Abstract
Background
Effects of hydrogen sulfide (H2S), a third gasotransmitter of the gut, are not well understood.
Aim
To determine effects/mechanisms of H2S action on contractile function in rat jejunal muscle.
Methods
Transmural strips of longitudinal muscle were evaluated. Response to sodium hydrosulfide (NaHS, H2S donor; 10−5-10−3M) was studied on spontaneous contractile activity and after precontraction (bethanechol, 3×10−6M). Atropine, propranolol, and phentolamine, tetrodotoxin, capsaicin, L-NG-nitro arginine (L-NNA), and glibenclamide were used to determine mechanisms. L-cysteine (10−4-10−2M; substrate for H2S production) and aminooxyacetic acid and DL-propargylglycine (inhibitors of enzymes generating H2S endogenously) were used to study endogenous production. Aminooxyacetic acid, DL-propargylglycine, L-NNA, and VIP antagonist [D-p-Cl-Phe6,Leu17]-VIP were used to study H2S release during electrical field stimulation (EFS) and interaction with VIP and nitric oxide. Immunohistofluorescence of ileal whole mounts was performed for endogenous H2S-producing enzymes.
Results
Cystathionine-β-synthase and cystathionine-γ-lyase were expressed only in myenteric plexus. NaHS suppressed spontaneous and stimulated contractile activity (p<0.01). Glibenclamide prevented some suppression by NaHS (p=0.01) of stimulated contractile activity but did not prevent suppression of spontaneous contractile activity. Other drugs had no effect on spontaneous contractile activity but increased inhibitory effects of NaHS on spontaneous and stimulated contractile activity (p<0.05). L-cysteine had no effects on contractile activity. Inhibitors altered basal and stimulated activity suggesting endogenous release of H2S.
Conclusions
H2S presumably suppresses contractile activity in jejunum by direct effects on smooth muscle. Mechanism(s) of inhibition remains unclear, because blocking known neurotransmitters enhanced H2S-induced suppression, while blocking ATP-sensitive K+-channels did not block H2S-induced inhibition.
Keywords: ATP-sensitive K+-channels, enteric nervous system, hydrogen sulfide, motility, primary afferent nerve fibers, small intestine
After nitric oxide (NO) and carbon monoxide (CO) were identified as gas “transmitters”, hydrogen sulfide (H2S) was identified as the third “gasotransmitter” [1]. Because gasotransmitters are freely permeable across cell membranes and NO and CO act on intracellular targets, gasotransmitters offer a new paradigm to classic signal transduction by neuronally released “neurotransmitters” which act via membrane-bound receptors. Intracellular mechanisms of signaling are well-studied for NO and CO, but mechanisms mediating effects of H2S are not understood in gastrointestinal tissues.
H2S has been well studied in vascular smooth muscle, where H2S opens ATP-sensitive K+-channels causing hyperpolarization, closing of voltage-gated Ca2+-channels, and muscular relaxation [1,2]. In gastrointestinal smooth muscle, the other gasotransmitters NO and CO, released from the enteric nervous system (ENS), suppress contractile activity. Evidence suggests that H2S is an endogenous gasotransmitter capable of suppressing contractile activity in the gut. Cystathionine-β-synthase (CBS) and cystathionine-γ-lyase (CSE), enzymes which catalyze endogenous production of H2S from L-cysteine [1], are co-expressed in submucous and myenteric plexuses of guinea pig colon and submucous plexus of human colon [3]. Guinea pig ileum incubated with L-cysteine has been reported to generate H2S that is inhibited by antagonists of CBS [4]. In vascular smooth muscle, NO interacts with H2S by amplifying the relaxant effects of H2S, stimulating the activity of CSE, and increasing expression of CSE [2,4-6]. In other tissues, several studies with H2S show excitation of primary afferent nerve fibers in stimulating Cl−-secretion from guinea pig and human colon by an axon reflex [3] and “pro-contractile” effects in rat bladder [7] and guinea pig airways [8].
In the gut, mechanisms of H2S in modulating contractile activity are not understood. In mouse and human colon, H2S suppresses spontaneous contractile activity via ATP-sensitive K+ channels, these effects are inhibited by glibenclamine [9], In guinea pig antrum, concentrations of H2S (<0.3 mM NaHS) increase muscle tension and decrease contractile amplitude; greater concentrations of HsS suppress contractile amplitude but do not affect tension [10]. Both effects appear mediated by potassium channels. Two studies using rat, rabbit, and guinea pig ileum suggested an inhibitory effect of H2S on contractile activity independent of ATP-sensitive K+ channels [4,5]. Gallego et al [9] found suppression of spontaneous activity in mouse jejunum by H2S that was unaffected by inhibiting nitric oxide synthase, neuronal activity by tetrodotoxin, purinergic receptors by PPADS, or primary visceral afferent nerves by capsaicin. The role of potassium channels is not well studied as in stomach [10] and colon [9]. The role of HsS in the control of longitudinal muscle contractility is also not well studied.
Our aim was to determine effects of exogenously applied and endogenously released H2S in rat intestinal smooth muscle. We studied jejunal longitudinal muscle as part of our systematic approach to understanding inhibitory neurotransmitters in all muscular layers of the small intestine. By using targeted antagonists, we explored involvement of the ENS, primary afferent nerve fibers, NO, and direct effects on smooth muscle in the response to H2S. We also evaluated exogenously applied L-cysteine as the substrate for endogenous H2S production and explored expression of CBS and CSE immunohistochemically. Our hypothesis was that H2S acts as an endogenous suppressor of contractile activity in rat jejunal smooth muscle by a direct effect on smooth muscle.
MATERIALS AND METHODS
Animal Use
The Mayo Clinic Institutional Animal Care and Use Committee approved this study.
Recording of Contractile Activity
Male Lewis rats (Harlan Sprague-Dawley, Indianapolis, IN) weighing 275–350 g were used. Responses to exogenous sodium hydrosulfide (NaHS; H2S-donor) and L-cysteine (substrate for endogenous H2S production) were studied in six muscle strips from each of eight rats. Eight other rats were used to study endogenous H2S release during EFS in four muscle strips/rat.
Under anesthesia by inhalation of 2% isoflurane (Abbott Laboratories, North Chicago, IL), and maintained by intraperitoneal sodium pentobarbital (30-50 mg/kg; AmproPharmacy, Arcadia, CA), a jejunal segment just distal to ligament of Treitz was harvested into chilled, modified, Krebs-Ringer’s bicarbonate solution (mmol/L: NaCl 116.4, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 23.8, calcium disodium edetate 0.26, and glucose 11.1) pre-oxygenated with 95% oxygen/5% carbon dioxide (Praxair, Burr Ridge, IL). Full-thickness muscle strips (8 mm long, 2 mm wide) cut in the orientation of the longitudinal muscle were suspended in 10-ml tissue chambers filled with Krebs-Ringer’s bicarbonate solution (37.5°) bubbled continuously with 95% oxygen/5% carbon dioxide. The ends of muscle strips were attached to a hook and a noncompliant force transducer (Kulite Semiconductors Products, Inc., Leonia, NJ) respectively to measure isometric force.
A Grass 7D polygraph (Grass Instrument Co, Quincy, MA) monitored real time contractile activity. Digital data saved onto a personal computer were analyzed later using dedicated software (MP-100A-CE and AcqKnowledge; Biopac Systems, Inc., Goleta, CA).
Experimental Design
After equilibration for 60-80 min with 15-min bath washouts, optimal length (L0) of each muscle strip was achieved by stretching every 10 min to a length beyond which stretching did not increase amplitude or contractile frequency as described previously [11]. Experiments were performed at L0. All strips developed spontaneous contractile activity.
Four muscle strips per rat were subjected to three different, escalating concentrations of NaHS (10−5, 10−4, 10−3 M) for 5-min with bath washout between doses. NaHS effects were completely reversible after washout and no tachyphylaxis occurred when dose responses were repeated. NaHS, a well-established donor of H2S, pH 7.4 and 37.5 °C provides 18.5% of NaHS as H2S in solution [12]. Because NaHS is as effective as bubbling H2S, use of NaHS is easier and allows more accurate estimation of H2S concentration in the bath solution [13]. NaHS (10−4 M and 10−3 M) was also studied 90 s after precontraction with bethanechol (3×10−6 M) with washout between doses.
Thereafter, the effect of 30-min preincubation with selected inhibitory agents (see below) was evaluated on a) spontaneous contractile activity, b) the response to NaHS (10−3M), and c) the response to NaHS (10−4M and 10−3M) given 90 s after precontraction with the cholinergic agonist bethanechol (3×10−6M). Each inhibitory agent was evaluated in each of two muscle strips from 8 rats. All doses were based on prior work with these agents. The following agents were evaluated separately. Tetrodotoxin (TTX; 10−6M) inhibits voltage-gated sodium channels in nerve cells, preventing membrane depolarization and the resultant release of neurotransmitters from the ENS. The combination of atropine (10−7M), phentolamine (10−7M), and propranolol (5×10−6M) were used to establish non-adrenergic, non-cholinergic (NANC) conditions. The NO synthase inhibitor L-NG-nitro arginine (L-NNA; 10−4M) was used to block production of NO. Glibenclamide (10−4M) was used to block ATP-sensitive K+ channel activity. Endogenous generation of H2S from CBS and CSE was inhibited by aminooxyacetic acid (AOAA, 5×10−4M) and DL-propargylglycine (PPG; 10−3M), respectively. The muscle strips were pretreated with capsaicin (10−5M), a transient receptor potential vanilloid receptor 1 (TRPV-1) agonist, for 30 min to desensitize primary afferent nerve fibers [3,8,14-16]; successful desensitization was confirmed, because temporary procontractile effects of capsaicin (10−5 M) were not elicited by repeated capsaicin (10−5 M) 15 and 30 min after the first dose.
In two other muscle strips, responses to L-cysteine (10−4, 10−3, 10−2 M), substrate for endogenous H2S production, was studied for 5min with bath washouts between doses. Because L-cysteine is an agonist of N-methyl-D-aspartate receptors, lack of responses to L-cysteine in presence of the N-methyl-D-aspartate receptor antagonist D-(−)-2-amino-5-phosphopentanoic acid (10−4 M) were confirmed.
In four other strips, EFS at 3, 6, and 50 Hz was evaluated at constant delivered voltage (10 V), pulse width (0.5 msec), and duration (10 s) under NANC conditions (10−7 M atropine, 10−7 M phentolamine, 5×10−6 M propranolol). Between each EFS, 10 min were allowed for spontaneous activity to recover; the bath solution was changed after each series of stimulations. The first series examined EFS under NANC conditions (control conditions). The second series studied EFS in the presence of L-NNA (10−4 M) and the vasoactive intestinal polypeptide (VIP) antagonist [D-p-Cl-Phe6,Leu17]-VIP (10−6 M). After bath washout, the third series studied EFS 15 min after AOAA (5×10−4 M) and PPG (10−3 M). The fourth series studied EFS responses in presence of L-NNA (10−4 M), VIP-antagonist (10−6 M), AOAA (5×10−4 M), and PPG (10−3 M). The VIP-antagonist and L-NNA were used to block the dominant NANC inhibitory neurotransmitters NO and VIP to unmask subtle effects of endogenously released H2S.
After experiments, muscle strips blotted on filter paper were weighed to normalize contractile data/mg weight.
Immunofluorescence Microscopy
Jejunal segments were pinned on Sylgard (Dow Corning Corporate, Midland, MI) and fixed in 4% paraformaldehyde in 0.1 M phosphate buffer for 2 h. After removing the mucosa, 1×1 cm whole mounts cut and washed in phosphate buffered saline (PBS) for 5 min were incubated for 2 h in PBS containing 4% normal goat serum and 0.5% Triton X-100 (blocking solution), washed, and incubated overnight with primary antibody solution containing 4% normal goat serum, 0.5% Triton X-100, and monoclonal mouse anti-CSE antibody (1:300) or anti-CBS antibody (1:1000) (ABNOVA Corp, Taipei City, Taiwan; Catalog Numbers: H00001491-M01 and H00000875-M01, respectively). Antibodies were raised against full or partial recombinant CSE and CBS, respectively and specificity has been confirmed by the manufacturer by single band staining in Western blots and abolished immunoreactivity in iRNA knock down animals. After three 15-min washes in 0.1 M PBS, tissue was incubated with secondary antibody, a Cy3-labeled, goat anti-mouse IgG (1:800) (Jackson ImmunoResearch, West Grove, PA) for 2 h, mounted on slides, dried on a slide warmer, and coverslipped. Micrographs were obtained using a confocal laser scanning microscope (Carl Zeiss LSM 5 Pascal, Version 3.2, Heidelberg, Germany).
Data Analysis
Total contractile activity was evaluated and quantitated as area under the (contractile) curve. Because NaHS decreased amplitude, frequency, and baseline tone, we only quantitated total contractile activity. The effects of NaHS as well as the other antagonists (TTX, NANC conditions, L-NNA, glibenclamide, capsaicin, L-cysteine, and VIP antagonist) on baseline contractile activity were measured for a 5-min period and were compared to baseline activity during an equally long interval immediately before drug administration. For AOAA and PPG, the effect on spontaneous activity was measured for 15 min and compared to the 5 min immediately before antagonists. After bethanechol precontraction, the response to NaHS was measured for the next 5 min and compared to the 90 s of precontraction secondary to the bethanechol directly before NaHS administration and adjusted to the percentage of baseline activity (see below). For NaHS dose-responses and responses to NaHS after precontraction without antagonist, the mean responses of four strips were calculated (Fig. 1). Responses to NaHS in the presence of antagonists were compared to control responses (without antagonist) in the same strips where antagonists were studied to avoid confounding variations in control responses between strips. Responses to NaHS under control conditions did not differ between pairs of strips (Table 1). Responses are given in % change from baseline activity (defined as 0%) and represent contractile activity/5 min or 90 s (whichever appropriate) before drug administration. Positive and negative values represent increases and decreases in total activity, respectively.
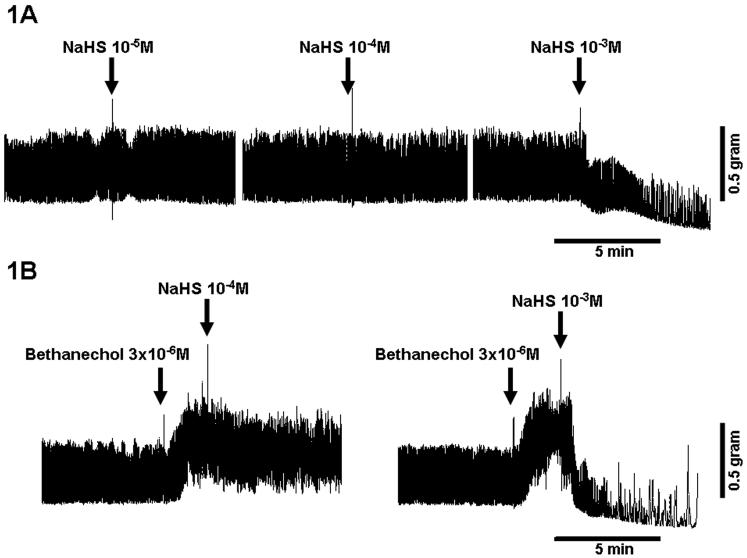
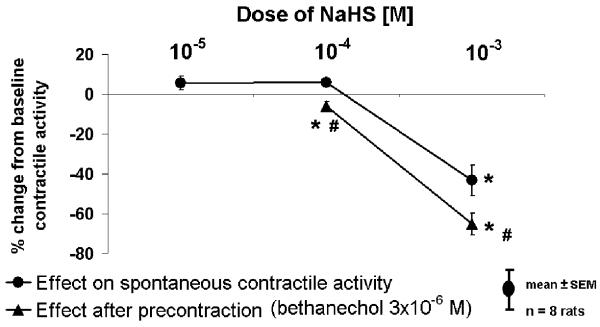
Figure 1.
Effect of NaHS (10−3M) on A) spontaneous contractile activity and on B) bethanechol-stimulated contractile activity. C) Effect of NaHS (10−5-10−3 M) on spontaneous (circles) and stimulated contractile activity (triangles; after precontraction with bethanechol [3×10−6 M]). *p<0.01 compared to baseline contractile activity. #p<0.01 compared to response without precontraction.
NaHS (10−3 M) inhibited spontaneous contractile activity; this effect was more pronounced after precontraction of muscle strips with bethanechol (3×10−6 M). After precontraction, NaHS (10−4 M) caused an inhibitory response.
Table 1.
Effect of NaHS (10−3 M) on spontaneous and stimulated contractile activity (after precontraction with bethanechol (3×10−6 M)) without and with antagonists of pathways potentially involved in the HsS effect
| Antagonist | Effect on spontaneous contractile activity† |
Effect on stimulated contractile activity† |
||||
|---|---|---|---|---|---|---|
| Control response to NaHS |
Response to NaHS with antagonist |
p | Control response to NaHS |
Response to NaHS with antagonist |
p | |
| NANC conditions |
−47±14 | −74±14 * | <0.02 | - | - | - |
| TTX | −34±12 | −81±8 * | <0.01 | −62±7 | −74±3 * | <0.05 |
| Capsaicin | −34±12 | −66±12 * | <0.01 | −62±7 | −69±8 | 0.09 |
| L-NNA | −53±10 | −77±10 * | <0.03 | −69±9 | −80±5 * | <0.01 |
| Glibenclamide | −53±10 | −68±6 | 0.23 | −69±9 | −42±7 * | <0.01 |
Percent change from baseline contractile activity; ; n=8 rats
Differs from control response under same condition (spontaneous or stimulated contractile activity; ANOVA)
NANC: non-adrenergic, non-cholinergic
TTX: tetrodotoxin
L-NNA: L-NG-nitro arginine
The responses to EFS were determined only during the 10-s stimulation; “off contractions” immediately after terminating EFS were not evaluated. We used 3 and 6 Hz as inhibitory and 50 Hz as non-inhibitory EFS frequencies and neuronally mediated EFS-effects were confirmed by preliminary studies using TTX to prevent release of neurotransmitters from the ENS [17,18]. Because early and late EFS responses with antagonists vary [19,20], EFS responses were analyzed as follows: contractile activity over entire 10 s, the first 4 s, and the last 6 s of EFS. Contractile activity was expressed as percent of baseline activity for the 20 s before EFS. The duration of complete inhibition of activity during EFS was evaluated visually.
Data are mean±SEM. Analysis of variance (ANOVA) examined the effects of drugs or EFS on contractile activity or duration of EFS inhibition. Repeated measures ANOVA was used for repeated measurements within the same rat and were treated as repeated factors (e.g. response to NaHS with antagonists or various EFS). When the overall main effect was statistically significant analyzed by ANOVA, post-hoc pairwise comparisons were performed using paired t-tests (for repeated factors) or two-sample t-tests (for independent group factors). T-tests analyzed single comparisons (e.g. versus baseline activity). Bonferroni corrections were applied to multiple comparisons. ANOVA on Ranks evaluated data not normally distributed.
Drugs
AOAA, atropine sulfate, bethanechol, capsaicin, L-cysteine, glibenclamide, L-NG-nitro arginine, phentolamine hydrochloride, PPG, DL-propargylglycine, DL-propranolol hydrochloride, NaHS, TTX, and VIP antagonist were purchased from Sigma-Aldrich (St. Louis, MO) and Triton X-100 from Fisher Scientific (Fair Lawn, NJ). L -cysteine (10−2 M) dissolved in 0.5 N HCl was diluted with 0.1 N HCl, while glibenclamide and capsaicin were dissolved in dimethyl sulfoxide; 0.5 N HCl and dimethyl sulfoxide vehicles did not affect spontaneous activity or bath pH. Other drugs were dissolved in purified water.
RESULTS
Response to Exogenous H2S Donor NaHS
NaHS at a dose of 10−3 M (approximately 180 μM H2S) suppressed spontaneous activity and bethanechol-stimulated contractile activity (p<0.01) by suppressing spontaneous contractions by decreasing amplitude, frequency, and basal tone (Fig 1A); doses of 10−5 and 10−4 M had no measureable effect (Fig. 1C). In pilot studies, doses of 10−2M inhibited contractions entirely and decreased resting tension. Bethanechol precontraction increased the relative suppression by 10−3 M NaHS (p<0.01). After bethanechol precontraction, a lesser dose of NaHS (10−4 M) also caused net suppression (p<0.01).
Neural pathways
Neither NANC conditions nor TTX nor L-NNA affected baseline spontaneous activity (ANOVA, p=NS). NANC conditions increased the suppressing effects of NaHS (10−3 M, p<0.02; ANOVA, Table 1). Similarly, TTX augmented the suppressing effects of NaHS (10−3 M) on spontaneous (p<0.01; ANOVA) and bethanechol precontraction activity (p<0.05; ANOVA). Capsaicin caused a short-lasting excitation in spontaneous contractile activity over the 5-min baseline interval before administering NaHS (18±8%; p=0.07; ANOVA) and also increased the suppressing effects of NaHS (10−3 M) on spontaneous activity (p<0.01; ANOVA), but effects of NaHS on bethanechol-stimulated activity were unchanged (p=0.09; ANOVA) in the presence of capsaicin. As with NANC, TTX, and capsaicin, L-NNA increased the suppressing effects of NaHS (10−3 M) on spontaneous activity and also the bethanechol-stimulated activity (p≤0.03; ANOVA each). L-NNA increased the inhibitory effects of NaHS (10−4M) from −4±4% to −20±5% (p≤0.01; ANOVA); other antagonists at this lesser dose of NaHS had no effect.
ATP-sensitive K+-channels
Glibenclamide alone suppressed spontaneous activity profoundly (−45±3%; p<0.0001; ANOVA); however, glibenclamide did not prevent or alter the subsequent suppressing effects of NaHS (10−3 M) on spontaneous activity (p=0.23; ANOVA). Glibenclamide prevented, but only in part, the suppression of bethanechol-stimulated activity by NaHS (10−3 M; p<0.01; ANOVA), but an overall dominant suppressing response remained (compared to baseline) (p<0.02, ANOVA).
Endogenous H2S Production
L-cysteine did not alter spontaneous activity at any dose administered (10−4 M: −2±3%, 10−3 M: 4±5%, 10−2 M: −4±4%; p=NS; ANOVA); both AOAA and PPG suppressed spontaneous activity (−15±3%; p<0.01).
Response to EFS
The 10-s response to EFS was quantitated as the total (10 s), early (first 4 s), and late (last 6 s) of response according to our previous studies on jejunal muscle [18,19]. EFS at 3 and 6 Hz inhibited contractile activity quantitated for the entire 10 s as well as for first 4 s and last 6 s of EFS (p<0.003; Fig. 2 A, B). Combined application of the VIP-antagonist and L-NNA reversed EFS-induced inhibition over the entire 10 s and last 6 s but did not affect the EFS-induced inhibition for first 4 s, consistent with previous work [18]. When 3 and 6 Hz were evaluated in presence of the inhibitors of the endogenous, HsS-generating enzymes AOAA and PPG, EFS-induced inhibition was unaffected. Combining the VIP-antagonist, L-NNA, AOAA, and PPG blocked inhibition over the entire 10 s and last 6 s of EFS, but AOAA and PPG added no effect over VIP-antagonist and L-NNA alone, and inhibition during first 4 s persisted.
Figure 2.
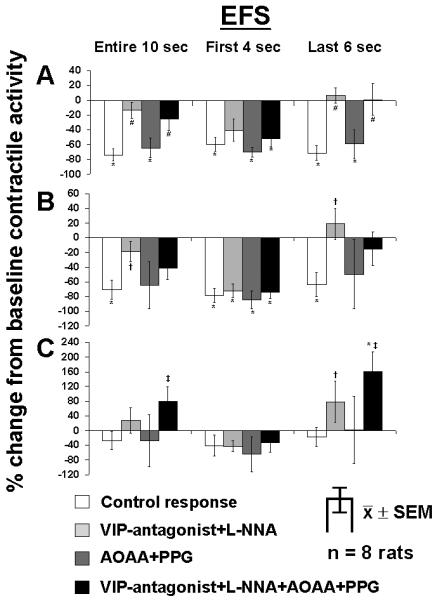
Effect of the VIP-antagonist (10−6 M) combined with L-NNA (10−4 M), the combination of AOAA (5×10−4 M) and PPG (10−3 M), and the combination of all 4 antagonists on the EFS response at A) 3 Hz, B) 6 Hz, and C) 50 Hz for the entire 10 s, the first 4 s, and the last 6 s of EFS. *p<0.05 compared to baseline contractile activity defined as 0% (ANOVA). #p<0.05 compared to control response and response with AOAA and PPG (ANOVA). †p<0.05 compared to control response (ANOVA). ‡p<0.05 compared to all other responses during the same interval (ANOVA).
The combination of the VIP-antagonist and L-NNA without and with AOAA and PPG reversed the EFS-induced inhibition during the entire 10 s and the last 6 s of EFS at 3 and 6 Hz. The VIP-antagonist and L-NNA increased the contractile response at 50 Hz during the entire 10 last 6 s of EFS; the combination of the VIP-antagonist, L-NNA, AOAA, and PPG increased the contractile response during the entire 10 s and the last 6 s of EFS compared to the combination of VIP antagonist and L-NNA
When evaluated at 50 Hz, the VIP antagonist and L-NNA increased net activity both over the entire 10 s and last 6 s of EFS (Fig. 2 C); AOAA and PPG alone had no effect on contractile activity at 50 Hz; however, combining AOAA and PPG with the VIP antagonist and L-NNA increased net activity both over entire 10 s and last 6 s of EFS compared to VIP antagonist and L-NNA alone (p<0.05; Fig. 2 C).
Durations of the initial EFS-induced inhibition under control conditions were lessened by the VIP antagonist and L-NNA together and combined with AOAA and PPG; AOAA and PPG alone, however, had no effect (Table 2).
Table 2.
Effect of VIP-antagonist, L-NNA, AOAA, and PPG on the duration of inhibition during the 10 s of EFS at 3, 6, and 50 Hz.
| Duration of inhibition [s] | ||||
|---|---|---|---|---|
| Frequency | Control response | VIP-antagonist + L-NNA |
AOAA + PPG | VIP-antagonist + L-NNA + AOAA + PPG |
| 3 Hz | 6.0±0.3 | 3.8 ±0.3# | 6.6±0.5 | 4.5±0.5# |
| 6 Hz | 6.6±0.2 | 4.4±0.3# | 7.5±0.7 | 5.2±0.3# |
| 50 Hz | 4.9±0.6 | 3.5±0.4# | 5.9±0.6 | 3.9±0.4# |
Differs from control response and response with AOAA and PPG (p<0.05; ANOVA); there was no difference between VIP antagonists and L-NNA and the combination of all four antagonists.
VIP-antagonist
L-NNA: L-NG-nitro arginine
AOAA: aminooxyacetic acid
PPG: DL-propargylglycine
Immunofluorescence Microscopy
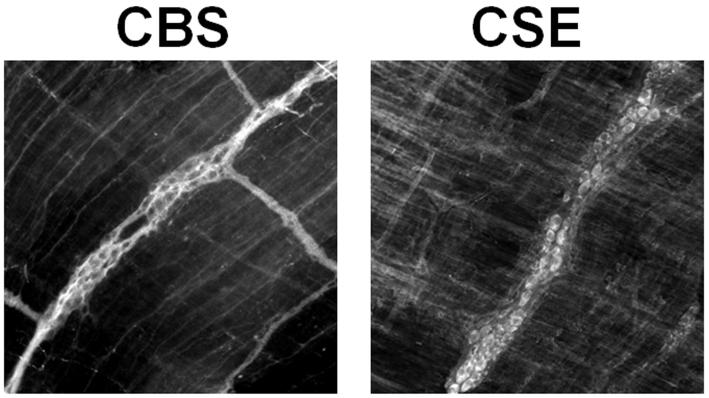
In the myenteric plexus, immunoreactivities for both CBS and CSE were both localized in most cell bodies and fibers of myenteric neurons; smooth muscle cells did not appear to stain (Fig. 3). Neuron-specific staining was confirmed by the typical distribution of cell bodies representing the net-like pattern of the myentric plexus.
Figure 3.
Immunofluorescence for cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) in whole mounts of rat jejunum.
Immunofluorescence for CBS and CSE was present in neurons of the myenteric plexus.
DISCUSSION
Our study was designed to determine effects and mechanisms of action of H2S on contractile activity in rat jejunal longitudinal smooth muscle. Using targeted inhibitors of specific neural pathways, our aim was to delineate potential pathways described previously in other tissues that might mediate the effects of HsS, such as neural pathways, nitrergic pathways, or ATP-sensitive K+-channels. We also explored endogenous H2S production and endogenous release of H2S during EFS, and studied expression (immunohistochemistry) of H2S-producing enzymes CBS and CSE in enteric neurons. In summary, NaHS, a donor of H2S, suppressed contractile activity, an effect that was not mediated via the ENS, primary afferent nerve fibers, or NO; in contrast, ATP-sensitive K+-channels appeared involved, at least in part, only in the NaHS -induced suppression of stimulated contractile activity. Although CBS and CSE were expressed in myenteric neurons, exogenous L-cysteine, at concentrations (10−2M) capable of stimulating H2S synthesis in mouse colonic muscle [21] and guinea pig ileum [4], did not suppress contractile activity by the anticipated endogenous production of H2S. Consistent with the mainly inhibitory actions of endogenous H2S and findings in the rat ileum [5], inhibition of H2S-synthesizing enzymes augmented contractile activity caused by 50 Hz EFS, suggesting H2S is released and under dynamic control during EFS. This said, effects of H2S released during EFS were limited and were not the dominant inhibitory effectors during low frequency EFS, because they were only present after inhibiting the NO and VIP signaling pathways.
To the best of our knowledge, effects of H2S on contractile activity in rat jejunal longitudinal muscle are unexplored. H2S suppressed spontaneous and stimulated contractile activity in rabbit and rat ileum [4,5] and mouse jejunum [22]; however, unlike vascular smooth muscle [2,13], little work has focused on H2S modulation of intestinal contractile activity. We showed suppression of spontaneous and bethanechol-stimulated activity by NaHS in rat longitudinal jejunal muscle with a steep, concentration-response curve. While some reports suggest tissue and blood levels of H2S are at μM concentrations and support the physiologic nature of H2S signaling [1], recent reports question these measurements [23] and suggest resting levels of H2S at nM concentrations. This said, the concentrations of H2S that caused readily reversible responses in our study are consistent with concentrations that cause effects in other systems [4,5] suggesting that physiologic responses to H2S occur at high μM concentrations. In many regards, this finding is consistent with other neurotransmitters (e.g. acetylcholine at nicotinic receptors), and suggest control of localized concentrations of H2S contribute to dynamic H2S signaling, perhaps by local synthesis and release, rather than by synaptic transmission. Under physiologic conditions, 18.5% of NaHS in solution exists as H2S [12]; thus, H2S concentrations in our chamber solution should not have exceeded 185 μM, and in the transmural muscle strips themselves, we assume tissue levels were even less. Therefore, we maintain that the observed NaHS-induced inhibitory responses are a physiologic effect not caused by H2S toxicity; quick, complete recovery of contractile activity after bath washout and repeated applications of NaHS further supports this concept.
Mechanisms mediating H2S relaxation of gastrointestinal smooth muscle are not understood. In vascular smooth muscle, H2S has been well characterized, HsS opens ATP-sensitive K+-channels causing cellular hyperpolarization. Our work and others have studied the role of the ENS when exploring mechanisms of inhibition of gastrointestinal smooth muscle. We used TTX to study H2S-induced effects on global neurotransmitter release from ENS. Because TTX blocks release of neurotransmitters virtually non-selectively and did not prevent H2S-induced suppression, it seems unlikely that H2S induces a neurally mediated suppression of contractile activity, a finding supported by observations that H2S did not evoke Ca2+ mobilization in cultured, enteric neurons [3]. H2S-induced suppression of spontaneous and stimulated contractile activity was augmented by TTX pretreatment, which suggests that the inhibitory effects of H2S apparently generated in myenteric neurons are in part suppressed on an ongoing basis by tonic neural input from ENS on smooth muscle cells despite the lack of any noticeable effect of TTX on spontaneous contractile activity. This observation appears novel and important concerning modulatory control of motility by interaction of H2S and the ENS. Neural pathways mediating this effect appear to involve cholinergic, adrenergic, and nitrergic mechanisms, because effects of exogenous H2S were enhanced by NANC conditions and L-NNA as well.
Most prior, albeit limited, work on effects of H2S on contractile activity of gastrointestinal smooth muscle have concentrated on potassium channels or calcium influx, based on the work in vascular smooth muscle. Recent work reported since completion of our study showed that H2S-mediated relaxation of precontracted gastric fundic muscle in mouse [24] was not mediated by potassium channel activity, neural effects, nitrergic pathways, Na+/K+ ATPase, or nifedipine; rather, this effect appeared to be mediated by activation of myosin light chain phosphatase activity. A similar effect through myosin light chain kinase activity was not, however, seen in mouse distal colonic smooth muscle [25]. These observations reinforce the variations in pathways mediating the effects of HsS in different tissues.
Work in other tissues suggests H2S exerts effects via afferent nerve fibers. Transient receptor potential vanilloid 1 (TRPV-1) receptors on primary afferent nerve fibers mediated the H2S-induced activation of submucous ganglia to stimulate Cl−-secretion in guinea pig and human colon [3] and H2S-mediated pro-contractile effects in rat urinary bladder [7,16] and guinea pig airways [8]. We could find no evidence for involvement of TRPV-1 receptors or primary afferent nerve fibers in rat jejunum, because capsaicin-desensitization had no effect on H2S-induced suppression of contractile activity, consistent with work in jejunum of TRPV-1 knockout mice [22]. Interestingly, capsaicin increased the suppressing effects of exogenous H2S on spontaneous activity, supporting the concept that primary afferent nerve fibers may also modulate contractile activity of gut muscle via the ENS, because these nerve fibers do not synapse on smooth muscle. Differences in H2S effects in gastrointestinal muscle compared to smooth muscle of urinary bladder and respiratory airways further support the diverse mechanisms of H2S in different regions.
Any interaction between H2S and NO in the gut remains poorly understood. In the vasculature, NO regulates H2S production by stimulating H2S production acutely and by increasing CSE activity and up-regulating CSE expression chronically [2,26]. Inhibiting NO release by inhibiting NO-synthase attenuated H2S-induced inhibitory responses in vascular smooth muscle [2]. In the gastrointestinal tract, the NO-donor sodium nitroprusside increased H2S production from rat ileum [6] and amplified the suppression by H2S on EFS-induced excitation in guinea pig ileum [5]; in contrast, inhibiting NO synthase had no effect on H2S-induced suppression of contractile activity in guinea pig ileum [5] or mouse jejunum [9]. Findings in guinea pig ileum are similar to our findings in rat jejunal longitudinal muscle, where blocking endogenous NO production by L-NNA increased the suppressing effects of exogenous H2S on spontaneous and stimulated contractile activity. These observations suggest interaction between NO and H2S-induced suppression of contractile activity; however, we did not study concomitant exogenous NO or involvement of downstream mechanisms of NO signaling, such that the mechanisms of this interaction remain to be elucidated.
In vascular smooth muscle, H2S induces relaxation by hyperpolarizing smooth muscle via opening of ATP-sensitive K+-channels [2]. Although ATP-sensitive K+-channels are expressed in gut smooth muscle cells [22], this pathway did not mediate H2S–induced relaxation in guinea pig ileum [5]; glibenclamide, an antagonist of ATP-sensitive K+-channels, suppressed spontaneous activity in rat jejunal longitudinal muscle but did not prevent or decrease NaHS-induced suppression of spontaneous activity; glibenclamide did prevent, but only in part, the NaHS-induced suppression of stimulated contractile activity, suggesting involvement of ATP-sensitive K+-channels in this effect of NaHS. Given, however, that glibenclamide inhibited spontaneous activity and that NaHS had a greater effect on stimulated contractile activity, the effects of glibenclamide on NaHS-induced inhibition of stimulated contractile activity may have been nonspecific. Even if specific, ATP-sensitive K+-channels were not the major effector mechanism of NaHS, because inhibitory responses persisted after glibenclamide pretreatment, consistent with guinea pig ileum [5] but different from guinea pig antrum [27] and human and rat colon [9]. Whether other K+ channels are involved is unknown as of yet.
In conclusion, we demonstrated that enzymes for endogenous H2S production are expressed in myenteric neurons of rat jejunum. H2S produced and released endogenously appears to act as a gasotransmitter capable of suppressing contractile activity in rat jejunum. ATP-sensitive K+-channels may contribute to a small part to effects of NaHS but only on contractile activity stimulated by a cholinergic agonist. Our work suggests the inhibitory effects of NaHS on smooth muscle contractile activity in rat jejunal longitudinal muscle occurs primarily by an as yet undetermined direct effect on smooth muscle cells not mediated by ATP-sensitive K+ channels but possibly modulated by other mechanisms.
ACKNOWLEDGEMENTS
The authors want to thank Judith A. Duenes for the technical support and Deborah I. Frank for the assistance in the preparation of this manuscript.
Supported by grant DK R01 39337-18 from the National Institutes of Health, United States Public Health Services (MGS) and grant KA 2329/1-1 from the Deutsche Forschungsgemeinschaft, Germany (MSK).
Abbreviations used in this manuscript
- ANOVA
analysis of variance
- ATP
adenosine triphosphate
- AOAA
aminooxyacetic acid
- CBS
cystathionine β-synthase
- CO
carbon monoxide
- CSE
cystathionine γ-lyase
- ENS
enteric nervous system
- HCl
hydrochloric acid
- H2S
hydrogen sulfide
- L-NNA
L-NG-nitro arginine
- NaHS
sodium hydrosulfide
- NANC
non-adrenergic non-cholinergic
- NO
nitric oxide
- PBS
phosphate buffered saline
- PPG
DL-propargylglycine
- TRPV-1
transient receptor potential vanilloid 1
- TTX
tetrodotoxin
- VIP
vasoactive intestinal polypeptide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Wang R. Two’s company, three’s a crowd: Can h2s be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 2.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of h(2)s as a novel endogenous gaseous k(atp) channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schicho R, Krueger D, Zeller F, et al. Hydrogen sulfide is a novel prosecretory neuromodulator in the guinea-pig and human colon. Gastroenterology. 2006;131:1542–1552. doi: 10.1053/j.gastro.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 4.Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- 5.Teague B, Asiedu S, Moore PK. The smooth muscle relaxant effect of hydrogen sulphide in vitro: Evidence for a physiological role to control intestinal contractility. Br J Pharmacol. 2002;137:139–145. doi: 10.1038/sj.bjp.0704858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao W, Ndisang JF, Wang R. Modulation of endogenous production of h2s in rat tissues. Can J Physiol Pharmacol. 2003;81:848–853. doi: 10.1139/y03-077. [DOI] [PubMed] [Google Scholar]
- 7.Patacchini R, Santicioli P, Giuliani S, Maggi CA. Pharmacological investigation of hydrogen sulfide (h2s) contractile activity in rat detrusor muscle. Eur J Pharmacol. 2005;509:171–177. doi: 10.1016/j.ejphar.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Trevisani M, Patacchini R, Nicoletti P, et al. Hydrogen sulfide causes vanilloid receptor 1-mediated neurogenic inflammation in the airways. Br J Pharmacol. 2005;145:1123–1131. doi: 10.1038/sj.bjp.0706277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallego D, Clave P, Donovan J, et al. The gaseous mediator, hydrogen sulphide, inhibits in vitro motor patterns in the human, rat and mouse colon and jejunum. Neurogastroenterol Motil. 2008;20:1306–1316. doi: 10.1111/j.1365-2982.2008.01201.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhao P, Huang X, Wang ZY, et al. Dual effect of exogenous hydrogen sulfide on the spontaneous contraction of gastric smooth muscle in guinea-pig. Eur J Pharmacol. 2009;616:223–228. doi: 10.1016/j.ejphar.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Murr MM, Miller VM, Sarr MG. Contractile properties of enteric smooth muscle after small bowel transplantation in rats. Am J Surg. 1996;171:212–217. doi: 10.1016/S0002-9610(99)80102-0. discussion 217-218. [DOI] [PubMed] [Google Scholar]
- 12.Dombkowski RA, Russell MJ, Olson KR. Hydrogen sulfide as an endogenous regulator of vascular smooth muscle tone in trout. Am J Physiol Regul Integr Comp Physiol. 2004;286:R678–685. doi: 10.1152/ajpregu.00419.2003. [DOI] [PubMed] [Google Scholar]
- 13.Zhao W, Wang R. H(2)s-induced vasorelaxation and underlying cellular and molecular mechanisms. Am J Physiol Heart Circ Physiol. 2002;283:H474–480. doi: 10.1152/ajpheart.00013.2002. [DOI] [PubMed] [Google Scholar]
- 14.Holzer P. Capsaicin: Cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- 15.Holzer P. Capsaicin as a tool for studying sensory neuron functions. Adv Exp Med Biol. 1991;298:3–16. doi: 10.1007/978-1-4899-0744-8_1. [DOI] [PubMed] [Google Scholar]
- 16.Patacchini R, Santicioli P, Giuliani S, Maggi CA. Hydrogen sulfide (h2s) stimulates capsaicin-sensitive primary afferent neurons in the rat urinary bladder. Br J Pharmacol. 2004;142:31–34. doi: 10.1038/sj.bjp.0705764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balsiger BM, Ohtani N, Anding WJ, Duenes JA, Sarr MG. Chronic extrinsic denervation after small bowel transplantation in rat jejunum: Effects and adaptation in nitrergic and non-nitrergic neuromuscular inhibitory mechanisms. Surgery. 2001;129:478–489. doi: 10.1067/msy.2001.112070. [DOI] [PubMed] [Google Scholar]
- 18.Kasparek MS, Fatima J, Iqbal CW, Duenes JA, Sarr MG. Role of vip and substance p in nanc innervation in the longitudinal smooth muscle of the rat jejunum - influence of extrinsic denervation. J Surg Res. 2007;141:22–30. doi: 10.1016/j.jss.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 19.Kasparek MS, Fatima J, Iqbal CW, Sarr MG. Effects of extrinsic denervation on innervation with vip and substance p in circular muscle of rat jejunum. Neurogastroenterol Motil. 2009;20:808–817. doi: 10.1111/j.1365-2982.2008.01083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasparek MS, Linden DR, Kreis ME, Sarr MG. Gasotransmitters in the gastrointestinal tract. Surgery. 2008;143:455–459. doi: 10.1016/j.surg.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linden DR, Sha L, Mazzone A, et al. Production of the gaseous signal molecule hydrogen sulfide in mouse tissues. J Neurochem. 2008;106:1577–1585. doi: 10.1111/j.1471-4159.2008.05502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franck H, Puschmann A, Schusdziarra V, Allescher HD. Functional evidence for a glibenclamide-sensitive k+ channel in rat ileal smooth muscle. Eur J Pharmacol. 1994;271:379–386. doi: 10.1016/0014-2999(94)90797-8. [DOI] [PubMed] [Google Scholar]
- 23.Linden DR, Levitt MD, Farrugia G, Szurszewski JH. Endogenous production of h2s in the gastrointestinal tract: Still in search of a physiologic function. Antioxid Redox Signal. 2010;12:1135–1146. doi: 10.1089/ars.2009.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhaese I, Lefebvre RA. Myosin light chain phosphatase activation is involved in the hydrogen sulfide-induced relaxation in mouse gastric fundus. Eur J Pharmacol. 2009;606:180–186. doi: 10.1016/j.ejphar.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Dhaese I, Van Colen I, Lefebvre RA. Mechanisms of action of hydrogen sulfide in relaxation of mouse distal colonic smooth muscle. Eur J Pharmacol. 2009 doi: 10.1016/j.ejphar.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 26.Wang R. The gasotransmitter role of hydrogen sulfide. Antioxid Redox Signal. 2003;5:493–501. doi: 10.1089/152308603768295249. [DOI] [PubMed] [Google Scholar]
- 27.Dominy JE, Stipanuk MH. New roles for cysteine and transsulfuration enzymes: Production of h2s, a neuromodulator and smooth muscle relaxant. Nutr Rev. 2004;62:348–353. doi: 10.1111/j.1753-4887.2004.tb00060.x. [DOI] [PubMed] [Google Scholar]