Abstract
Background and Importance
We present a unique case of an anterior skull base von Hippel-Lindau disease (VHL)-associated microcystic neoplasm. To determine the lesion’s relationship with VHL and its appropriate management, we discuss its salient clinical, pathologic, and molecular features.
Clinical Presentation
A 36-year-old female with VHL presented with a 3-month history of phantosmia. Serial magnetic resonance imaging studies revealed a lesion within the ethmoid and frontal sinus region that was first evident 18 months before symptom development and demonstrated progressive growth over the interval period. The lesion was resected through a transbasal approach. Histopathologic and immunohistochemical analysis revealed a microcystic lesion composed of bland clear cells and underlying endothelial cells consistent with a VHL-associated microcystic neoplasm that are not known to metastasize. Molecular testing demonstrated loss of heterozygosity of the VHL locus, verifying the tumor as a VHL related neoplasm.
Conclusion
Because primary VHL-associated microcystic tumors in the anterior skull base have not been described previously, the natural history of these tumors remains unclear. Based on the benign features of these lesions, they can be managed conservatively with close observation and surgical intervention reserved for those that produce symptoms.
Keywords: Microcystic adenoma, Skull base tumor, von Hippel-Lindau disease
Introduction
von Hippel-Lindau disease (VHL) is an autosomal dominant multiple neoplasia syndrome with an incidence of approximately 1 in 39,000 births.1, 2 VHL is caused by a germline mutation of the VHL tumor suppressor gene located on 3p25.3 Individuals with VHL are predisposed to develop a number of neoplasms and benign cysts. Common visceral neoplasms include renal cell carcinomas (RCCs), pheochromocytomas, pancreatic neuroendocrine tumors, pancreatic microcystic adenomas, as well as broad ligament and epididymal cystadenomas.4 Within the central nervous system (CNS), hemangioblastomas frequently develop in the cerebellum, retina, spinal cord and/or brainstem (60 to 80% of VHL patients)4 and endolymphatic sac tumors can be found in approximately 15% of VHL patients.5
Recent histopathologic studies have identified a new category of neoplasms associated with VHL. These VHL-associated microcystic neoplasms are characterized by clear epithelial cells and numerous endothelial cells and lack metastatic potential.6–9 While VHL-associated microcystic neoplasms have been described in the gallbladder6 and lung9 of VHL patients, they have not been described elsewhere. Here, we present a VHL patient that underwent resection of a growing and symptomatic sinonasal tumor. Histopathologic features of the lesion were consistent with a VHL-associated microcystic adenoma and loss of heterozygosity (LOH) analysis confirmed its association with VHL.
Case Presentation
Clinical findings
A 36-year-old woman presented for evaluation with a 3-month history of olfactory hallucinations. Twelve years before presentation, the patient was diagnosed with VHL based on clinical criteria and confirmed through genetic testing (Table 1).10 Her CNS manifestations of disease, identified by ocular examination and contrast-enhanced MR-imaging, included hemangioblastomas of the retina, cerebellum, spinal cord and brainstem. Visceral manifestations VHL in this patient, identified through contrast-enhanced computed tomography (CT) scanning of the chest, abdomen and pelvis, included renal and pancreatic cysts, but no malignancies.
Table 1.
Clinical Diagnosis of von-Hippel Lindau Disease (VHL)
| Additional finding needed for diagnosis | |
|---|---|
| 2 or more central nervous system (CNS) and retinal hemangioblastomas | None |
| Family Medical History of VHL | CNS Hemangioblastoma or Retinal Hemangiblastoma or Pheochromocytoma or Clear Cell Renal Carcinoma |
| 1 CNS hemangioblastoma | Pheochromocytoma or Clear Cell Renal Carcinoma or Neuroendocrine tumor or Epididymal/Broad ligament adenoma |
Imaging
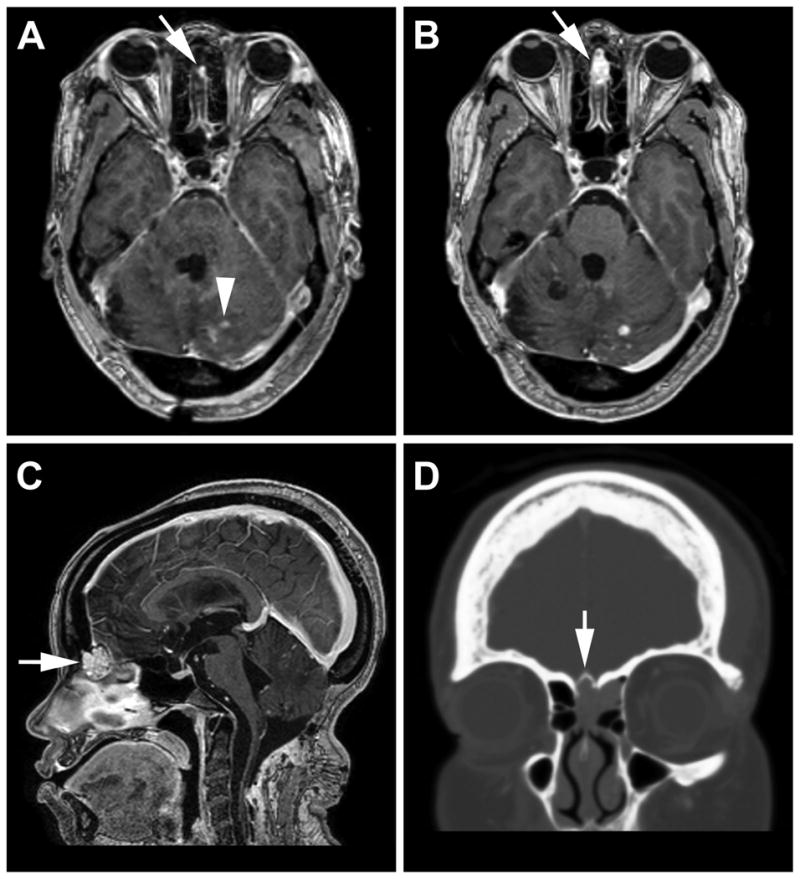
Craniospinal magnetic resonance (MR)-imaging at the time of symptom development revealed an enhancing sinonasal lesion (2.2 cm3). The lesion had grown over the preceding 18 months (0.2 cm3 on first MRI that demonstrated the lesion) disproportionately faster than other lesions within the CNS (Fig. 1A-C). CT revealed the lesion was eroding and expanding the anterior cribiform plate with extension into the left ethmoid sinus (Fig. 1D). Annual and preoperative CT scans of the chest, abdomen and pelvis obtained for visceral surveillance during the observation period revealed no changes in the multiple renal and pancreatic cysts.
Figure 1.
A) Eighteen months before symptom development, T1-weighted post-contrast magnetic resonance imaging revealed a 0.2 cm3 enhancing sinonasal mass (arrow) as well as multiple contrast enhancing tumors in the posterior fossa consistent with hemangioblastomas (arrowhead). One and a half years later, follow-up imaging (B, C) show progression of the sinonasal lesion to 2.2 cm3. D) CT imaging shows the mass eroding into the anterior cribiform plate (arrow) with lateral extension into the left frontoethmoidal recess.
Intervention
Because tumors within the anterior skull base have not been previously reported in association with VHL, the malignant potential of sporadic tumors that arise in this area, and implications for subsequent operative management, an endoscopic nasal biopsy was performed. Histologic analysis of biopsy specimens revealed the presence of a clear cell neoplasm. The locally aggressive nature of the lesion, marked progression over a short period, and new-onset of symptoms led us to recommend surgical resection.
Resection of lesion
The lesion was resected via a transbasal approach.11 In situ, the tumor was observed to be a red-grey soft tissue mass with distinct margins within the frontal and ethmoid sinuses. Although the tumor had eroded the anterior portion of the cribiform plate, no intradural extension was evident. An en bloc resection of the tumor was performed without complication. After surgery, the patient reported no further olfactory hallucinations and has remained neurologically stable with the exception of anosmia. MR-imaging performed at 6 months follow-up revealed no evidence of recurrence.
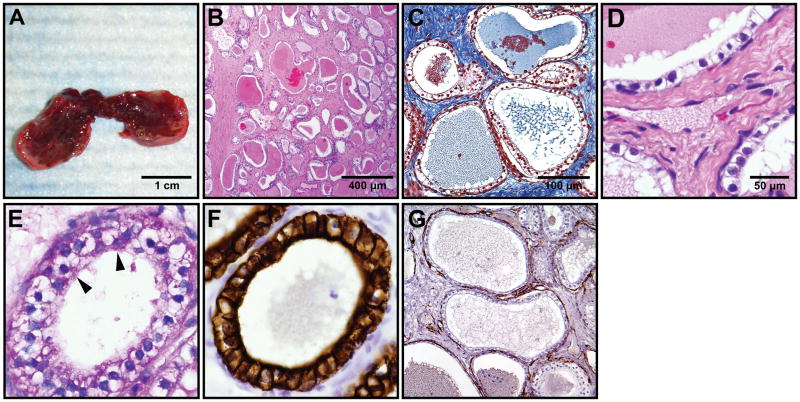
Gross and microscopic findings
On gross examination, the resected tumor was a firm red-tan homogenous mass measuring 2.0 × 1.0 × 0.5 cm with multiple small cysts visible upon bisection (Fig. 2A). Histopathologic evaluation revealed a submucosal well-circumscribed microcystic lesion abutting the normal sinus mucosa without direct invasion. The lesion was composed of numerous microcysts separated by prominent fibrous stroma (Fig. 2B-C). The cysts were lined by a single layer of bland cuboidal cells with prominent clear cytoplasm (Fig. 2D). Mitoses, nuclear atypia, necrosis and hemorrhage were absent. Periodic acid-Schiff staining revealed prominent glycogen in the cytoplasm of the cells (Fig. 2E). Stain for CD31 (Fig. 2F) demonstrated a rich network of capillaries lined by endothelial cells closely associated with the clear cells.
Figure 2.
Gross findings, Histopathology and immunochistochemistry of a VHL-associated microcystic lesion of the sinonasal region.
A) Gross examination of the well circumscribed, bisected specimen reveals the cystic quality of the lesion. B) Low power view of numerous microcysts in dense fibrous stroma (H&E; 100 x). C) Prominent fibrosis surrounding microcysts (Masson’s trichrome stain; 400 x). D) High power view of the cysts lined by bland epithelial cells with clear cytoplasm and intermixed endothelial cells (H&E; 600 x). E) Prominent glycogen in the epithelial cells (Periodic acid-Schiff stain; 600 x). F) Positive cytokeratin 7 in the epithelial cells (Cytokeratin7; 400 x). G) Rich capillary network underlying the cysts (CD31 stain; 400X)
Immunohistochemistry analysis
To further characterize the lesion, immunohistochemical staining was performed for the following markers: epithelial membrane antigen (EMA), neuron specific enolase (NSE), CD10, α-Inhibin, S-100 protein, and the cytokeratins AE1/AE3, MAK-6, and cytokeratin 7. A comparative summary of the staining results are displayed in Table 2. Clear cells in the lesion were negative for CD10, NSE, and S-100 protein, but strongly positive for EMA, α-inhibin, and cytokeratin stains (Fig. 2G).
Table 2.
a. Immunohistochemistry summary.
| Stain | Tumor | Metastatic RCCb (reference) | Hemangioblastomab (reference) | Pancreatic Microcystic Adenomab (reference) | |||
|---|---|---|---|---|---|---|---|
| EMA | + | + | (16, 17) | − | (16, 17) | + | (23, 24) |
| NSE | − | − | (17, 25) | +/− | (17, 25) | +/− | (7, 23) |
| CD10 | − | + | (14, 15) | − | (15) | N/A | |
| α-Inhibin | + | − | (26) | + | (15, 26) | + | (7) |
| Cytokeratin 7 | + | − | (27) | N/A | + | (23, 28) | |
| MAK-6 | + | N/A | N/A | + | (12) | ||
| AE1 / AE3 | + | + | (18) | − | (18, 19) | + | (12, 23, 24) |
| S-100 | − | +/− | (25) | +/− | (17, 19) | − | (8) |
EMA denotes epithelial membrane antigen and NSE, neuron specific enolase.
Staining patterns were derived from literature and were noted as + if ≥75% of reported samples stained positively, − if ≤25% of samples stained positively, and +/− for staining rates in between.
Molecular analysis
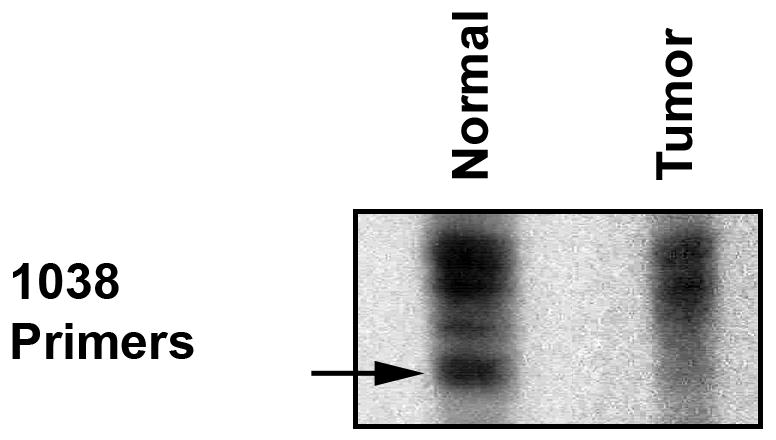
To determine whether the tumor was associated with VHL, we evaluated for LOH of the VHL gene in microdissected specimens as previously described.12 DNA isolated from the patient’s leukocytes revealed the microsatellite marker D3S1038, which flanks the VHL gene, was polymorphic (Fig. 3). Tumor DNA obtained from microdissected cysts showed LOH of the VHL gene based on autoradiographic densitometry analysis using ImageJ software (ImageJ software, version 1.43, Bethesda, MD).
Figure 3.
Loss of heterozygosity analysis. DNA isolated from patient’s leukocytes demonstrated a polymorphism for the microsatellite marker D3S1038. Tumor DNA shows loss of one allele (arrow). Densitometric analysis using ImageJ, showed less than 20% of the PCR product compared to the normal control.
Discussion
Sinonasal tumors
When encountering sporadic masses involving the anterior skull base, the differential diagnosis includes primary carcinomas derived from the sinus mucosa, esthesioneuroblastoma, lymphomas and nasopharyngeal carcinoma. In this particular case, these possibilities were excluded based on the endoscopic nasal biopsy that showed clear tumor cells commonly observed in VHL lesions.
The sinus tumor shared positive EMA and cytokeratin stains with VHL-associated microcystic adenoma of the pancreas (Table 2). Its similarity with RCC and hemangioblastoma on the basis of clear cell morphology warranted a differential diagnosis of metastatic RCC, ectopic hemangioblastoma, and RCC metastasis to a hemangioblastoma.13 The tumor was negative for CD10, a highly sensitive and specific marker for primary and metastatic RCC.14, 15 This finding ruled out metastatic RCC in this case. Although α-Inhibin stain was positive in tumor cells, as seen with hemangioblastoma, the cells were also positive for EMA16, 17 and cytokeratins18, 19, excluding the diagnosis of hemangioblastoma is this case.
Cellular proliferations that typically occur in the setting of VHL comprise a spectrum of clear cell neoplasms that vary in anatomic distribution and malignant potential. Recently, VHL-related tumors, termed VHL-associated microcystic neoplasms, have been reported to arise ectopically from the gallbladder6 and the lung9. These tumors are histologically similar to VHL pancreatic microcystic adenomas and clear cell RCCs. The tumor described in this report is histologically and morphologically consistent with a VHL-associated microcystic neoplasm, demonstrating clear epithelial cells filled with glycogen, prominent fibrosis, and a rich network of endothelial cells underlying the epithelial components. The congruent tumor cell phenotypes may be due to similar epigenetic influences from local epithelium on a common precursor cell, analogous to the cell origin of VHL CNS hemangioblastomas.20
Clinical implications
The rarity of ectopic VHL-associated microcystic neoplasms currently precludes determination of their natural history. However, based on the 2 previous reports and current report, we can draw several conclusions. First, these tumors can arise in diverse anatomic locations including areas that may secondarily impact the nervous system. Second, these tumors exhibit a wide spectrum of growth rates. Previously, the authors reported that the tumor found within the lung had only grown 0.5 cm in 5 years. In contrast, the present case involving a tumor of the anterior skull base exhibited a greater than 10-fold increase in size over 1.5 years. Third, although VHL-associated microcystic neoplasms share a clear cell histology with RCC in VHL, they are well circumscribed, lack nuclear atypia and mitosis, and contain well organized microcysts separated by prominent fibrous stroma; features that are more characteristic of benign lesions. In these ways, the clinical picture of VHL-associated microcystic neoplasms is similar to other common benign VHL tumors, such as hemangioblastomas that also exhibit variable growth rates and produce morbidity mainly through mass effect.21, 22 These findings indicate that VHL-associated microcystic neoplasms can be managed conservatively with close observation and surgical resection reserved for symptomatic lesions.
Acknowledgments
Disclosure of funding:
This research was supported by the Intramural Research Program of the National Institutes of Neurological Disorders and Stroke at the National Institutes of Health. The authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
References
- 1.Maher ER, Iselius L, Yates JR, et al. Von Hippel-Lindau disease: a genetic study. J Med Genet. 1991 Jul;28(7):443–447. doi: 10.1136/jmg.28.7.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neumann HP, Wiestler OD. Clustering of features of von Hippel-Lindau syndrome: evidence for a complex genetic locus. Lancet. 1991 May 4;337(8749):1052–1054. doi: 10.1016/0140-6736(91)91705-y. [DOI] [PubMed] [Google Scholar]
- 3.Latif F, Tory K, Gnarra J, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993 May 28;260(5112):1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- 4.Lonser RR, Glenn GM, Walther M, et al. von Hippel-Lindau disease. Lancet. 2003;361(9374):2059–2067. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]
- 5.Lonser RR, Kim HJ, Butman JA, Vortmeyer AO, Choo DI, Oldfield EH. Tumors of the endolymphatic sac in von Hippel-Lindau disease. N Engl J Med. 2004 Jun 10;350(24):2481–2486. doi: 10.1056/NEJMoa040666. [DOI] [PubMed] [Google Scholar]
- 6.Sinkre PA, Murakata L, Rabin L, Hoang MP, Albores-Saavedra J. Clear Cell Carcinoid Tumor of the Gallbladder: Another Distinctive Manifestation of Von Hippel-Lindau Disease. Am J Surg Pathol. 2001;25(10):1334–1339. doi: 10.1097/00000478-200110000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Kosmahl M, Wagner J, Peters K, Sipos B, Kloppel G. Serous cystic neoplasms of the pancreas: an immunohistochemical analysis revealing alpha-inhibin, neuron-specific enolase, and MUC6 as new markers. Am J Surg Pathol. 2004 Mar;28(3):339–346. doi: 10.1097/00000478-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Compagno J, Oertel JE. Microcystic adenomas of the pancreas (glycogen-rich cystadenomas): a clinicopathologic study of 34 cases. Am J Clin Pathol. 1978 Mar;69(3):289–298. doi: 10.1093/ajcp/69.1.289. [DOI] [PubMed] [Google Scholar]
- 9.Klein J, Zhuang Z, Lubensky I, Colby TV, Martinez F, Jr, Leslie KO. Multifocal microcysts and papillary cystadenoma of the lung in von Hippel-Lindau disease. Am J Surg Pathol. 2007 Aug;31(8):1292–1296. doi: 10.1097/PAS.0b013e3180377aaf. [DOI] [PubMed] [Google Scholar]
- 10.LAMIELL JM, SALAZAR FG, HSIA YE. Von Hippel-Lindau Disease Affecting 43 Members of a Single Kindred. Medicine (Baltimore) 1989;68(1):1–29. doi: 10.1097/00005792-198901000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Persing JA, Jane JA, Levine PA, Cantrell RW. The versatile frontal sinus approach to the floor of the anterior cranial fossa. Technical note. J Neurosurg. 1990 Mar;72(3):513–516. doi: 10.3171/jns.1990.72.3.0513. [DOI] [PubMed] [Google Scholar]
- 12.Mohr VH, Vortmeyer AO, Zhuang Z, et al. Histopathology and Molecular Genetics of Multiple Cysts and Microcystic (Serous) Adenomas of the Pancreas in von Hippel-Lindau Patients. Am J Pathol. 2000 November 1;157(5):1615–1621. doi: 10.1016/S0002-9440(10)64799-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarrell ST, Vortmeyer AO, Linehan WM, Oldfield EH, Lonser RR. Metastases to hemangioblastomas in von Hippel–Lindau disease. J Neurosurg. 2006;105(2):256–263. doi: 10.3171/jns.2006.105.2.256. [DOI] [PubMed] [Google Scholar]
- 14.Avery AK, Beckstead J, Renshaw AA, Corless CL. Use of Antibodies to RCC and CD10 in the Differential Diagnosis of Renal Neoplasms. Am J Surg Pathol. 2000;24(2):203–210. doi: 10.1097/00000478-200002000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Jung S-M, Kuo T-t. Immunoreactivity of CD10 and inhibin alpha in differentiating hemangioblastoma of central nervous system from metastatic clear cell renal cell carcinoma. Mod Pathol. 2004;18(6):788–794. doi: 10.1038/modpathol.3800351. [DOI] [PubMed] [Google Scholar]
- 16.Andrew SM, Gradwell E. Immunoperoxidase labelled antibody staining in differential diagnosis of central nervous system haemangioblastomas and central nervous system metastases of renal carcinomas. J Clin Pathol. 1986 Aug;39(8):917–919. doi: 10.1136/jcp.39.8.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gouldesbrough DR, Bell JE, Gordon A. Use of immunohistochemical methods in the differential diagnosis between primary cerebellar haemangioblastoma and metastatic renal carcinoma. J Clin Pathol. 1988 Aug;41(8):861–865. doi: 10.1136/jcp.41.8.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinbreck NMD, Marie BMD, Bressenot AMD, et al. Immunohistochemical Markers to Distinguish Between Hemangioblastoma and Metastatic Clear-cell Renal Cell Carcinoma in the Brain: Utility of Aquaporin1 Combined With Cytokeratin AE1/AE3 Immunostaining. Am J Surg Pathol. 2008;32(7):1051–1059. doi: 10.1097/PAS.0b013e3181609d7d. [DOI] [PubMed] [Google Scholar]
- 19.Frank TS, Trojanowski JQ, Roberts SA, Brooks JJ. A detailed immunohistochemical analysis of cerebellar hemangioblastoma: an undifferentiated mesenchymal tumor. Mod Pathol. 1989 Nov;2(6):638–651. [PubMed] [Google Scholar]
- 20.Park DM, Zhuang Z, Chen L, et al. von Hippel-Lindau Disease-Associated Hemangioblastomas Are Derived from Embryologic Multipotent Cells. PLoS Med. 2007;4(2):e60. doi: 10.1371/journal.pmed.0040060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammel PR, Vilgrain V, Terris B, et al. Pancreatic involvement in von Hippel-Lindau disease. Gastroenterology. 2000;119(4):1087–1095. doi: 10.1053/gast.2000.18143. [DOI] [PubMed] [Google Scholar]
- 22.Wanebo JE, Lonser RR, Glenn GM, Oldfield EH. The natural history of hemangioblastomas of the central nervous system in patients with von Hippel-Lindau disease. J Neurosurg. 2003 Jan;98(1):82–94. doi: 10.3171/jns.2003.98.1.0082. [DOI] [PubMed] [Google Scholar]