Abstract
Rationale
Chronic cocaine exposure has been associated with progressive brain structural and functional changes. Clarifying mechanisms underlying cocaine’s progressive brain effects may help in the development of effective cocaine abuse treatments.
Objectives
We used a controlled squirrel monkey model of chronic cocaine exposure (45 mg/kg/week for 9 months) combined with ultra-high magnetic field (9.4 T) proton magnetic resonance spectroscopy to prospectively measure putamen metabolite changes.
Methods
Proton metabolites were measured with a STEAM sequence, quantified with LCModel using a simulated basis set, and expressed as metabolite/total creatine (tCr) ratios.
Results
We found cocaine-induced time-dependent changes in putamen glutamate/tCr and glutamine/tCr metabolite ratios suggestive of altered glutamate compartmentalization, neurotransmission, and metabolism. By contrast, saline-treated monkeys exhibited no metabolite changes over time. The time course of cocaine-induced metabolite abnormalities we detected is consistent with the apparent time course of glutamate abnormalities identified in a cross-sectional study in human cocaine users, as well as with microdialysis findings in rodent models of repeated cocaine exposure.
Conclusions
Together, these findings suggests that this squirrel monkey model may be useful for characterizing glutamatergic changes associated with cocaine exposure and for determining efficacies of treatments designed to mitigate cocaine-induced glutamatergic system dysfunction.
Keywords: Cocaine, Glutamate, Magnetic resonance spectroscopy, Animal model, Neuroimaging
Introduction
Chronic cocaine exposure has been associated with abnormal brain structure, function, chemistry, and cognition. A number of cocaine-associated brain and cognitive changes appear to be progressive in nature, with cumulative dose- or duration-effect relationships having been reported between lifetime cocaine exposure and brain structural changes (Liu et al. 1998; Matochik et al. 2003; O’Neill et al. 2001; Pascual-Leone et al. 1991; Sim et al. 2007), neurochemistry (Yang et al. 2009), cerebrovascular dysfunction (Kaufman et al. 1998), and cognitive abnormalities (Ardila et al. 1991; Beatty et al. 1995; Bolla et al. 2000, 1999; Carpenter and Hittner 1997; Colzato et al. 2007; O’Malley et al. 1992; Rosselli and Ardila 1996). The findings from these and other studies suggest that characterizing mechanisms underlying progression of cocaine’s brain and behavioral effects may help in the development of effective cocaine abuse treatments.
A major impediment to determining which effects to characterize is the inability to definitively link human chronic cocaine use to specific biological or behavioral changes; human cocaine users typically are exposed to other psychoactive substances that moderate cocaine’s effects (e.g., alcohol, smoking, and/or other illicit drugs) (Brecht et al. 2008; Regier et al. 1990), to unknown and potentially psychoactive and/or toxic adulterants in illicit drug samples (Cunningham et al. 1984; Klatt et al. 1986; Neiman et al. 2000), and/or may have confounding premorbid/comorbid psychiatric (Regier et al. 1990), or general health conditions (Chen et al. 1996). While confounding effects can be reduced by conducting within-subjects repeated-measures studies, there is a high loss-to-follow-up rate in most human longitudinal substance abuse research studies. This has motivated the development of animal models of chronic cocaine exposure.
Results from controlled studies in rodents and nonhuman primates have been informative. Rodent studies indicated that chronic cocaine exposure progressively altered cerebrovascular and brain structure (Barroso-Moguel et al. 2002; Barroso-Moguel et al. 1997) and neural responsivity to acute cocaine challenge (Febo et al. 2005), and it reduced frontal cortex neuronal and oligodendrocyte densities, the degree of which correlated with working memory impairments (George et al. 2008). Controlled nonhuman primate cocaine administration studies confirm that chronic cocaine exposure changes brain chemistry and metabolism and, in some cases, appears to do so in a progressive manner (Beveridge et al. 2006, 2004, 2005; Bradberry 2000; Daunais et al. 1997; Fagergren et al. 2003; Freeman et al. 2001; Letchworth et al. 2001; Macey et al. 2003; Moore et al. 1998a, b; Nader et al. 2002, 2006; Porrino et al. 2002, 2004). Thus, human and animal models consistently demonstrate that chronic cocaine exposure causes progressive brain damage and dysfunction. Recently, it was suggested that nonhuman primate models may more closely parallel findings in humans than rodent models (Bradberry 2008).
Presently, we used in vivo proton magnetic resonance spectroscopy (MRS) at 9.4 T, which resolves glutamate, glutamine, and GABA in addition to other metabolites that can be detected at lower magnetic field strengths, to characterize effects of chronic cocaine exposure in nonhuman primates (squirrel monkeys). Because MRS is noninvasive, it is suitable for longitudinal studies and for studies in humans. MRS has been used previously in cross-sectional studies to document cocaine’s acute (Christensen et al. 2000), chronic (Chang et al. 1999, 1997; Ke et al. 2004; Li et al. 1999; Meyerhoff et al. 1999; O’Neill et al. 2001; Streeter et al. 2005; Yang et al. 2009), and prenatal (Smith et al. 2001) effects in humans. To our knowledge, MRS has not been applied in nonhuman primate studies of cocaine’s effects. This study reports single-voxel MRS findings in putamen obtained at baseline (prior to cocaine/ saline exposure) and serially for up to 9 months of daily cocaine or saline exposure. The putamen was selected because previous nonhuman primate chronic cocaine exposure studies documented time-dependent changes in dopamine transporter levels (Letchworth et al. 2001) and local cerebral glucose utilization (Porrino et al. 2004). Prior MRS studies of cocaine’s chronic effects reported abnormal N-acetylaspartate (NAA) levels which may reflect neuronal or mitochondrial loss (Chang et al. 1999; Li et al. 1999) as well as abnormal myo-inositol (Ino) levels, which may reflect glial activation (Chang et al. 1999). More recently, abnormal glutamate (Glu) levels were reported (Yang et al. 2009) that may reflect abnormalities in glutamate neurotransmission and/or bioenergetics (Gruetter et al. 1998); moreover, there was an apparent time dependence to the Glu effect such that longer cocaine use careers were associated with higher Glu levels (Yang et al. 2009). Additionally, we reported finding reduced GABA levels which may reflect abnormal inhibitory neurotransmission (Ke et al. 2004) and also reported that GABA levels normalized with effective cocaine-dependence treatment (Streeter et al. 2005). Accordingly, we explored with a well-controlled nonhuman primate model of chronic cocaine exposure whether cocaine would induce time-dependent changes in putamen Glu, GABA, and other metabolite ratios.
Methods and materials
Animals
Eight adult male squirrel monkeys (Saimiri boliviensis, 4–5 years old, weighing 700–1,000 g at the start of the study) were included in this study. Monkeys were housed in stainless steel cages and had ad libitum access to water and ad libitum access to food (commercial monkey pellets, LabDiet 4045, supplemented with fresh fruits, peanuts, and carrots). After an initial quarantine period, monkeys were singly housed for 4–6 weeks in adjacent cages to acclimate to the vivarium and then pair housed for the duration of the cocaine/saline administration part of the experiment. The vivarium maintained a 12-h light/dark cycle with lights on from 7 AM to 7 PM. Prior to beginning the study, monkeys were trained to be hand caught to voluntarily allow chain attachment and leave cages on a pole, without sedation, for drug infusions. Monkeys were administered cocaine (N=4) or saline (N=4) via intramuscular (i.m.) injections, alternating left and right thighs daily, three times per day, 5 days per week, for 9 months. Cocaine-exposed monkeys received an initial cocaine dose of 1 mg/kg/injection for 1 week followed by 2 mg/kg/ injections during week 2. They were maintained chronically on 3 mg/kg/injections (45 mg/kg/week) for 8.5 months, to simulate weekly cocaine self-administered doses reported in human studies (Liu et al. 1998). All procedures were conducted in compliance with established guidelines as described in the National Institutes of Health Guide for Care and Use of Laboratory Animals. In addition, all procedures were reviewed and approved by the McLean Hospital Institutional Animal Care and Use Committee.
Magnetic resonance scans
Magnetic resonance (MR) scans were acquired using a 9.4 T horizontal bore system (Varian Direct Drive, Varian Inc, Palo Alto, CA, USA) using the software version Vnmrj 2.3A. A 12-cm inner diameter gradient insert was used with maximum gradient strength of 40 G/cm and minimum rise time of 140 μs. A custom-made proton (400 MHz) quadrature volume coil was used in this study. The coil was geometrically designed for squirrel monkey brain scans to optimize magnetic field homogeneity across the monkey brain. Each coil channel was tuned and matched separately.
MR imaging and proton spectroscopy (MRS) scans were acquired at baseline, before exposure to cocaine or saline and serially after 1, 3, 6, and 9 months of chronic cocaine/saline exposure. For scanning, monkeys were transported from the vivarium to the scanner suite, sedated with i.m. ketamine (25 mg/kg) using the pole and collar injection technique, intubated, and anesthetized and maintained with 1.5–2% isoflurane gas. A circulating warm-water blanket was used to maintain body temperature during scans. Monkeys were scanned in the prone position in an MRI-compatible monkey holder, which enabled reproducible head positioning in serial scans. Vital signs including heart rate, respiration rate, body temperature, and oxygen saturation (SPO2) were monitored and maintained during scans. To avoid a potentially confounding effect of acute cocaine administration on MRS measures (Christensen et al. 2000), spectra were acquired at least 2.5 h after cocaine injections, by when cocaine’s acute cardiovascular effects (Schindler et al. 2001) and striatal dopamine elevations (Bauzo et al. 2009; Czoty et al. 2000; Kimmel et al. 2005) have resolved.
Monkeys were scanned with the following pulse sequences: a gradient-echo imaging sequence (repetition time (TR)/echo time (TE)=200 ms/4.75 ms, flip angle=15°, Field of view = 60 × 60 mm, acquisition matrix = 256 × 256, number of averages=4) was used to acquire structural images in coronal and sagittal planes for MRS voxel placement; a T2-weighted fluid-attenuated inversion recovery (FLAIR) sequence (TR/TE=7,000 ms/80 ms, inversion time= 2,400 ms) was used to detect structural abnormalities; and a STEAM (STimulated Echo Acquisition Mode) proton MRS sequence was used to acquire single-voxel proton spectra (TE/mixing time TM/TR of 9.7/7/ 4,000 ms and 128 signal averages). The MRS voxel dimension was 6×6×6 mm (0.216 cm3) and it was positioned over the right putamen (Fig. 1). A 3D gradient-echo automatic voxel shimming scheme was applied resulting in unsuppressed water line widths of 11–13 Hz. A VAPOR scheme (Tkac et al. 1999) was used to suppress the water signal. For each scan, a two-iteration optimization for VAPOR scheme was performed to minimize the water peak.
Fig. 1.
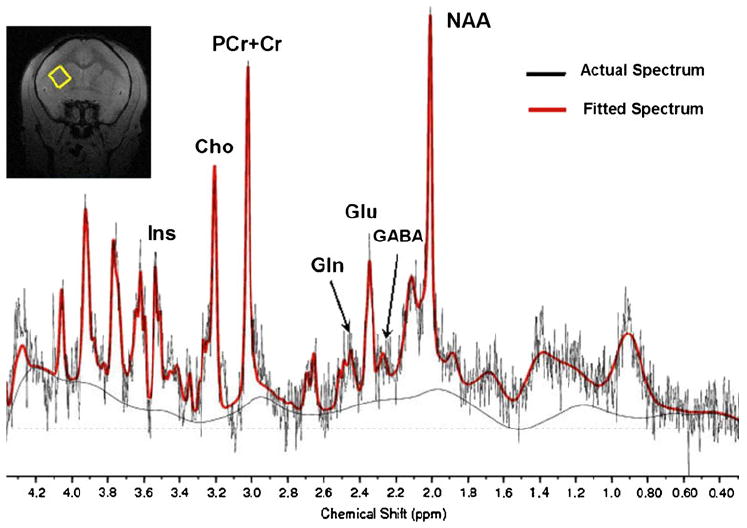
A typical proton spectrum (with no line broadening) is shown. Metabolites highlighted are myo-inositol (Ins), choline-containing compounds (Cho), total creatine (tCr = phosphocreatine (PCr) + creatine (Cr)), Glutamine (Gln), Glutamate (Glu), γ-aminobutyric acid (GABA), and N-acetylaspartate (NAA). The red trace shows the LCModel fit of the data
MRS data analysis
MRS data was processed using customized Matlab (Mathworks, Natick, MA, USA) script. Initial-phase correction (Waddell et al. 2007) was performed before LCModel raw data was generated. Resultant spectra were automatically fitted in LCModel 6.1 (Provencher 1993) using a simulated basis set. The simulated basis set was generated using a density-matrix simulation in GAVA (Soher et al. 2007), including metabolites frequently measured in vivo as suggested in the LCModel manual (http://s-provencher.com/pages/lcm-manual.shtml). The chemical shifts and coupling constants of the metabolites included in this basis set are based on previous literature (Govindaraju et al. 2000). Other essential parameters including TE and TM were identical to parameters used in in vivo scans. Spectral fitting was performed automatically to avoid operator bias. Metabolite ratios for different metabolites were referenced to total creatine (tCr). A 2-factor repeated measures analysis of variance (ANOVA) was used to detect effects of treatment and time. Statistical analyses were performed in ezANOVA (http://www.cabiatl.com/mricro/ezanova/index.html) and Prism (GraphPad Software, La Jolla, CA, USA).
Results
Saline and cocaine monkey group baseline weights averaged 815±30 and 790±143 g, respectively. Monkeys in both treatment groups maintained body weights throughout the study (one-way within subject ANOVA, F4,12=1.06 p<0.42 for saline controls and F4,12=1.36 p<0.30 for cocaine monkeys). Monkey behaviors during and after drug injections were observed (but were not video recorded or scored formally or blindly). During drug injection, weeks 1–2, cocaine-treated monkeys exhibited short-term continuous motion in their home cages, and transient muscle twitching and rapid eye movements that lasted up to 1 h after cocaine treatment. At week 3 and beyond, cocaine-treated monkeys exhibited episodes of stereotypic behaviors including muscle contractions and fixed glances lasting up to 1 h. These behaviors were not observed in saline-treated monkeys. All four cocaine-treated animals readily presented themselves for cocaine injections with minimal delay, while saline-treated monkeys did not.
Examination of gradient echo and FLAIR structural images in all monkeys at all time points did not reveal any gross structural abnormalities or evidence of white matter hyperintensities, indicating that the chronic cocaine exposure paradigm used in this study did not induce development of structural abnormalities for up to 9 months of exposure. A typical proton spectrum is shown in Fig. 1. Metabolites of interest were well resolved at 9.4 T without spectral editing. The Cramer-Rao Lower Bounds (CRLBs) for glutamate (Glu), N-acetylaspartate (NAA), myo-inositol (Ins), and total creatine (tCr = phosphocreatine (PCr) + creatine (Cr)) in all monkeys were less than 10%. The CRLBs of γ-aminobutyric acid (GABA) and glutamine (Gln) were less than 20%.
To analyze for metabolite ratio main effects, two-way (treatment, time) within-subjects repeated measures ANOVAs were run for all metabolite ratios. For NAA, Ins, and GABA metabolite ratios, there were no significant main effects of treatment, time point, or treatment × time point interactions (Fs<1.9, Ps>0.14). However, significant treatment × time interaction effects were found for glutamate (Glu/tCr, F4,24=15.4, P<0.0001) and glutamine (Gln/tCr, F4,24=3.0, P<0.039) metabolite ratios (Fig. 2). Post-hoc pairwise two-sided t test comparisons revealed that in cocaine-treated monkeys, 1-month Glu/tCr ratios were reduced from baseline (t(3)=4.91, P<0.02) and 6 and 9 month Glu/tCr ratios were increased from baseline (t(3)= 5.35, P<0.02 and t(3)=9.81, P<0.003, respectively, Fig. 2). The Glu/tCr metabolite ratio in 9-month cocaine-treated monkeys also was greater than either the baseline or the 9-month ratios in saline-treated monkeys (t(6)>4.27, P<0.006, Fig. 2). Post-hoc pairwise two-sided t test comparisons also revealed that in cocaine-treated monkeys, 9-month Gln/tCr ratios were increased versus cocaine (t(3)= 4.73, P<0.02) and saline group baselines (t(6)=4.35, P<0.005) and versus 9-month ratios in saline-treated monkeys (t(6)=3.38, P<0.02, Fig. 2).
Fig. 2.
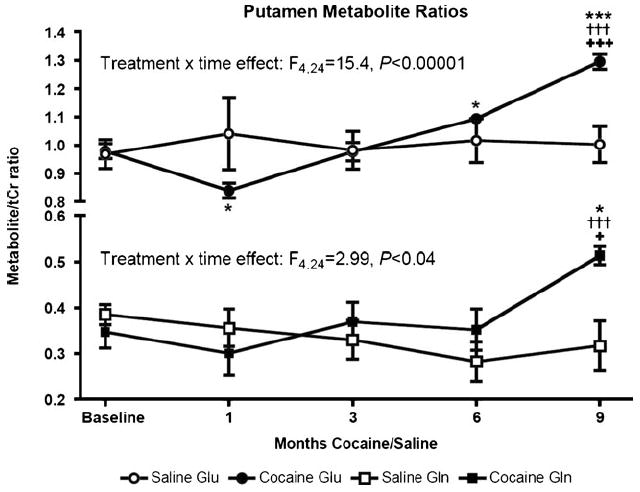
Time courses of Glu/tCr and Gln/tCr metabolite ratios in monkeys administered saline (N=4) and cocaine (N=4). Shown are means and standard errors of the mean. Post-hoc pair-wise two-sided t test contrasts legend: ***P<0.005 vs. cocaine group baseline; *P<0.05 vs. cocaine group baseline; †††P< 0.005 vs. saline group baseline; +++P<0.01 vs. saline group 9-month measurement; +P<0.05 vs. saline group 9-month measurement
Discussion
Using a well-controlled model of chronic cocaine exposure unconfounded by polydrug abuse, contaminants, or premorbid/comorbid health or psychiatric conditions, we found cocaine-induced time-dependent changes in putamen Glu/tCr and Gln/tCr metabolite ratios. In cocaine-treated monkeys, the Glu/tCr ratio declined at 1-month cocaine exposure and thereafter exhibited a linear increase with exposure duration up to 9 months (Fig. 2). This glutamate effect parallels the apparent time course of anterior cingulate cortex glutamate metabolite abnormalities (initial decrease followed by progressive increase with increased cocaine use duration), identified in a human cross-sectional study involving subjects reporting 6–20 years cocaine use (Yang et al. 2009). Notably, that study also reported a selective effect of cocaine on glutamate metabolite ratios. Although our study measured proton metabolites in putamen, the comparability of the time course of our findings to those reported by Yang et al. Yang et al. (2009) suggests that our squirrel monkey model may have promise for studying cocaine-induced glutamate metabolite abnormalities and treatments intended to mitigate them.
Proton MRS is not sensitive enough to detect the synaptic and extracellular glutamate (low micromolar concentrations) levels and changes typically reported in neurochemical studies of cocaine’s acute and chronic effects. Rather, proton MRS glutamate measurements reflect primarily intracellular glutamate (low millimolar range), which is present in neuronal and glial metabolic and neurotransmitter pools (Gruetter et al. 1998). The Glu/tCr reduction we detected after 1 month of cocaine exposure may reflect decreased intracellular glutamate in metabolic pools. In this regard, rhesus monkeys self-administering cocaine for an extended period (100 days) exhibited reduced putamen glucose utilization when measured shortly after the last cocaine self-administration session (Porrino et al. 2004). The Glu/tCr ratio increases we detected at 6 and 9 months cocaine exposure, in combination with the increased Gln/tCr ratios we detected at 9 months, may reflect increased intracellular glutamate in metabolic and neurotransmitter pools, since a glutamine increase could imply increased glutamate neurotransmission.
Although our measurements cannot differentiate which pools intracellular glutamate and glutamine signals arise from, our findings are consistent with reports of abnormal glutamate compartmentalization and release in striatal regions and in several other brain areas as a consequence of chronic or repeated cocaine exposure. For example, in nucleus accumbens, repeated cocaine exposures decreased activity of the cystine–glutamate antiporter, which extrudes glial intracellular glutamate into the extracellular space (Baker et al. 2002a). That effect would be expected to increase glial intracellular glutamate levels. If the effect occurs in dorsal striatum, in which extracellular glutamate levels also have been shown to be maintained by the glial cystine–glutamate antiporter (Baker et al. 2002b), this could increase glial glutamate concentration and the proton MRS glutamate signal. Indeed, recent evidence suggests that chronic cocaine exposure decreases basal striatal extracellular glutamate levels (Yablonsky-Alter et al. 2009) which likely reflects a striatal glutamate homeostasis abnormality similar to the one reported in nucleus accumbens. Repeated cocaine exposure also has been associated with increased cocaine-induced glutamate release in striatum (Lee et al. 2008; Yablonsky-Alter et al. 2009) and nucleus accumbens (Suto et al. 2010) when cocaine was administered soon after the last repeated cocaine dose. Similar effects were observed in medial prefrontal cortex in rats administered KCl to evoke neuronal glutamate release soon after the last repeated dose of cocaine (Xie and Steketee 2008). Together, these findings suggest that repeated cocaine exposures increase neuronal glutamate concentrations in several brain areas. This effect, in combination with reduced activity of the glial cystine–glutamate transporter, would be expected to increase proton MRS glutamate signals concentrations in those brain areas. Thus, our findings of increased putamen Glu/tCr and Gln/tCr metabolite ratios with chronic cocaine exposure may reflect chronic cocaine-induced glutamate compartmentalization abnormalities.
The present findings are interesting in light of the fact that it has been shown in squirrel monkeys that cocaine self-administration is reduced by antagonists of mGluR5/N-methyl-D-aspartate (Platt et al. 2008) and mGluR2/3 (Bauzo et al. 2009) glutamate receptors. The efficacies of these antagonists may be related to their ability to block glutamate receptors that might otherwise be overstimulated by increased evoked glutamate release in subjects exposed repeatedly to chronic cocaine (Lee et al. 2008; Yablonsky-Alter et al. 2009). Given that certain cocaine abuse treatments under development are designed to correct glutamate homeostasis abnormalities, including N-acetylcysteine and ceftriaxone (Kalivas et al. 2009; Knackstedt et al. 2010; Sari et al. 2009), it appears that proton MRS and possibly other MRS modalities (e.g., carbon-13 MRS) may be useful for further characterizing effects of chronic cocaine and how novel therapeutic drugs alter glutamate compartmentalization.
Based on our human study findings (Ke et al. 2004; Streeter et al. 2005), we anticipated that we also would observe cocaine-induced GABA metabolite changes over time, which we did not detect. This apparent discrepancy could result from a number of factors including species differences, from the fact that our human GABA findings were observed in a different brain area (prefrontal cortex), from one or more of the potentially confounding effects in human studies (e.g., the majority of cocaine abusers in our human studies also had lifetime histories of alcohol abuse or dependence), or from the fact that cocaine exposure durations in the present study were shorter (extrapolated to several human years) than in our human studies, in which subjects reported mean cocaine use durations exceeding 15 years (Ke et al. 2004; Streeter et al. 2005). In fact, most human studies reporting cocaine’s chronic effects on brain structure, function, or cognition typically enrolled users reporting 10 to 20-year exposure durations. Thus, it is possible that with continued cocaine exposure, other proton metabolite abnormalities could emerge along with brain structural changes.
Limitations
Because at the time of study initiation, we had not implemented magnetic resonance spectroscopic imaging, which enables proton MRS data to be acquired from multiple voxels simultaneously, and because of limits on anesthesia time in this study, we only acquired single voxel proton MRS data from putamen. Thus, it is unclear whether the metabolite effects we observed are selective for putamen or are generalizable to other areas such as anterior cingulate cortex. However, as noted above, we selected putamen because it had been shown previously to exhibit time-dependent effects of cocaine in chronic self-administration studies in macaques (Letchworth et al. 2001; Porrino et al. 2004).
In addition, we expressed metabolites as ratios to tCr, a common practice in this area of research (Christensen et al. 2000; Ke et al. 2004; Li et al. 1999; Meyerhoff et al. 1999; Smith et al. 2001; Streeter et al. 2005; Yang et al. 2009). Since our findings could reflect changes in the denominator (tCr), we cannot rule out the possibility that observed effects result from tCr changes over time. However, we believe this is highly unlikely because the stability of the Ins/tCr, Cho/tCr, GABA/tCr, and NAA/tCr ratios over time in both saline and cocaine-treated groups implies stability of the four numerators in those ratios as well as of the denominator (tCr), as any cocaine-induced tCr change would have had to have been opposed by equivalent and parallel changes in Ins, Cho, GABA, and NAA. Additional support for tCr stability comes from human studies reporting that chronic cocaine exposure does not alter gray matter tCr metabolite levels (Li et al. 1999; O’Neill et al. 2001).
As noted above, MRS measures reflect intracellular metabolite levels, not extracellular levels often reported in studies of cocaine’s neurochemical effects. Thus, by itself, MRS does not provide a detailed picture of all cellular environments. Yet, MRS is noninvasive, it is an accepted research method used in human and animal models of a number of brain disorders, it has been used to study cocaine’s acute and chronic effects (Chang et al. 1999, 1997; Christensen et al. 2000; Ke et al. 2004; Li et al. 1999; Meyerhoff et al. 1999; O’Neill et al. 2001; Smith et al. 2001; Streeter et al. 2005; Yang et al. 2009), and our findings closely parallel those observed in a human chronic cocaine study (Yang et al. 2009), suggesting that our model may be useful for further characterizing glutamate changes associated with chronic cocaine exposures (Lee et al. 2008; Suto et al. 2010; Xie and Steketee 2008; Yablonsky-Alter et al. 2009).
A fourth limitation is that monkeys were anesthetized with isoflurane to undergo scans, while humans typically are scanned without anesthesia and animal microdialysis studies typically are performed without anesthesia. Thus, we cannot rule out an anesthesia confound in our study. A fifth limitation of this study is that we administered cocaine passively (noncontingently), while most human cocaine exposures are self-administered (contingently administered). Although the evidence is mixed, some differences in cocaine’s effects have been reported in nonhuman primates and rodents depending upon whether it is administered contingently or noncontingently. For example, in rhesus monkeys, cerebral metabolic rates for glucose differed in a number of brain areas including putamen depending upon contingency (Lyons et al. 1996; Porrino et al. 2002). By contrast, self- and passively administered cocaine had similar behavioral effects in rhesus macaques performing complex operant behavioral tasks (Winsauer et al. 2003). In squirrel monkeys, the kinetics of cocaine-induced caudate nucleus extracellular dopamine (DA) increases differed in monkeys self-administering cocaine versus when they were passively administered the same cocaine doses (Kimmel et al. 2005). Rodent striatal DA transporter binding was increased after chronic contingent versus noncontingent cocaine administration, although no striatal glutamate transporter (sodium dependent excitatory amino acid transporter) differences were detected (Miguens et al. 2008). Self-administered cocaine induced greater nucleus accumbens DA increases than passively administered doses in the same rats (Hemby et al. 1997). Noncontingent cocaine administration more effectively induced behavioral and neurochemical sensitization in rats (Lecca et al. 2007). Recently, it was reported (Suto et al. 2010) that cocaine increased nucleus accumbens glutamate in rats challenged soon after a repeated cocaine dosing regimen only in rats self-administering the drug, and not in those receiving yoked cocaine. Although we did not track or try to shape behavior in our study, monkeys administered daily cocaine undoubtedly learned some associations between the act of being administered cocaine and any reward that would follow. The four monkeys receiving cocaine injections readily presented themselves for cocaine injections, while saline-treated monkeys did not. Thus, although they were not working to receive cocaine, it seems possible that over time, cocaine-treated monkeys in this study may have experienced some of cocaine’s effects associated with contingent administration. These and other issues suggest that it will be important to extend our model to include studies in squirrel monkeys chronically self-administering cocaine to characterize proton metabolite changes in putamen and other brain areas as well as to characterize other brain effects.
Another limitation of this study is that it included only male subjects, and thus it is not possible to determine whether sex differences exist, as has been suggested by prior work (Chang et al. 1999). Furthermore, we did not control for effects of cocaine withdrawal by standardizing timing between cocaine administration and MRS scans. Thus, we cannot rule out the possibility that withdrawal effects contribute to the present results.
Notwithstanding these limitations, the present results suggest that this controlled squirrel monkey model of human chronic cocaine abuse may have utility for studying glutamate homeostatic and other cerebral metabolite abnormalities associated with chronic cocaine abuse, as well as the efficacy of experimental treatments designed to mitigate cocaine-induced glutamatergic and other brain dysfunction. Future studies are planned to determine whether chronic cocaine administration, including self-administration, induces metabolite changes in putamen and other brain areas, and whether experimental treatments for cocaine dependence that moderate glutamate transport including N-acetylcysteine and ceftriaxone can alter brain glutamate homeostasis as measured with proton MRS.
Acknowledgments
This project was supported in part by the Counter-Drug Technology Assessment Center (CTAC), an office within the Office of National Drug Control Policy (ONDCP), via Contract Number DABK39-03-C-0075 awarded by the Army Contracting Agency. The content of the information does not necessarily reflect the position or the policy of the Government and no official endorsement should be inferred. This project also was sponsored in part by NIH grants S10RR019356, R01DA09448, and K02DA017324, by a grant from Varian, Inc., and by gifts from John and Virginia B. Taplin. We thank Nicolas Bolo, Amy C. Janes, and Bonnie Adams for their assistance with this study.
Footnotes
A portion of these results has been reported in abstract form at the 72nd Annual Meeting of the College on Problems of Drug Dependence in Scottsdale, AZ, USA in June, 2010.
References
- Ardila A, Rosselli M, Strumwasser S. Neuropsychological deficits in chronic cocaine abusers. Int J Neurosci. 1991;57:73–79. doi: 10.3109/00207459109150348. [DOI] [PubMed] [Google Scholar]
- Baker DA, Shen H, Kalivas PW. Cystine/glutamate exchange serves as the source for extracellular glutamate: modifications by repeated cocaine administration. Amino Acids. 2002a;23:161–162. doi: 10.1007/s00726-001-0122-6. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002b;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso-Moguel R, Villeda-Hernandez J, Mendez-Armenta M, Rios C. Brain capillary lesions produced by cocaine in rats. Toxicol Lett. 1997;92:9–14. doi: 10.1016/s0378-4274(97)00027-1. [DOI] [PubMed] [Google Scholar]
- Barroso-Moguel R, Mendez-Armenta M, Villeda-Hernandez J, Nava-Ruiz C, Santamaria A. Brain lesions induced by chronic cocaine administration to rats. Progr Neuro Psychopharmacol Biol Psychiatr. 2002;26:59–63. doi: 10.1016/s0278-5846(01)00229-9. [DOI] [PubMed] [Google Scholar]
- Bauzo RM, Kimmel HL, Howell LL. Interactions between the mGluR2/3 agonist, LY379268, and cocaine on in vivo neurochemistry and behavior in squirrel monkeys. Pharmacol Biochem Behav. 2009;94:204–210. doi: 10.1016/j.pbb.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty WW, Katzung VM, Moreland VJ, Nixon SJ. Neuropsychological performance of recently abstinent alcoholics and cocaine abusers. Drug Alcohol Depend. 1995;37:247–253. doi: 10.1016/0376-8716(94)01072-s. [DOI] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Nader MA, Porrino LJ. Functional effects of cocaine self-administration in primate brain regions regulating cardiovascular function. Neurosci Lett. 2004;370:201–205. doi: 10.1016/j.neulet.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Nader MA, Porrino LJ. Effects of chronic cocaine self-administration on norepinephrine transporters in the nonhuman primate brain. Psychopharmacology (Berl) 2005;180:781–788. doi: 10.1007/s00213-005-2162-1. [DOI] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Daunais JB, Nader MA, Porrino LJ. Chronic cocaine self-administration is associated with altered functional activity in the temporal lobes of non human primates. Eur J Neurosci. 2006;23:3109–3118. doi: 10.1111/j.1460-9568.2006.04788.x. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Rothman R, Cadet JL. Dose-related neurobehavioral effects of chronic cocaine use. J Neuropsychiatry Clin Neurosci. 1999;11:361–369. doi: 10.1176/jnp.11.3.361. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Funderburk FR, Cadet JL. Differential effects of cocaine and cocaine alcohol on neurocognitive performance. Neurology. 2000;54:2285–2292. doi: 10.1212/wnl.54.12.2285. [DOI] [PubMed] [Google Scholar]
- Bradberry CW. Acute and chronic dopamine dynamics in a nonhuman primate model of recreational cocaine use. J Neurosci. 2000;20:7109–7115. doi: 10.1523/JNEUROSCI.20-18-07109.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry CW. Comparison of acute and chronic neurochemical effects of cocaine and cocaine cues in rhesus monkeys and rodents: focus on striatal and cortical dopamine systems. Rev Neurosci. 2008;19:113–128. doi: 10.1515/revneuro.2008.19.2-3.113. [DOI] [PubMed] [Google Scholar]
- Brecht ML, Huang D, Evans E, Hser YI. Polydrug use and implications for longitudinal research: ten-year trajectories for heroin, cocaine, and methamphetamine users. Drug Alcohol Depend. 2008;96:193–201. doi: 10.1016/j.drugalcdep.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter KM, Hittner JB. Cognitive impairment among the dually-diagnosed: substance use history and depressive symptom correlates. Addiction. 1997;92:747–759. [PubMed] [Google Scholar]
- Chang L, Mehringer CM, Ernst T, Melchor R, Myers H, Forney D, Satz P. Neurochemical alterations in asymptomatic abstinent cocaine users: a proton magnetic resonance spectroscopy study. Biol Psychiatry. 1997;42:1105–1114. doi: 10.1016/s0006-3223(97)00135-2. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Strickland T, Mehringer CM. Gender effects on persistent cerebral metabolite changes in the frontal lobes of abstinent cocaine users. Am J Psychiatr. 1999;156:716–722. doi: 10.1176/ajp.156.5.716. [DOI] [PubMed] [Google Scholar]
- Chen K, Scheier LM, Kandel DB. Effects of chronic cocaine use on physical health: a prospective study in a general population sample. Drug Alcohol Depend. 1996;43:23–37. doi: 10.1016/s0376-8716(96)01285-9. [DOI] [PubMed] [Google Scholar]
- Christensen JD, Kaufman MJ, Frederick B, Rose SL, Moore CM, Lukas SE, Mendelson JH, Cohen BM, Renshaw PF. Proton magnetic resonance spectroscopy of human basal ganglia: response to cocaine administration. Biol Psychiatry. 2000;48:685–692. doi: 10.1016/s0006-3223(00)00897-0. [DOI] [PubMed] [Google Scholar]
- Colzato LS, van den Wildenberg WP, Hommel B. Impaired inhibitory control in recreational cocaine users. PLoS ONE. 2007;2:e1143. doi: 10.1371/journal.pone.0001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham EE, Venuto RC, Zielezny MA. Adulterants in heroin/cocaine: implications concerning heroin-associated nephropathy. Drug Alcohol Depend. 1984;14:19–22. doi: 10.1016/0376-8716(84)90014-0. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Justice JB, Jr, Howell LL. Cocaine-induced changes in extracellular dopamine determined by microdialysis in awake squirrel monkeys. Psychopharmacology (Berl) 2000;148:299–306. doi: 10.1007/s002130050054. [DOI] [PubMed] [Google Scholar]
- Daunais JB, Nader MA, Porrino LJ. Long-term cocaine self-administration decreases striatal preproenkephalin mRNA in rhesus monkeys. Pharmacol Biochem Behav. 1997;57:471–475. doi: 10.1016/s0091-3057(96)00432-7. [DOI] [PubMed] [Google Scholar]
- Fagergren P, Smith HR, Daunais JB, Nader MA, Porrino LJ, Hurd YL. Temporal upregulation of prodynorphin mRNA in the primate striatum after cocaine self-administration. Eur J Neurosci. 2003;17:2212–2218. doi: 10.1046/j.1460-9568.2003.02636.x. [DOI] [PubMed] [Google Scholar]
- Febo M, Segarra AC, Nair G, Schmidt K, Duong TQ, Ferris CF. The neural consequences of repeated cocaine exposure revealed by functional MRI in awake rats. Neuropsychopharmacology. 2005;30:936–943. doi: 10.1038/sj.npp.1300653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman WM, Nader MA, Nader SH, Robertson DJ, Gioia L, Mitchell SM, Daunais JB, Porrino LJ, Friedman DP, Vrana KE. Chronic cocaine-mediated changes in non-human primate nucleus accumbens gene expression. J Neurochem. 2001;77:542–549. doi: 10.1046/j.1471-4159.2001.00252.x. [DOI] [PubMed] [Google Scholar]
- George O, Mandyam CD, Wee S, Koob GF. Extended access to cocaine self-administration produces long-lasting prefrontal cortex-dependent working memory impairments. Neuropsychopharmacology. 2008;33:2474–2482. doi: 10.1038/sj.npp.1301626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Seaquist ER, Kim S, Ugurbil K. Localized in vivo 13C-NMR of glutamate metabolism in the human brain: initial results at 4 tesla. Dev Neurosci. 1998;20:380–388. doi: 10.1159/000017334. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology (Berl) 1997;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Suppl 1):169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MJ, Levin JM, Ross MH, Lange N, Rose SL, Kukes TJ, Mendelson JH, Lukas SE, Cohen BM, Renshaw PF. Cocaine-induced cerebral vasoconstriction detected in humans with magnetic resonance angiography. JAMA. 1998;279:376–380. [PubMed] [Google Scholar]
- Ke Y, Streeter CC, Nassar LE, Sarid-Segal O, Hennen J, Yurgelun-Todd DA, Awad LA, Rendall MJ, Gruber SA, Nason A, Mudrick MJ, Blank SR, Meyer AA, Knapp C, Ciraulo DA, Renshaw PF. Frontal lobe GABA levels in cocaine dependence: a two-dimensional, J-resolved magnetic resonance spectroscopy study. Psychiatr Res. 2004;130:283–293. doi: 10.1016/j.pscychresns.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Kimmel HL, Ginsburg BC, Howell LL. Changes in extracellular dopamine during cocaine self-administration in squirrel monkeys. Synapse. 2005;56:129–134. doi: 10.1002/syn.20135. [DOI] [PubMed] [Google Scholar]
- Klatt EC, Montgomery S, Namiki T, Noguchi TT. Misrepresentation of stimulant street drugs: a decade of experience in an analysis program. J Toxicol Clin Toxicol. 1986;24:441–450. doi: 10.3109/15563658608992606. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca D, Cacciapaglia F, Valentini V, Acquas E, Di Chiara G. Differential neurochemical and behavioral adaptation to cocaine after response contingent and noncontingent exposure in the rat. Psychopharmacology (Berl) 2007;191:653–667. doi: 10.1007/s00213-006-0496-y. [DOI] [PubMed] [Google Scholar]
- Lee DK, Bian S, Rahman MA, Shim YB, Shim I, Choe ES. Repeated cocaine administration increases N-methyl-d-aspartate NR1 subunit, extracellular signal-regulated kinase and cyclic AMP response element-binding protein phosphorylation and glutamate release in the rat dorsal striatum. Eur J Pharmacol. 2008;590:157–162. doi: 10.1016/j.ejphar.2008.06.048. [DOI] [PubMed] [Google Scholar]
- Letchworth SR, Nader MA, Smith HR, Friedman DP, Porrino LJ. Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys. J Neurosci. 2001;21:2799–2807. doi: 10.1523/JNEUROSCI.21-08-02799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SJ, Wang Y, Pankiewicz J, Stein EA. Neurochemical adaptation to cocaine abuse: reduction of N-acetyl aspartate in thalamus of human cocaine abusers. Biol Psychiatry. 1999;45:1481–1487. doi: 10.1016/s0006-3223(98)00230-3. [DOI] [PubMed] [Google Scholar]
- Liu X, Matochik JA, Cadet JL, London ED. Smaller volume of prefrontal lobe in polysubstance abusers: a magnetic resonance imaging study. Neuropsychopharmacology. 1998;18:243–252. doi: 10.1016/S0893-133X(97)00143-7. [DOI] [PubMed] [Google Scholar]
- Lyons D, Friedman DP, Nader MA, Porrino LJ. Cocaine alters cerebral metabolism within the ventral striatum and limbic cortex of monkeys. J Neurosci. 1996;16:1230–1238. doi: 10.1523/JNEUROSCI.16-03-01230.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey DJ, Smith HR, Nader MA, Porrino LJ. Chronic cocaine self-administration upregulates the norepinephrine transporter and alters functional activity in the bed nucleus of the stria terminalis of the rhesus monkey. J Neurosci. 2003;23:12–16. doi: 10.1523/JNEUROSCI.23-01-00012.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage. 2003;19:1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ, Bloomer C, Schuff N, Ezekiel F, Norman D, Clark W, Weiner MW, Fein G. Cortical metabolite alterations in abstinent cocaine and cocaine/alcohol-dependent subjects: proton magnetic resonance spectroscopic imaging. Addiction Biol. 1999;4:405–419. doi: 10.1080/13556219971399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguens M, Del Olmo N, Higuera-Matas A, Torres I, Garcia-Lecumberri C, Ambrosio E. Glutamate and aspartate levels in the nucleus accumbens during cocaine self-administration and extinction: a time course microdialysis study. Psychopharmacology (Berl) 2008;196:303–313. doi: 10.1007/s00213-007-0958-x. [DOI] [PubMed] [Google Scholar]
- Moore RJ, Vinsant SL, Nader MA, Porrino LJ, Friedman DP. Effect of cocaine self-administration on dopamine D2 receptors in rhesus monkeys. Synapse. 1998a;30:88–96. doi: 10.1002/(SICI)1098-2396(199809)30:1<88::AID-SYN11>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Moore RJ, Vinsant SL, Nader MA, Porrino LJ, Friedman DP. Effect of cocaine self-administration on striatal dopamine D1 receptors in rhesus monkeys. Synapse. 1998b;28:1–9. doi: 10.1002/(SICI)1098-2396(199801)28:1<1::AID-SYN1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Nader MA, Daunais JB, Moore T, Nader SH, Moore RJ, Smith HR, Friedman DP, Porrino LJ. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology. 2002;27:35–46. doi: 10.1016/S0893-133X(01)00427-4. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Ehrenkaufer R, Mach RH. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Neiman J, Haapaniemi HM, Hillbom M. Neurological complications of drug abuse: pathophysiological mechanisms. Eur J Neurol. 2000;7:595–606. doi: 10.1046/j.1468-1331.2000.00045.x. [DOI] [PubMed] [Google Scholar]
- O’Malley S, Adamse M, Heaton RK, Gawin FH. Neuropsychological impairment in chronic cocaine abusers. Am J Drug Alcohol Abuse. 1992;18:131–144. doi: 10.3109/00952999208992826. [DOI] [PubMed] [Google Scholar]
- O’Neill J, Cardenas VA, Meyerhoff DJ. Separate and interactive effects of cocaine and alcohol dependence on brain structures and metabolites: quantitative MRI and proton MR spectroscopic imaging. Addiction Biol. 2001;6:347–361. doi: 10.1080/13556210020077073. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Dhuna A, Anderson DC. Longterm neurological complications of chronic, habitual cocaine abuse. Neurotoxicology. 1991;12:393–400. [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. Attenuation of cocaine self-administration in squirrel monkeys following repeated administration of the mGluR5 antagonist MPEP: comparison with dizocilpine. Psychopharmacology (Berl) 2008;200:167–176. doi: 10.1007/s00213-008-1191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Miller MD, Smith HR, Friedman DP, Daunais JB, Nader MA. Metabolic mapping of the effects of cocaine during the initial phases of self-administration in the nonhuman primate. J Neurosci. 2002;22:7687–7694. doi: 10.1523/JNEUROSCI.22-17-07687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci. 2004;24:3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Rosselli M, Ardila A. Cognitive effects of cocaine and polydrug abuse. J Clin Exp Neuropsychol. 1996;18:122–135. doi: 10.1080/01688639608408268. [DOI] [PubMed] [Google Scholar]
- Sari Y, Smith KD, Ali PK, Rebec GV. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2009;29:9239–9243. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Zheng JW, Goldberg SR. Effects of cocaine and cocaine metabolites on cardiovascular function in squirrel monkeys. Eur J Pharmacol. 2001;431:53–59. doi: 10.1016/s0014-2999(01)01406-6. [DOI] [PubMed] [Google Scholar]
- Sim ME, Lyoo IK, Streeter CC, Covell J, Sarid-Segal O, Ciraulo DA, Kim MJ, Kaufman MJ, Yurgelun-Todd DA, Renshaw PF. Cerebellar gray matter volume correlates with duration of cocaine use in cocaine-dependent subjects. Neuropsychopharmacology. 2007;32:2229–2237. doi: 10.1038/sj.npp.1301346. [DOI] [PubMed] [Google Scholar]
- Smith LM, Chang L, Yonekura ML, Gilbride K, Kuo J, Poland RE, Walot I, Ernst T. Brain proton magnetic resonance spectroscopy and imaging in children exposed to cocaine in utero. Pediatrics. 2001;107:227–231. doi: 10.1542/peds.107.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soher BJ, Young K, Bernstein A, Aygula Z, Maudsley AA. GAVA: spectral simulation for in vivo MRS applications. J Magn Reson. 2007;185:291–299. doi: 10.1016/j.jmr.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter CC, Hennen J, Ke Y, Jensen JE, Sarid-Segal O, Nassar LE, Knapp C, Meyer AA, Kwak T, Renshaw PF, Ciraulo DA. Prefrontal GABA levels in cocaine-dependent subjects increase with pramipexole and venlafaxine treatment. Psychopharmacology (Berl) 2005;182:516–526. doi: 10.1007/s00213-005-0121-5. [DOI] [PubMed] [Google Scholar]
- Suto N, Ecke LE, You ZB, Wise RA. Extracellular fluctuations of dopamine and glutamate in the nucleus accumbens core and shell associated with lever-pressing during cocaine self-administration, extinction, and yoked cocaine administration. Psychopharmacology (Berl) 2010;211:267–275. doi: 10.1007/s00213-010-1890-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkac I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999;41:649–656. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Waddell KW, Avison MJ, Joers JM, Gore JC. A practical guide to robust detection of GABA in human brain by J-difference spectroscopy at 3 T using a standard volume coil. Magn Reson Imaging. 2007;25:1032–1038. doi: 10.1016/j.mri.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsauer PJ, Moerschbaecher JM, Molina PE, Roussell AM. Contingent and noncontingent cocaine administration in rhesus monkeys: a comparison of the effects on the acquisition and performance of response sequences. Behav Pharmacol. 2003;14:295–306. doi: 10.1097/01.fbp.0000081785.35927.08. [DOI] [PubMed] [Google Scholar]
- Xie X, Steketee JD. Repeated exposure to cocaine alters the modulation of mesocorticolimbic glutamate transmission by medial prefrontal cortex Group II metabotropic glutamate receptors. J Neurochem. 2008;107:186–196. doi: 10.1111/j.1471-4159.2008.05593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonsky-Alter E, Agovic MS, Gashi E, Lidsky TI, Friedman E, Banerjee SP. Cocaine challenge enhances release of neuroprotective amino acid taurine in the striatum of chronic cocaine treated rats: a microdialysis study. Brain Res Bull. 2009;79:215–218. doi: 10.1016/j.brainresbull.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Salmeron BJ, Ross TJ, Xi ZX, Stein EA, Yang Y. Lower glutamate levels in rostral anterior cingulate of chronic cocaine users - A (1)H-MRS study using TE-averaged PRESS at 3 T with an optimized quantification strategy. Psychiatr Res. 2009;174:171–176. doi: 10.1016/j.pscychresns.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


