Abstract
Purpose
To determine intraindividual variability in concentrations of homocysteine and related thiols in follicular fluids of particular follicles after ovarian stimulation and assess the differences between follicles with/or without oocytes.
Methods
HPLC-FD analysis of plasma and follicular fluid cysteine, homocysteine, cysteinylglycine and glutathione in women undergoing IVF.
Results
In blood plasma, the homocysteine, cysteine, and cysteinylglycine concentrations decreased significantly during stimulation with rec FSH (p < 0.001). We found significant differences in concentrations of cysteine and glutathione between follicles with or without retrieved oocytes. High intraindividual variability in concentrations of thiols was determined.
Conclusions
The concentration variability of thiols between single follicles is very high and we recommend mean at least from 3 follicles with/or without oocytes for characterization of each woman. It is the best to examine individual follicles for further research and analysis of fertility outcomes.
Keywords: Homocysteine, Thiols, Follicular fluid, Oocytes
Introduction
Hyperhomocysteinemia is associated with many pathologies, such as cardiovascular diseases, neurodegenerative disorders (e.g. Alzheimer’s disease), hypothyroidism, birth defects and late pregnancy complications, such as pre-eclampsia, intrauterine grow retardation, preterm birth and intrauterine fetal death and abruption of placenta [1–6].
Both male and female factors are concerned in the causes of human infertility and changes of homocysteine (Hcy) levels in body fluids were observed in fertility disorders in both sexes [7–10].
Exogenous estrogens have been shown to affect several endocrine and metabolic pathways including homocysteine metabolism. Plasma estrogen and progesterone levels are higher in premenopausal women compared with postmenopausal women. Estrogen treatment of post-menopausal women significantly decreases the total Hcy levels [11]. Previous studies showed that the levels are significantly lower in the luteal phase than in the follicular phase of the menstrual cycle of premenopausal women [12]. And moreover, blood plasma total Hcy decreases during pregnancy from preconception onwards [13, 14].
A significant negative correlation between follicular fluid Hcy levels and oocyte and embryo quality was demonstrated in women undergoing assisted reproduction [8]. Ovarian stimulation deranges blood and follicular fluid biomarkers of the Hcy pathway and affects the Hcy concentration in follicular fluid [15]. High ovarian follicular fluid Hcy and folate levels may have detrimental effect on follicular development [17], Hcy inhibits the methylations and has a negative effect on IVF outcome [18]. Elevated Hcy may also suppress the synthesis of estradiol and consequently influence the oocyte maturation and fertilization in women with polycystic ovary syndrome [17, 18]. Because the cystathionine-beta-synthase is only poorly expressed or absent in the human oocyte [19], the remethylation pathway is the only alternative to remove Hcy.
Metabolism of Hcy is closely related to the metabolism of other thiols—cysteine (Cys), cysteinylglycine (CG) and glutathione (GSH). Cysteine generates in the transsulfuration pathway of Hcy metabolism and meets CG and GSH in gamma-glutamyl cycle. GSH is a very potent antioxidant protecting from oxidative stress and through direct conjugation, it can detoxify many organic and inorganic xenobiotics and carcinogens.
The goals of this study were to measure the levels of total Cys, Hcy, CG and GSH in blood plasma and follicular fluids of women undergoing assisted reproduction techniques on the day of oocyte retrieval, assess differences in thiol concentrations between the individual follicles, determine the differences between follicles with and without oocytes, and define recommendations for further analyses.
Materials and methods
Patients and samples
Forty-nine women (22–44 years old) undergoing ovarian stimulation (recFSH, GnRH agonists in long protocol) were enrolled in this study. All of them signed an informed consent for their participation in the experiment. Venous blood samples were drawn from each woman just before FSH application and before oocyte retrieval in vacutainer tubes containing anticoagulant. Blood samples were centrifuged at 2 000 g for 10 min. and plasma samples were stored at −80°C until assayed.
Particular follicular fluid concentrations were examined after the FSH stimulation. After the oocyte retrieval for the IVF or ICSI procedure, samples of follicular fluids from 2–8 follicles were centrifuged separately at 4 000 g for 10 min, then frozen without preservatives and stored at −80°C until analysis. Only follicular fluids without blood contamination were used. The presence of blood was tested by HemoPhan Test stripes (Lachema, Czech Republic). In total, 279 (169 with and 110 without oocyte) follicular fluid samples were collected and further assayed.
Biochemical assays
A method of high-performance liquid chromatography with fluorescence detection (HPLC-FD) and preceeding derivatization was used for determination of thiols in blood plasma and follicular fluid.
We used 25 μl of 0.5 mmol/l N-Acetyl-L-cysteine (Fluka, Switzerland) as an internal standard, which was added in the first step to 100 μl of each sample. Next, 25 μl of 10% solution of Tributylphosphine (TBF; Sigma Aldrich, Canada) in N,N-dimethylformamide (DMF; Sigma Aldrich, Germany) was added. After 30 min reduction at 4°C, samples were deproteinated by 100 μl of 70% (v/v) perchloric acid (PCA; Fluka, Switzerland), 0.6 mol/l PCA containing 1 mmol/l EDTA·Na2·2H2O (Fluka, Switzerland), for 10 min and centrifuged at 21 910 g for 15 min. Subsequently, 25 μl of the deproteinated sample was derivatized with 7-fluorobenzofurazane-4-sulfonic acid (SBD-F; Fluka, Switzerland) for 1 h at 60°C by adding 50 μl of borate buffer (125 mmol/l Na2B4O7 ·10 H2O (Fluka, Germany) and 4 mmol/l EDTA·Na2·2H2O (Fluka, Switzerland), pH 10.1) and 25 μl of SBD-F solution (1 mg/ml of borate buffer; 125 mmol/l Na2B4O7·10 H2O and 4 mmol/l EDTA·Na2·2H2O, pH 9.5). The derivatization was stopped by an ice-bath and the samples were stored at 4°C until the next day HPLC analysis.
20 μl of each derivatized sample was injected on the column. We used a gradient elution at flow rate of 1 ml/min: 0.1 M phosphate buffer, pH 3.0 : acetonitrile (ACN; Scharlau, Spain) 100:3 (v/v) for 1.9 min, 0.1 M phosphate buffer, pH 3.0 : ACN 100:7 (v/v) for another 6.1 min, and 0.1 M phosphate buffer, pH 3.0 : ACN 100:3 (v/v) for last 2 min of analysis.
We applied a solvent delivery system (UFLC prominence liquid chromatograph LC-20 AD, Shimadzu, USA) and fluorescence detector (RF 10AXL, Shimadzu, USA) operating at an excitation wavelength of 385 nm and an emission wavelength of 515 nm. We used the separating column Supelcosil LC-18, 150 × 4.6 mm × 5 μm particle size (Supelco, USA) and the guard column C18, 4 × 3 mm (Phenomenex, USA). Obtained data were analysed with the Clarity chromatography data system (DataApex, Czech Republic). Concentrations of homocysteine were calculated using a calibration curve, which was obtained from homocysteine standard solutions (Sigma Aldrich, England) of concentrations 3.125 μmol/l, 6.25 μmol/l, 12.5 μmol/l, 25 μmol/l, and 50 μmol/l for follicular fluid and 6.25 μmol/l, 12.5 μmol/l, 25 μmol/l, 50 μmol/l and 100 μmol/l for blood plasma. In the same way, the concentrations of other thiols were calculated; the concentrations of standard solutions were 12.5 μmol/l, 25 μmol/l, 50 μmol/l, 100 μmol/l and 200 μmol/l (follicular fluid) and 25 μmol/l, 50 μmol/l, 100 μmol/l, 200 μmol/l and 400 μmol/l (blood plasma) for Cys, 6.25 μmol/l, 12.5 μmol/l, 25 μmol/l, 50 μmol/l and 100 μmol/l (follicular fluid) and 12.5 μmol/l, 25 μmol/l, 50 μmol/l, 100 μmol/l and 200 μmol/l (blood plasma) for CG and 1.5625 μmol/l, 3.125 μmol/l, 6.25 μmol/l, 12.5 μmol/l and 25 μmol/l (follicular fluid) and 3.125 μmol/l, 6.25 μmol/l, 12.5 μmol/l, 25 μmol/l, and 50 μmol/l (blood plasma) for GSH.
All samples were analysed in duplicate in the same run. The limit of detection (signal/noise 5 : 1) was 0.2 μmol/l.
Statistical analysis
Statistical significance of changes from the start of FSH stimulation to the date of follicle puncture was analyzed by means of Wilcoxon paired test. Non-parametric Spearman correlation was used for the analysis of dependance on age and intraindividual variability was measured using the ratio of difference between average value of follicles with and without eggs against range of individual follicles values. The values of mean, median, standard deviation and range of values of follicles were computed from the patient’s follicles and the median supplemented by 5th and 95th percentile of these patient’s characteristics was then computed. Statistical significance of thiols between follicles with and without eggs was analyzed by means of Wilcoxon paired test.
Results
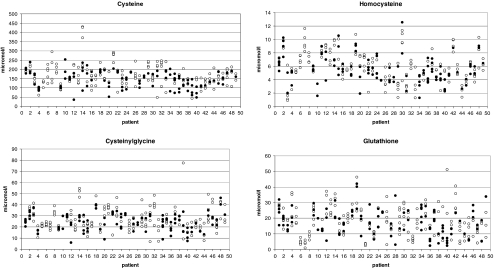
The concentrations of cysteine, homocysteine, cysteinylglycine and glutathione in blood are presented in Table 1. The differences between thiol levels in follicles with or without oocytes are shown in Table 2. Intraindividual variability of thiols in follicular fluid with or without oocytes is presented in Table 3 and individual values of follicular thiols concentrations are shown in Fig. 1.
Table 1.
Concentrations of thiols in blood plasma
| Analyte | Before FSH stimulation (μmol/l) a | After FSH stimulation (μmol/l) a | Differences between concentrations (μmol/l) a | p-value |
|---|---|---|---|---|
| Cys | 242.6 (184.0; 424.4) | 221.0 (148.9; 332.3) | −27.4 (−145.6; 42.7) | < 0.001 |
| Hcy | 10.4 (6.6; 17.0) | 8.1 (5.2; 12.7) | −2.1 (−6.4; 0.8) | < 0.001 |
| CG | 51.8 (31.1; 104.6) | 40.7 (28.9; 54.1) | −10.1 (−58.4; 7.3) | < 0.001 |
| GSH | 8.2 (3.6; 16.2) | 7.4 (3.6; 12.1) | −1.1 (−11.6; 6.5) | 0.251 |
aData are median (5th; 95th percentile)
Table 2.
Differences between concentrations of thiols in follicles with and without oocytes
| Analyte | Follicles without oocytes (μmol/l) b | Follicles with oocytes (μmol/l) b | p-value |
|---|---|---|---|
| Cys | 155.0 | 149.4 | 0.022 |
| Hcy | 6.1 | 5.2 | 0.262 |
| CG | 25.0 | 24.1 | 0.190 |
| GSH | 17.6 | 18.0 | 0.027 |
bData are median
Table 3.
Concentrations of thiols in follicular fluid and intraindividual variability
| Follicles | Mean (μmol/l) c | Median (μmol/l) c | SD (μmol/l) c | Range (μmol/l) c |
|---|---|---|---|---|
| Without oocytes | ||||
| Cys | 157.9 (100.0; 214.1) | 155.0 (95.6; 231.7) | 26.8 (12.7; 79.6) | 56.8 (20.1; 167.6) |
| Hcy | 6.0 (3.2; 9.1) | 6.1 (2.8; 9.1) | 0.9 (0.2; 2.8) | 1.9 (0.5; 5.1) |
| CG | 25.4 (15.9; 43.3) | 25.0 (15.4; 43.3) | 4.6 (1.5; 12. 4) | 9.6 (4.0; 27.0) |
| GSH | 17.0 (4.8; 31.5) | 17. 6 (4.7; 30.5) | 5.3 (0.9; 11.5) | 12.0 (1.5; 21.9) |
| With oocytes | ||||
| Cys | 147.6 (93.0; 211.9) | 149.4 (91.2; 209.4) | 22.2 (1.3; 63.1) | 46.2 (1.9; 138.1) |
| Hcy | 5.2 (2.6; 9.1) | 5.2 (2.5; 9.4) | 0.8 (0.1; 2.8) | 1.8 (0.2; 4.7) |
| CG | 24.1 (13.2; 38.7) | 24.1 (13.2; 39.2) | 3.4 (0.6; 9.7) | 6.4 (0.9; 18.9) |
| GSH | 18.0 (6.1; 33.9) | 18.0 (6.1; 33.9) | 4.2 (0.5; 10.6) | 7.9 (0.8; 20.5) |
cData are median (5th; 95th percentile)
Fig. 1.
Individual values of follicular thiol’s concentrations. Note: ● follicles with an oocyte, ○ follicles without an oocyte
The Hcy, Cys and CG concentrations in blood plasma decreased significantly after the stimulation with rec FSH (2300 ± 600 IU; achieved estradiol level 15.7 ± 11.3 nmol/l) (p < 0.001; Table 1). The decrease of GSH concentration was also observed but this did not reach a statistical significance (p = 0.251).
We found significant differences in Cys (p = 0.022) and GSH (p = 0.027) levels between follicles with or without oocytes (Table 2). The mean ± SD value of Cys in follicles without oocyte was 157.9 ± 26.8 μmol/l with a median of 155.0 μmol/l while the mean ± SD value in follicles with oocyte was 147.6 ± 22.2 μmol/l with a median of 149.4 μmol/l. The mean ± SD value of GSH in follicles without oocyte was 17.0 ± 5.3 μmol/l with a median of 17.6 μmol/l while the mean ± SD value in follicles with oocyte was 18.0 ± 4.2 μmol/l with a median of 18.0 μmol/l. Hcy and CG concentration was higher in follicles without oocyte (mean ± SD = 6.0 ± 0.9 μmol/l and 25.4 ± 4.6 μmol/l) than in follicles containing the oocyte (mean ± SD = 5.2 ± 0.8 μmol/l and 24.1 ± 3.4 μmol/l), but the difference was not statistically significant (p = 0.262 and p = 0.190, respectively).
High concentration variability of all thiols was found in different follicles of each woman, regardless of whether they contained the oocyte or not. Concentrations of thiols in individual follicles of each patient are shown in Fig. 1.
Discussion
Ovarian stimulation deranges blood and follicular fluid biomarkers of the Hcy pathway and affects the Hcy concentration in follicular fluid [15]. It was demonstrated that ovarian stimulation in women undergoing IVF treatment is associated with a decrease of total Hcy in blood. It may be due to estradiol mediated induction of general enzyme activity and in particular activity of cystathionin-β-synthase through which the catabolism of Hcy might be increased [8, 16, 17]. This study also confirmed the decrease of homocysteine in the blood plasma after ovarian stimulation.
We found similar decrease in blood plasma Cys concentration. The mechanism is probably analogous to the Hcy decline and observed decrease of blood plasma CG concentration in the present study appears to be based on the same mechanism. The observed changes in GSH blood levels are not statistically significant. In human organism, GSH is synthesized in a gamma-glutamyl cycle. Except its role as a potent antioxidant, preventing damage of important cellular components caused by reactive oxygen species (ROS), GSH is a substrate in conjugation reactions. To conclude, estradiol mediated induction of enzyme activities can affect both the enzymes of GSH synthesis—gamma-glutamylcysteine synthetase and glutathione synthetase—and enzymes related to GSH function—glutathione S-transferase, glutathione peroxidases and glutathione reductase.
The presence of Hcy in follicular fluid was demonstrated already in 1993 [20]. More recently, new studies assessed the concentration of Hcy in follicular fluid [17, 18] and our levels are a little lower than the published with mean ± SD value of 6.0 ± 0.9 μmol/l and median of 6.1 μmol/l in follicles without oocyte and 5.2 ± 0.8 μmol/l and median of 5.2 μmol/l in follicles containing the oocyte. It was also reported that low follicular fluid Hcy concentration is associated with a higher degree of the oocyte maturation [20] and that there is a significant association between follicular fluid Hcy levels and the grade of oocytes. High follicular fluid Hcy levels are negatively correlated with oocyte fertilization and the quality of embryo, which indicates that follicular fluid Hcy may play an important role in the development of oocytes and fertilization [18]. Hcy is a well-known inhibitor of methylation reactions and this could explain why high Hcy levels negatively affect methylations and consequently the oocyte and embryo development. This effect on methylation reaction should be considered mainly because of the trend to culture early human embryos in medium lacking essential amino acids including methionine. So, methionine is not available during the first days of in-vitro culture when methylation is of major importance [21].
On the other hand, lower concentrations of Hcy in more mature follicles can be due to physiological transudation process, which is more expressed in the latter stages of maturity and can cause dilution of some metabolites.
The presence of other thiols in follicular fluid of oocyte-containing follicles was also already demonstrated [8]. Unfortunately, we cannot compare this data to our results because of different units of concentration. Also, we measured thiols concentrations in follicles without oocytes. The assessed values are 157.9 ± 26.8 μmol/in follicles without oocyte and 147.6 ± 22.2 μmol/l in follicles with oocyte for Cys, 25.4 ± 4.6 μmol/l in follicles without oocyte and 24.1 ± 3.4 μmol/l in follicles with oocyte for CG and 17.0 ± 5.3 μmol/l in follicles without oocyte and 18.0 ± 4.2 μmol/l l in follicles with oocyte for GSH.
This is the first study focused on the intraindividual variability of thiols concentrations in folicullar fluid between single follicles and reveals that this variability, especially in some of patients, is very high. We consider these findings to be the most important. Our recommendation is to observe one and the same oocyte-containing follicle and potencial pregnancy originated from this oocyte for analysis of fertility outcomes. Only when this is not possible, use the mean from at least three oocyte-containing follicles for characterization of each woman. This variability should also be taken into account when evaluating the fertility outcomes of the woman.
Conclusion
Because of the very high intraindividual variability of concentrations of thiols (Hcy, Cys, CG and GSH) in follicular fluids from particular follicles we recommend to examine the individual oocyte-containing follicle for further research and analysis of fertility outcomes. It is practical to use the mean from at least three follicles only if this is not possible.
Acknowledgements
This study was supported by grant no. NS/9661-4 of the Ministry of Health of the Czech Republic.
Footnotes
In the opinion of the authors, the first two authors are “Joint first Authors“.
All authors refuse any commercial interest, financial interest, and/or other relationship with manufacturers of pharmaceuticals, laboratory supplies, and/or medical devices and with commercial providers of medically related services. The study was supported by grant no. NS/9661-4 of the Ministry of Health of the Czech Republic.
Capsule
Study reveals a high intraindividual variability in concentrations of homocysteine and related thiols in follicular fluids. The same follicle should be observed for further analysis.
References
- 1.Unfried G, Greismacher A, Weismuller W, Huber JC, Temper CB. The C677T polymorphism of the methylenetetrahydrofolate reductase gene and idiopathic recurrent miscarriage. Obstet Gyn. 2002;99:614–619. doi: 10.1016/S0029-7844(01)01789-6. [DOI] [PubMed] [Google Scholar]
- 2.Calle M, Usandizaga R, Sancha M, Magdaleno F, Herranz A, Cabrillo E. Homocysteine, folic acid and B-group vitamins in obstetrics and gynaecology. Eur J Obstet Gynecol Reprod Biol. 2003;107:125–134. doi: 10.1016/S0301-2115(02)00305-6. [DOI] [PubMed] [Google Scholar]
- 3.Quablan HS, Eid SS, Ababneh HA, Amarin ZO, Smadi AZ, Al-Khafaji FF, Khader YS. Acquired and inherited thrombophilia: implication in recurrent IVF and embryo transfer failure. Hum Reprod. 2006;21:2694–2698. doi: 10.1093/humrep/del203. [DOI] [PubMed] [Google Scholar]
- 4.Tamura T, Picciano MF. Folate and human reproduction. Am J Clin Nutr. 2006;83:993–1016. doi: 10.1093/ajcn/83.5.993. [DOI] [PubMed] [Google Scholar]
- 5.Makedos G, Papanicolaou A, Hitoglou A, Kalagiannidis I, Makedos A, Vrazioti V, Goutzioulis M. Homocysteine, folic acid and B12 serum levels in pregnancy complicated with preeclampsia. Arch Gyneco Obstet. 2007;275(2):121–124. doi: 10.1007/s00404-006-0223-2. [DOI] [PubMed] [Google Scholar]
- 6.Hoque M, Bulbul T, Mahal M, Islam NF, Ferdausi M. Serum homocysteine in pre-eclampsia and eclampsia. Bangladesh Med Res Counc Bull. 2008;34:16–20. doi: 10.3329/bmrcb.v34i1.1165. [DOI] [PubMed] [Google Scholar]
- 7.Andersen AN, Goosens V, Gianaroli L, Felberbaum R, Mouzon J, Nygren KG. Assisted reproductive technology in Europe, 2003. Results generated from European register by ESHRE. Hum Reprod. 2007;22:1513–1525. doi: 10.1093/humrep/dem053. [DOI] [PubMed] [Google Scholar]
- 8.Ebisch IMW, Peters WHM, Thomas CMG, Wetzels AMM, Peer PGM, Steegers-Theunissen RPM. Homocysteine, glutathione and related thiols affect fertility parameters in the (sub)fertile couple. Hum Reprod. 2006;21:1725–1733. doi: 10.1093/humrep/del081. [DOI] [PubMed] [Google Scholar]
- 9.Boxmeer JC, Smith M, Weber RF, Lindemans J, Romijn JC, Eijkemans MJ, Macklon NS, Steegers-Theunissen RP. Seminal plasma cobalamin significantly correlates with sperm concentration in men undergoing IVF or ICSI procedures. J Androl. 2007;28(4):521–527. doi: 10.2164/jandrol.106.001982. [DOI] [PubMed] [Google Scholar]
- 10.Crha I, Kralikova M, Melounova J, Ventruba P, Zakova J, Beharka R, Husicka R, Pohanka M, Huser M. Seminal plasma homocysteine, folate and cobalamin in men with obstructive and non-obstructive azoospermia. J Assist Reprod Genet. 2010;27:533–538. doi: 10.1007/s10815-010-9458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madsen JS, Kristensen SR, Klitgaard NA, Bladbjerg EM, Abrahamsen B, Stilgren L, Jespersen J. Effect of long-term hormone replacement therapy on plasma homocysteine in postmenopausal women: a randomized controlled study. Am J Obstet Gynecol. 2002;187:33–39. doi: 10.1067/mob.2002.123030. [DOI] [PubMed] [Google Scholar]
- 12.Tallova J, Tomandl J, Bicikova M, Hill M. Changes of plasma total homocysteine levels during the menstrual cycle. Eur J Clin Invest. 1999;29:1041–1044. doi: 10.1046/j.1365-2362.1999.00572.x. [DOI] [PubMed] [Google Scholar]
- 13.Cikot RJ, Steegers-Theunissen RP, Thomas CM, Boo TM, Merkus HM, Steegers EA. Longitudinal vitamin and homocysteine levels in normal pregnancy. Br J Nutr. 2001;85:49–58. doi: 10.1079/BJN2000209. [DOI] [PubMed] [Google Scholar]
- 14.Murphy MM, Scott JM, McPartlin JM, Fernandez-Ballart JD. The pregnancy-related decrease in fasting plasma homocysteine is not explained by folic acid supplementation, hemodilution or a decrease in albumin in a longitudinal study. Am J Clin Nutr. 2001;76:614–619. doi: 10.1093/ajcn/76.3.614. [DOI] [PubMed] [Google Scholar]
- 15.Sato A, Otsu E, Negishi H, Utsunomiya T, Arima T. Aberant DNA methylation of imprinted loci in superovulated oocytes. Hum Reprod. 2007;22:26–35. doi: 10.1093/humrep/del316. [DOI] [PubMed] [Google Scholar]
- 16.Forges T, Monnier-Barbarino P, Alberto JM, Guéant-Rodriguez RM, Daval JL, Guéant JL. Impact of folate and homocysteine metabolism on human reproductive health. Hum Reprod update. 2007;13(3):225–238. doi: 10.1093/humupd/dml063. [DOI] [PubMed] [Google Scholar]
- 17.Boxmeer JC, Steegers-Theunissen RPM, Lindemans J, Wildhagen MF, Martini E, Steegers EAP, Macklon NS. Homocysteine metabolism in the pre-ovulatory follicle during ovarian stimulation. Hum Reprod. 2008;23:2570–2576. doi: 10.1093/humrep/den292. [DOI] [PubMed] [Google Scholar]
- 18.Berker B, Kaya C, Aytac R, Satıroglu H. Homocysteine concentrations in follicular fluid are associated with poor oocyte and embryo qualities in polycystic ovary syndrome patients undergoing assisted reproduction. Hum Reprod. 2009;24:2293–2302. doi: 10.1093/humrep/dep069. [DOI] [PubMed] [Google Scholar]
- 19.Benkhalifa M, Montjean D, Cohen-Bacrie P, Menezo Y. Imprinting: RNA expression for homocysteine recycling in the human oocyte. Fertil Steril. 2010;93:1585–1590. doi: 10.1016/j.fertnstert.2009.02.081. [DOI] [PubMed] [Google Scholar]
- 20.Steegers-Theunissen RPM, Steegers EA, Thomas CM, Hollanders HM, Peereboom-Stegeman JH, Tribels FJ, Eskes TK. Study on the presence of homocysteine in ovarian follicular fluid. Fertil Steril. 1993;60:1006–1010. doi: 10.1016/s0015-0282(16)56401-2. [DOI] [PubMed] [Google Scholar]
- 21.Menezo Y, Elder K, Benkhalifa M, Dale B. DNA methylation and gene expression in IVF. Reprod Biomed Online. 2010;20:709–710. doi: 10.1016/j.rbmo.2010.02.016. [DOI] [PubMed] [Google Scholar]