Abstract
Purpose
Infantile spasms are the signature seizures of West syndrome. The conventional treatments for infantile spasms, such as adrenocorticotropic hormone (ACTH) and vigabatrin, are not always effective, especially in symptomatic infantile spasms (SIS). We tested the efficacy of carisbamate, a novel neurotherapeutic drug, to suppress spasms in the multiple-hit rat model of SIS and compared it with phenytoin to determine if its effect is via sodium-channel blockade.
Methods
Sprague-Dawley rats received right intracerebral infusions of doxorubicin and lipopolysaccharide at postnatal day 3 (PN3) and intraperitoneal p-chlorophenylalanine at PN5. A single intraperitoneal injection of carisbamate was administered at PN4, after the onset of spasms, at the following doses: 10mg/kg (CRS-10); 30 mg/kg (CRS-30); 60 mg/kg (CRS-60) and was compared to vehicle-injected group (VEH). Video-monitoring of PN6–7 CRS-60 or VEH injected pups was also done.
Key findings
Carisbamate acutely reduced both behavioral spasms (CRS-30 and CRS-60 groups only) and electroclinical spasms, during the first 2–3 post-injection hours, without detectable toxicity or mortality. In contrast, phenytoin (20 or 50 mg/kg) failed to suppress spasms.
Significance
Our findings provide preclinical evidence that carisbamate displays acute anticonvulsive effect on spasms through a sodium channel-independent mechanism. As spasms in the multiple-hit rat model are refractory to ACTH and transiently sensitive to vigabatrin, carisbamate may constitute a candidate new therapy for SIS, including the ACTH-refractory spasms. Further confirmation with clinical studies is needed.
Keywords: West syndrome, treatment, anticonvulsant, EEG, phenytoin
INTRODUCTION
Infantile spasms (IS) are seizures that occur in specific epileptic encephalopathies of infancy, which may lead to poor epilepsy or cognitive outcomes. The majority of IS are linked to pre-existing structural/metabolic brain pathologies, comprising the symptomatic or structural/metabolic IS group (SIS) (Berg et al. 2010; Pellock et al. 2010). Currently recommended treatments for IS [adrenocorticotropic hormone (ACTH), high dose glucocorticoids, vigabatrin] have a short-term efficacy between 40–80 % (Mackay et al. 2004). However, many patients with IS fail to achieve complete seizure control or spasms recur. Furthermore, serious adverse effects, like hypertension, infection, adrenal insufficiency and electrolyte disturbances associated with hormonal therapies, or vigabatrin-related visual field deficits may limit their use. The existing rodent models of IS include acute [postnatal NMDA injection with or without prenatal betamethasone (Mares et al. 1992; Velisek et al. 2007); γ-butyrolactone induced spasms in a Down’s syndrome mouse model (Cortez et al. 2009)] and chronic models [tetrodotoxin model (Lee et al. 2008); conditional knockout (Marsh et al. 2009) and knockin (Price et al. 2009) models of the aristaless related homeobox gene; multiple-hit model of SIS (Scantlebury et al. 2010)]. Critical reviews of these models have been provided in recent publications (Stafstrom 2009; Chudomelova et al. 2010). In this study, we have used the multiple-hit rat model of SIS for two reasons. First, it is the only available chronic model of IS with well-defined pharmacosensitivity. Spasms in this model are ACTH-refractory and transiently responsive to vigabatrin. Hence, new candidate therapies with efficacy in this model could potentially be of benefit also in the more refractory population of patients with IS. Second, it recapitulates the chronicity of the epilepsy phenotype and dyscognitive features of patients with IS, which may allow, in the future, to examine not only the acute efficacy but also the sustained antiepileptic, antiepileptogenic as well as disease-modifying effects of a drug.
Here we are testing the preclinical efficacy of a broad-spectrum antiepileptic drug, carisbamate (S-2-O-carbamoyl-1-o-chlorophenyl-ethanol; RWJ-333369; Johnson & Johnson) in the treatment of IS. Carisbamate has been shown to have antiepileptic and potentially antiepileptogenic effects in animal and in vitro models of generalized or limbic epilepsy (Deshpande et al. 2008; Francois et al. 2008; Grabenstatter et al. 2008). Clinical trials have demonstrated efficacy in two of the four cohorts of patients with focal onset seizures (Faught et al. 2008; Bialer et al. 2010; Sperling et al. 2010) as well as in epilepsy patients with photosensitivity (Trenite et al. 2007). However, its efficacy on IS has not been explored.
We show that carisbamate acutely and dose-dependently suppresses behavioral and electroclinical spasms in the multiple-hit model for 2–3 hours following a single injection. In contrast, phenytoin was ineffective, suggesting that suppression of spasms by carisbamate is not via sodium channel blockade. The immediate efficacy of carisbamate on spasms in the multiple-hit model of SIS, in which spasms are ACTH-refractory and transiently sensitive to vigabatrin (Scantlebury et al. 2010), may have significant repercussions, if confirmed in human clinical trials, as early cessation of spasms has been proposed to improve long-term clinical outcomes (Lux et al. 2005)
METHODS
Multiple-hit rat model of IS
We used male offspring of timed pregnant Sprague-Dawley rats (Taconic Farms). Animal preparation and surgical procedures were as described before (Scantlebury et al. 2010). At postnatal day 3 (PN3), doxorubicin (right intracerebroventricular) and lipopolysaccharide (right intraparietal) were infused stereotactically, under isoflurane anesthesia. Rats tested at PN6–7 also received p-chlorophenylalanine intraperitoneally (i.p.) at PN5. The administration of p-chlorophenylalanine is not required for the expression of spasms, which are semiologically the same compared to spasms appearing prior to its administration, but it increases their frequency. Animal care and use conformed to institutional policy and guidelines of the American Association for the Accreditation of Laboratory Animal Care. All procedures and experiments were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Monitoring
At PN4, rats were separated for video-monitoring as described (Scantlebury et al. 2010). The monitoring session consisted of 1 pre-injection and 5 post-injection hours. Rats were sacrificed at the conclusion of the studies for histology to ensure appropriate targeting of injections. Epidural EEG and subcutaneous nuchal electromyography (EMG) electrodes (Pinnacle Technology, Lawrence, Kansas) were placed in different rats at PN5 or 6 as described (Scantlebury et al. 2010) and video-EEG monitoring (1 hour pre- and 5 hours post-injection) was done a day later. In the video-monitored rats, “behavioral spasms” were considered the sudden and synchronous high amplitude movements of all limbs and body to a flexion or extension posture. Flexion or extension events that had asynchronous limb movements or appeared as an attempt of the pup to reposition were excluded to minimize false positive events. In the video-EEG experiments, “electroclinical spasms” were behavioral spasms that were also associated with electrodecremental responses (background attenuation) with or without bursts of polyspikes and sharp wave discharges or paroxysmal fast rhythmic activities (Scantlebury et al. 2010).
Drug treatments
Drugs and their vehicles were given i.p. after the onset of spasms. Carisbamate (Johnson & Johnson Pharmaceutical Research & Development, LLC, Raritan, NJ, U.S.A.) was given at the following doses: 10 mg/kg (CRS-10), 30 mg/kg (CRS-30), or 60 mg/kg (CRS-60). Vehicle-treated controls (VEH) received equal volumes of 0.5% methylcellulose vehicle (VEH). Different rats received phenytoin (5,5-Diphenylhydantoin sodium salt 20 or 50 mg/kg i.p.: PHT-20 and PHT-50 groups respectively) dissolved in aqueous solution with 0.075M NaOH (SIGMA-ALDRICH, St Louis, MO, U.S.A.) or its vehicle (VEH; alkalinized saline). These doses generate therapeutic (PHT-20) or supratherapeutic (PHT-50) phenytoin levels (Bittigau et al. 2002).
Data analysis
Scoring of spasms and electrographic ictal and interictal events was done by an investigator blinded to treatment (T.O.). We considered “electroclinical spasms” the flexion/extension spasms associated with paroxysmal fast activity or polyspikes with or without voltage attenuation (decremental responses) (Fig. 1B). “Electrographic ictal-like patterns” resembled the above ictal EEG patterns without being associated with behavioral spasms or EMG discharges. Interictal epileptiform discharges (IEDs) included interictal spikes, polyspikes or sharp waves with amplitudes at least twice the background activity and duration less than 200 ms, which disrupted background activities.
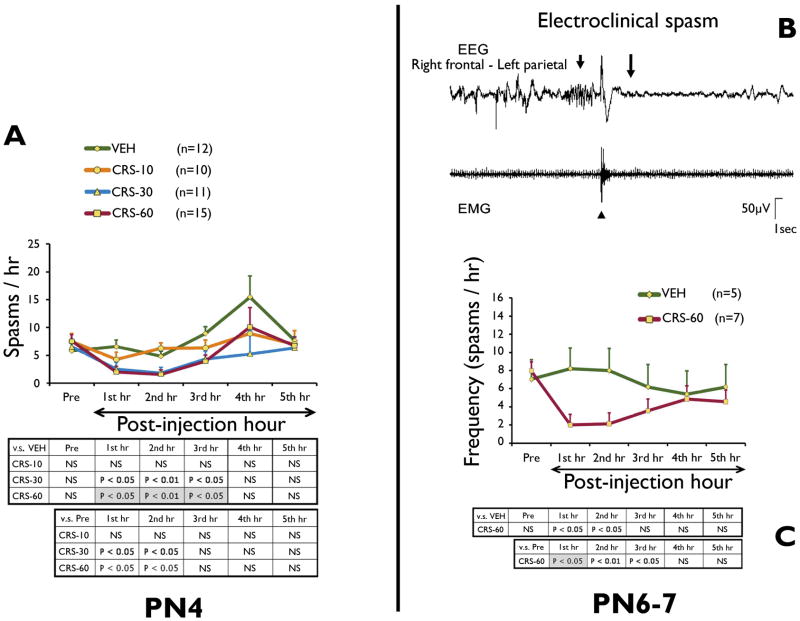
Figure 1. Carisbamate suppresses acutely behavioral and electroclinical spasms in the multiple-hit model, in a dose-dependent manner.
Panel A: Video-monitoring of PN4 DLP pups treated with carisbamate (CRS-10, CRS-30, and CRS-60 groups) or vehicle (VEH), given after the onset of spasms, shows dose-dependent acute reduction in the frequency of spasms Compared to VEH group, both CRS-30 and CRS-60 groups had significantly less frequent spasms during the first 3 post-injection hours. CRS-10 dose had no effect on spasms. Compared to pre-injection doses, both CRS-30 and CRS-60 caused an immediate reduction in the frequencies of spasms during the first post-injection hours. The increased frequency of spasms of VEH group during the 4th post-injection hour may be due either to random clustering or stress-induced increase of spasms.
Panel B: Example of epidural EEG and nuchal EMG recordings of an electroclinical spasm in the multiple-hit model. The ictal EEG pattern consists of (a) paroxysmal fast activities (8–12 Hz, short arrow) that precedes the EMG burst of the clinical spasm (arrowhead), (b) a period of voltage attenuation (onset at long arrow) and subsequent return of baseline activities.
Panel C: Video-EEG monitoring of PN6–7 DLP pups shows significant reduction in the frequency of electroclinical spasms compared to both VEH (effect significant during the first 2 post-injection hours) as well as compared to pre-injection frequencies (effect significant during the first 3 hours). The tables demonstrate the significant intergroup differences using Student’s t-test (white cells) as well as Tukey HSD post hoc (gray highlighted cells).
Multifactorial ANOVA followed by post hoc Tukey HSD (honestly significant difference) test were performed (JMP 7 software, SAS Institute Inc., Cary NC). As multiple comparisons may reduce the sensitivity of detecting significant intergroup differences, Student’s t-test comparisons were also utilized to explore potential intergroup differences. Results are expressed as Least Square Means (LSMean) ± standard errors (SE). Statistically significant differences had P < 0.05.
RESULTS
Carisbamate suppresses behavioral spasms acutely at PN4
Two-way ANOVA showed significant main effects for both carisbamate (F = 5.67, P = 0.001) and time after injection (F=7.61, P < 0.001). No significant pre-injection differences in the frequencies of spasms were found (Fig. 1A).
At PN4, carisbamate reduced behavioral spasms dose-dependently. CRS-30 and CRS-60 groups decreased spasms both compared to pre-injection baseline (first 2 post-injection hours) and post-injection VEH group (first 3 post-injection hours). No significant effects were found for CRS-10 group.
Carisbamate suppresses electroclinical spasms acutely at PN6–7, but not the interictal discharges
At PN6–7, carisbamate (CRS-60 protocol) significantly reduced the frequency of electroclinical spasms compared to both VEH group (first 2 post-injection hours) and the pre-injection frequencies (first 3 post-injection hours) (F=11.57, P=0.012, ANOVA) (Fig. 1C). Pre-injection frequencies of spasms were similar among groups (Fig. 1C).
Carisbamate had no significant effect on the frequencies of electrographic ictal-like patterns [F = 0.069; P = 0.79, ANOVA] or IEDs [F = 1.35; P = 0.25, ANOVA].
Carisbamate: tolerability and effects on survival
No significant sedation was seen, as assessed by their baseline activity and the neurodevelopmental assessment (weights, open field activity, surface righting time, negative geotaxis) after carisbamate injection at 2hrs post-injection or a day after injection. After the injection of carisbamate or vehicle at PN4, all rats of groups CRS-10, -30 and VEH survived. Only 1/17 CRS-60 pups died among those observed for 24 hours.
Phenytoin does not suppress spasms
Phenytoin had no significant impact on the frequency of behavioral spasms at PN4 (Fig. 2). This suggests that carisbamate-mediated suppression of spasms is not via sodium channel blockade.
Figure 2. Phenytoin has no significant effect on spasms.
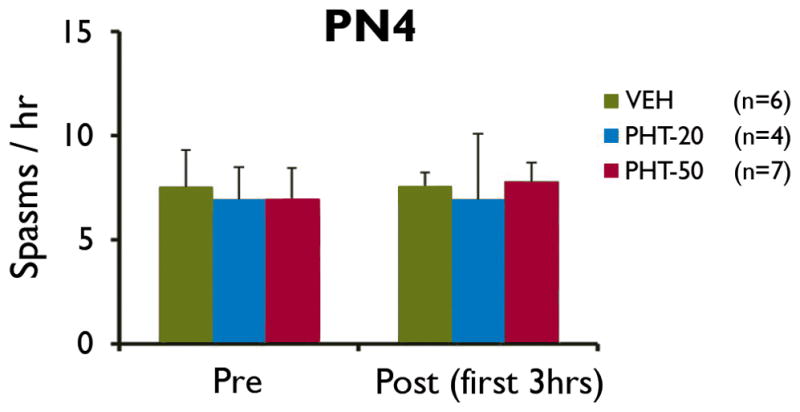
Administration of phenytoin (PHT-20, PHT-50) at PN4, after the onset of spasms, did not have an effect on the frequencies of behavioral spasms compared to vehicle-treated pups (VEH).
DISCUSSION
We show that carisbamate, given after the onset of spasms, suppresses acutely both behavioral (PN4) and electroclinical spasms (PN6–7) in the multiple-hit rat model of SIS, dose-dependently. The carisbamate effect is not via voltage-sensitive sodium channel blockade, as phenytoin was ineffective on spasms. The acute antiepileptic effect of carisbamate on IS, warrants further evaluation of repetitive, prolonged carisbamate administration to confirm if suppression of spasms is sustained and followed by improved long-term neurodevelopmental outcomes in DLP pups, since clinical studies have linked early cessation of IS with improved long-term cognitive outcome (Lux et al. 2004).
A single injection of carisbamate improved EEG, by reducing the electroclinical spasms, but not the electrographic ictal-like EEG patterns or IEDs. This contrasts with its effects in the genetic absence epilepsy rats (GAERS) and the low magnesium in vitro model, in which carisbamate suppressed IEDs acutely, suggesting antiepileptogenic effects (Deshpande et al. 2008; Francois et al. 2008). Such findings support the distinct pathogenetic mechanisms implicated in IS as opposed to other types of seizures and interictal epileptic discharges. It is possible, however, that suppression of interictal epileptiform activities and EEG normalization, in the multiple-hit model of IS, may require longer exposure to carisbamate.
Our findings extend the spectrum of seizure and epilepsy syndromes, upon which carisbamate has shown preclinical efficacy, to include IS. In prior preclinical studies, carisbamate acutely suppressed limbic, myoclonic, tonic, wild running seizures, bursts of spike-wave discharges in GAERS, delayed kindling acquisition, reduced the frequency of spontaneous seizures in the kainic acid model and the development of spontaneous seizures in the lithium-pilocarpine model (White et al. 2006; Andre et al. 2007; Klein et al. 2007; Novak et al. 2007; Francois et al. 2008; Grabenstatter et al. 2008; Bialer et al. 2010). Antiepileptogenic effects were therefore suggested in the lithium-pilocarpine model (Andre et al. 2007). In contrast, no significant antiepileptic or antiepileptogenic effects were found in the fluid percussion post-traumatic rat epilepsy model, in which the observed neocortical and limbic seizures are also refractory to carbamazepine, an approved antiepileptic drug for focal onset epilepsies (Eastman et al. 2010; Eastman et al. 2011). Collectively, these preclinical studies support a broad-spectrum antiepileptic efficacy for carisbamate, but also acknowledge the presence of refractory types of epilepsy, as is the case with all existing antiepileptic drugs.
Clinical trials have provided efficacy data for generalized epilepsy with photoparoxysmal responses (Trenite et al. 2007) but also positive and negative results for focal-onset epilepsies (Faught et al. 2008; Bialer et al. 2010; Sperling et al. 2010). Two of the three published cohorts of patients with focal epilepsies studied in randomized, double-blind, placebo-controlled, add-on phase II or phase III clinical trials demonstrated efficacy for carisbamate, at doses equal or higher than 300 mg daily (Faught et al. 2008; Sperling et al. 2010). No significant effect was seen in the third cohort (Sperling et al. 2010), whereas seizure reduction only with very high doses was reported in a fourth, yet unpublished phase III clinical trial with higher doses of the drug (EPY-3013) (Bialer et al. 2010). Factors contributing to the modest effects of carisbamate in the adjunctive treatment of focal onset seizures in clinical as opposed to preclinical trials include species and population biological differences, study design (add-on rather than first treatment; drug interactions; inclusion of medically refractory patients in clinical trials), model-related differences, or heterogeneity of focal epilepsy syndromes and their etiologies in humans. Given the low incidence of IS in humans, multicenter studies will be required to proper adjust for these potential confounders.
The carisbamate doses required to suppress spasms in the multiple-hit rat model are comparable to those required to decrease spontaneous seizures in the kainic acid model (Grabenstatter et al. 2008), spike-wave discharges in the GAERS model and audiogenic seizures in Wistar rats (Francois et al. 2008), delay kindling acquisition and reduce post-kindling seizures (Klein et al. 2007), but lower than those exhibiting anti-epileptogenic effects in the lithium-pilocarpine model (Andre et al. 2007). The onset and duration of the therapeutic effect of a single carisbamate injection on spasms were also similar to those reported in adult GAERS (Francois et al. 2008), but shorter lasting than its effect on the kainic acid model of epilepsy (Grabenstatter et al. 2008), reflecting model or age-specific differences. However, there are no clinical studies in human infants to permit safe extrapolations of carisbamate dose equivalence.
Carisbamate suppressed spasms at doses that did not produce any significant sedation, mortality, or abnormalities in neurodevelopmental reflexes in the multiple-hit rat model. Our observations are in agreement with those in GAERS adult rats in which ataxia and sedation were only observed if carisbamate exceeded 120mg/kg (Francois et al. 2008). In the clinical trials, dropout rates due to adverse effects (somnolence, dizziness) ranged between 4–6% of patients treated with 300–400 mg po daily and increased significantly higher than in the placebo group only when carisbamate exceeded 750 mg/day (Trenite et al. 2007; Faught et al. 2008; Sperling et al. 2010). Good tolerability with only mild drug-related side effects (headache, dizziness) was seen in healthy or nonepileptic controls (Levy et al. 2008; Zannikos et al. 2009; Elble et al. 2010). Further documentation of short and long-term safety profile in both pups and human infants with IS is warranted.
The suppressing effect of a single carisbamate injection on spasms lasted only up to 3 hours, with the higher doses. Elimination half life and drug metabolism differs among species, being shorter in adult rats (5.6 – 6.6 hours) than in adult humans (10.9 – 13.3 hours) (Mamidi et al. 2007; Mannens et al. 2007; Levy et al. 2008; Zannikos et al. 2009). We anticipate therefore that the duration of biological effect of carisbamate in humans would be double than the one observed in rodents. Pharmacokinetic/pharmacodynamic studies of carisbamate in infants, whether rodents or humans, need to be done, both with single and repetitive dosing, given the well established age-related differences seen with other drugs (Kaye et al. 2001; Allegaert et al. 2008) and the potential for self- or drug-related induction of carisbamate metabolism (Chien et al. 2006; Bialer et al. 2010; Sperling et al. 2010; Eastman et al. 2011).
Although sodium channel blockade has been one of the proposed mechanisms of action for carisbamate (Liu et al. 2009; Whalley et al. 2009), suppression of spasms by carisbamate was not reproduced by the sodium channel blocker phenytoin. This is in agreement with the ineffectiveness of phenytoin as a therapy for infants with IS (Haines et al. 1994; Mackay et al. 2004). Similar dissociation between carisbamate and phenytoin effects has been described for the spontaneous recurrent epileptiform discharges in cultured hippocampal neurons (Deshpande et al. 2008), whereas carisbamate has exhibited antiepileptic effects in other models of seizures that do not respond to sodium channel blockers, such as in GAERS, 6Hz seizure model or the lamotrigine-resistant amygdala kindled model (White et al. 2006; Francois et al. 2008). Carisbamate has been reported to increase Cl- conductances, via a pathway antagonized by the GABAA receptor antagonist picrotoxin (Whalley et al. 2009), although so far only at very high carisbamate concentrations (Whalley et al. 2009). Further studies are needed to decipher the carisbamate targets amidst the networks that control spasms, including, but not limited to, GABAA receptors and/or Cl- channels or cotransporters. Other possible mechanisms worth exploring include effects on neurovascular interactions or histopathology (i.e. gliosis, inflammation, cellular injury). Neuroprotective effects of carisbamate on CA1, piriform and endorhinal cortex have been proposed in the lithium-pilocarpine model (Andre et al. 2007), whereas no effect on post-traumatic cerebral edema was seen in the fluid percussion model (Keck et al. 2007).
As spasms in the rat multiple-hit model are ACTH-refractory and only transiently sensitive to vigabatrin (Scantlebury et al. 2010), carisbamate appears to be a promising therapy also for medically-refractory IS. Further evaluation of the effects of repetitive administration of carisbamate in chronic models of IS will be required to determine if these antiepileptic effects are sustained and potentially can lead to disease modification. Our results need further confirmation in human patients with IS. The recent conflicting efficacy data derived from phase II and III clinical trials in patients with focal-onset seizures have generated hesitation in proceeding with further clinical trials on the antiepileptic effects of carisbamate. The scarcity of available therapies with acute efficacy on IS, however, makes it urgent to explore new potential therapies for this devastating epileptic syndrome of infants.
Acknowledgments
This work was supported by a grant from Johnson & Johnson Pharmaceutical Research & Development, LLC, Raritan, NJ, USA. Additional funding support was received from NINDS/NICHD grant NS62947; NINDS research grants NS20253, NS58303, NS45243 grants from People Against Childhood Epilepsy, the International Rett Syndrome Foundation, and the Heffer Family Foundation. We would like to acknowledge the excellent technical support by Qianyun Li, Wei Liu and Hong Wang. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Footnotes
CONFLICTS OF INTEREST
This work was supported by a grant from Johnson & Johnson Pharmaceutical Research & Development, LLC, Raritan, NJ, USA. Dr Moshé has received consultancy fees from Eisai. There is no other conflict of interest in the work reported in the present manuscript.
References
- Allegaert K, Verbesselt R, Naulaers G, van den Anker JN, Rayyan M, Debeer A, de Hoon J. Developmental pharmacology: neonates are not just small adults. Acta Clin Belg. 2008;63(1):16–24. doi: 10.1179/acb.2008.003. [DOI] [PubMed] [Google Scholar]
- Andre V, Dube C, Francois J, Leroy C, Rigoulot MA, Roch C, Namer IJ, Nehlig A. Pathogenesis and pharmacology of epilepsy in the lithium-pilocarpine model. Epilepsia. 2007;48(Suppl 5):41–47. doi: 10.1111/j.1528-1167.2007.01288.x. [DOI] [PubMed] [Google Scholar]
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, Engel J, French J, Glauser TA, Mathern GW, Moshe SL, Nordli D, Plouin P, Scheffer IE. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51(4):676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. Progress report on new antiepileptic drugs: A summary of the Tenth Eilat Conference (EILAT X) Epilepsy Res. 2010:89–124. doi: 10.1016/j.eplepsyres.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Bittigau P, Sifringer M, Genz K, Reith E, Pospischil D, Govindarajalu S, Dzietko M, Pesditschek S, Mai I, Dikranian K, Olney JW, Ikonomidou C. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci U S A. 2002;99(23):15089–15094. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien S, Bialer M, Solanki B, Verhaeghe T, Doose DR, Novak G, Yao C. Pharmacokinetic interaction study between the new antiepileptic and CNS drug RWJ-333369 and carbamazepine in healthy adults. Epilepsia. 2006;47(11):1830–1840. doi: 10.1111/j.1528-1167.2006.00815.x. [DOI] [PubMed] [Google Scholar]
- Chudomelova L, Scantlebury MH, Raffo E, Coppola A, Betancourth D, Galanopoulou AS. Modeling new therapies for infantile spasms. Epilepsia. 2010;51(Suppl 3):27–33. doi: 10.1111/j.1528-1167.2010.02605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez MA, Shen L, Wu Y, Aleem IS, Trepanier CH, Sadeghnia HR, Ashraf A, Kanawaty A, Liu CC, Stewart L, Snead OC., 3rd Infantile spasms and Down syndrome: a new animal model. Pediatr Res. 2009;65(5):499–503. doi: 10.1203/PDR.0b013e31819d9076. [DOI] [PubMed] [Google Scholar]
- Deshpande LS, Nagarkatti N, Sombati S, DeLorenzo RJ. The novel antiepileptic drug carisbamate (RWJ 333369) is effective in inhibiting spontaneous recurrent seizure discharges and blocking sustained repetitive firing in cultured hippocampal neurons. Epilepsy Res. 2008;79(2–3):158–165. doi: 10.1016/j.eplepsyres.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CL, Verley DR, Fender JS, Stewart TH, Nov E, Curia G, D’Ambrosio R. Antiepileptic and antiepileptogenic performance of carisbamate after head injury in the rat: blind and randomized studies. J Pharmacol Exp Ther. 2011;336(3):779–790. doi: 10.1124/jpet.110.175133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CL, Verley DR, Fender JS, Temkin NR, D’Ambrosio R. ECoG studies of valproate, carbamazepine and halothane in frontal-lobe epilepsy induced by head injury in the rat. Exp Neurol. 2010;224(2):369–388. doi: 10.1016/j.expneurol.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elble RJ, Biondi DM, Ascher S, Wiegand F, Hulihan J. Carisbamate in essential tremor: brief report of a proof of concept study. Mov Disord. 2010;25(5):634–638. doi: 10.1002/mds.22872. [DOI] [PubMed] [Google Scholar]
- Faught E, Holmes GL, Rosenfeld WE, Novak G, Neto W, Greenspan A, Schmitt J, Yuen E, Reines S, Haas M. Randomized, controlled, dose-ranging trial of carisbamate for partial-onset seizures. Neurology. 2008;71(20):1586–1593. doi: 10.1212/01.wnl.0000334751.89859.7f. [DOI] [PubMed] [Google Scholar]
- Francois J, Boehrer A, Nehlig A. Effects of carisbamate (RWJ-333369) in two models of genetically determined generalized epilepsy, the GAERS and the audiogenic Wistar AS. Epilepsia. 2008;49(3):393–399. doi: 10.1111/j.1528-1167.2007.01278.x. [DOI] [PubMed] [Google Scholar]
- Grabenstatter HL, Dudek FE. A new potential AED, carisbamate, substantially reduces spontaneous motor seizures in rats with kainate-induced epilepsy. Epilepsia. 2008;49(10):1787–1794. doi: 10.1111/j.1528-1167.2008.01657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines ST, Casto DT. Treatment of infantile spasms. Ann Pharmacother. 1994;28(6):779–791. doi: 10.1177/106002809402800616. [DOI] [PubMed] [Google Scholar]
- Kaye CM, Allen A, Perry S, McDonagh M, Davy M, Storm K, Bird N, Dewit O. The clinical pharmacokinetics of a new pharmacokinetically enhanced formulation of amoxicillin/clavulanate. Clin Ther. 2001;23(4):578–584. doi: 10.1016/s0149-2918(01)80061-8. [DOI] [PubMed] [Google Scholar]
- Keck CA, Thompson HJ, Pitkanen A, LeBold DG, Morales DM, Plevy JB, Puri R, Zhao B, Dichter M, McIntosh TK. The novel antiepileptic agent RWJ-333369-A, but not its analog RWJ-333369, reduces regional cerebral edema without affecting neurobehavioral outcome or cell death following experimental traumatic brain injury. Restor Neurol Neurosci. 2007;25(2):77–90. [PMC free article] [PubMed] [Google Scholar]
- Klein BD, Smith MD, White HS. The novel neuromodulator carisbamate delays the acquisition of rat amygdala kindling and maintains acute antiepileptic activity when evaluated in post-kindled rats. Epilepsia. 2007;48(S6):367–368. [Google Scholar]
- Lee CL, Frost JD, Jr, Swann JW, Hrachovy RA. A new animal model of infantile spasms with unprovoked persistent seizures. Epilepsia. 2008;49(2):298–307. doi: 10.1111/j.1528-1167.2007.01377.x. [DOI] [PubMed] [Google Scholar]
- Levy R, Ragueneau-Majlessi I, Solanki B, Zannikos P, Yao C, Novak G. Pharmacokinetics, safety, and tolerability of the new antiepileptic carisbamate in the elderly. Epilepsy Res. 2008;79(1):22–30. doi: 10.1016/j.eplepsyres.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yohrling GJ, Wang Y, Hutchinson TL, Brenneman DE, Flores CM, Zhao B. Carisbamate, a novel neuromodulator, inhibits voltage-gated sodium channels and action potential firing of rat hippocampal neurons. Epilepsy Res. 2009;83(1):66–72. doi: 10.1016/j.eplepsyres.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Lux AL, Edwards SW, Hancock E, Johnson AL, Kennedy CR, Newton RW, O’Callaghan FJ, Verity CM, Osborne JP. The United Kingdom Infantile Spasms Study comparing vigabatrin with prednisolone or tetracosactide at 14 days: a multicentre, randomised controlled trial. Lancet. 2004;364(9447):1773–1778. doi: 10.1016/S0140-6736(04)17400-X. [DOI] [PubMed] [Google Scholar]
- Lux AL, Edwards SW, Hancock E, Johnson AL, Kennedy CR, Newton RW, O’Callaghan FJ, Verity CM, Osborne JP. The United Kingdom Infantile Spasms Study (UKISS) comparing hormone treatment with vigabatrin on developmental and epilepsy outcomes to age 14 months: a multicentre randomised trial. Lancet Neurol. 2005;4(11):712–717. doi: 10.1016/S1474-4422(05)70199-X. [DOI] [PubMed] [Google Scholar]
- Mackay MT, Weiss SK, Adams-Webber T, Ashwal S, Stephens D, Ballaban-Gill K, Baram TZ, Duchowny M, Hirtz D, Pellock JM, Shields WD, Shinnar S, Wyllie E, Snead OC., 3rd Practice parameter: medical treatment of infantile spasms: report of the American Academy of Neurology and the Child Neurology Society. Neurology. 2004;62(10):1668–1681. doi: 10.1212/01.wnl.0000127773.72699.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamidi RN, Mannens G, Annaert P, Hendrickx J, Goris I, Bockx M, Janssen CG, Kao M, Kelley MF, Meuldermans W. Metabolism and excretion of RWJ-333369 [1,2-ethanediol, 1-(2-chlorophenyl)-, 2-carbamate, (S)-] in mice, rats, rabbits, and dogs. Drug Metab Dispos. 2007;35(4):566–575. doi: 10.1124/dmd.106.012336. [DOI] [PubMed] [Google Scholar]
- Mannens GS, Hendrickx J, Janssen CG, Chien S, Van Hoof B, Verhaeghe T, Kao M, Kelley MF, Goris I, Bockx M, Verreet B, Bialer M, Meuldermans W. The absorption, metabolism, and excretion of the novel neuromodulator RWJ-333369 (1,2-ethanediol, [1-2-chlorophenyl]-, 2-carbamate, [S]-) in humans. Drug Metab Dispos. 2007;35(4):554–565. doi: 10.1124/dmd.106.011940. [DOI] [PubMed] [Google Scholar]
- Mares P, Velisek L. N-methyl-D-aspartate (NMDA)-induced seizures in developing rats. Brain Res Dev Brain Res. 1992;65(2):185–189. doi: 10.1016/0165-3806(92)90178-y. [DOI] [PubMed] [Google Scholar]
- Marsh E, Fulp C, Gomez E, Nasrallah I, Minarcik J, Sudi J, Christian SL, Mancini G, Labosky P, Dobyns W, Brooks-Kayal A, Golden JA. Targeted loss of Arx results in a developmental epilepsy mouse model and recapitulates the human phenotype in heterozygous females. Brain. 2009;132(Pt 6):1563–1576. doi: 10.1093/brain/awp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak GP, Kelley M, Zannikos P, Klein B. Carisbamate (RWJ-333369) Neurotherapeutics. 2007;4(1):106–109. doi: 10.1016/j.nurt.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellock JM, Hrachovy R, Shinnar S, Baram TZ, Bettis D, Dlugos DJ, Gaillard WD, Gibson PA, Holmes GL, Nordli DR, O’Dell C, Shields WD, Trevathan E, Wheless JW. Infantile spasms: A U.S. consensus report. Epilepsia. 2010 doi: 10.1111/j.1528-1167.2010.02657.x. [DOI] [PubMed] [Google Scholar]
- Price MG, Yoo JW, Burgess DL, Deng F, Hrachovy RA, Frost JD, Jr, Noebels JL. A triplet repeat expansion genetic mouse model of infantile spasms syndrome, Arx(GCG)10+7, with interneuronopathy, spasms in infancy, persistent seizures, and adult cognitive and behavioral impairment. J Neurosci. 2009;29(27):8752–8763. doi: 10.1523/JNEUROSCI.0915-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scantlebury MH, Galanopoulou AS, Chudomelova L, Raffo E, Betancourth D, Moshe SL. A model of symptomatic infantile spasms syndrome. Neurobiol Dis. 2010;37(3):604–612. doi: 10.1016/j.nbd.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling MR, Greenspan A, Cramer JA, Kwan P, Kalviainen R, Halford JJ, Schmitt J, Yuen E, Cook T, Haas M, Novak G. Carisbamate as adjunctive treatment of partial onset seizures in adults in two randomized, placebo-controlled trials. Epilepsia. 2010;51(3):333–343. doi: 10.1111/j.1528-1167.2009.02318.x. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE. Infantile spasms: a critical review of emerging animal models. Epilepsy Curr. 2009;9(3):75–81. doi: 10.1111/j.1535-7511.2009.01299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenite DG, French JA, Hirsch E, Macher JP, Meyer BU, Grosse PA, Abou-Khalil BW, Rosenfeld WE, van Gerven J, Novak GP, Parmeggiani L, Schmidt B, Gibson D, Guerrini R. Evaluation of carisbamate, a novel antiepileptic drug, in photosensitive patients: an exploratory, placebo-controlled study. Epilepsy Res. 2007;74(2–3):193–200. doi: 10.1016/j.eplepsyres.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Velisek L, Jehle K, Asche S, Veliskova J. Model of infantile spasms induced by N-methyl-D-aspartic acid in prenatally impaired brain. Ann Neurol. 2007;61(2):109–119. doi: 10.1002/ana.21082. [DOI] [PubMed] [Google Scholar]
- Whalley BJ, Stephens GJ, Constanti A. Investigation of the effects of the novel anticonvulsant compound carisbamate (RWJ-333369) on rat piriform cortical neurones in vitro. Br J Pharmacol. 2009;156(6):994–1008. doi: 10.1111/j.1476-5381.2008.00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HS, Srivastava A, Klein B, Zhao B, Choi YM, Gordon R, Lee SJ. The novel investigational neuromodulator RWJ 333369 displays a broad-spectrum anticonvulsant profile in rodent seizure and epilepsy models. Epilepsia. 2006;47(S4):200–201. [Google Scholar]
- Zannikos P, Novak G, Yao C, Verhaeghe T, Franc MA, Solanki B, Bialer M. Pharmacokinetics of carisbamate (RWJ-333369) in healthy Japanese and Western subjects. Epilepsia. 2009;50(8):1850–1859. doi: 10.1111/j.1528-1167.2009.02081.x. [DOI] [PubMed] [Google Scholar]