Abstract
DCs are critical in initiating immune responses by cross-priming of tumor antigens to T cells. Previous results showed that NK cells inhibited DC-mediated cross-presentation of tumor Ags both in vivo and in vitro. In this study, enhanced Ag presentation was observed in draining lymph nodes in TRAIL−/− and DR5−/− mice compared to that of wild-type mice. NK cells inhibit DC cross-priming of tumor antigens in vitro, but not direct presentation of endogenous antigens. NK cells lacking TRAIL but not perforin were not able to inhibit DC cross-priming of tumor Ags. DCs that lack expression of TRAIL receptor DR5 were less susceptible to NK cell-mediated inhibition of cross-priming and cross-linking of DR5 receptor led to reduced generation of MHC class I-antigen peptide complex, followed by attenuated cross-priming of CD8+ T cells. In addition, key molecules involved in the TRAIL/DR5 pathway during DC/NK cell interactions were determined. In summary, these data indicate a novel alternative pathway for DC/NK cell interactions in anti-tumor immunity and may reflect homeostasis of both DCs and NK cells for regulation of CD8+ T cell function in physiological conditions.
Keywords: DC, natural killer, cross-presentation, arginase, iNOS, tumor immunity
Introduction
Natural killer cells are an important component of the innate immune system and play critical roles in immune surveillance against tumor cells (1). NK cells recognize tumor cells through both inhibitory and stimulating receptors. Tumor cells that down-regulate MHC expression or express high levels of ligands for NK cell activating receptors become susceptible to NK-mediated lysis. In addition, NK cells can also regulate adaptive immunity, partially through interactions with DCs (2, 3).
DCs are the most efficient professional APCs for initiating antigen-specific T cell responses. Cross-priming has been suggested to be a major pathway for tumor antigens to be presented to naïve CD8+ T cells because tumor cells usually lack co-stimulatory molecules as well as adhesion molecules (4). During cross-priming, antigens derived from dead or dying cancer cells are captured by DCs, which process these antigens and present tumor-derived peptides on their MHC class I molecules (4, 5). Tumor antigen peptide-bearing DCs then activate naïve CD8+ T cells, a critical step in the development of effective anti-tumor immunity.
To date, most studies on NK/DC cross-talk are performed in infectious or immunogenic tumor models (often in conjunction with adjuvants). During infection or in the presence of adjuvants with pathogen-associated molecular patterns, DCs and/or NK cells can be activated, promoting a mutually beneficial DC/NK interaction (6–8). Interplay between NK cells and DC have been shown both in vitro and in vivo. Upon stimulation, DCs produce cytokines such as IFN-α/β, IL-12, IL-15 and IL-18, which stimulate NK cells (9–11). Activated NK cells release cytokines, such as IFN-γ, which promote antigen priming activity by DCs (9). Under certain circumstances, NK cells have been shown to kill immature DC through TRAIL (12). In mice, trimerized TRAIL binds to TRAIL receptor (DR5/TRAIL receptor 2), which results in apoptosis of transformed cells (13). DR5 has also been shown to negatively regulate innate immune responses in DCs (14). Although TRAIL and its receptors have been shown to play important roles in immunosuppressive, immunoregulatory and immune-effector functions (15), little is known about the interaction while DC and NK cells interplay in less-immunogenic tumor models. Our previous results have shown that NK cells inhibited DC activation of antigen-specific CD8+ T cells both in vitro and in vivo using less-immunogenic tumor cells (16). In this study, we examined regulatory mechanisms of TRAIL/DR5 cross-linking when NK cells reduced cross-priming by DCs in vitro and in vivo. Our results reveal a novel alternative pathway for DC/NK cell interactions in anti-tumor immunity, and this pathway may reflect a means for regulation of CD8+ T cell activation in the absence of acute inflammation. This mechanism may be used by tumor cells to limit the induction of immune T cells against tumor antigens.
Materials and Methods
Mice and cell lines
C57BL/6 (B6, wildtype, wt) were purchased from NCI (Frederick, MD). Perforin knockout mice C57BL/6-Prf1tm1Sdz/J (Pfp−/−) were obtained from the Jackson Laboratory (Bar Harbor, ME). TRAIL knockout mice (TRAIL−/−) were obtained from Dr. Jacques Peschon (Amgen, Seattle, WA). TRAIL receptor DR5 knockout mice (DR5−/−) were obtained from Dr. Tak Mak (the University Health Network, Toronto, Canada). Male antigen HY-TCR transgenic mice (TCR HY) and chicken OVA-specific, MHC class II-restricted TCR transgenic OT-II mice were obtained from Dr. Randolph J. Noelle (Dartmouth Medical School). OVA-specific H-2Kb restrict TCR transgenic OT-I mice, were obtained from Jackson lab. B6.Rag1−/− mice were bred and maintained at Dartmouth Medical School. Animals used in experiments were between 7 and 12 weeks of age. All experiments were conducted according to protocols approved by Dartmouth College’s Institutional Animal Care and Use Committee. Mouse T cell line lymphoma RMA/tOVA (H-2b), melanoma B16F10/tOVA (H-2b) and mastocytoma P815/tOVA (H-2d) cells that express a non-secreted truncated form of OVA were used for in vivo and in vitro experiments, respectively. H-2Kb-restreiced B3Z T cell hybridoma cells that recognize the OVA257–264 epitope were obtained from Dr. Nilabh Shastri (University of California at Berkley). Upon activation, B3Z cells express the LacZ gene. All cells were grown in RPMI medium 1640 supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT), 100 U/ml penicillin, 100 μg/ml streptomycin, 1 mM pyruvate, 10 mM Hepes, 0.1 mM non-essential amino acids and 50 μM 2-mercaptoethanol.
Tumor inoculation
RMA/tOVA cells (106) or B16F10/tOVA cells (5 × 105) were injected subcutaneously into right flank of mice (day 0). For depletion of NK cells, mice were injected i.p. with 50 μg of anti-NK1.1 antibody PK136 (days −2, +3). Control mice were given mouse gamma globulin (Jackson ImmunoResearch Laboratory, West Grove, PA).
Cell preparation
Enrichment of DCs from DLNs, preparation of bone marrow (BM)-derived DCs, H-2Kb restricted OVA-specific OT-I T cells and H-Y T cells were performed as described (16). Purified NK cells were obtained from B6.Rag1−/− spleen cells using magnetic cell sorting kit (Miltenyi Biotec Inc., Auburn, CA) and FITC-labeled anti-DX5 mAbs according to the manufacturer’s instructions. NK cell purity was >90%. OVA-reactive OT-II cells (I-Ab restricted) were purified in a similar way, except that anti-CD4 mAbs were used. To prepare splenic DCs, spleens were digested with DNase (100 μg/ml, Sigma-Aldrich, St. Louis, MO) and collagenase (1 mg/ml, Sigma) at 37°C for 45 min, and light-density cells were collected after centrifugation over Nycodenz density gradient (w/v=14.5%, 1.077 g/cm3). Cells at the interface were stained with anti-CD11c+-FITC and then purified by a magnetic cell sorting kit (Miltenyi Biotec Inc., Auburn, CA) according to the manufacturer’s instructions.
Ex vivo antigen presentation assay
Nycodenz-enriched LN cells were cocultured with B3Z cells (105) at a ratio of 1:5 (LN cells:B3Z) in 96-well, round-bottom plates for 24 h and stained for LacZ+ cells as described previously (17).
Cross-linking of DR5 with plate-bound anti-DR5 mAb
Functional grade of anti-DR5 mAbs (MD5-1, 10 μg/ml, eBioscience, San Diego, CA) or control hamster mAbs in PBS were coated on non-tissue culture-treated 96-well plates or 24-well plates. The plates were washed twice with PBS, and then CD11c+ BM-DCs in complete media were cross-linked for 4 h or 24 h.
In vitro antigen presentation assays
Purified CFSE-labeled OT-I or OT-II T cells (5 × 104) were cultured with either 2 × 104 BM-DCs or splenic DCs and 2 × 104 irradiated P815/tOVA (120 Gys) in a total of 200 μl of complete medium. In some cases, DCs were pulsed with OVA257–264 peptide (10−12 – 10−10 M) for 45 min at 37°C in complete medium and then washed three times before culture with OT-I T cells. In some wells, 2–50 × 103 NK cells were added to cell cultures. To stimulate H-Y specific T cells, DCs from B6 male mice were used. Proliferation of T cells (loss of CFSE labeling in T cells) was determined by flow cytometry after 65 to 96 hr of culture. Conjugation of OVA to latex beads (polybeads, polyscience, Warrington, PA) was performed as described previously (18). In some studies, 50 μM L-NIL, 50 μM nor-NOHA, 200 μM 1-MT, 10 μg/ml anti-IL-10 mAbs or 10 μg/ml mIgG2a mAbs were added to wells to analyze specific mechanisms.
Phagocytosis assay
BM-DCs were cross-linked with control mAbs or anti-DR5 mAbs for 24 hr, and then PKH26-labeled irradiated P815/tOVA cells were added to the wells. After 30 min to 24 hr incubation, cells were washed with quenching buffer (2 mM EDTA/PBS), stained with APC-conjugated anti-CD11c mAbs on ice, and then analyzed by flow cytometry.
Real-time RT-PCR
BM-DCs were cross-linked for 4 hr to 24 hr with control mAbs- or anti-DR5 mAbs-coated wells or in control wells (PBS). Total RNA was isolated from the cells by using the RNeasy kit (Qiagen, Valencia, CA). cDNA was synthesized by using random hexamers and M-MLV Reverse Transcriptase (Invitrogen). Real-time PCR was performed with SYBRR Green PCR Master Mix (Applied biosystems, Carlsbad, CA) and the following primers: GAPDH (F: CAGTCTGGAGAAAGCCAAGG, R: GCATTTCCAGCCAGACAGAT), IL-10 (F: CCAAGCCTTATCGGAAATGATC, R: ATTTTCACAGGGGAGAAATCG), iNOS (F: GGCAGCCTGTGAGACCTTTG, R: TGCATTGGAAGTGAAGCGTTT), arginase-1 (F: TGAACACGGCAGTGGCTTTA, R: CAGTCCCTGGCTTATGGTTACC), IDO1 (F: CAGTCTGGAGAAAGCCAAGG, R: GCATTTCCAGCCAGACAGAT) and galectin7-1 (F: TCAAACCTGGGGAATGTCTCAA, R: GCGAGGATTGAAGTGTAGGCAC). Amplification of GAPDH was done in each experiment. Each Ct value of the samples was standardized by GAPDH Ct value (ΔCt), and each ΔCt value was normalized to that of 4 hr-incubated DC ΔCt value (ΔΔCt). Results were shown as relative expression (1/2ΔΔCt).
Flow cytometry
To check for NK cell depletion, spleen cells were stained with FITC-anti-CD49b (DX5; BD Biosciences, San Jose, CA) and PE-anti-CD3ε (145-2C11; BD Biosciences), or isotype Ig controls. Before staining, cells were blocked with anti-mouse CD16/32 and mouse IgG. TRAIL expression on NK cells and DR5 expression on DCs were detected by PE-anti-TRAIL mAbs (N2B2; eBioscience) and biotin-anti-DR5 followed by streptavidin-PE, respectively. To gate on OT-I and OT-II cells, APC-anti-CD8 (H35-17.2; eBioscience) and anti-CD4 (GK1.5; eBioscience) were used, respectively. Annexin V and propidium iodide (PI) staining were performed using Annexin V-FITC apoptosis detection kit (Sigma). Surface expression of Kb-OVA complex on DCs (gated on CD11c+ cells) was determined using anti-H-2Kb-OVA mAb 25-D1.16 (eBioscience). Cells were analyzed using a FACSCalibur (BD Biosciences) and CellQuest software (BD Biosciences). In some cases, data were acquired using Accuri C6 flow cytometer (Accuri, Ann Arbor, MI), and acquired data were analysed using FlowJo software (Tree Star, Ashland, OR).
Statistical analysis
Differences between groups were analyzed using the Student’s t-test. Values p < 0.05 were considered significant.
Results
NK cells inhibit DC cross-priming through TRAIL/DR5 in vivo
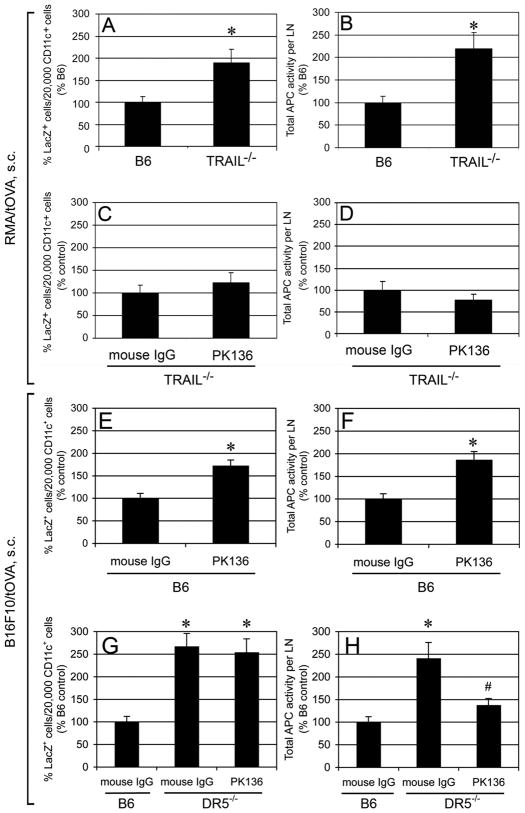
To investigate the role of TRAIL in DC cross-priming in vivo, experiments were designed to determine whether TRAIL affects DC cross-presentation of tumor antigens in vivo using TRAIL−/− mice. Wt B6 mice or TRAIL−/− mice were inoculated with truncated OVA-expressing tumor cells such as RMA/tOVA and B16F10/tOVA. RMA (H-2b) is a T cell lymphoma cell line, whereas B16F10 (H-2b) is a mouse melanoma cell line, and both cell lines are originated from C57BL/6. Nycodenz gradient-enriched DCs (~40% CD11c+) from tumor DLNs were cocultured with OVA-specific B3Z T cells overnight. LacZ+ cell counts that represent the amount of OVA antigen presentation were determined (17). All antigen presentation in this assay is due to the CD11chi cells, as previously shown (16). This assay allows the determination of DC Ag-presentation on a per cell basis and as the total Ag presentation in the tumor DLN. As shown in Fig. 1A and 1B, DCs from TRAIL−/− mice-derived DLNs showed 2-fold higher Ag presentation of RMA/tOVA cells on both a per cell basis and total activity in the DLNs compared with those from wt B6 mice. Our previous data showed that NK cell depletion in wt B6 mice resulted in higher CD8+ T cell priming by DLN DCs in vivo (16). If cells other than NK cells used TRAIL to inhibit DC cross-priming, we would expect higher cross-priming by DLN DCs in TRAIL−/−mice after NK cell depletion. However, NK cell depletion in TRAIL−/− mice did not significantly increase CD8+ T cell priming compared to control Abs-treated TRAIL−/− mice (Fig. 1C, 1D). The negative regulation of cross-priming by NK cells was also observed in a different tumor model by using B16F10/tOVA melanoma cells (Fig. 1E, 1F). This indicates that NK cell are negative regulators of DC cross-priming in this melanoma model, consistent with the finding in the RMA lymphoma system (16).
FIGURE 1. DC cross-presentation of tumor antigens was inhibited by NK cells through TRAIL/DR5 in vivo.
B6 mice, TRAIL−/− mice or DR5−/− mice were inoculated with 106 RMA/tOVA cells (A–D) or 5 × 105 B16F10/tOVA cells (E–H). Anti-NK1.1 or control mouse γ-globulin was injected into mice i.p. at days −2 and +3 relative to tumor inoculation at day 0 (C–H). 5 days after the inoculation, DC-enriched LNs were cocultured with B3Z cells for 24 hr. Results are expressed as percentage of LacZ+ B3Z cells per 2 × 104 CD11c+ cells and total APC activity per LN. Cumulative data from three independent experiments are shown as mean ± SEM. * p < 0.05, # p < 0.05, compared to DR5−/− mouse IgG, but p > 0.05, compared to B6 mouse IgG.
Next experiments were designed to determine whether DR5, a TRAIL receptor, was engaged in DC cross-priming, DLN DCs from DR5−/− mice were compared with DCs from wt B6 mice in the capability of cross-presentation of tumor-associated Ags. It was found that DR5−/− mice-derived DLNs exhibited more than 2-fold higher Ag presentation on both a per cell basis and total activity in the DLNs compared with those from wt B6 mice (Fig. 1G, 1H). Similar to TRAIL−/− mice, NK cell depletion in DR5−/− mice did not increase CD8+ T cell priming compared to control mAbs-treated TRAIL−/− mice (Fig. 1G, H). Collectively, these results indicate that NK cells delay the development of specific CTLs in RMA lymphoma and B16F10 melanoma.
NK cells inhibit DC cross-priming through TRAIL/DR5 in vitro
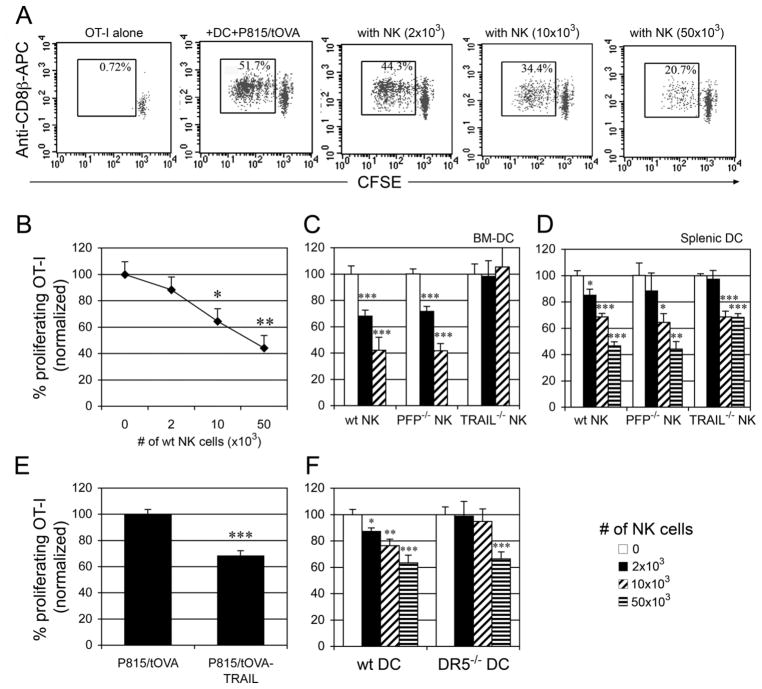
NK-DC interactions are generally thought to result in mutual cell activation (2, 3). However, previous data have shown that tumor Ag cross-presentation by DCs was reduced in the presence of NK cells in an in vitro co-culture system (16). When OVA-specific H-2Kb-restricted OT-I T cells (CFSE labeled) were cocultured with splenic DCs plus irradiated OVA-expressing P815 cells (P815/tOVA, H-2d) in the presence of NK cells, splenic DC-mediated cross-priming was reduced by NK cells in a dose-dependent manner (Fig. 2A, 2B). Although these tumor cells express OVA, they cannot activate OT-I T cells because they do not express the appropriate MHC class I molecules. To determine which mechanisms NK cells use to inhibit DC cross-presentation function, we tested NK cells purified from TRAIL−/− and perforin−/− mice. It has been reported that TRAIL and perforin are involved in NK cell-mediated killing of immature DCs (12). As shown in Fig. 2C, perforin−/− NK cells showed a similar ability as B6 NK cells to inhibit cross-priming by BM-DCs in a dose-dependent manner, indicating that perforin was not required for inhibition of DC cross-priming. In contrast, TRAIL−/− NK cells had a reduced ability to inhibit DC cross-priming (Fig. 2C), suggesting that TRAIL was involved in inhibiting DC-mediated cross-priming. Similar results were observed using splenic DCs (Fig. 2D), but more NK cells were needed to achieve significant inhibitory effects on splenic DC-mediated cross-priming. This difference may be due to the distinct intrinsic features between BM-DCs and splenic DCs. TRAIL−/− NK cells have been shown to exert both perforin- and FasL-dependent cytotoxicity similar to wildtype NK cells (19, 20). Thus, cross-priming by DCs is likely regulated by TRAIL itself rather than through an indirect effect of TRAIL deficiency on NK cell development or function. To confirm the vital role of TRAIL in inhibiting DC cross-priming, we generated P815/tOVA cells expressing TRAIL (P815/tOVA-TRAIL). In comparison to P815/tOVA cells, the use of P815/tOVA-TRAIL cells significantly reduced OT-I proliferation in the absence of NK cells, supporting the critical role of TRAIL in negative regulation of DC cross-priming (Fig. 2E). Expression of TRAIL on NK cells was also determined after co-culture with DCs or DCs with irradiated P815/tOVA cells. Without stimulation, resting NK cells do not express TRAIL (Table I). After co-culture with DCs or DCs plus tumor cells, NK cells expressed TRAIL at the cell surface (Table I). As shown in Fig. 2F, addition of NK cells (2–10 × 103) did not inhibit DR5−/− DC-mediated cross-priming. These results are similar to those using TRAIL−/− NK cells (Fig. 2C, 2D), indicating that TRAIL/DR5 interactions play a key role in negative regulation of DC cross-priming.
FIGURE 2. NK cells inhibit DC cross-priming through TRAIL/DR5.
(A) CFSE-labeled OVA-specific OT-I T cells (5 × 104) were co-cultured with splenic DCs (2 × 104), P815/t-OVA cells (2 × 104) in the presence of NK cells. After 2.5 days, proliferation of T cells was determined by flow cytometry as a reduction in CFSE labeling of CD8β+ T cells. (B) A representative result of four independent experiments is shown as mean ± SEM. (C, D) CFSE-labeled OT-I T cells were cocultured for 2.5 days with P815/tOVA cells and either BM-DCs (C) or splenic DCs (D) in the presence of NK cells from B6, PFP−/− or Trail−/− mice. (E) CFSE-labeled OT-I T cells were cocultured for 2.5 days with splenic DCs and P815/tOVA cells or the cells expressing TRAIL. (F) CFSE-labeled OT-I T cells were cocultured for 2.5 days with P815/tOVA cells and splenic DCs from B6 or DR5−/− mice in the presence of NK cells. Numbers represent normalized percentage of proliferating T cells (no NK = 100%). Cumulative data from three independent experiments are shown as mean ± SEM. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Table I.
NK cells express TRAIL after co-culture with DC.
NK cells, NK cells + DCs and NK cells + DCs + P815/tOVA cells
TRAIL expression on NK cells (gated on NK1.1+) was determined by flow cytometry after co-culture with DC and/or tumor cells for 24–72 hr. The percentage of TRAIL+ NK cells is shown. The values represent means of two independent experiments.
NK cells inhibit DC priming of CD4+ T cells with cell-associated antigens but not soluble antigens
To determine whether NK inhibition of DC function is restricted to cross-priming, we examined the capability of the DCs to present cell-associated OVA as well as soluble OVA protein by the MHC class II pathway to CD4+ OT-II cells. Exogenous Ags need to be endocytosed and processed before presenting to MHC class II-restricted CD4+ T cells. DC-mediated MHC class II priming of CD4+ T cells to tumor cell-associated Ags was also inhibited by NK cells, although to a lesser extent than presentation to CD8+ T cells (Fig. 3A). Only marginal, but consistent, inhibition (<15%) was observed in DC presentation of soluble OVA proteins to CD4+ T cells (Fig. 3B). Thus, NK cells inhibited the presentation by DCs of cell-associated Ags but not soluble Ags to T cells.
FIGURE 3. NK cells inhibit DC-mediated MHC class II-restricted priming of CD4+ T cells with tumor antigens but not soluble antigens.
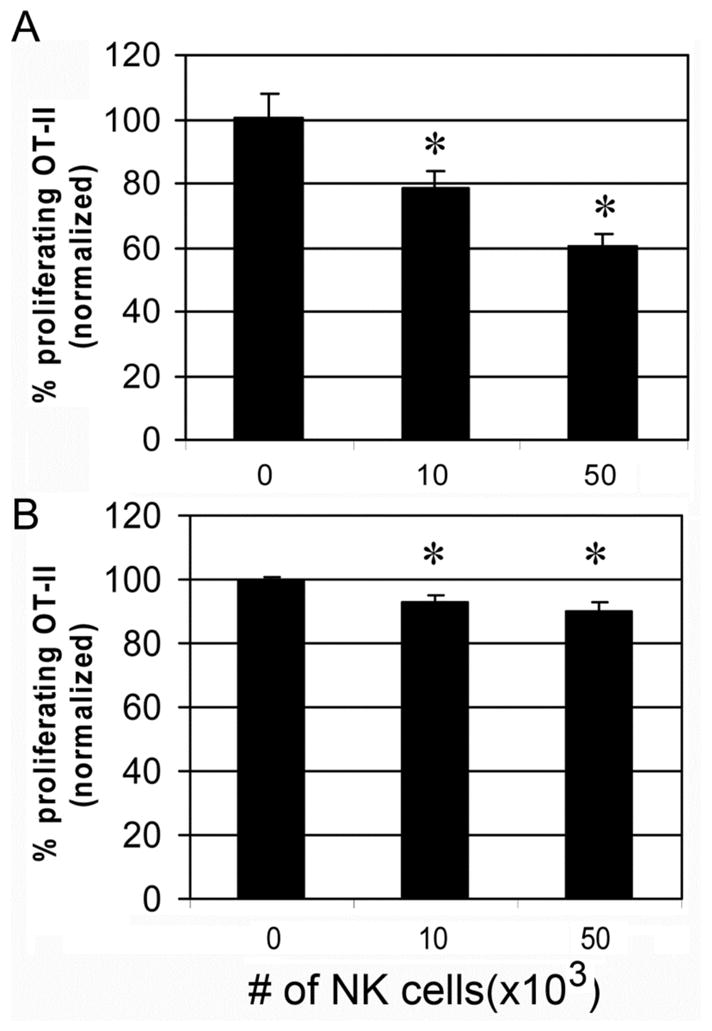
CFSE-labeled OVA-specific OT-II (CD4+, I-Ab restricted) T cells were cocultured with splenic DCs and (A) γ-ray irradiated tumor cells (P815/t-OVA), or (B), soluble OVA protein (100 μg/ml). After 2.5 days, proliferation of CD4+ T cells was determined by flow cytometry. Numbers represent normalized percentage of proliferating T cells (no NK = 100%). Cumulative data from three independent experiments are shown as mean ± SEM. * p < 0.05.
NK cells do not inhibit direct priming by DCs
To test the effect of NK cells on endogenous Ag presentation on MHC class I, T cells purified from H-Y TCR transgenic mice, which are specific for the male minor histocompatibility Ag HY, were co-cultured with male DCs in the presence of female NK cells. Robust proliferation (~100%) of H-Y transgenic T cells was observed, and NK cells did not inhibit the T cell proliferation (Fig. 4A, 4B). To confirm this, DCs were pulsed with OVA peptide (10−12 to 10−10 M) before coculture with OT-I T cells. OVA peptide-pulsed DCs can directly present peptide to OT-I cells without uptake or processing of Ags. NK cells did not inhibit peptide-pulsed DC-induced T cell proliferation (Fig. 4C) or direct stimulation of OT-I T cells by peptide or anti-CD3 mAbs, whether in the presence of absence of P815 tumor cells (Fig. S1).
FIGURE 4. NK cells do not inhibit DC direct priming.
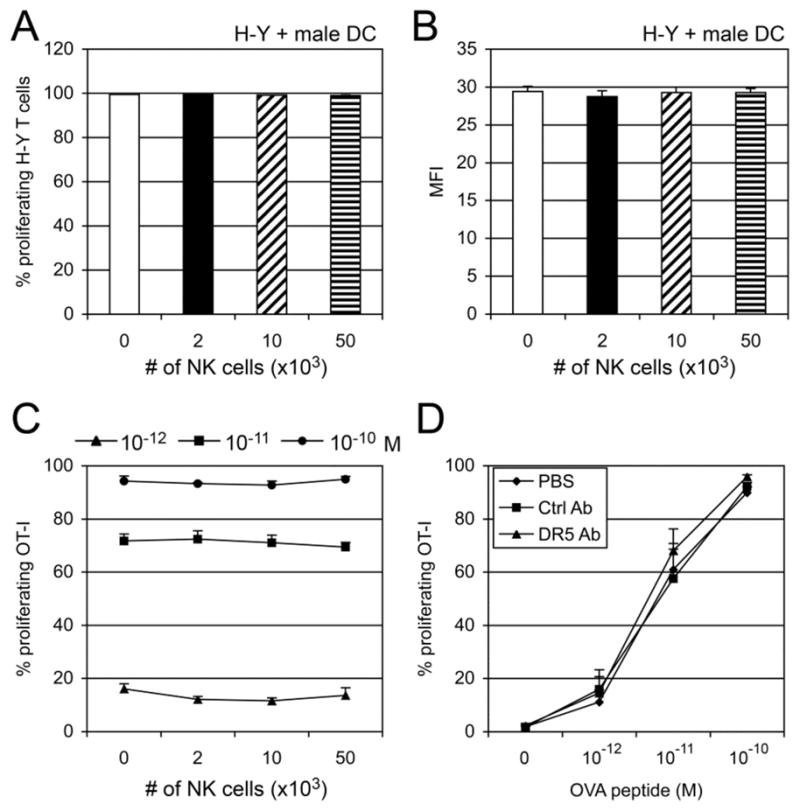
(A & B) CFSE-labeled H-Y-specific T cells were co-cultured with male splenic DCs for 2.5 days in the presence of NK cells (2–50 × 103). Percentage (A) or MFI (B) of proliferating T cells (gated on CD8β+ cells) is shown as mean ± SEM of triplicates. (C) OVA peptide (SIINFEKL, 10−10–10−12 M)-pulsed BM-DCs (2 × 104) were co-cultured with CFSE-labeled OT-I T cells (5 × 104) for 2.5 days in the presence or absence of NK cells (2–50 × 103/well). Numbers represent percentage of proliferating T cells. A representative result of two independent experiments is shown as mean ± SEM of triplicates. (D) CD11c+ BM-DCs (5 × 105) were cultured for 24 hr in control mAbs or anti-DR5 mAbs-coated 24-well plates or in control wells (PBS). The cells (2 × 104) were pulsed with OVA peptide (10−10–10−12 M), and then co-cultured with CFSE-labeled OT-I T cells. Proliferation of CD8β+ T cells was determined by flow cytometry. Cumulative data from three independent experiments are shown as mean ± SEM.
Primary non-activated NK cells do not induce cell death of DCs
There are several possibilities that may account for NK cell inhibition of DC cross-priming. First, NK cells may kill DCs, which can happen when NK cells are pre-activated (e.g. with IL-2) and cultured at higher NK:DC ratios (21). Second, NK cells may affect DC uptake of Ags. To address these possibilities, we first identified the stage(s) at which NK cells affect DC cross-priming. In our in vitro experiments, freshly isolated, non-activated NK cells were used. These fresh NK cells did not kill P815/tOVA tumor cells (Fig. 5A). To examine whether non-activated NK cells induced death of DCs, NK cells (105) were co-cultured with DCs (5 × 104) for 24 and 48 hr before being subjected to propidium iodide (PI) and annexin-V staining. Compared with DCs alone, there was no significant (p > 0.05) increase in either apoptosis (Annexin-Vstaining positive, PI staining negative) or necrosis (Annexin-V staining positive, PI staining positive) of DCs (CD11c+ cells) after 24h of coculture with NK cells (Fig. 5B). Interestingly, 48 hr after NK and DC coculture, the percentages of both apoptotic and necrotic DCs were reduced when NK cells were present in these cultures compared with DCs alone (Fig. 5B), suggesting that NK cells may provide some survival factors to DCs. These data indicate that NK cells were not killing DCs, but they were likely affecting Ag uptake and/or Ag processing.
FIGURE 5. Resting NK cells do not kill tumor cells or DCs.
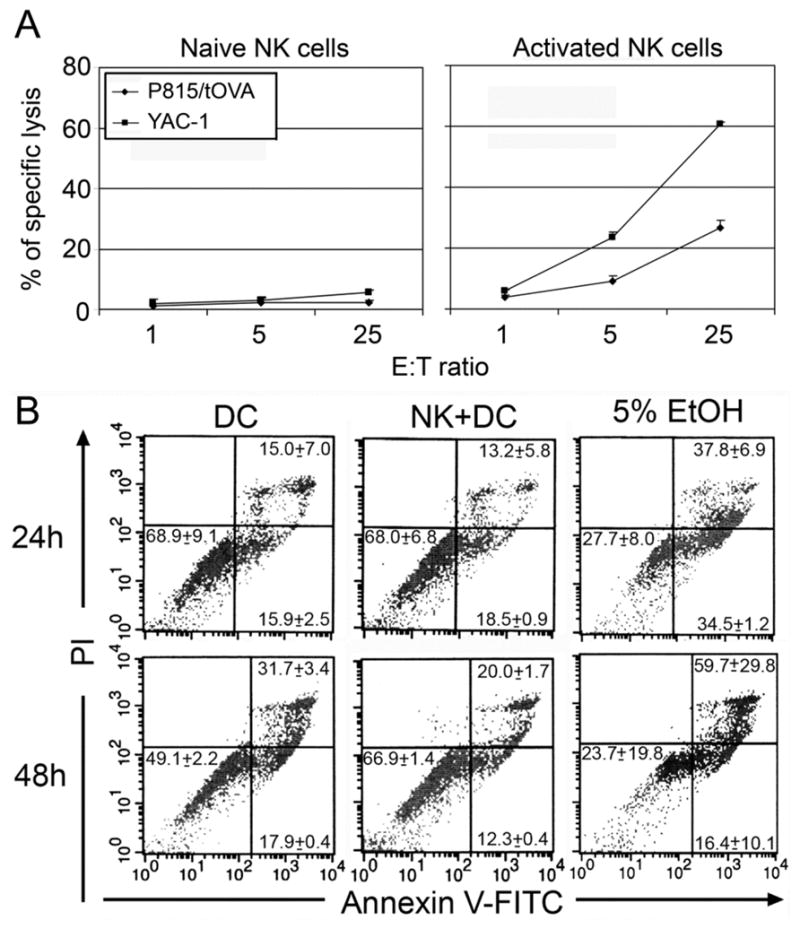
A, Splenocytes from RAG-I−/− mice were incubated with or without IL-4 (1,000 U) for 4 days, and then cocultured with P815/tOVA cells or YAC-1 cells at ratios from 1:1 to 25:1 in 5-h 51Cr release assays. Cumulative data from three independent experiments are shown as mean ± SEM. B, primary non-activated NK cells do not induce cell death of DCs. DCs (BM-DCs, 5×104) alone or NK (105) plus DCs were cultured in 96-well plates for either 24 hr or 48 hr before annexin V and PI staining. The data shown are gated on DCs (APC-anti-CD11c+). As positive control, 5% EtOH was added 24h before cell staining. A representative result of two independent experiments is shown as mean ± SEM of triplicates.
DR5 inhibits DC cross-priming in vitro by reducing phagocytic activity of tumor cells
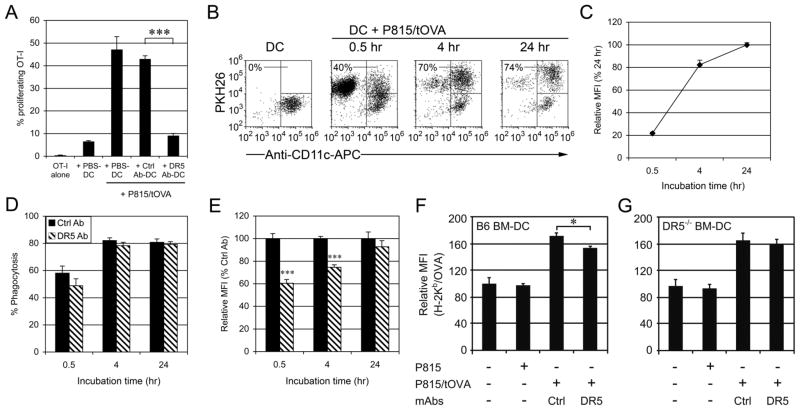
To get insight into the molecular mechanisms of DR5-mediated negative regulation of cross-priming, BM-DCs were cultured for 24 hr in anti-DR5 mAbs (MD5-1)-coated wells (anti-DR5-treated DC), control mAbs-coated wells (ctrl Ab-treated DC) or non-Ab-coated wells (PBS-treated DC). It was found that ctrl Ab-treated DCs as well as PBS-treated DCs effectively cross-primed OT-I T cells in the presence of P815/tOVA cells, but anti-DR5-treated DCs significantly attenuated the cross-presentation (Fig. 6A), which is consistent with the inhibitory effects of NK cells (Fig. 2). To examine whether this inhibitory effect was restricted to the cross-priming of tumor cell-associated Ags, the DCs were pulsed with OVA peptide and cocultured with OT-I T cells. The results showed that DR5 did not affect the capability of Ag presentation by OVA peptide-pulsed DCs (Fig. 4D), indicating that DR5 attenuated DC cross-presentation of cellular Ags but not DC direct peptide presentation.
FIGURE 6. DC Cross-priming of tumor cells is inhibited by cross-linking with anti-DR5 mAbs.
(A) CD11c+ BM-DCs (5 × 105) were cultured for 24 hr in control mAbs or anti-DR5 mAbs-coated 24-well plates or in control wells (PBS). The DCs (2 × 104) were then cocultured for 4 days with CFSE-labeled OVA-specfic OT-I T cells (5 × 104) and P815/tOVA cells (2 × 104). Numbers represent percentage of proliferating T cells. A representative result of four independent experiments is shown as mean ± SEM of triplicate. (B) BM-DCs (5 × 104) were cultured for 24 hr in control mAbs or anti-DR5 mAbs-coated 96-well plates and then incubated with P815/tOVA cells (5 × 104, PKH26+). Percentages refer to PKH26+ cells within the CD11c+ gated cells (DCs). The MFI of PKH26 (C, E) and percentages of PKH26 positive DCs (D) are indicated for each time point (30 min, 1 hr and 4 hr). Cumulative data from three independent experiments are shown as mean ± SEM. Wt (F) or DR5−/− (G) BM-DCs alone or DCs with tumor cells were cocultured for 24 h before staining with 25-D1.16 and anti-CD11c-mAbs. A representative result of two independent experiments is shown as mean ± SEM of triplicates. *, p < 0.05; ***, p < 0.001.
As the first step in cross-priming of tumor cell-associated Ags, DCs need to phagocytose tumor cells. To analyze this step, BM-DCs were cocultured with PKH26+ P815/tOVA cells for different periods of time and then analyzed by flow cytometry. The CD11c+PKH26+ population represents the DCs that phagocytosed tumor cells (Fig. 6B). Both percentage of phagocytosis and mean fluorescent intensity (MFI) of PKH26 were enhanced in a time-dependent manner (Fig. 6B, 6C). It was found that percentages of phagocytosis positive cells showed no significant difference between ctrl Ab-treated DCs and anti-DR5-treated DCs (Fig. 6D). Intriguingly, the uptake of P815/tOVA by anti-DR5-treated DCs was attenuated in earlier time points (30 min and 4 hr), compared to that of ctrl Ab-treated DCs (Fig. 6E), suggesting that phagocytic activity of individual DCs for tumor cells was delayed by DR5 cross-linking.
Because the TRAIL/DR5 pathway showed negative regulation of DC cross-priming both in vivo and in vitro, the activation of DR5 on the generation of MHC-Ag peptide complexes on the cell surface of DCs was determined. To this end, BM-DCs were cultured in the anti-DR5 mAbs-coated wells in the presence of irradiated P815/tOVA cells for 24 h. Cross-linking of DR5 with anti-DR5 mAbs resulted in lower amounts of Kb-OVA complexes compared with control mAb-treated DCs (Fig. 6F). In addition, treatment anti-DR5 mAbs did not affect DR5−/− DCs ability to present Kb-OVA complexes on cell surfaces (Fig. 6G), indicating that DR5 reduced cross-presentation activity of tumor cells-associated Ags.
DR5 does not inhibit DC priming of soluble OVA as well as OVA-coated latex beads
Cross-priming by DCs is strictly regulated by distinct Ags as well as differential receptors (22, 23). Based on this hypothesis, cross-priming must depend on specific routes of Ag processing mechanisms that can be regulated separately (24). To address the processing routes for specific Ags, the effects of DR5-cross-linking on presentation of soluble OVA protein as well as OVA-coupled latex beads were tested. DCs effectively presented soluble OVA protein to OT-I T cells in a dose dependent manner, and cross-priming against soluble Ag was not altered by DR5 cross-linking (Fig. 7A). In addition, OVA-coupled beads induced DC cross-priming to OT-I T cells, but DR5 did not reduce cross-priming of the bead Ags (Fig. 7B). These data indicate that DR5-mediated inhibition of Ags cross-priming was restricted to tumor cell-associated Ags.
FIGURE 7. DR5 cross-linking did not inhibit DC cross-priming of soluble OVA and OVA-coupled latex beads.
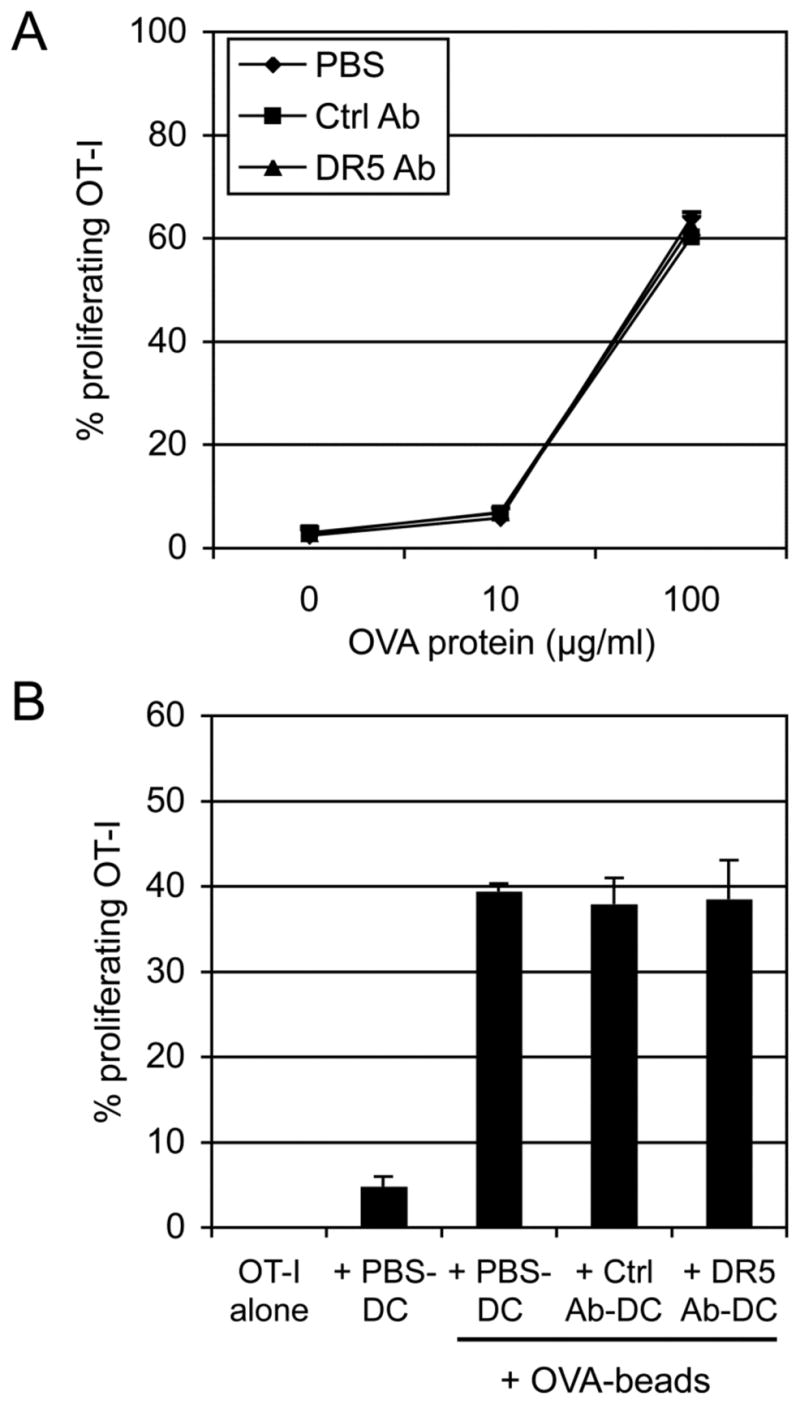
(A) CD11c+ BM-DCs (5 × 105) were cultured in control mAbs or anti-DR5 mAbs-coated 24-well plates or in control wells (PBS). The cells (2 × 104) were then cocultured for 2.5 days with indicated dose of soluble OVA protein and CFSE-labeled OT-I T cells (5 × 104). (B) OVA-coupled beads were cocultured for 4 days with the DCs and CFSE-labeled OT-I T cells. Numbers represent percentage of proliferating CD8β+ T cells. Cumulative data from two independent experiments are shown as mean ± SEM.
Arginase-1 is involved in DR5-mediated inhibition of DC cross-priming
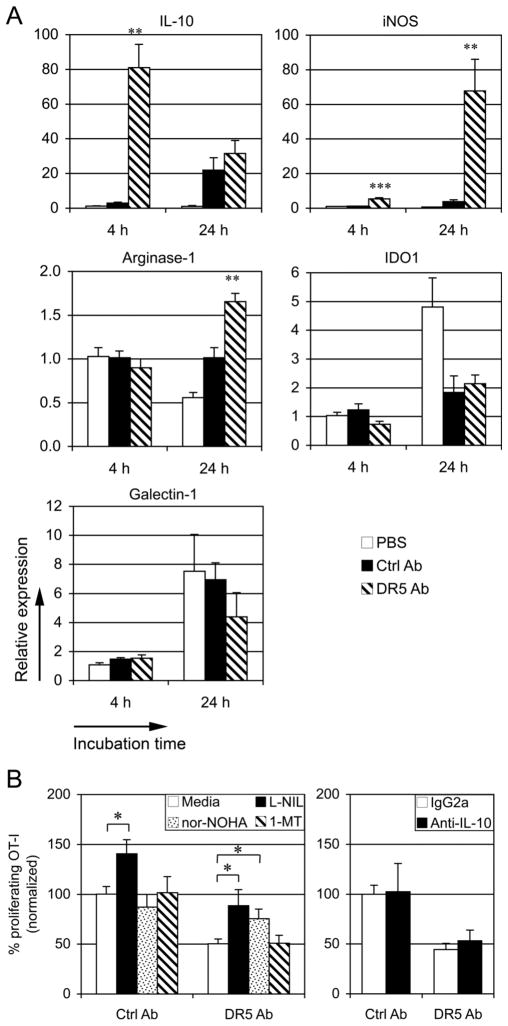
The tumor microenvironment is abundant in molecules suppressing differentiation and function of both DCs and T cells, including vascular endothelial growth factor, IL-6, IL-10, TGF-β, M-CSF, arginase, IDO, PGE2, cyclooxygenase-2, and NOS (25, 26). Because interaction of TRAIL and TRAIL receptors has been shown to regulate production of cytokines and chemokines in an NF-κB-dependent manner (27, 28), it is possible that the suppressive cytokines and chemokines were induced by cross-linking of anti-DR5 mAbs and involved in the inhibitory effects of DC cross-priming as secondary factors. As shown in Fig 8A,mRNA expression of IL-10, iNOS, and arginase-1 in BM-DCs was upregulated in response to cross-linking of anti-DR5 mAbs. The upregulation of IL-10 in DCs was induced by DR5 at an early time point (4 hr), whereas iNOS and arginase-1 were induced after 24 hr. Cross-linking with anti-DR5 mAbs on DCs did not affect the mRNA expression of IDO1 and galectin-1 (Fig. 8A). To assess whether the inhibitory molecules were involved in the DR5-mediated inhibition of DC cross-priming, experiments were carried out to determine whether inhibitors specific for these molecules affected DC cross-presentation. The results showed that the iNOS inhibitor L-NIL significantly enhanced both Ctrl Ab-treated DC and anti-DR5-treated DC cross-priming (Fig. 8B). On the other hand, the arginase inhibitor nor-NOHA did not affect Ctrl Ab-treated DC cross-priming, but significantly increased anti-DR5-treated DC cross-priming (Fig. 8B). The IDO inhibitor 1-MT and the anti-IL-10 blocking Abs had no effect on the DC cross-priming (Fig. 8B). Taken together, these data suggest that DR5-mediated induction of ariginase-1 negatively regulate DC cross-priming of tumor associated Ags to CD8+ T cells.
FIGURE 8. Ariginase-1 is involved in DR5-mediated inhibition of DC cross-priming.
(A) CD11c+ BM-DCs (5 × 104) were cultured for 4 hr and 24 hr in control mAbs or anti-DR5 mAbs-coated 96-well plates or in control wells (PBS). The expression of indicated genes were quantified by real-time RT-PCR (PBS at 4 hr = 1). Cumulative data from two independent experiments are shown as mean ± SEM. (B) CFSE-labeled OVA-specific OT-I T cells were cocultured with control mAbs- or anti-DR5 mAbs-cross-linked BM-DCs and γ-ray irradiated P815/tOVA in the presence of inhibitors, including 50 μM L-NIL, 50 μM nor-NOHA, 200 μM 1-MT, media control, or in the presence of blocking mAbs against IL-10 (10 μg/ml) or control mAbs. After 4 days, proliferation of CD8β+ T cells was determined by flow cytometry (Media or IgG2a = 100%). Cumulative data from three independent experiments are shown as mean ± SEM. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Discussion
The data presented in this study demonstrate that freshly isolated NK cells inhibit DC cross-priming but not direct priming in a TRAIL/DR5-dependent manner. NK cell-derived TRAIL but not perforin was the key mechanism for inhibition of DC-mediated cross-priming both in vitro and in vivo. Consistent with these data, the TRAIL receptor DR5-deficient DCs were less susceptible to NK-mediated inhibition on DC cross-priming. In addition, cross-linking with anti-DR5 mAbs attenuated the capability of DC cross-priming by reducing phagocytosis and through the induction of arginase.
NK cells have been shown to be important not only in elimination of tumors and virus-infected cells but also in modulation of adaptive immunity. One of the mechanisms that NK cells regulate adaptive immunity is through cross-talk with DCs. DCs can activate NK cells via production of IL-12, IL-15, type I interferons (IFN-α/β) (10, 11). Activated NK cells kill tumor cells via perforin and/or death receptor-dependent manner thus accelerating antigen release (29, 30). At the same time, TNF-α and IFN-γ production by activated NK cells and cell-cell contacts promote effective maturation of DCs (7, 21). In some cases, NK cells may limit DC activity by killing immature DCs through either TRAIL-mediated or NKp30-mediated mechanism (12, 31). Another possible regulatory role played by NK cells is mediated through the interaction between the class Ib MHC molecule Qa-1-Qdm on activated T cells and CD94/NKG2A inhibitory receptors expressed by NK cells in some autoimmune disease models, such as experimental autoimmune encephalomyelitis (32).
Activated, but not resting, human NK cells have been shown to be capable of killing immature autologous DCs at a high NK/DC ratio (21). Hayakawa et al. showed that freshly purified hepatic NK cells mediated lysis of immature DCs in vitro in a TRAIL-dependent manner, although data suggest that perforin may be partially involved in the elimination (12). In their study, hepatic NK cells were used that constitutively express TRAIL (12, 20). In this study, purified splenic NK cells were used, and these resting splenic NK cells do not express TRAIL. After coculture with DCs, TRAIL expression was induced (Table I). In addition, using OVA peptide-pulsed DCs, we demonstrate that as long as OVA peptide-MHC complexes were expressed on DCs, NK cells did not inhibit DC-induced T cell proliferation, indicating that freshly isolated, non-activated NK cells do not kill DCs. Moreover, in two tumor models in vivo, we demonstrate that NK cells inhibited cross-priming of tumor Ags in the DLN.
Multiple factors can affect DC cross-priming. Molecules that stimulate DCs, such as CD40 ligand and IFN-α/β, have been shown to enhance cross-priming (5, 33). The immunogenic properties of antigens might also affect DC cross-priming of T cells (34, 35). In addition, another study suggests that activated tumor-infiltrating NK cells are crucial for cross-priming of B16 melanoma cells by TLR9-activated plasmacytoid DCs (36). In our experiments, less-immunogenic RMA, B16F10 and P815 cells were used, and these tumor cells are rather resistant to primary resting NK cells. The lack of TLR signals may account for the discrepancy of results using different models. TLR activation may cause regression of tumors by increasing vascular permeability and by recruiting leukocytes, resulting in tumor lysis by infiltrating NK and cytotoxic T cells (37). Furthermore, DC/NK contact is essential for the induction of polyI:C-mediated antitumor NK cells (38). However under physiological conditions, the function of resting NK cells in anti-tumor immunity remains to be elucidated. Present study indicates the unique homeostasis that resting NK cells negatively regulate establishment of tumor immunity by immature DCs.
There are at least two distinct pathways by which the DC cross-priming occurs: the phagosome-to-cytosol pathway and the vacuolar pathway (Fig. 9) (5). It is possible that NK cells may affect DC cross-priming during steps from antigen transfer from the phagosome to cytosol, antigen degradation by the proteasome, peptide transport by TAP and peptide loading in the ER (Fig. 9). Presentation of native OVA-coupled beads is TAP-dependent and implicated in the phagosome-to-cytosol pathway (39), whereas soluble Ag is found to be cross-presented in the vacuolar pathway (40), and both pathway contributed to the presentation of some Ags such as polylactic-coglycolic acid-OVA (41, 42). DR5 did not inhibit cross-priming of soluble OVA as well as OVA-coupled beads in this study, indicating that DR5-mediated inhibition of cross-priming is restricted to tumor cell-associated Ags. We showed that DR5−/− DCs are resistant to NK cell inhibition of DC cross-priming in comparison to wt B6 DCs and that DR5-cross-linking reduced generation of Kb-OVA complex on DCs, further supporting the critical role of TRAIL/DR5 regulating DC cross-priming of tumor Ags. Although T cells may express DR5, which could lead to blockade of T cell cycle progression or T cell death (43, 44), and T cell proliferation was used as readout for DC cross-priming, NK cells did not inhibit peptide-pulsed DC-induced or anti-CD3 mAb-induced direct T cell proliferation indicates that NK cells did not directly affect T cell proliferation, as has been previously reported (16).
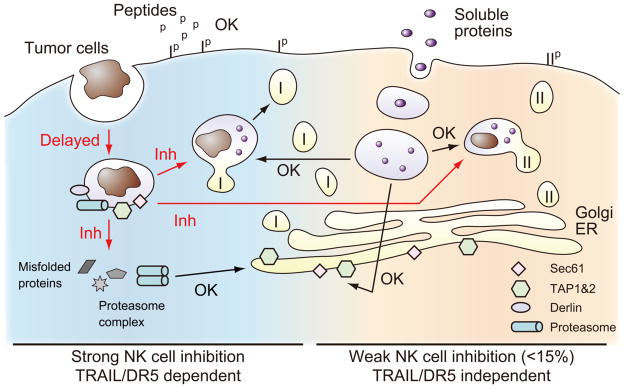
FIGURE 9. Summary of NK cell-mediated effects on Ag presentation by DCs.
NK cell effects on presentation of peptides, soluble proteins, and tumor cells has been evaluated. Key molecules involved in cross-presentation are indicated (Tap, Derlin, Sec61 and proteasome). The pathways inhibited by NK cells are indicated as red arrows “Inh”, and non-altered pathways as black arrows “OK”. MHC class I is indicated as “I”, and MHC class II as “II”. The exact location of proteins and vesicles are only approximate, and they are meant to show general pathways. See text for details.
DR5 can function as a negative regulator of DCs rather than inducing apoptosis of DCs (14). DR5−/− DCs producedmore IL-12 and TNF-α upon stimulation by TLR agonists. The role of DR5 in TLR signaling is likely due to the regulation of IκB-α degradation or stability, leading the induction of immunoregulatory molecules. In this study, enhanced expression of IL-10, iNOS and arginase-1 in DCs by DR5-cross-linking indicates that DR5 pathway can create immunosuppressive circumstances that protect the tumors from immune attack. The imbalances of immunological reactions in the tumor microenvironment may result in immune tolerization (25). So far, roles of arginase-1 in DC cross-priming have not been well defined, although this molecule has been demonstrated to inhibit T cell effector functions (45). Our data showed that the reduced cross-priming by DR5-cross-linking was specifically reversed by the arginase-1 inhibitor nor-NOHA. Thus, these data support the concept that activation of arginase-1 downstream of DR5 leads to suppression of DC-cross-priming for tumor cells.
Tumor cells are “altered self” (46). The immune system can efficiently recognize immunogenic tumor cells when a proinflammatory environment is induced (2, 47). However, some less-immunogenic tumor cells can evade immunosurveillance via weak self-antigen expression or local immune suppressive mechanisms (46). Without proinflammatory signals, a NK/DC positive feedback loop may not be efficiently initiated, so that tumor-induced tolerance may prevail (8, 48). The detailed mechanisms for DC cross-tolerance induction remain unclear, and DC-derived signals are likely a key step in the initiation of immunity or maintenance of tolerance. Under normal circumstances, tissues are being remodeled, cell death occurs, and macrophages and other leukocytes repair and help maintain tissue architecture and function. DCs are constantly bringing antigens into LNs derived from self-antigens, which should not induce an immune response in the absence of an inflammatory trigger. The data presented in this study suggest that DCs may use NK cells to help regulate this process. Tumor cells can take advantage of this NK cell-mediated inhibition of DC cross-priming and use it to prevent or delay the induction of adaptive immunity. Thus, the data may reflect a normal immune homeostatic mechanism that regulates the initiation of adaptive immunity within LNs. Our results support the hypothesis that NK cells negative regulate DC-mediated cross-priming through TRAIL/DR5. These data provide new insights into NK-mediated regulation of adaptive immunity.
Supplementary Material
Acknowledgments
We thank Drs. Jacques Peschon (Amgen, Seattle, WA) and Tak Mak (the University Health Network, Toronto, Canada) for providing TRAIL−/− and DR5−/− mice, respectively; Dr. Randolph J. Noelle for providing OT-II and H-Y transgenic mice; Alice Givan and Gary Ward for assistance with flow cytometry; and the staff of the Animal Resources Center for assistance with animal care.
Abbreviation used in this paper
- DC
dendritic cell
- DLN
draining lymph node
- NOS
nitric oxide synthase
Footnotes
This work was supported by grants from the National Institutes of Health (CA101748), a special fellowship (3187-07) from the Leukemia and Lymphoma Society, and funds from the department of Microbiology and Immunology.
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
- 1.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 2.Degli-Esposti MA, Smyth MJ. Close encounters of different kinds: dendritic cells and NK cells take centre stage. Nat Rev Immunol. 2005;5:112–124. doi: 10.1038/nri1549. [DOI] [PubMed] [Google Scholar]
- 3.Walzer T, Dalod M, Robbins SH, Zitvogel L, Vivier E. Natural-killer cells and dendritic cells: “l’union fait la force”. Blood. 2005;106:2252–2258. doi: 10.1182/blood-2005-03-1154. [DOI] [PubMed] [Google Scholar]
- 4.Tacken PJ, I, de Vries J, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007;7:790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- 5.Shen L, Rock KL. Priming of T cells by exogenous antigen cross-presented on MHC class I molecules. Curr Opin Immunol. 2006;18:85–91. doi: 10.1016/j.coi.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Munz C, Steinman RM, Fujii S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J Exp Med. 2005;202:203–207. doi: 10.1084/jem.20050810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327–333. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zitvogel L, Terme M, Borg C, Trinchieri G. Dendritic cell-NK cell crosstalk: regulation and physiopathology. Curr Top Microbiol Immunol. 2006;298:157–174. doi: 10.1007/3-540-27743-9_8. [DOI] [PubMed] [Google Scholar]
- 9.Ferlazzo G, Munz C. NK cell compartments and their activation by dendritic cells. J Immunol. 2004;172:1333–1339. doi: 10.4049/jimmunol.172.3.1333. [DOI] [PubMed] [Google Scholar]
- 10.Mortier E, Woo T, Advincula R, Gozalo S, Ma A. IL-15Ralpha chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J Exp Med. 2008;205:1213–1225. doi: 10.1084/jem.20071913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayakawa Y, Screpanti V, Yagita H, Grandien A, Ljunggren HG, Smyth MJ, Chambers BJ. NK cell TRAIL eliminates immature dendritic cells in vivo and limits dendritic cell vaccination efficacy. J Immunol. 2004;172:123–129. doi: 10.4049/jimmunol.172.1.123. [DOI] [PubMed] [Google Scholar]
- 13.Finnberg N, Gruber JJ, Fei P, Rudolph D, Bric A, Kim SH, Burns TF, Ajuha H, Page R, Wu GS, Chen Y, McKenna WG, Bernhard E, Lowe S, Mak T, El-Deiry WS. DR5 knockout mice are compromised in radiation-induced apoptosis. Mol Cell Biol. 2005;25:2000–2013. doi: 10.1128/MCB.25.5.2000-2013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diehl GE, Yue HH, Hsieh K, Kuang AA, Ho M, Morici LA, Lenz LL, Cado D, Riley LW, Winoto A. TRAIL-R as a negative regulator of innate immune cell responses. Immunity. 2004;21:877–889. doi: 10.1016/j.immuni.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Falschlehner C, Schaefer U, Walczak H. Following TRAIL’s path in the immune system. Immunology. 2009;127:145–154. doi: 10.1111/j.1365-2567.2009.03058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barber MA, Zhang T, Gagne BA, Sentman CL. NK cells negatively regulate antigen presentation and tumor-specific CTLs in a syngeneic lymphoma model. J Immunol. 2007;178:6140–6147. doi: 10.4049/jimmunol.178.10.6140. [DOI] [PubMed] [Google Scholar]
- 17.Shastri N, Gonzalez F. Endogenous generation and presentation of the ovalbumin peptide/Kb complex to T cells. J Immunol. 1993;150:2724–2736. [PubMed] [Google Scholar]
- 18.Savina A, Vargas P, Guermonprez P, Lennon AM, Amigorena S. Measuring pH, ROS production, maturation, and degradation in dendritic cell phagosomes using cytofluorometry-based assays. Methods Mol Biol. 2010;595:383–402. doi: 10.1007/978-1-60761-421-0_25. [DOI] [PubMed] [Google Scholar]
- 19.Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ, Smyth MJ. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J Immunol. 2002;168:1356–1361. doi: 10.4049/jimmunol.168.3.1356. [DOI] [PubMed] [Google Scholar]
- 20.Takeda K, Cretney E, Hayakawa Y, Ota T, Akiba H, Ogasawara K, Yagita H, Kinoshita K, Okumura K, Smyth MJ. TRAIL identifies immature natural killer cells in newborn mice and adult mouse liver. Blood. 2005;105:2082–2089. doi: 10.1182/blood-2004-08-3262. [DOI] [PubMed] [Google Scholar]
- 21.Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002;195:335–341. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burgdorf S, Kautz A, Bohnert V, Knolle PA, Kurts C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science. 2007;316:612–616. doi: 10.1126/science.1137971. [DOI] [PubMed] [Google Scholar]
- 23.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, Steinman RM, Nussenzweig MC. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 24.Kurts C, Robinson BW, Knolle PA. Cross-priming in health and disease. Nat Rev Immunol. 2010;10:403–414. doi: 10.1038/nri2780. [DOI] [PubMed] [Google Scholar]
- 25.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 26.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang W, Wang W, Zhang Y, Liu S, Liu Y, Zheng D. TRAIL receptor mediates inflammatory cytokine release in an NF-kappaB-dependent manner. Cell Res. 2009;19:758–767. doi: 10.1038/cr.2009.57. [DOI] [PubMed] [Google Scholar]
- 28.Leverkus M, Sprick MR, Wachter T, Denk A, Brocker EB, Walczak H, Neumann M. TRAIL-induced apoptosis and gene induction in HaCaT keratinocytes: differential contribution of TRAIL receptors 1 and 2. J Invest Dermatol. 2003;121:149–155. doi: 10.1046/j.1523-1747.2003.12332.x. [DOI] [PubMed] [Google Scholar]
- 29.Zamai L, Ahmad M, Bennett IM, Azzoni L, Alnemri ES, Perussia B. Natural killer (NK) cell-mediated cytotoxicity: differential use of TRAIL and Fas ligand by immature and mature primary human NK cells. J Exp Med. 1998;188:2375–2380. doi: 10.1084/jem.188.12.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smyth MJ, Takeda K, Hayakawa Y, Peschon JJ, van den Brink MR, Yagita H. Nature’s TRAIL--on a path to cancer immunotherapy. Immunity. 2003;18:1–6. doi: 10.1016/s1074-7613(02)00502-2. [DOI] [PubMed] [Google Scholar]
- 31.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Munz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195:343–351. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu L, Ikizawa K, Hu D, Werneck MB, Wucherpfennig KW, Cantor H. Regulation of activated CD4+ T cells by NK cells via the Qa-1-NKG2A inhibitory pathway. Immunity. 2007;26:593–604. doi: 10.1016/j.immuni.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basta S, Alatery A. The cross-priming pathway: a portrait of an intricate immune system. Scand J Immunol. 2007;65:311–319. doi: 10.1111/j.1365-3083.2007.01909.x. [DOI] [PubMed] [Google Scholar]
- 34.Guan H, Moretto M, Bzik DJ, Gigley J, Khan IA. NK cells enhance dendritic cell response against parasite antigens via NKG2D pathway. J Immunol. 2007;179:590–596. doi: 10.4049/jimmunol.179.1.590. [DOI] [PubMed] [Google Scholar]
- 35.Kang HK, Lee HY, Lee YN, Jo EJ, Kim JI, Aosai F, Yano A, Kwak JY, Bae YS. Toxoplasma gondii-derived heat shock protein 70 stimulates the maturation of human monocyte-derived dendritic cells. Biochem Biophys Res Commun. 2004;322:899–904. doi: 10.1016/j.bbrc.2004.07.205. [DOI] [PubMed] [Google Scholar]
- 36.Liu C, Lou Y, Lizee G, Qin H, Liu S, Rabinovich B, Kim GJ, Wang YH, Ye Y, Sikora AG, Overwijk WW, Liu YJ, Wang G, Hwu P. Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice. J Clin Invest. 2008;118:1165–1175. doi: 10.1172/JCI33583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9:57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 38.Seya T, Shime H, Ebihara T, Oshiumi H, Matsumoto M. Pattern recognition receptors of innate immunity and their application to tumor immunotherapy. Cancer Sci. 2010;101:313–320. doi: 10.1111/j.1349-7006.2009.01442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wick MJ, Pfeifer JD. Major histocompatibility complex class I presentation of ovalbumin peptide 257–264 from exogenous sources: protein context influences the degree of TAP-independent presentation. Eur J Immunol. 1996;26:2790–2799. doi: 10.1002/eji.1830261135. [DOI] [PubMed] [Google Scholar]
- 40.Chen L, Jondal M. Alternative processing for MHC class I presentation by immature and CpG-activated dendritic cells. Eur J Immunol. 2004;34:952–960. doi: 10.1002/eji.200324359. [DOI] [PubMed] [Google Scholar]
- 41.Shen L, Sigal LJ, Boes M, Rock KL. Important role of cathepsin S in generating peptides for TAP-independent MHC class I crosspresentation in vivo. Immunity. 2004;21:155–165. doi: 10.1016/j.immuni.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Rock KL, Shen L. Cross-presentation: underlying mechanisms and role in immune surveillance. Immunol Rev. 2005;207:166–183. doi: 10.1111/j.0105-2896.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- 43.Song K, Chen Y, Goke R, Wilmen A, Seidel C, Goke A, Hilliard B. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is an inhibitor of autoimmune inflammation and cell cycle progression. J Exp Med. 2000;191:1095–1104. doi: 10.1084/jem.191.7.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 45.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 46.Houghton AN, Guevara-Patino JA. Immune recognition of self in immunity against cancer. J Clin Invest. 2004;114:468–471. doi: 10.1172/JCI22685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Avogadri F, Martinoli C, Petrovska L, Chiodoni C, Transidico P, Bronte V, Longhi R, Colombo MP, Dougan G, Rescigno M. Cancer immunotherapy based on killing of Salmonella-infected tumor cells. Cancer Res. 2005;65:3920–3927. doi: 10.1158/0008-5472.CAN-04-3002. [DOI] [PubMed] [Google Scholar]
- 48.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.