Abstract
Recent studies have shown that IL-17 can contribute beneficially to pathogen defense, but also that excessive IL-17 levels are associated with chronic inflammation and autoimmune disorders. So far, the role of IL-17 in viral infections and type 1 diabetes is ambiguous. In this study, we used IL-17A eGFP bicistronic reporter mouse strains to analyze in situ production of IL-17A. Upon Klebsiella pneumonia bacterial infection, CD4+ and γδ T cells produce IL-17A. In contrast, CD4+ or CD8+ T cells do not produce IL-17A in response to acute or protracted viral infection with lymphocytic choriomeningitis virus (LCMV), or during autoimmune diabetes development in the CD8-driven LCMV-induced model of type 1 diabetes. We conclude that viral elimination and type 1 diabetes can occur in the absence of detectable IL-17A production, suggesting IL-17A is not essential in these settings.
Free-form keywords: Type 1 diabetes, viral infection, Th17, Tc17, reporter
Introduction
Upon activation by antigen-presenting cells, CD4 helper T cells undergo clonal expansion and differentiation into cytokine-secreting effector T cells. Besides Th1, Th2 and Tfh, a subset of polarized effector cells, Th17, is characterized by the production of interleukin (IL)-17 and other cytokines (1). IL-17 producing CD8 T cells (Tc17) have also been described and shown to harbor colitogenic properties (2). We have begun to appreciate the importance of Th17 in host defense against certain pathogens (3), especially in bacterial (e.g. Klebsiella pneumonia (4)) and fungal (e.g. Candida albicans (5)) infections. IL-17 can be protective by initiating granulopoiesis and orchestrating neutrophil trafficking (6). Data on the role of IL-17 in anti-viral immunity on the other hand is rather limited. For example, following primary challenge with influenza A, Th17 and Tc17 effector cells are found in the lung and blocking IL-17 during influenza A infection increases weight loss and reduces survival (7). Moreover, IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge (8). On the contrary, Th17 cells can also contribute to viral persistence, as is the case for Theiler's murine encephalomyelitis virus (9). For other viruses, the data at best suggest an indirect link between IL-17 and infection. As such, during the immune response to LCMV (Lymphocytic Choriomenigitis Virus) infection, cytotoxic T cells produce IFNγ and TNFα, but CD4 T cell-derived IL-21 (10) is crucial to control LCMV infection (11–13). IL-21 can initiate and amplify Th17 differentiation in vitro (14, 15), but it remains to be seen whether IL-17 is also produced during LCMV infection.
Unregulated Th17 responses or overwhelming IL-17 production from T cells and other sources is associated with chronic (autoimmune) inflammation and severe immunopathologic conditions (16) with evidence that Th17 cells are involved in rheumatoid arthritis, psoriasis, multiple sclerosis (17)and inflammatory bowel disease. Recent studies also provide evidence of increased frequencies of IL-17-secreting cells in the lymphocyte population of longterm T1D patients (18) as well as children with T1D (19). Studies in mouse models of type 1 diabetes report conflicting results. In an early study, Vukkadapu et al. showed that levels of serum IL-17 are elevated in very young NOD mice, but not at later ages or at diabetes onset (20). In contrast, recently it was shown that the levels of IL-17A/F transcripts increase as diabetes develops (21). Th17 cells are also elevated in the gut of young NOD mice, a phenomenon that can be ended by an anti-diabetogenic diet (22). Blockade of IL-17A by anti-IL-17A mAb delays diabetes in NOD when administered late prediabetic (10 weeks), but not at young age (23). Moreover, Jain et al. (24) reported that treatment with a fusion protein consisting of IgG and GAD peptide 206–220 confers diabetes protection to hyperglycemic NOD mice, correlating with a reduced number of IL-17-producing cells present in the spleen and induction of IFNγ-producing cells. While these data support a role for IL-17 in type 1 diabetes etiology or pathogenesis, data from transfer models shed a different light on this. Th17 generated from ‘islet-specific’ BDC2.5 TCR transgenic CD4 T cells induce diabetes but convert to Th1 upon transfer to NOD/SCID (21, 25). Interestingly, blockade of IFNγ but not IL-17A delayed Th17 transfer-induced diabetes (25), indicating that IL-17 is not essential for the diabetogenic properties of the transferred BDC2.5 effector cells. Recent studies even show a protective role for IL-17 producing γδ T cells in NOD mice (26). Also, transfer of bulk splenocytes isolated from CFA-treated NOD mice and activated in the presence of TGF-β and IL-6 delayed diabetes in the NOD/SCID transfer model (27). Similarly, IL-17–producing CD8+ T cells differentiated with TGF-β and IL-6 are not diabetogenic, whereas IL-23–treated cells potently induce diabetes (28).
Here, we assessed the expression of IL-17A during autoimmune diabetes development in the CD8-driven RIP-LCMV model. We used an eGFP reporter system to allow eventually the real-time in vivo imaging of IL-17A producing cells within the pancreas using 2-photon imaging technology, and the purification of IL-17A-producing cells from diabetic mice for further functional studies. For this purpose, we used transgenic models based on IRES-driven enhanced green fluorescent protein IL-17A (IL-17A eGFP) bicistronic reporter strains (29). Our results confirm that the IL-17A eGFP reporter system can be used for direct detection of IL-17A and show that IL-17A is produced by T cells during bacterial Klebsiella pneumonia infection. However, IL-17A remained undetectable during viral LCMV infection and during the development of autoimmune diabetes in the CD8-driven RIP-LCMV model.
Materials and Methods
Mice and infections
IL-17A eGFP knock-in mice (IL-17A eGFP; allele symbol: Il17a<tm1.1Flv>; allele accession ID: MGI:5006665) are described elsewhere (29) and were used as such or crossed to RIP-LCMV-GP (C57BL/6) mice to generate IL-17A eGFP RIP-LCMV-GP mice. IL-17A eGFP Smarta mice were obtained by crossing IL-17A eGFP mice with Smarta/SjL (C57BL/6) mice. The LCMV gp61-80 specific CD4 TCR Tg Smarta cells, capable of reporting in situ IL-17 production, were used for transfers to the LCMV-induced RIP-LCMV-GP (C57BL/6) model of type 1 diabetes.
Plaque-purified viral isolates of the Armstrong and clone 13 strains of LCMV were propagated in BHK-21 cells. The titers of viral stocks were determined by plaque assay. To establish acute infections, B6 mice received 104 pfu LCMV-Armstrong (low dose) or 2×106 pfu LCMV-Armstrong (high dose) in a volume of 0.1 ml by i.p. or i.v. inoculation respectively. Protracted infections were established by i.v. inoculation with 2×106 pfu LCMV-Clone 13 in a volume of 0.1 ml. For diabetes experiments, mice were infected i.p. with 10^4 pfu LCMV Armstrong. All animal experiments were done with permission from the Institutional Animal Care and Use Committee (IACUC).
Klebsiella pneumoniae infection
K. pneumoniae ATCC 43816 was incubated overnight with aeration in brain heart infusion media and then diluted in sterile PBS to the desired dose. IL17A-IRES-eGFP Mice were lightly anesthetized using methoxyflurane and then intranasally instilled with 1×104 CFU in a volume of 50 μl. Mice were sacrificed at the indicated time post-infection and lung, liver and spleens were harvested and processed as described previously. Expression of IL17A (eGFP) was analyzed by flow cytometry.
Diabetes induction and blood glucose values
For diabetes induction, RIP-LCMV-GP or derived strains were infected with low-dose (104 pfu) LCMV Armstrong 53b by i.p. inoculation. Blood glucose was monitored with OneTouch Ultra at 3-day intervals. Diabetes onset was dated on the first of 2 consecutive readings >300mg/dl.
In vitro Th17/Tc17 culture conditions
Spleens were removed from 8 week old mice and whole splenocytes were cultured at 1x10^6 cells/ml after RBC removal in RPMI1640 medium in the presence of anti-CD3 (1 μg/ml plate-bound, 145-2C11, BD) and anti-CD28 (10 μg/ml plate-bound, 37.51, BD) as well as IL-6 (20ng/ml, PeproTech), IL-23 (20ng/ml, PeproTech), TGF-β (1 ng/ml, RnD), anti-IFNγ (5 μg/ml, BD), anti-IL-4 (5 μg/ml, BD) and anti-IL-2 (5 μg/ml, JES6-1A12, BD). Cells were harvested at day 3, stained for CD4 or CD8 and samples were acquired on an LSRII flow cytometer.
Cell preparations
Spleen, pancreas and pancreas-draining lymph node cells were isolated from (recipient) mice at indicated times. Spleen and pancreatic LN were mechanically disrupted and washed twice. Cells from the pancreas were prepared as follows: after mincing and incubating in 1mg/ml Collagenase P (Roche) + 1μg/ml DNAse (Sigma) for 20 min at 37°C, the mixture was passed through a 350μm screen and washed twice with HBSS. Density centrifugation for 15 min at 800× g using Histopaque 1077 (Sigma) was followed by two washes with HBSS. Single cell suspension were obtained by incubation in non-enzymatic cell dissociation buffer (Invitrogen) for 6 min at 37°C. Cells were washed twice before staining or restimulation assays.
Flow cytometry staining and fixation
Antibodies used for flow cytometry were from eBioscience: CD25 (PC61), CD44 (IM7), CD62L (MEL-14), CD4 (RM-4.5), CD8 (53-6.7). Neo-autoantigen-specific CD8 T cells were detected by incubating for 1 hour at RT with Pro5 MHC Pentamer (ProImmune, UK) LCMV/gp33-41/H-2Db or LCMV/np396-404/H-2Db. For intracellular staining of cytokines using mAbs, in vitro restimulation was necessary (data not shown) and done by PMA and ionomycin for 3 hours, gp33-41 peptide for 4 hours or gp61-80 peptide for 7 hours. For PMA/ionomycin and gp33-41 stimulation, Brefeldin A was added immediately, for gp61-80 after 2 hours of culture. For intracellular cytokine staining or overnight (16hrs) storage, fixation tests showed that resuspending and incubating in BD Cytofix for 15 minutes at RT is superior to 1% PFA for 15 minutes at 6°C (see Supplemental Figure 1). After 2 washes, cells were stored until acquisition, or incubated with anti-IL17A, anti-TNFα or anti-IFNγ mAbs in BD CytoPerm buffer for 45 minutes at 6°C, washed twice and immediately acquired on an LSRII flow cytometer (BD Biosciences) and analyzed using FlowJo software.
Results
In vitro validation of IL-17A eGFP reporter mice: Th17/Tc17 cultures
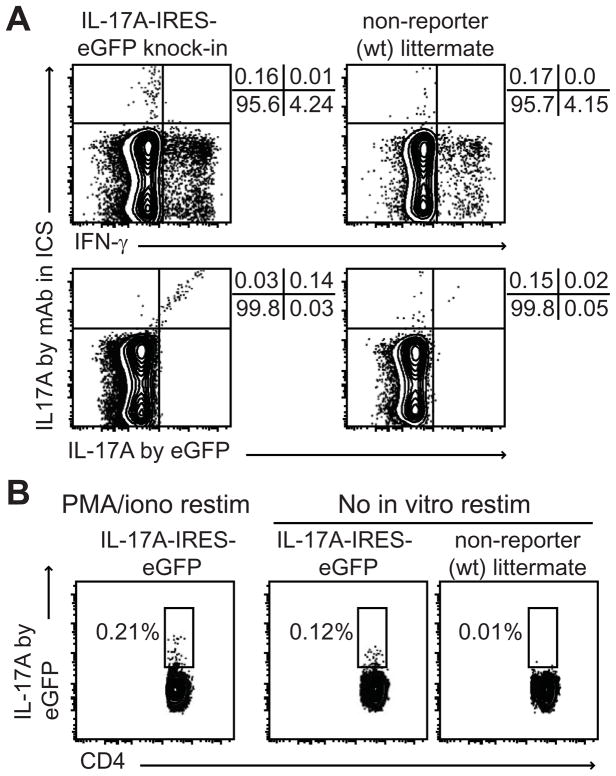
Recently, novel reporter strains of mice have become available to track in vivo expression of IL-17 isoforms. For example, the Cre-loxP-based strain IL-17F-CreEYFP (30) or IRES-driven red fluorescent protein (RFP) IL-17F reporter mice (31) accurately and sensitively report IL-17F. Here, we track in situ production of IL-17A using recently generated IL-17A eGFP mice (29). We first determined the sensitivity of the IL-17A eGFP reporter strain by stimulating splenocytes from homozygous (IL-17KI), heterozygous (IL-17Het) IL-17A eGFP or wild-type littermates (WT) under Th17 conditions for 3 days. We found clear and strong IL-17A/eGFP expression by both CD4 T cells (± 63% of CD4+) and CD8 T cells (± 72% of CD8+) in the cultures of IL-17KI and IL-17 Het splenocytes, but not WT splenocytes (Supplementary Fig. 1A). We also found that fixation using 1–2% paraformaldehyde is not ideal for eGFP-reporter signal detection. However, formaldehyde-based fixation (e.g. BD Cytofix) maintains the signal intensity of eGFP, and allows detection of intracellular molecules on permeabilized cells as well as overnight (16hrs) storage (Supplementary Figure 1B and 1C). Also, while detection of the eGFP signal can be done right after culture without the need for an in vitro restimulation, restimulation is needed for intracellular cytokine detection (ICS) by mAb (data not shown and Supplementary Fig. 1C). This is because visualization of IL-17A expression via detection of the eGFP signal is not only more sensitive than the antibody-based detection approach, but also does not require an accumulation of cytokines because eGFP produced in parallel with the cytokine is not immediately secreted during the effector T cell response.
From this we concluded that the IL-17A eGFP reporter is capable of strongly reporting IL-17 expression and that the eGFP signal can be used with caution in combination with formaldehyde fixation for the duration of standard intracellular staining protocols used in some of the experiments below.
Direct ex vivo validation of IL-17A eGFP reporter mice: Klebsiella pneumoniae infection
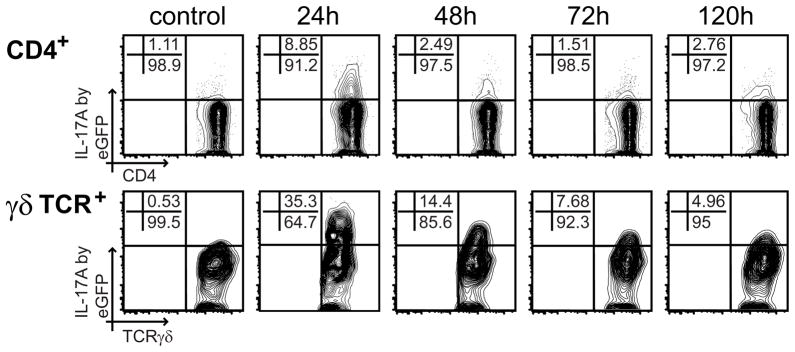
It has been previously established that IL-17A production is induced in response to infection with K. pneumoniae (32, 33). This in turn leads to the production of chemokines in infected tissues followed by the recruitment of neutrophils. To validate the IL-17A eGFP mouse directly ex vivo, we infected IL-17A eGFP mice with K. pneumoniae and examined the expression of eGFP in lung lymphocytes as K. pneumoniae infection initiates in the lung (Fig. 1). Kinetics of eGFP expression in the infected mice revealed a peak of IL17A expression in both CD4+αβ andγδ T cells at 24 hours after K. pneumoniae infection. However, a direct comparison between CD4+αβ and γδ T cells indicated an increased frequency of eGFP+ cells in the γδ T cell (35%) over the CD4+αβ (8%) compartment (Fig. 1). Interestingly, a gradual reduction of eGFP expressing cells in both CD4+αβ and γδ T cell compartments was observed at later time points.
FIGURE 1.
IL-17A expression in CD4 and γδ T cells in the lung peaks 24h after infection with Klebsiella pneumoniae. IL-17A knock-in mice were infected intranasally with 104 Klebsiella pneumoniae. At the indicated time points, animals were sacrificed, lungs harvested and analyzed for the presence of eGFP+ cells. The isolated cells were gated on CD4+ (A) and γδ T cells (B). A representative FACS plot for each analyzed time point is shown.
eGFP signal corresponds with detection of IL-17A by intracellular cytokine staining
Having shown that eGFP can be detected directly ex vivo, we validated that this eGFP signal reports IL-17A similar to standard ICS detection methods. The IL-17A eGFP signal corresponds with IL-17A detection by the conventional intracellular staining for cytokines, at least from in vitro cultures (Supplementary Fig. 1C). We next determined whether this also holds true for the direct ex vivo measurement. We infected IL-17A eGFP or non-reporter littermate mice with 104 pfu LCMV and harvested splenocytes after 1 day. In vitro restimulation by PMA/ionomycin in the presence of Brefeldin A revealed comparable frequencies of IFNγ+ (4.24 vs. 4.15%) and IL- 17A+ (0.16 vs. 0.17%) in CD4 T cells from reporter versus non-reporter mice (Figure 2A), indicating that the reporter mice respond similarly to viral infection. The data also showed that CD4 T cells make very little IL-17A at day 1 post infection (also see below). Moreover, IL-17A detection by eGFP reporter signal or by ICS largely overlap (Figure 2A). This confirms the accuracy and usefulness of the IL-17A eGFP reporter system. We also found that the use of pharmacological stimulation of cells in vitro overestimates the frequency of IL-17A positive cells directly ex vivo (Figure 2B). This discrepancy underscores the difference of both methods: eGFP reporters allow detection of actual producers, in vitro restimulation reveals the frequency of cells that have the capacity to produce cytokine. These ex vivo data, together with the in vitro data above, validate the use of IL-17A eGFP reporter mice for the detection of IL-17A production by cells.
FIGURE 2.
IL17A-IRES-eGFP mice accurately report IL-17 production capacity of CD4 T cells. A Comparison of IL-17A detection by eGFP signal or by intracellular cytokine staining (ICS) for IL-17A using mAb. Splenocytes from day 1 LCMV-infected mice were restimulated in vitro for 3 hrs with PMA/ionomycin + Brefeldin A. Cells were fixed with BDFix, then stained for intracellular IL-17A and IFNγ by mAb in BDPerm buffer. B In vitro restimulation by PMA/ionomycin overestimates the in vivo production of IL-17A cytokine by CD4 T cells. eGFP levels were measured by flow cytometry after in vitro restimulation with PMA/ionomycin + Brefeldin A, or without restimulation.
Lack of T cell-derived IL-17A during acute and protracted LCMV infection
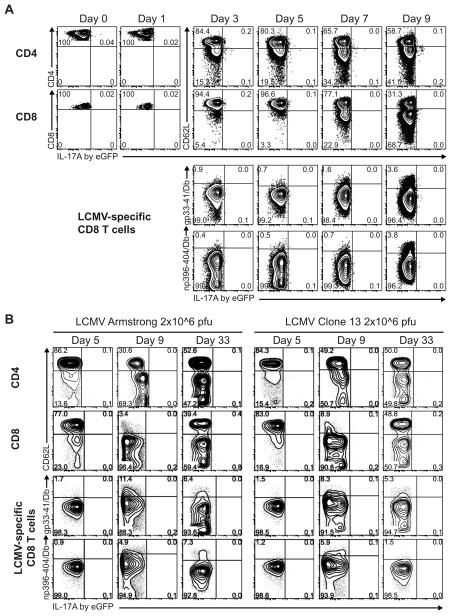
CD4 T cells can augment the functional activity of pathogen-specific CD8 T cells (34, 35). The main inflammatory cytokines produced by CD8 T cells are IFNγ and TNFα, but IL-21 is crucial to control LCMV infection (10–13) and can initiate and amplify Th17 differentiation (14, 15). So, to determine whether Th17 or Tc17 are present at any stage during an anti-LCMV T cell response, we infected IL-17A eGFP C57BL/6 mice and assessed the in situ production of IL-17A at various time points after infection. Prior to infection, splenocytes from IL-17A eGFP reporter mice produce negligible fractions of IL-17A directly ex vivo (<0.05%; Fig. 3A), or upon in vitro restimulation (<0.2%; Supplementary Fig. 2, day 0). In response to an acute infection with a low dose of LCMV Armstrong, CD4 T cells become activated, as determined by the increase in CD44 (data not shown) and the loss of CD62L expression, but fail to produce IL-17A, as measured directly ex vivo (Fig. 3A, top row). To show that this is not an issue with the IL-17A eGFP reporter system, standard ICS with mAbs and in vitro restimulation with LCMV- gp61-80 peptide or PMA/ionomycin showed that CD4 T cells increased the production of IFNγ, but not of IL-17A, after LCMV infection (Supplementary Fig. 2).
FIGURE 3.
IL-17A is not produced by T cells during an anti-LCMV response. A The in situ production of IL-17A by splenic T cells was determined at various time points following acute low dose LCMV infection (104 pfu LCMV Armstrong; panel A), acute high dose LCMV infection (2×106 pfu LCMV Armstrong; panel B, left side), or protracted infection (2×106 pfu LCMV Clone 13; panel B, right side) of IL-17A eGFP C57BL/6 mice. Cells were freshly stained for the indicated surface markers and freshly acquired without prior in vitro restimulation, inhibition of protein secretion, cell fixation or permeabilization. Graphs shown are gated on CD4 T cells (top rows) or CD8 T cells (lower three rows). n=4 per time point per infection condition. gp33-41/Db and np396-404/Db are pentamers to detect gp33-41-specific and np396-404-specific CD8 T cells, respectively. Numbers in graphs denote the percentages of cells in the respective gates.
Similarly, acute infection with high dose LCMV Armstrong or a protracted infection with Clone 13 also did not reveal IL-17A/eGFP signal directly ex vivo in CD4 T cells during the expansion phase (day 5), peak (day 9) or after contraction (day 33) of the response (Fig. 3B, top row). Moreover, both bulk and LCMV-specific CD8 T cells failed to produce IL-17A during the expansion, peak or contraction phases of the anti-LCMV response, independent of the acute or protracted character of the infection (Fig. 3A-B). We assessed LCMV-specific CD8 T cells only from day 3 onwards, because we could not get sufficient frequencies of these antigen-specific cells before that time point.
From this we conclude that CD4 T cells do not differentiate into Th17 cells and CD8 T cells do not become Tc17 cells during acute or protracted LCMV infection.
T cells in diabetic mice lack in situ IL-17A expression
On the transcript level, IL-17A and especially IL-17F increase as NOD mice develop diabetes (21). Serum levels of IL-17 protein however, are elevated in very young NOD mice and decline with age (20). We were interested in studying the role of IL-17A production during the diabetic effector phase in the pancreas for real-time imaging studies, but also to find out if IL-17A producing cells were pathogenic and how their diabetogenic potential could be curbed. Despite the lack of IL-17A production directly ex vivo during the systemic response to LCMV infection, we anticipated, based on the data in NOD mice and EAE (3), that organ-specific autoimmunity would create more favorable conditions for IL-17A production. Indeed, the activation of T cells in the pancreatic LN and the specific microenvironment in the pancreas both can alter the T cell response during diabetogenesis. In the RIP-LCMV mouse model for virally induced type 1 diabetes (36), mice express viral antigens from LCMV under the control of the rat insulin promoter (RIP) selectively on pancreatic β-cells and, in some lines, in the thymus (37). In C57BL/6 RIP-LCMV-glycoprotein (RIP-LCMV-GP), used in the study presented here, the viral antigen is not expressed in the thymus and infection with LCMV induces rapid CD8 T cell-dependent diabetes. We crossed the RIP-LCMV-GP strain to IL-17A eGFP reporter mice to track the in situ production by conventional CD4 T cells, Tregs, as well as auto-antigen-specific CD8 T cells during virally-induced T1D.
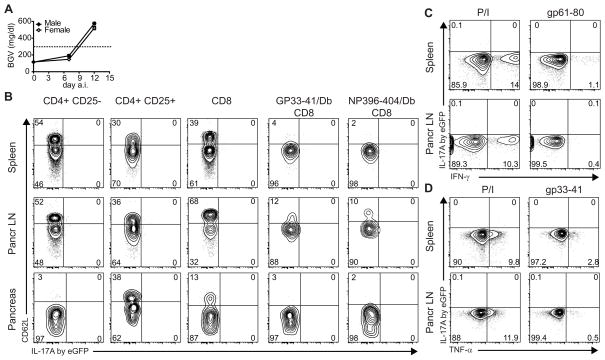
We found that both male and female IL-17A eGFP × RIP-LCMV-GP become hyperglycemic upon LCMV infection and develop diabetes with comparable kinetics of wild-type RIP-LCMV-GP C57BL/6 mice (Fig. 4A and data not shown). Next, we harvested spleens, pancreatic LNs and pancreata from diabetic IL-17A eGFP RIP-LCMV-GP mice to determine in situ IL-17A expression by CD4 (Th17) or CD8 (Tc17) T cells. We could not detect in situ IL-17A expression by conventional CD4 T cells (CD4+CD25−) or regulatory T cells (CD4+CD25+) from spleen, pancreatic LN or pancreas, regardless of the expression of CD62L (Fig. 4B) or CD44 (data not shown). Moreover, autoantigen (GP)-specific CD8 T cells, detected by GP33-41/Db pentamer, did not produce IL-17A/eGFP, despite being activated (CD62Llow) (Fig. 4B). Similarly, LCMV-specific CD8 T cells, detected by NP396-404/Db pentamer, lacked IL-17A/eGFP signal. These data indicate that IL-17A is not present in situ and is unlikely to be required for diabetes development in the RIP-LCMV model.
FIGURE 4.
T cells do not produce IL-17A in diabetic RIP-LCMV-GP mice. IL-17A eGFP reporter RIP-LCMV-GP mice were infected with 104 pfu LCMV Armstrong (n=8). A Blood glucose levels of male (black symbols), or female (open symbols) IL-17A eGFP reporter RIP-LCMV-GP mice at indicated time points a.i. B IL-17A/eGFP expression in various T cell types from spleen (upper row), pancreatic LN (middle row), or pancreas (lower row) of diabetic mice (day 14 a.i.). Cells were freshly stained and acquired without prior in vitro restimulation, inhibition of protein secretion, cell fixation or permeabilization. Graphs shown are gated on the cell type indicated on top of the column. Values <0.1 are depicted as zero. C, D Lack of IL-17A expression by restimulated CD4 T cells (C) and CD8 T cells (D) from diabetic RIP-LCMV-GP mice. IL-17A eGFP RIP-LCMV-GP mice were infected with 104 pfu LCMV Armstrong (n=6). At day 14 post infection (recent onset diabetes), spleen (top row) and pancreatic LN (bottom row) were harvested and, to allow IFN-γ/TNF-α visualization, were restimulated in vitro with PMA and ionomycin (P/I), gp33-41 peptide or gp61-80 peptide in the presence of Brefeldin A, as indicated. Representative graphs shown are gated on CD8 or CD4 T cells in panel C or D, respectively.
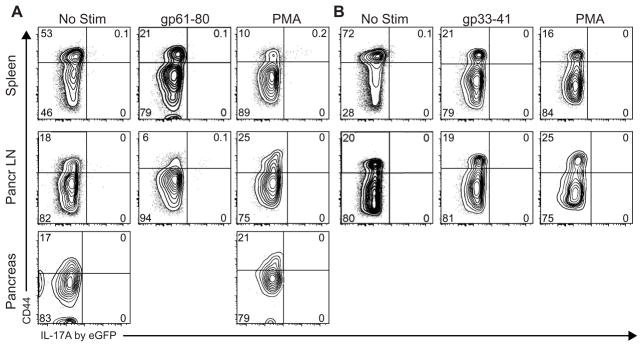
Direct ex vivo measurement of cells isolated from diabetic RIP-LCMV-GP mice did not reveal IL-17A/eGFP signal. It can be argued that in vitro restimulation can increase the IL-17A/eGFP signal. Therefore, we restimulated cells from spleen and pancreatic LN pharmacologically using PMA/ionomycin, or auto-antigen-specifically using MHC I-restricted gp33-41 or MHC II-restricted gp61-80 peptides. Next, we determined whether IL-17A was produced via eGFP signal detection, in combination with TNFα and IFNγ detection via standard intracellular staining. As expected, CD4 T cells produced IFNγ in response to PMA/ionomycin and, to a lesser extent, to gp61-80 peptide (Fig. 4C). However, CD4s did not show any measurable IL-17A/eGFP signal (Fig. 4C). Moreover, we found that both polyclonal and antigen-specific restimulation elicited TNFα production by CD8 T cells from spleen and pancreatic LN (Fig. 4D). However, as in CD4 T cells, we could not detect IL-17A/eGFP in CD8 T cells (Fig. 4D). Since the formaldehyde fixation preserves the eGFP signal during an additional ICS (see Supplementary Fig. 1), we conclude that T cells from diabetic RIP-LCMV-GP mice do not produce IL-17A, even after restimulation in vitro.
T cells from pre-diabetic RIP-LCMV-GP mice do not produce IL-17A
To address whether IL-17A is produced earlier in the diabetes development, we induced diabetes in IL-17A eGFP × RIP-LCMV-GP mice and analyzed IL-17A/eGFP expression at day 7, i.e. when autoantigen specific T cells can be detected in the pancreas and pancreatic LN (data not shown). Detection of IL-17A/eGFP was performed directly ex vivo as well as upon in vitro restimulation with PMA/ionomycin or with autoantigen-specific peptides. We found that CD4 T cells did not express any IL-17A/eGFP when freshly measured after isolation from day 7 pre-diabetic mice (Fig. 5A, No Stim). Moreover, autoantigen-specific restimulation of CD4 T cells in vitro by MHC II-restricted gp61-80 peptide did not elicit IL-17A/eGFP signal in spleen or in pancreatic LN (Fig. 5A). Even strong stimulation with PMA/ionomycin did not produce strong IL-17A/eGFP signals in CD4 from spleen, pancreatic LN or pancreas, regardless of whether they were of naïve (CD44Low) or effector/memory (CD44High) phenotype (Fig. 5A). Similarly, CD8 T cells from pre-diabetic animals did not produce IL-17A in situ or upon stimulation with MHC I-restricted gp33-41 peptide or PMA/ionomycin (Fig. 5B). This indicates that during the effector response leading up to diabetes in the RIP-LCMV model, IL-17A is unlikely to play a driving role in the autoimmune process.
FIGURE 5.
T cells do not produce IL-17A in pre-diabetic RIP-LCMV-GP mice. IL-17A eGFP RIP-LCMV-GP mice were infected with 104 pfu LCMV Armstrong (n=6). At day 7 post infection (before diabetes onset), IL-17A/eGFP production by A CD4 T cells and B CD8 T cells from spleen (top row), pancreatic LN (middle row) and pancreas (bottom row) was determined after in vitro restimulation in the presence of Brefeldin A with mock, with PMA/ionomycin, gp33-41 peptide or gp61-80 peptide as indicated. Next, cells were acquired fresh without cell fixation or permeabilization. Representative graphs shown are gated on CD4 T cells or CD8 T cells in panel A and B, respectively.
Transferred auto-antigen specific CD4 T cells do not become Th17 during virally-induced diabetes
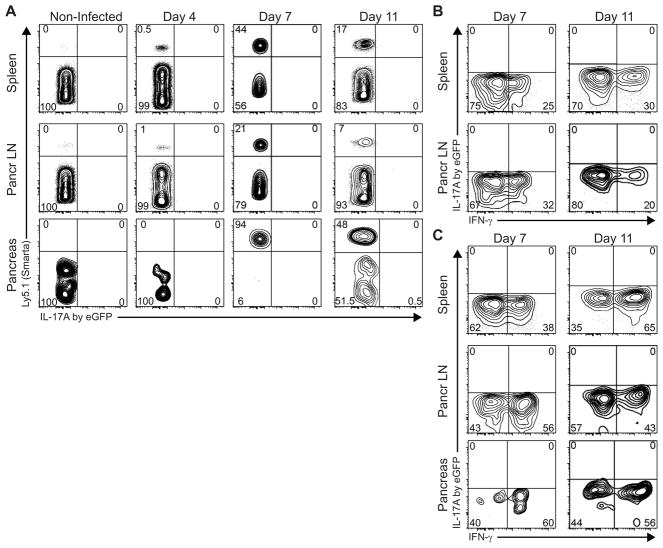
We further substantiated that auto-antigen specific CD4 T cells do not produce IL-17A during diabetes development by transferring auto-antigen-specific IL-17A eGFP reporter Smarta CD4 T cells into RIP-LCMV mice. Smarta T cells are CD4 TCR transgenic T cells derived from a transgenic mouse expressing an MHC II-restricted TCR with specificity for an LCMV glycoprotein (GP)-derived T helper cell epitope (gp61-80/I-Ab specific)(38). Here, we crossed B6.SjL × Smarta, containing Ly5.1 allelically-marked Smarta T cells, to the IL-17A eGFP reporter mouse strain. Next, transfer of purified Ly5.1+ IL-17A eGFP Smarta CD4 T cells allowed us to track CD4 T cells with a specificity for beta-cell autoantigen and assess in vivo IL-17A production accurately and without manipulations or artifacts.
On day 4, 7, and 11 after diabetes induction, we harvested spleen, pancreatic LN and pancreas from recipient RIP-LCMV-GP mice. As expected, the Ly5.1+ Smarta CD4 population was readily detectable at day 4, peaked at day 7 and contracted by day 11 (Fig. 6A). However, we could not detect any IL-17A/eGFP in host CD4 T cells (Ly5.1−) or transferred Smarta (Ly5.1+) cells recovered from spleen, pancreatic LN or pancreas (Fig. 6A). In vitro restimulation showed that recovered Smarta CD4 T cells produced IFNγ, but not IL-17A/eGFP in response to their cognate antigen gp61-80, at both days 7 and 11 (Fig. 6B). Similarly upon PMA/ionomycin restimulation, Smarta T cells recovered from spleen, pancreatic LN or pancreas produced mainly IFNγ, but not IL-17A/eGFP (Fig. 6C). Taken together, we conclude that autoantigen specific CD4 T cells do not differentiate into Th17 during activation and clonal expansion in the type 1 diabetogenic process.
FIGURE 6.
Transferred Smarta cells do not produce IL-17A during diabetogenesis. Ly5.1+-allelically marked IL-17A eGFP reporter Smarta CD4 T cells were transferred on day -1 to LCMV-infected RIP-LCMV-GP mice (n=4 per time point). A In situ IL-17A production was assessed at indicated time points in spleen, pancreatic LN and pancreas by flow cytometry. Cells were stained fresh for surface markers and acquired fresh without prior in vitro restimulation, inhibition of protein secretion, cell fixation or permeabilization. Representative graphs are shown and displayed as IL-17A/eGFP versus Ly5.1 of the live CD4+CD8- lymphocyte gate. B,C Transferred Smarta cells do not produce IL-17 upon in vitro restimulation. Cells recovered as in A were restimulated in vitro in the presence of Brefeldin A with gp61-80 peptide (B) or PMA and ionomycin (C) before fixation, permeabilization and intracellular staining for IFNγ. Representative graphs (IFNγ versus IL-17A/eGFP) shown are gated on live Ly5.1+ (B6.SjL Smarta) CD4+CD8− lymphocytes. Frequencies <0.1% are rounded to zero.
Discussion
Scientists now value the role of IL-17 in the clearance of pathogens, especially in bacterial or fungal infections (3). Here, we first validated the use of an IL-17A eGFP bicistronic reporter mouse strain (29) for IL-17A detection directly ex vivo upon bacterial infection with Klebsiella pneumonia. We next assessed the in situ production of IL-17A during infection with LCMV, an RNA arenavirus. The CD8 immune response to LCMV is characterized by an initial primary expansion of LCMV-specific, IFNγ positive T cells, peaking at 7–9 days after inoculation. This is followed by a contraction phase leaving a resting pool of LCMV memory CTLs that are maintained at a similar frequency for a long period of time (39). The main inflammatory cytokines secreted by cytotoxic T cells are IFNγ and TNFα. CD4, on the other hand, produce IL-21 during LCMV infection that was recently shown to be crucial to control LCMV infection (10–13) and can initiate and amplify Th17 differentiation (14, 15). Therefore, it was reasonable to speculate that Th17 cells may have a role in immunity to LCMV. We addressed this by using a cytokine reporter system for IL-17A in favor of IL-17F, because IL-17F is expressed by only a subpopulation of IL-17A-expressing Th17 cells. However, in contrast to our anticipation and the data acquired in a bacterial infection setting, we could not detect T cell-derived IL-17A at any stage of the immune response to acute low or high dose LCMV Armstrong infection, or protracted infection by LCMV Clone 13.
Our data on LCMV infection resemble the data on neurotropic coronavirus infection in that IL-17 levels did not exceed background (40). However, data on other viral infections show that Th17 can affect to viral clearance as well as immunopathology, depending on the virus. An example of IL-17 contributing to viral clearance is the action of Tc17 and Th17 cells in response to influenza A virus (7, 8). In contrast, Th17 promote viral persistence after Theiler's murine encephalomyelitis virus infection (9). IL-17 can also contribute to immunopathology. As such, elevated levels of IL-17 have been found in the cornea of mice infected with herpes simplex virus-1 (HSV-1), a DNA virus (41), but the increased immunopathological lesions upon HSV-1 infection of p19−/− mice which lack IL-23, and thus Th17 responses, indicate that IL-17 might serve by dampening inflammatory immune responses (42). In other viral infections, IL-17 or Th17 have been detected, but their role remains to be determined. For example, hepatitis C virus (HCV) infection of individuals induces Ag-specific Th17, probably to combat viral infection (43). However, HCV-nonstructural protein-4 (NS4)-induced TGF-β and IL-10 inhibit these responses which may represent another example of an evasion strategy of viruses to subvert protective immune responses (43). Moreover, both CD4+ and CD4− T cells from HIV-infected individuals can produce IL-17 following in vitro stimulation with PMA and ionomycin, but the specificity of these cells is unknown (44). Interestingly, acute pathogenic lentiviral simian immunodeficiency virus (SIV) infection of pigtailed macaques, in contrast to non-pathogenic infection of African green monkeys, results in selective loss of Th17 cells that is an independent predictor of increased systemic immune activation (45). Importantly, this difference was explained by bacterial translocation and compromised colonic mucosal integrity (46). This implies that effects of viral infections on IL-17 responses are not necessary direct host-to-pathogen reactions, but can be a result of indirect effects involving non-viral microbial stimuli. With regards to LCMV infection, a recent report showed that both Eomesodermin and t-bet block differentiation of CD8 T cells into IL-17 producing cells (47). Consequently our data imply that the differentiation pathways governed by these transcription factors are not simultaneously impaired at any stage during acute or protracted infection. Taken together, we conclude that it is very unlikely that T cell-derived IL-17A plays a role in acute or protracted LCMV infection.
Besides a role in host defense, there is evidence that Th17 cells, or at least unregulated IL-17 production, is involved in rheumatoid arthritis, psoriasis, multiple sclerosis, and inflammatory bowel disease (16). However, the role of IL-17 in autoimmune diabetes is less clear. Recently, it was shown that treatment with anti-IL-17A mAb prevents diabetes in NOD mice when administered at late pre-diabetic stage (10 weeks), but not early stages (23). In contrast, transferred BDC2.5 Th17 are diabetogenic in NOD and NOD/SCID mice, but convert to IFNγ producing Th1 cells that induce diabetes (21, 25). This ‘Th17-induced’ diabetes is not inhibited by anti-IL-17 treatment, but by anti-IFNγ treatment (25), suggesting it is not the Th17 cell that is diabetogenic. Further to this, recent evidence even suggests IL-17 producing γδ T cells (26) or Th17 (27) can be protective in type 1 diabetes, perhaps after regulation in the gut (29). Here, using IL-17A reporter mouse strains, we show that CD4 or CD8 T cells do not produce IL-17A in situ before or after diabetes onset in a virally-induced model of type 1 diabetes. This was regardless of the naïve or memory phenotype of the cells, or bystander or auto-antigen-specificity of the CD4 or CD8 T cells. Importantly, this was not because of a technical detection limitation of the IL-17A reporter because IL-17A remained undetected after in vitro restimulation by eGFP signal or standard ICS methods. Of note, other cytokine reporter mice for IFNγ (Yeti, (48)), IL-4 (4get, (49)), or IL-10 (Tiger, (50)) do not require in vitro restimulation to report the in situ production of the respective cytokine. This indicates that T cells simply are not skewed towards the Th17 or Tc17 lineage. Our data showing the lack of IL-17A protein but abundance of IFNγ suggest that IL-17A is unlikely to be an essential molecule in type 1 diabetes. This statement is further supported by the data from the labs of Anne Cooke (25) and Chen Dong(21), showing that transferred Th17 cells convert to IFNγ producing Th1 cells before inducing diabetes in a NOD/SCID transfer model. Our data might also imply that the reported increased levels of IL-17A transcripts in NOD (21) do not translate into more IL-17A protein. Taken together, our data support a role for IL-17A in bacterial infections, but suggest that IL-17A is not essential in viral elimination or autoimmune diabetes.
Supplementary Material
Acknowledgments
The authors are grateful to Malina McClure and the staff at La Jolla Institute for Allergy and Immunology Animal Facility for assistance with animal care, and Priscilla Colby for administrative assistance.
This work was supported by research grants from The Brehm Center for T1D Research and Analysis, and NIH Program grant P01 AI058105. T.V.B. is a fellow of the Belgian American Educational Foundation and supported by an ADA mentor grant.
Footnotes
Disclosures
The authors have no conflicting financial interests.
References
- 1.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 2.Huber M, Heink S, Grothe H, Guralnik A, Reinhard K, Elflein K, Hunig T, Mittrucker HW, Brustle A, Kamradt T, Lohoff M. A Th17-like developmental process leads to CD8(+) Tc17 cells with reduced cytotoxic activity. Eur J Immunol. 2009;39:1716–1725. doi: 10.1002/eji.200939412. [DOI] [PubMed] [Google Scholar]
- 3.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 4.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 6.von Vietinghoff S, Ley K. Homeostatic regulation of blood neutrophil counts. J Immunol. 2008;181:5183–5188. doi: 10.4049/jimmunol.181.8.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamada H, Garcia-Hernandez Mde L, Reome JB, Misra SK, Strutt TM, McKinstry KK, Cooper AM, Swain SL, Dutton RW. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J Immunol. 2009;182:3469–3481. doi: 10.4049/jimmunol.0801814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKinstry KK, Strutt TM, Buck A, Curtis JD, Dibble JP, Huston G, Tighe M, Hamada H, Sell S, Dutton RW, Swain SL. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J Immunol. 2009;182:7353–7363. doi: 10.4049/jimmunol.0900657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou W, Kang HS, Kim BS. Th17 cells enhance viral persistence and inhibit T cell cytotoxicity in a model of chronic virus infection. J Exp Med. 2009;206:313–328. doi: 10.1084/jem.20082030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holm C, Nyvold CG, Paludan SR, Thomsen AR, Hokland M. Interleukin-21 mRNA expression during virus infections. Cytokine. 2006;33:41–45. doi: 10.1016/j.cyto.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324:1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frohlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, Marsland BJ, Oxenius A, Kopf M. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324:1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- 14.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 16.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 17.Matusevicius D, Kivisakk P, He B, Kostulas N, Ozenci V, Fredrikson S, Link H. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler. 1999;5:101–104. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- 18.Bradshaw EM, Raddassi K, Elyaman W, Orban T, Gottlieb PA, Kent SC, Hafler DA. Monocytes from patients with type 1 diabetes spontaneously secrete proinflammatory cytokines inducing Th17 cells. J Immunol. 2009;183:4432–4439. doi: 10.4049/jimmunol.0900576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Honkanen J, Nieminen JK, Gao R, Luopajarvi K, Salo HM, Ilonen J, Knip M, Otonkoski T, Vaarala O. IL-17 immunity in human type 1 diabetes. J Immunol. 185:1959–1967. doi: 10.4049/jimmunol.1000788. [DOI] [PubMed] [Google Scholar]
- 20.Vukkadapu SS, Belli JM, Ishii K, Jegga AG, Hutton JJ, Aronow BJ, Katz JD. Dynamic interaction between T cell-mediated beta-cell damage and beta-cell repair in the run up to autoimmune diabetes of the NOD mouse. Physiol Genomics. 2005;21:201–211. doi: 10.1152/physiolgenomics.00173.2004. [DOI] [PubMed] [Google Scholar]
- 21.Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol. 2009;39:216–224. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alam C, Valkonen S, Palagani V, Jalava J, Eerola E, Hanninen A. Inflammatory tendencies and overproduction of IL-17 in the colon of young NOD mice are counteracted with diet change. Diabetes. 59:2237–2246. doi: 10.2337/db10-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emamaullee JA, Davis J, Merani S, Toso C, Elliott JF, Thiesen A, Shapiro AM. Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice. Diabetes. 2009;58:1302–1311. doi: 10.2337/db08-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain R, Tartar DM, Gregg RK, Divekar RD, Bell JJ, Lee HH, Yu P, Ellis JS, Hoeman CM, Franklin CL, Zaghouani H. Innocuous IFNgamma induced by adjuvant-free antigen restores normoglycemia in NOD mice through inhibition of IL-17 production. J Exp Med. 2008;205:207–218. doi: 10.1084/jem.20071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bending D, De La Pena H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, Cooke A. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009 doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han G, Wang R, Chen G, Wang J, Xu R, Wang L, Feng J, Li X, Guo R, Fu L, Shen B, Li Y. Interleukin-17-producing gammadelta+ T cells protect NOD mice from type 1 diabetes through a mechanism involving transforming growth factor-beta. Immunology. 129:197–206. doi: 10.1111/j.1365-2567.2009.03166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nikoopour E, Schwartz JA, Huszarik K, Sandrock C, Krougly O, Lee-Chan E, Singh B. Th17 polarized cells from nonobese diabetic mice following mycobacterial adjuvant immunotherapy delay type 1 diabetes. J Immunol. 184:4779–4788. doi: 10.4049/jimmunol.0902822. [DOI] [PubMed] [Google Scholar]
- 28.Ciric B, El-behi M, Cabrera R, Zhang GX, Rostami A. IL-23 drives pathogenic IL-17-producing CD8+ T cells. J Immunol. 2009;182:5296–5305. doi: 10.4049/jimmunol.0900036. [DOI] [PubMed] [Google Scholar]
- 29.Esplugues E, Huber S, Gagliani N, Hauser AE, Town T, Wan YY, WOC, Rongvaux A, Rooijen NV, Haberman AM, Iwakura Y, Kuchroo VK, Kolls JK, Bluestone JA, Herold KC, Flavell RA. Control of TH17 cells occurs in the Small Intestine. Nature. 2011 doi: 10.1038/nature10228. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Croxford AL, Kurschus FC, Waisman A. Cutting edge: an IL-17F-CreEYFP reporter mouse allows fate mapping of Th17 cells. J Immunol. 2009;182:1237–1241. doi: 10.4049/jimmunol.182.3.1237. [DOI] [PubMed] [Google Scholar]
- 31.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, Feng XH, Jetten AM, Dong C. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001;25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 33.Happel KI, Zheng M, Young E, Quinton LJ, Lockhart E, Ramsay AJ, Shellito JE, Schurr JR, Bagby GJ, Nelson S, Kolls JK. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J Immunol. 2003;170:4432–4436. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 35.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oldstone MB, Nerenberg M, Southern P, Price J, Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991;65:319–331. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- 37.von Herrath MG, Dockter J, Oldstone MB. How virus induces a rapid or slow onset insulin-dependent diabetes mellitus in a transgenic model. Immunity. 1994;1:231–242. doi: 10.1016/1074-7613(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 38.Oxenius A, Bachmann MF, Zinkernagel RM, Hengartner H. Virus-specific MHC-class II-restricted TCR-transgenic mice: effects on humoral and cellular immune responses after viral infection. Eur J Immunol. 1998;28:390–400. doi: 10.1002/(SICI)1521-4141(199801)28:01<390::AID-IMMU390>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 39.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 40.Kapil P, Atkinson R, Ramakrishna C, Cua DJ, Bergmann CC, Stohlman SA. Interleukin-12 (IL-12), but not IL-23, deficiency ameliorates viral encephalitis without affecting viral control. J Virol. 2009;83:5978–5986. doi: 10.1128/JVI.00315-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Molesworth-Kenyon SJ, Yin R, Oakes JE, Lausch RN. IL-17 receptor signaling influences virus-induced corneal inflammation. J Leukoc Biol. 2008;83:401–408. doi: 10.1189/jlb.0807571. [DOI] [PubMed] [Google Scholar]
- 42.Kim B, Sarangi PP, Azkur AK, Kaistha SD, Rouse BT. Enhanced viral immunoinflammatory lesions in mice lacking IL-23 responses. Microbes Infect. 2008;10:302–312. doi: 10.1016/j.micinf.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rowan AG, Fletcher JM, Ryan EJ, Moran B, Hegarty JE, O'Farrelly C, Mills KH. Hepatitis C virus-specific Th17 cells are suppressed by virus-induced TGF-beta. J Immunol. 2008;181:4485–4494. doi: 10.4049/jimmunol.181.7.4485. [DOI] [PubMed] [Google Scholar]
- 44.Maek ANW, Buranapraditkun S, Klaewsongkram J, Ruxrungtham K. Increased interleukin-17 production both in helper T cell subset Th17 and CD4-negative T cells in human immunodeficiency virus infection. Viral Immunol. 2007;20:66–75. doi: 10.1089/vim.2006.0063. [DOI] [PubMed] [Google Scholar]
- 45.Favre D, Lederer S, Kanwar B, Ma ZM, Proll S, Kasakow Z, Mold J, Swainson L, Barbour JD, Baskin CR, Palermo R, Pandrea I, Miller CJ, Katze MG, McCune JM. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009;5:e1000295. doi: 10.1371/journal.ppat.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lederer S, Favre D, Walters KA, Proll S, Kanwar B, Kasakow Z, Baskin CR, Palermo R, McCune JM, Katze MG. Transcriptional profiling in pathogenic and non-pathogenic SIV infections reveals significant distinctions in kinetics and tissue compartmentalization. PLoS Pathog. 2009;5:e1000296. doi: 10.1371/journal.ppat.1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Intlekofer AM, Banerjee A, Takemoto N, Gordon SM, Dejong CS, Shin H, Hunter CA, Wherry EJ, Lindsten T, Reiner SL. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science. 2008;321:408–411. doi: 10.1126/science.1159806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, Gapin L, Kronenberg M, Locksley RM. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198:1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 50.Kamanaka M, Kim ST, Wan YY, Sutterwala FS, Lara-Tejero M, Galan JE, Harhaj E, Flavell RA. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity. 2006;25:941–952. doi: 10.1016/j.immuni.2006.09.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.