Scholar]
- 12.Pepling ME, Wilhelm JE, O’Hara AL, Gephardt GW, Spradling AC. Mouse oocytes within germ cell cysts and primordial follicles contain a Balbiani body. Proc Natl Acad Sci USA. 2007;104:187–92. doi: 10.1073/pnas.0609923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santos TA, El Shourbagy A, St John JC. Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil Steril. 2006;85:584–91. doi: 10.1016/j.fertnstert.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 2010. doi:10.1016/j.mito.2010.09.012 [DOI] [PubMed]
- 15.Davis AF, Clayton DA. In situ localization of mitochondrial DNA replication in intact mammalian cells. J Cell Biol. 1996;135:883–93. doi: 10.1083/jcb.135.4.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Facucho-Oliveira JM, Alderson J, Spikings EC, Egginton S, St John JC. Mitochondrial DNA replication during differentiation of murine embryonic stem cells. J Cell Sci. 2007;120:4025–34. doi: 10.1242/jcs.016972. [DOI] [PubMed] [Google Scholar]
- 17.Tyynismaa H, Suomalainen A. Mouse models of mtDNA replication diseases. Methods. 2010;51:405–10. [DOI] [PubMed]
- 18.Seo AY, Joseph AM, Dutta D, Hwang JC, Aris JP, Leeuwenburgh C. New insights into the role of mitochondria in aging: mitochondrial dynamics and more. J Cell Sci. 2010;123(Pt 15):2533-42. [DOI] [PMC free article] [PubMed]
- 19.Igosheva N, Abramov AY, Poston L, Eckert JJ, Fleming TP, Duchen MR et al. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS One. 2010;5(4):e10074. [DOI] [PMC free article] [PubMed]
- 20.Thundathil J, Filion F, Smith LC. Molecular control of mitochondrial function in preimplantation mouse embryos. Mol Reprod Dev. 2005;71:405–13. doi: 10.1002/mrd.20260. [DOI] [PubMed] [Google Scholar]
- 21.Reynier P, May-Panloup P, Chretien MF, Morgan CJ, Jean M, Savagner F, et al. Mitochondrial DNA content affects the fertilizability of human oocytes. Mol Hum Reprod. 2001;7:425–9. doi: 10.1093/molehr/7.5.425. [DOI] [PubMed] [Google Scholar]
- 22.May-Panloup P, Chretien MF, Jacques C, Vasseur C, Malthiery Y, Reynier P. Low oocyte mitochondrial DNA content in ovarian insufficiency. Hum Reprod (Oxford, England) 2005;20:593–7. doi: 10.1093/humrep/deh667. [DOI] [PubMed] [Google Scholar]
- 23.Wai T, Ao A, Zhang X, Cyr D, Dufort D, Shoubridge EA. The role of mitochondrial DNA copy number in mammalian fertility. Biology of reproduction. 2010;83(1): 52–62. [DOI] [PMC free article] [PubMed]
- 24.Larsson NG, Wang J, Wilhelmsson H, Oldfors A, Rustin P, Lewandoski M, et al. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet. 1998;18:231–6. doi: 10.1038/ng0398-231. [DOI] [PubMed] [Google Scholar]
- 25.Hance N, Ekstrand MI, Trifunovic A. Mitochondrial DNA polymerase gamma is essential for mammalian embryogenesis. Hum Mol Genet. 2005;14:1775–83. doi: 10.1093/hmg/ddi184. [DOI] [PubMed] [Google Scholar]
- 26.Huo L, Scarpulla RC. Mitochondrial DNA instability and peri-implantation lethality associated with targeted disruption of nuclear respiratory factor 1 in mice. Mol Cell Biol. 2001;21:644–54. doi: 10.1128/MCB.21.2.644-654.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ristevski S, O’Leary DA, Thornell AP, Owen MJ, Kola I, Hertzog PJ. The ETS transcription factor GABPalpha is essential for early embryogenesis. Mol Cell Biol. 2004;24:5844–9. doi: 10.1128/MCB.24.13.5844-5849.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lodish H, Berk A, Zipursky LS, Matsudaira P, Baltimore D, Darnell J. Molecular Cell Biology. In: Cellular Energetics: Glycolysis, Aerobic Oxidation, and Photosynthesis: W. H. FREEMAN, 2000.
- 29.Crane FL. The evolution of coenzyme Q. BioFactors (Oxford, England) 2008;32:5–11. doi: 10.1002/biof.5520320102. [DOI] [PubMed] [Google Scholar]
- 30.Wilding M, Fiorentino A, Simone ML, Infante V, Matteo L, Marino M, et al. Energy substrates, mitochondrial membrane potential and human preimplantation embryo division. Reprod Biomed Online. 2002;5:39–42. doi: 10.1016/S1472-6483(10)61595-7. [DOI] [PubMed] [Google Scholar]
- 31.Barbehenn EK, Wales RG, Lowry OH. The explanation for the blockade of glycolysis in early mouse embryos. Proc Natl Acad Sci USA. 1974;71:1056–60. doi: 10.1073/pnas.71.4.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dunning KR, Cashman K, Russell DL, Thompson JG, Norman RJ, Robker RL. Beta-oxidation is essential for mouse oocyte developmental competence and early embryo development. Biology of reproduction. 2010;83(6):909–18. [DOI] [PubMed]
- 33.Wilding M, Matteo L, Dale B. The maternal age effect: a hypothesis based on oxidative phosphorylation. Zygote (Cambridge, England) 2005;13:317–23. doi: 10.1017/S0967199405003382. [DOI] [PubMed] [Google Scholar]
- 34.Seifer DB, DeJesus V, Hubbard K. Mitochondrial deletions in luteinized granulosa cells as a function of age in women undergoing in vitro fertilization. Fertil Steril. 2002;78:1046–8. doi: 10.1016/S0015-0282(02)04214-0. [DOI] [PubMed] [Google Scholar]
- 35.Blerkom J, Davis PW, Lee J. ATP content of human oocytes and developmental potential and outcome after in-vitro fertilization and embryo transfer. Hum Reprod (Oxford, England) 1995;10:415–24. doi: 10.1093/oxfordjournals.humrep.a135954. [DOI] [PubMed] [Google Scholar]
- 36.Wilding M, Placido G, Matteo L, Marino M, Alviggi C, Dale B. Chaotic mosaicism in human preimplantation embryos is correlated with a low mitochondrial membrane potential. Fertil Steril. 2003;79:340–6. doi: 10.1016/S0015-0282(02)04678-2. [DOI] [PubMed] [Google Scholar]
- 37.Jansen RP, Boer K. The bottleneck: mitochondrial imperatives in oogenesis and ovarian follicular fate. Mol Cell Endocrinol. 1998;145:81–8. doi: 10.1016/S0303-7207(98)00173-7. [DOI] [PubMed] [Google Scholar]
- 38.Voisine C, Craig EA, Zufall N, Ahsen O, Pfanner N, Voos W. The protein import motor of mitochondria: unfolding and trapping of preproteins are distinct and separable functions of matrix Hsp70. Cell. 1999;97:565–74. doi: 10.1016/S0092-8674(00)80768-0. [DOI] [PubMed] [Google Scholar]
- 39.Acton BM, Jurisicova A, Jurisica I, Casper RF. Alterations in mitochondrial membrane potential during preimplantation stages of mouse and human embryo development. Mol Hum Reprod. 2004;10:23–32. doi: 10.1093/molehr/gah004. [DOI] [PubMed] [Google Scholar]
- 40.Johnson MT, Freeman EA, Gardner DK, Hunt PA. Oxidative metabolism of pyruvate is required for meiotic maturation of murine oocytes in vivo. Biol Reprod. 2007;77:2–8. doi: 10.1095/biolreprod.106.059899. [DOI] [PubMed] [Google Scholar]
- 41.Thouas GA, Trounson AO, Wolvetang EJ, Jones GM. Mitochondrial dysfunction in mouse oocytes results in preimplantation embryo arrest in vitro. Biol Reprod. 2004;71:1936–42. doi: 10.1095/biolreprod.104.033589. [DOI] [PubMed] [Google Scholar]
- 42.Takeuchi T, Neri QV, Katagiri Y, Rosenwaks Z, Palermo GD. Effect of treating induced mitochondrial damage on embryonic development and epigenesis. Biol Reprod. 2005;72:584–92. doi: 10.1095/biolreprod.104.032391. [DOI] [PubMed] [Google Scholar]
- 43.Thouas GA, Trounson AO, Jones GM. Effect of female age on mouse oocyte developmental competence following mitochondrial injury. Biol Reprod. 2005;73:366–73. doi: 10.1095/biolreprod.105.040956. [DOI] [PubMed] [Google Scholar]
- 44.Yin H, Baart E, Betzendahl I, Eichenlaub-Ritter U. Diazepam induces meiotic delay, aneuploidy and predivision of homologues and chromatids in mammalian oocytes. Mutagenesis. 1998;13:567–80. doi: 10.1093/mutage/13.6.567. [DOI] [PubMed] [Google Scholar]
- 45.Ylikallio E, Tyynismaa H, Tsutsui H, Ide T, Suomalainen A. High mitochondrial DNA copy number has detrimental effects in mice. Human molecular genetics. 2010;19(13):2695–705. [DOI] [PubMed]
- 46.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–50. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 47.Qiang W, Weiqiang K, Qing Z, Pengju Z, Yi L. Aging impairs insulin-stimulated glucose uptake in rat skeletal muscle via suppressing AMPKalpha. Exp Mol Med. 2007;39:535–43. doi: 10.1038/emm.2007.59. [DOI] [PubMed] [Google Scholar]
- 48.Luce K, Weil AC, Osiewacz HD. Mitochondrial protein quality control systems in aging and disease. Adv Exp Med Biol. 2010;694:108–25. [DOI] [PubMed]
- 49.Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM et al. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 2010;141(2):280–9. [DOI] [PMC free article] [PubMed]
- 50.Tolkovsky AM. Mitophagy. Biochim Biophys Acta. 2009;1793:1508–15. doi: 10.1016/j.bbamcr.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 51.Mizushima N, Levine B. Autophagy in mammalian development and differentiation. Nat Cell Biol. 2010;12(9):823–30. [DOI] [PMC free article] [PubMed]
- 52.Mammucari C, Rizzuto R. Signaling pathways in mitochondrial dysfunction and aging. Mech Ageing Dev. 2010;131(7–8):536–43. [DOI] [PMC free article] [PubMed]
- 53.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 54.Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci USA. 1994;91:10771–8. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parsons TJ, Muniec DS, Sullivan K, Woodyatt N, Alliston-Greiner R, Wilson MR, et al. A high observed substitution rate in the human mitochondrial DNA control region. Nat Genet. 1997;15:363–8. doi: 10.1038/ng0497-363. [DOI] [PubMed] [Google Scholar]
- 56.Krishnan KJ, Reeve AK, Samuels DC, Chinnery PF, Blackwood JK, Taylor RW, et al. What causes mitochondrial DNA deletions in human cells? Nat Genet. 2008;40:275–9. doi: 10.1038/ng.f.94. [DOI] [PubMed] [Google Scholar]
- 57.Chen X, Prosser R, Simonetti S, Sadlock J, Jagiello G, Schon EA. Rearranged mitochondrial genomes are present in human oocytes. Am J Hum Genet. 1995;57:239–47. [PMC free article] [PubMed] [Google Scholar]
- 58.Keefe DL, Niven-Fairchild T, Powell S, Buradagunta S. Mitochondrial deoxyribonucleic acid deletions in oocytes and reproductive aging in women. Fertil Steril. 1995;64:577–83. [PubMed] [Google Scholar]
- 59.Kang D, Hamasaki N. Mitochondrial DNA in somatic cells: a promising target of routine clinical tests. Clin Biochem. 2005;38:685–95. doi: 10.1016/j.clinbiochem.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 60.Yu X, Wester-Rosenlof L, Gimsa U, Holzhueter SA, Marques A, Jonas L, et al. The mtDNA nt7778 G/T polymorphism affects autoimmune diseases and reproductive performance in the mouse. Hum Mol Genet. 2009;18:4689–98. doi: 10.1093/hmg/ddp432. [DOI] [PubMed] [Google Scholar]
- 61.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–23. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 62.Tarin JJ, Brines J, Cano A. Long-term effects of delayed parenthood. Hum Reprod. 1998;13:2371–6. doi: 10.1093/humrep/13.9.2371. [DOI] [PubMed] [Google Scholar]
- 63.Aagesen L, Grinsted J, Mikkelsen M. Advanced grandmaternal age on the mother’s side–a risk of giving rise to trisomy 21. Ann Hum Genet. 1984;48:297–301. doi: 10.1111/j.1469-1809.1984.tb00843.x. [DOI] [PubMed] [Google Scholar]
- 64.Malini SS, Ramachandra NB. Influence of advanced age of maternal grandmothers on Down syndrome. BMC Med Genet. 2006;7:4. doi: 10.1186/1471-2350-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.St John JC, Cooke ID, Barratt CL. Mitochondrial mutations and male infertility. Nat Med. 1997;3:124–5. doi: 10.1038/nm0297-124c. [DOI] [PubMed] [Google Scholar]
- 66.Smits LJ, Willemsen WN, Zielhuis GA, Jongbloet PH. Conditions at conception and risk of menstrual disorders. Epidemiology. 1997;8:524–9. doi: 10.1097/00001648-199709000-00009. [DOI] [PubMed] [Google Scholar]
- 67.Corbould A. Insulin resistance in skeletal muscle and adipose tissue in polycystic ovary syndrome: are the molecular mechanisms distinct from type 2 diabetes? Panminerva Med. 2008;50:279–94. [PubMed] [Google Scholar]
- 68.Ruiz-Pesini E, Diez C, Lapena AC, Perez-Martos A, Montoya J, Alvarez E, et al. Correlation of sperm motility with mitochondrial enzymatic activities. Clin Chem. 1998;44:1616–20. [PubMed] [Google Scholar]
- 69.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–81. doi: 10.1016/S0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 70.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–80. doi: 10.1016/S0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 71.Burstein EB, Y. Omari, S. Yavorska, T. Jurisicova, A. Casper, R.F.Mitochondria in the offspring of old mice exhibit alterations similar to those seen in their mothers In: DeCherney AH, ed. ASRM Annual Meeting. Denver, Colorado: Elsevier Inc., 2010:S57.
- 72.Cohen J, Scott R, Alikani M, Schimmel T, Munne S, Levron J, et al. Ooplasmic transfer in mature human oocytes. Mol Hum Reprod. 1998;4:269–80. doi: 10.1093/molehr/4.3.269. [DOI] [PubMed] [Google Scholar]
- 73.Muggleton-Harris A, Whittingham DG, Wilson L. Cytoplasmic control of preimplantation development in vitro in the mouse. Nature. 1982;299:460–2. doi: 10.1038/299460a0. [DOI] [PubMed] [Google Scholar]
- 74.Barritt J, Willadsen S, Brenner C, Cohen J. Cytoplasmic transfer in assisted reproduction. Hum Reprod Update. 2001;7:428–35. doi: 10.1093/humupd/7.4.428. [DOI] [PubMed] [Google Scholar]
- 75.Barritt JA, Brenner CA, Malter HE, Cohen J. Mitochondria in human offspring derived from ooplasmic transplantation. Hum Reprod (Oxford, England) 2001;16:513–6. doi: 10.1093/humrep/16.3.513. [DOI] [PubMed] [Google Scholar]
- 76.Chiaratti MR, Ferreira CR, Perecin F, Meo SC, Sangalli JR, Mesquita LG et al. Ooplast-mediated developmental rescue of bovine oocytes exposed to ethidium bromide. Reproductive biomedicine online. 2011;22(2):172–83. [DOI] [PubMed]
- 77.Harvey AJ, Gibson TC, Quebedeaux TM, Brenner CA. Impact of assisted reproductive technologies: a mitochondrial perspective of cytoplasmic transplantation. Curr Top Dev Biol. 2007;77:229–49. doi: 10.1016/S0070-2153(06)77009-0. [DOI] [PubMed] [Google Scholar]
- 78.Acton BM, Lai I, Shang X, Jurisicova A, Casper RF. Neutral mitochondrial heteroplasmy alters physiological function in mice. Biol Reprod. 2007;77:569–76. doi: 10.1095/biolreprod.107.060806. [DOI] [PubMed] [Google Scholar]
- 79.Moini H, Packer L, Saris NE. Antioxidant and prooxidant activities of alpha-lipoic acid and dihydrolipoic acid. Toxicol Appl Pharmacol. 2002;182:84–90. doi: 10.1006/taap.2002.9437. [DOI] [PubMed] [Google Scholar]
- 80.Yi X, Maeda N. Endogenous production of lipoic acid is essential for mouse development. Mol Cell Biol. 2005;25:8387–92. doi: 10.1128/MCB.25.18.8387-8392.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Santos-Ocana C, Do TQ, Padilla S, Navas P, Clarke CF. Uptake of exogenous coenzyme Q and transport to mitochondria is required for bc1 complex stability in yeast coq mutants. J Biol Chem. 2002;277:10973–81. doi: 10.1074/jbc.M112222200. [DOI] [PubMed] [Google Scholar]
- 82.Mitchell P. The protonmotive Q cycle: a general formulation. FEBS Lett. 1975;59:137–9. doi: 10.1016/0014-5793(75)80359-0. [DOI] [PubMed] [Google Scholar]
- 83.Bentinger M, Brismar K, Dallner G. The antioxidant role of coenzyme Q. Mitochondrion. 2007;7(Suppl):S41–50. doi: 10.1016/j.mito.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 84.Noia G, Romano D, Santis M, Mariorenzi S, Caruso A, Mancuso S. Coenzyme Q10 fetal plasma levels. Fetal Diagn Ther. 1998;13:127–30. doi: 10.1159/000020820. [DOI] [PubMed] [Google Scholar]
- 85.Compagnoni G, Giuffre B, Lista G, Mosca F, Marini A. CoQ10 plasmatic levels in breast-fed infants compared to formula-fed infants. Biol Neonate. 2004;86:165–9. doi: 10.1159/000079393. [DOI] [PubMed] [Google Scholar]
- 86.Pignatti C, Cocchi M, Weiss H. Coenzyme Q10 levels in rat heart of different age. Biochem Exp Biol. 1980;16:39–42. [PubMed] [Google Scholar]
- 87.Mas E, Mori TA. Coenzyme Q(10) and statin myalgia: what is the evidence? Curr Atheroscler Rep. 2010;12(6):407–13. [DOI] [PubMed]
- 88.Quinzii CM, Hirano M, DiMauro S. CoQ10 deficiency diseases in adults. Mitochondrion. 2007;7(Suppl):S122–6. doi: 10.1016/j.mito.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marriage BJ, Clandinin MT, Macdonald IM, Glerum DM. Cofactor treatment improves ATP synthetic capacity in patients with oxidative phosphorylation disorders. Mol Genet Metab. 2004;81:263–72. doi: 10.1016/j.ymgme.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 90.Burns J, Yokota T, Ashihara H, Lean ME, Crozier A. Plant foods and herbal sources of resveratrol. J Agric Food Chem. 2002;50:3337–40. doi: 10.1021/jf0112973. [DOI] [PubMed] [Google Scholar]
- 91.Casper RF, Quesne M, Rogers IM, Shirota T, Jolivet A, Milgrom E, et al. Resveratrol has antagonist activity on the aryl hydrocarbon receptor: implications for prevention of dioxin toxicity. Mol Pharmacol. 1999;56:784–90. [PubMed] [Google Scholar]
- 92.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–68. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci USA. 2008;105:9793–8. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]