Abstract
Purpose
We aimed to analyse the in vitro fertilization-embryo transfer (IVF-ET) outcomes of the patients with sleep disturbances who were administered melatonin.
Methods
A total of 60 patients with sleep disturbances were divided into two groups. The study group (group A, n = 30) had underwent the IVF-ET with melatonin administration and the control group (group B, n = 30) without melatonin. Sleeping status after melatonin administration and the IVF outcomes were compared between the two groups.
Results
Sleeping status change was not significant (p > 0.05). The mean number of the retrieved oocytes, the mean MII oocyte counts, the G1 embryo ratio were significantly higher in the melatonin administered group (group A) than that the non-administered group (group B); p = 0.0001; p = 0.0001; p < 0.05 respectively.
Conclusion
IVF patients with sleep disorders may benefit from melatonin administration in improving the oocyte and the embryo quality, but the sleeping problem itself may not be fixed.
Keywords: Melatonin, IVF, Sleep disorders
High levels of psychological stress, anxiety, insomnia, depression, and sexual function changes have been reported during in vitro fertilization–embryo transfer (IVF-ET) [1–6]. These are related to a number of stresses that are the results of both the diagnosis and treatment of infertility [7]. Furthermore, IVF is usually the final treatment option for infertile couples, and the unpredictable outcome of the IVF treatment may induce a depressive mood [3, 5, 7].
Insomnia and depression share a complex theoretical and clinical relationship. It is estimated that 60% of adults meeting the criteria for major depressive disorders (MDDs) complain of insomnia, while approximately 10%–20% of individuals diagnosed with insomnia meet the criteria for MDDs [8]. Sleep disturbances are a frequent but underestimated symptom in IVF patients and accompany the pronounced psychological problems [9].
Melatonin, or N-acetyl-5-methoxytryptamine, is mainly produced in the mammalian pineal gland and possesses multifunctional bioactivities, including anti-oxidative, anti-inflammatory, anti-apoptotic, endocrinologic, and behavioral effects. It is also the primary synchronizing agent of the circadian sleep pattern [10].
Besides melatonin improving disturbed sleep; it acts as a free radical scavenger and affects the redox status of tissues, the association of reactive oxygen species (ROS) with tissue injury under different circumstances may be mitigated with melatonin [11–13]. This protective effect of melatonin against free radical damage has been widely analyzed, and it is used for many diseases other than insomnia, including cancer, diabetes, viral infections, and Alzheimer’s disease [14–16].
The positive effect of melatonin on the reproductive system is well documented [17–25].
In this study, we aimed to search the effect of melatonin on an infertile group of patients with sleep disturbances. The IVF-ET outcomes of these patients were studied.
Materials methods
The present study is a prospective, single-center, randomized controlled clinical trial assessing the impact of melatonin administration on women undergoing IVF. This study was conducted between January 2010 and December 2010 in Zekai Tahir Burak Women’s Training and Research Hospital, IVF Department, Ankara, Turkey. All of the patients seen in the IVF Department are routinely evaluated by a psychologist or, if needed, a psychiatrist. They are evaluated and treated for sexual function, depression, sleep dysfunction, psychological stress, and anxiety.
Patient selection
A group of 66 IVF patients (median age: 30; range: 24–38 years) who were diagnosed to be in a disturbed sleep status by the psychologist were eligible for inclusion in this trial. Three patients were excluded before randomization due to refusal to participate or a history of more than 1 total fertilization failure cycles. After randomization, three others were excluded from the study population due to either incorrect ingestion of the melatonin or cancellation of the IVF cycle. Thus, 60 patients completed the study. All of the patients were being followed with the diagnosis of unexplained infertility, which was defined according to the World Health Organization (WHO) as those couples without any ovulatory problem, together with a normal hysterosalpingography or laparoscopy and a normal semen sample [26].
Exclusion criteria were existence of chronic drug usage, history of total fertilization failure cycles, hypertension, diabetes mellitus, uterine myoma and/or ovarian cyst, and smoking. The local ethical committee approved the study and written informed consent was taken from all of the patients before randomization.
Diagnosis of insomnia
Diagnosis of insomnia is primarily based on medical history, and the Pittsburg Sleep Quality Index (PSQI) was the main source utilized in analyzing the sleep disturbances [27]. The PSQI is a self-rated questionnaire assessing the individual’s perception of sleep quality. It consists of 19 questions that are grouped into seven components reflecting different aspects of sleep. Each question is scored on a scale ranging from 0 to 3, with 0 indicating the absence of sleep complaints and 3 indicating the most severe complaints. The seven components are then added to form the global score of the PSQI, ranging from 0 to 21, with higher scores indicating worse sleep quality. Scores >5 on the global score are a reflection of severe sleep problems. The PSQI is a reliable measure, with a test–retest correlation of 0.87 for the global scores and of 0.53–0.88 for the component scores for patients with primary insomnia. Good evidence of validity has been reported [27]. During the evaluation of these patients, the Turkish adaptation of this index was used [28].
Study design
Patients were divided into two groups according to a randomization table. The study group (Group A, n = 30) underwent IVF-ET with melatonin administration, while the control group (Group B, n = 30) underwent IVF-ET without melatonin. Daily melatonin dosage was 3 mg orally, taken at 22:00–23:00 pm, in accordance with the studies of Tamura et al. and Simpson et al. [24, 29]. We used the preparation Melatonina 3 mg tablets (Przedsiebiorstwo Farmaceutyczne, Zakroczym, Polland). Administration of melatonin had begun from the 3rd to the 5th day of the menstrual cycle until the human chorionic gonadotropin (hCG) injection day of the controlled ovarian hyperstimulation. We did not use a placebo in the control group due to logistic restraints. Re-analysis of the sleeping status was performed on the day after the embryo transfer. Duration of induction, the required total gonadotropin doses, the number of follicles with a diameter ≥16 mm, peak E2 level, endometrial thickness on the day of hCG injection, fertilization failure rate, number of oocytes retrieved, metaphase II (MII) oocyte ratio, grade I (GI), grade II (GII), and grade III (GIII) embryo rates, fertilization, implantation, and the clinical pregnancy rates were compared between the two groups. Our primary outcome measure was oocyte quality.
Stimulation protocol
All patients received a standard gonadotropin-releasing hormone (GnRH) agonist (leuprolide acetate) regimen starting on Day 21 of a spontaneous menstrual cycle. Leuprolide acetate (0.5 mg Lucrin, daily injection, Abbott, Istanbul) was administered for 10–14 days until complete pituitary desensitization was documented. Recombinant follicle-stimulating hormone stimulation (150 IU, recFSH) was initiated on the third day of subsequent withdrawal bleeding, and at that time, the dose of leuprolide acetate was decreased to 0.25 mg/day. Further recFSH doses were determined according to the standard criterion for follicular maturation assessed by ultrasound and serum E2 measurements. 250 μg recombinant hCG (r-hCG) (Ovitrelle, Merck Serono, Italy) was administered when at least three follicles had reached a diameter of 18 mm. Oocyte retrieval was performed 36 h later under transvaginal ultrasound guidance and intravenous (i.v.) sedation. All patients received luteal phase support of 90 mg/day of vaginally administered progesterone (Crinone gel, Merck Serono, Central Pharma, UK) starting from the day after oocyte retrieval. Embryo quality was assessed before embryo transfer, and a maximum of three embryos were transferred to each patient on the third day of oocyte retrieval using an Edwards Wallace catheter (Simcare Ltd, UK) under transabdominal sonographic guidance.
Luteal phase support was started on the day of oocyte pick-up with daily vaginal administration of 90 mg progesterone (Crinone gel, Merck Serono, Central Pharma, UK). Biochemical pregnancy was established when serum β-hCG level was 20 IU/L or more on the 12th day after embryo transfer, and clinical pregnancy was defined as the presence of a gestational sac on ultrasound performed at 6 weeks after embryo transfer.
Outcome variable and statistical analysis
When unexplained infertility prevalence of 20.7% among the infertile population [30] and 57.8% fertilization rate of unexplained infertile patients in IVF [31] were taken on reference, with a 95% confidence, an alpha of 0.05 and 85% power the sample size was calculated as 24 cases and 24 controls. 30 cases and 30 controls were evaluated for this study.
Statistical analysis was carried out using the Statistical Package for the Social Sciences software (SPSS, version 11.5). Data were presented as either median (range) or mean ± SD as appropriate. All variables were tested for normal distribution with Kolmogorov-Smirnov test, histogram, and P-P plots. Variables were compared with either independent samples t-test or Mann-Whitney U test depending on the normality of the data. All categorical variables were compared with Pearson chi-square and Fisher’s exact tests. A p value < 0.05 was considered as statistically significant.
Results
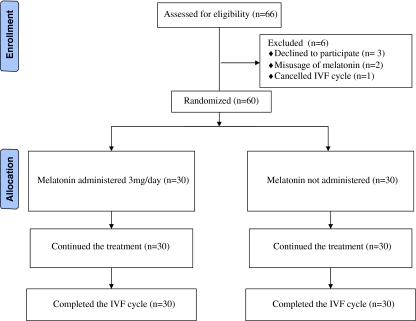
The flowchart of the randomized trial is presented in Fig. 1. Three patients were excluded before randomization due to refusal to participate or a history of more than one total fertilization failure cycles. After randomization, three others were excluded due to either incorrect melatonin ingestion or their cancellation of the IVF cycle. Thus, 60 patients completed the study. There was no difference in demographic and biochemical data between groups (Table 1).
Fig. 1.
Flow diagram of the patients who received melatonin and not received melatonin
Table 1.
Characteristics and outcome of patients who received melatonin (Group A) and not received (Group B) melatonin
| Group A (n = 30) | Group B (n = 30) | P value | |
|---|---|---|---|
| Age (years) | 30.1 ± 4.0 | 29.9 ± 3.6 | NS |
| BMI (kg/m²) | 25.7 ± 4.5 | 26.1 ± 3.5 | NS |
| Infertility duration(years) | 7.5 ± 3.9 | 6.4 ± 3.7 | NS |
| Duration of stimulation(days) | 11.1 ± 2.3 | 11.1 ± 1.9 | NS |
| Total gonadotropin used(IU) | 2323.4 ± 1454.3 | 2429.0 ± 1570.7 | NS |
| E2 level on hCG day(pg/mL) | 2206.4 ± 1158.3 | 1855.3 ± 984.2 | NS |
| Endometrium thickness on hCG day | 10.5 ± 1.9 | 10.2 ± 1.7 | NS |
Values are expressed as mean ± SD
NS not significant
The demographic data of the women who did (Group A) and did not (Group B) receive melatonin are summarized in Table 1. There were no significant differences between the two groups with respect to mean age, body mass index (BMI), the duration of infertility, total days of gonadotropin administration, the required total gonadotropin doses, peak 17β E2 levels, and the endometrial thickness on the day of hCG administration (p > 0.05).
Based on the re-analysis of the sleeping status in Group A (melatonin), 7 of 30 patients scored <5 (23.3%), while in Group B (no melatonin), 2 of the 30 patients scored <5 at the end of the treatment period (6.6%). The difference with respect to the improved scored between patients in Groups A and B was not statistically significant (p > 0.05).
The clinical outcomes of the two groups are shown in Table 2. The difference between the rates of the total fertilization failure between the groups was insignificant (p > 0.05). The mean number of the retrieved oocytes was significantly higher in the melatonin-administered group (Group A) than in the non-administered group (Group B) (11.5 ± 6.3 vs 6.9 ± 3.8; p = 0.0001). The mean MII oocyte counts were 9.0 ± 5.6 for Group A and 4.4 ± 3.3 for Group B (p = 0.0001). The transfer ratio of G1 embryos was also significantly higher in Group A than in Group B (69.3 vs 44.8, respectively; p < 0.05). The fertilization, implantation and clinical pregnancy rates were all similar between the two groups (p > 0.05).
Table 2.
Oocyte maturity and embryo score in patients who received melatonin (group A; n = 30) and not received melatonin (group B)
| Group A (n = 30) | Group B (n = 30) | P value | |
|---|---|---|---|
| Fertilization failure rate (%) | 10.0 | 17.5 | NS |
| No. of follicles ≥16 mm | 7.2 ± 4.5 | 5.5 ± 2.7 | NS |
| No. of retrieved oocytes | 11.5 ± 6.3 | 6.9 ± 3.8 | 0.0001 |
| No. of MII oocytes | 9.0 ± 5.6 | 4.4 ± 3.3 | 0.0001 |
| MII oocyte ratio (%) | 79.6 | 62.3 | 0.0001 |
| Fertilization rate (%) | 51.3 | 44.9 | NS |
| No. of embryos transfered | 1.2 ± 0.7 | 0.9 ± 0.5 | NS |
| Grade I embryo (%) | 69.3 | 44.8 | 0.05 |
| Grade II embryo (%) | 20.4 | 39.4 | NS |
| Grade III embryo (%) | 10.3 | 15.8 | NS |
| Fertilization rate (%) | 51.3 | 44.9 | NS |
| Implantation rate (%) | 28.5 | 27.0 | NS |
| Clinical pregnancy rate (%) | 24.4 | 24.3 | NS |
Values are expressed as mean ± SD. The embryos were scored according to Veeck’s criteria [40]
MII metaphase II; NS not significant
Discussion
Since assisted reproductive treatment (ART) is the final treatment option for infertile couples and requires daily hormone injections, ultrasound scans, semen analysis, and invasive procedures, high levels of psychological stress, anxiety, insomnia, depression, and sexual function changes are seen. Sleep disturbances that characterize insomnia, including difficulty initiating and maintaining sleep, early morning awakenings and excessive somnolence are frequent but underestimated symptoms in IVF patients.
In addition to melatonin being the primary synchronizing agent of the circadian sleep pattern, it also acts as a free radical scavenger by modulating expression of antioxidants and decreasing pro-apoptotic proteins, such as Bax [10–13]. Another use of melatonin as a drug to prevent free radical damage has been widely investigated in infertility treatments [11, 12, 23, 24].
Hemadi et al. concluded the improving effect of melatonin on the primordial follicles of the ovarian graft, as it facilitates their development to the next morphological stage [23]. Tamura et al. studied the melatonin effect on oocyte quality, quality of the cumulus-oocyte complex and the fertilization rate, and found it to have a favorable impact in IVF-ET patients. In his study, intrafollicular concentrations of 8-hydoxy-2′-deoxyguanosine (8-OHdG), which shows damaged DNA products, was found to be reduced with melatonin administration at a dose of 3 mg/day [24]. In a study of culture medium, melatonin was found to support mouse fertilization and early development of embryonic tissue [17]. Abecia et al. had shown similar results in his culture study, in which melatonin improved the development of thawed blastocysts with a higher hatching rate after 24 h [32]. However, some investigations have reported the negative effect of melatonin on the reproductive system. Karakaş et al. reported that melatonin hormone administration plays an inhibitory role on follicular development in female Syrian hamsters [33]. Reiter et al. found that when melatonin is continuously available, it counteracts the anti-gonadotrophic influence of the pineal gland in light-restricted or blinded hamsters [34]. Furthermore, chronically available melatonin negates the anti-gonadotrophic capability of acute melatonin injections. Forcada et al. reported that melatonin can restore the functionality of the neuroendocrine system, after it has been reduced by senescence [25].
One of the most frequently experienced challenges in IVF patients is the oocyte quality. ROS like OH, O2, and H2O2 were thought to be the responsible factors for disturbing the perifollicular microenvironment and causing the poor oocyte quality [35]. A balance between the ROS production and the antioxidant levels is important for oocyte maturation and fertilization [21]. Melatonin, being one of the free radical scavengers and antioxidant agents, has been found to have a positive effect on the oocyte quality of IVF-ET patients [23, 24, 36, 37]. Hemadi et al. concluded that melatonin has a promoting effect on the quality of the cumulus-oocyte complex [23]. The distribution of the follicle cells covering the oocyte was observed to be uniform and the mean survival of these follicles was improved with melatonin usage. Rizzo et al. studied the melatonin effect together with myo-inositol and folic acid, and they showed that the quality of the oocytes improved in IVF patients using melatonin, myoinositol and folic acid [36]. Tamura et al. also documented that melatonin increased the fertilization and pregnancy rates in patients with previous low-quality oocytes [24]. Our study had also supported the promoting effect of melatonin on oocyte quality. The oocytes of the patients who received melatonin had a higher MII ratio than those who did not receive melatonin, which may be the result of the repaired peri-oocyte microenvironment, decreased free radical content and increased antioxidant capacity as well as the concentration. Although melatonin did not correct the sleeping problems of the patients, its administration was useful for improving oocyte quality.
The quality of the embryos has also been found to be affected by ROS production [38]. Higher day 1 ROS levels in the endometrial culture media were related with delayed embryonic development and high fragmentation [39]. In our study, melatonin administration also improved the grades of the transferred embryos. This may again be through the aid of the antioxidant effect of melatonin. The higher quality oocytes were followed by the higher quality embryos in the melatonin-administered patients.
The sleep disturbances among our patients were mostly in the form of difficulties in initiating and maintaining sleep. Subjects estimated sleeping an average of 7.2 h per night, and most subjects reported that they also “sometimes” napped and felt rested during the day. Administration of melatonin throughout the IVF treatment was not successful in the recovery of these sleeping problems. This may be attributed to the fact that infertile patients with these problems may have a more resistant psychological state, or the problem may be heightened with the infertility treatment itself, or the dosage of melatonin used (3 mg/day) was not sufficient.
Our study has several weaknesses that should be mentioned. Because measurement of free radicals in the follicular fluid was not performed, we could not explain the pathophysiology of the antioxidant effect of melatonin. According to our results, we can only suggest that IVF patients with sleep disorders may benefit from melatonin administration for improving the oocyte and embryo quality. As the number of subjects in this study is small, we recommend further multicentric studies with larger numbers of subjects for further verification of the results of our trial, elucidation of the long-term consequences of the teratogenic effects and clarification of the suitable melatonin doses. The assessment and treatment of insomnia-related complaints and psychological counselling should be included in any overall plan of care designed to optimize quality of life as well as clinical outcome in IVF patients.
Acknowledgments
Conflicts of interest We confirm that the submitting authors have no conflicts of interest to declare.
Disclosure statement We declare that that this article has not been published elsewhere; it is not being considered for publication elsewhere; and it has been submitted with the full knowledge and approval of the institution or organization given as the affiliation of the authors.
Footnotes
Capsule Melatonin may be helpful in fixing the quality problems of the ocyte in IVF patients who suffer from sleep disturbances.
References
- 1.Pal L, Bevilacqua K, Zeitlian G, Shu J, Santoro N. Implications of diminished ovarian reserve (DOR) extend well beyond reproductive concerns. Menopause. 2008;15(6):1086–1094. doi: 10.1097/gme.0b013e3181728467. [DOI] [PubMed] [Google Scholar]
- 2.Thierring P, Beaurepaire J, Jones M, Sounders D, Tennant C. Mood state as a predictor of treatment outcome after in vitro fertilization/embryo transfer technology (IVF/ET) J Psychosom Res. 1993;37:481–491. doi: 10.1016/0022-3999(93)90004-Y. [DOI] [PubMed] [Google Scholar]
- 3.Cousineu TM, Domar A. Psychological impact of infertility. Best Pract Res Clin Obstet Gynecol. 2007;21:293–308. doi: 10.1016/j.bpobgyn.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Demyttenaere K, Bonte L, Gheldof M, Vervaeke M, Meuleman C, Vanderschuerem D, et al. Coping style and depression level influence outcome in in vitro fertilization. Fertil Steril. 1998;69:1026–1033. doi: 10.1016/S0015-0282(98)00089-2. [DOI] [PubMed] [Google Scholar]
- 5.Kee BS, Jung BJ, Lee SH. A study on psychological strain in IVF patients. J Assist Reprod Genet. 2000;17:445–448. doi: 10.1023/A:1009417302758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Csemiczky G, Landgren BM, Collins A. The influence of stress and state anxiety on the outcome of IVF treatment: psychological and endocrinological assessment of Swedish women entering IVF treatment. Acta Obstet Gynecol Scand. 2000;79:113–118. doi: 10.1034/j.1600-0412.2000.079002113.x. [DOI] [PubMed] [Google Scholar]
- 7.Klonoff-Cohen H, Chu E, Natarajan L, Sieber W. A prospective study of stress among women undergoing in vitro fertilization or gamate intrafallopian transfer. Fertil Steril. 2001;76:675–687. doi: 10.1016/S0015-0282(01)02008-8. [DOI] [PubMed] [Google Scholar]
- 8.Ohayon MM. Insomnia: a ticking clock for depression? J Psychiatr Res. 2007;41(11):893. doi: 10.1016/j.jpsychires.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Isaac F, Greenwood KM. The relationship between insomnia and depressive symptoms: genuine or artifact? Neuropsychiatr Dis Treat. 2011;7:57. doi: 10.2147/NDT.S16267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodson ER, Zee PC. Therapeutics for circadian rhythm sleep disorders. Sleep Med Clin. 2010;5:701–715. doi: 10.1016/j.jsmc.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan DX, Manchester LC, Sainz RM, Mayo JC, Leon J, Hardeland R, et al. Interaction between melatonin and nicotinamide nucleotide: NADH preservation in cells and in cell-free systems by melatonin. J Pineal Res. 2005;39:185–194. doi: 10.1111/j.1600-079X.2005.00234.x. [DOI] [PubMed] [Google Scholar]
- 12.Reiter RJ, Tan DX, Maldenado MD. Melatonin as an antioxidant: physiology versus pharmacology. J Pineal Res. 2005;39:215–216. doi: 10.1111/j.1600-079X.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira ZS, Markus RP. Melatonin inhibits nitric oxide production by microvascular endothelial cells in vivo and in vitro. Br J Pharmacol. 2007;151(2):195–205. doi: 10.1038/sj.bjp.0707225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pappolla MA, Chyan YJ, Poeggeler B, Frangione B, Wilson G, Ghiso J, et al. An assessment of the antioxidant and the antimyloidogenic properties of melatonin: implications for Alzheimer’s disease. J Neural Transm. 2000;107:203–231. doi: 10.1007/s007020050018. [DOI] [PubMed] [Google Scholar]
- 15.Bonilla E, Valero N, Chacin-Bonilla L, Medina-Leendertz S. Melatonin and viral infections. J Pineal Res. 2004;36:73–79. doi: 10.1046/j.1600-079X.2003.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hussain SA, Khadim HM, Khalaf BH, Ismail SH, Hussein KI, Sahib AS. Effects of melatonin and zinc on glycemic control in type 2 diabetic patients poorly controlled with metformin. Saudi Med J. 2006;27:1483–1488. [PubMed] [Google Scholar]
- 17.Ishizuka B, Kuribayashi Y, Murai K, Amemiya A, Itoh MT. The effect of melatonin on in vitro fertilization and embryo development in mice. J Pineal Res. 2000;28:48–51. doi: 10.1034/j.1600-079x.2000.280107.x. [DOI] [PubMed] [Google Scholar]
- 18.Unfer V, Raffone E, Rizzo P, Buffo S. Effect of a supplementation with myo-inositol plus melatonin on oocyte quality in women who failed to conceive in previous in vitro fertilization cycles for poor oocyte quality: a prospective, longitudinal, cohort study. Gynecol Endocrinol. 2011 Apr 5. [Epub ahead of print]. [DOI] [PubMed]
- 19.Berlinguer F, Leoni GG, Succu S, Spezzigu A, Madeddu M, Satta V, et al. Exogenous melatonin positively influences follicular dynamics, oocyte developmental competence and blastocyst output in a goat model. J Pineal Res. 2009;46:383–391. doi: 10.1111/j.1600-079X.2009.00674.x. [DOI] [PubMed] [Google Scholar]
- 20.Vázquez MI, Forcada F, Casao A, Abecia JA, Sosa C, Palacín I. Undernutrition and exogenous melatonin can affect the in vitro developmental competence of ovine oocytes on a seasonal basis. Reprod Domest Anim. 2010;45:677–684. doi: 10.1111/j.1439-0531.2008.01329.x. [DOI] [PubMed] [Google Scholar]
- 21.Tamura H, Nakamura Y, Korkmaz A, Manchester LC, Tan DX, Sugino N, et al. Melatonin and the ovary: physiological and pathophysiological implications. Fertil Steril. 2009;92(1):328–343. doi: 10.1016/j.fertnstert.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 22.Woo MMM, Tai CJ, Kang SK, Nathwani PM, Pang SF, Leung PCK. Direct action of melatonin in human granulosa-luteal cells. J Clin Endocrinol Metab. 2001;86:4789–4797. doi: 10.1210/jc.86.10.4789. [DOI] [PubMed] [Google Scholar]
- 23.Hemadi M, Abolhassani F, Akbari M, Sobhani A, Pasbakhsh P, Ährlund-Richter L, et al. Melatonin promotes the annulus-oocyte complexes quality of vitrified-thawed murine ovaries; with increased mean number of follicles survival and ovary size following heterotropic transplantation. Eur J Pharm. 2009;618:84–90. doi: 10.1016/j.ejphar.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 24.Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res. 2007;44:280–287. doi: 10.1111/j.1600-079X.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- 25.Forcada F, Abecia JA, Casao A, Cebrián-Pérez JA, Muiño-Blanco T, Palacín I. Effects of ageing and exogenous melatonin on pituitary responsiveness to GnRH in ewes during anestrus and the reproductive season. Theriogenology. 2007;67(4):855–862. doi: 10.1016/j.theriogenology.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 26.WHO Laboratory Manual for the examination and processing of human semen. 5. Geneva: World Health Organization; 2010. [Google Scholar]
- 27.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburg Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 28.Agargün MY, Kara H, Anlar O. Pittsburg uyku kalitesi indeksinin geçerliliği ve güvenilirliği. Turk Psikiatri Dergisi. 1996;7:107–115. [Google Scholar]
- 29.Simpson D, Curran MP. Ramelteon: a review of its use in insomnia. Drugs. 2008;68(13):1901–1919. doi: 10.2165/00003495-200868130-00011. [DOI] [PubMed] [Google Scholar]
- 30.Farhi J, Ben-Haroush A. Distribution of causes of infertility in patients attending primary fertility clinics in Israel. Isr Med Assoc J. 2011;13(1):51–54. [PubMed] [Google Scholar]
- 31.Gurgan T, Urman B, Yaralı H, Kisnisci H. The results of in vitro fertilization-embryo transfer in couples with unexplained infertility failing to conceive with superovulation and intrauterine insemination. Fertil Steril. 1995;64(1):93–97. [PubMed] [Google Scholar]
- 32.Abecia JA, Forcada F, Zuniga O. The effect of melatonin on the secretion of progesterone in sheep and on the development of ovine embryos in vitro. Vet Res Commun. 2002;26:151–158. doi: 10.1023/A:1014099719034. [DOI] [PubMed] [Google Scholar]
- 33.Karakaş A, Kaya A, Gündüz B. The effect of pinealectomy, melatonin and leptin hormones on ovarian follicular development in female Syrian hamsters (Mesocricetus auratus) Acta Biol Hung. 2010;61(4):380–390. doi: 10.1556/ABiol.61.2010.4.2. [DOI] [PubMed] [Google Scholar]
- 34.Reiter RJ, Rollag MD, Panke ES, Banks AF. Melatonin: reproductive effects. J Neural Transm Suppl. 1978;13:209–223. [PubMed] [Google Scholar]
- 35.Zuelka KA, Jones DP, Perreault SD. Glutathione oxidation is associated with altered microtubule function and disrupted fertilization in mature hamster oocytes. Biol Reprod. 1997;57:1413–1419. doi: 10.1095/biolreprod57.6.1413. [DOI] [PubMed] [Google Scholar]
- 36.Rizzo P, Raffone E, Benedetto V. Effect of the treatment with myo-inositol plus folic acid plus melatonin in comparison with a treatment with myo-inositol plus folic acid on oocyte quality and pregnancy outcome in IVF cycles. A prospective clinical trial. Eur Rev Med Pharmacol Sci. 2010;14:555–561. [PubMed] [Google Scholar]
- 37.Adriaens I, Jacquet P, Cortvrindt R, Janssen K, Smitz J. Melatonin has dose-dependent effects on folliculogenesis, oocyte maturation capacity and steroidogenesis. Toxicology. 2006;228:333–343. doi: 10.1016/j.tox.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 38.Paszkowski T, Clarke RN. Antioxidant capacity of preimplantation embryo culture medium declines following the incubation of poor quality embryos. Hum Reprod. 1996;11:2493–2495. doi: 10.1093/oxfordjournals.humrep.a019146. [DOI] [PubMed] [Google Scholar]
- 39.Harvey AJ, Kind KL, Thompson JG. REDOX regulation of early embryo development. Reproduction. 2002;123:479–486. doi: 10.1530/rep.0.1230479. [DOI] [PubMed] [Google Scholar]
- 40.Veeck LL. Oocyte assessment and biological performance. Ann NY Acad Sci. 1988;541:259–274. doi: 10.1111/j.1749-6632.1988.tb22263.x. [DOI] [PubMed] [Google Scholar]